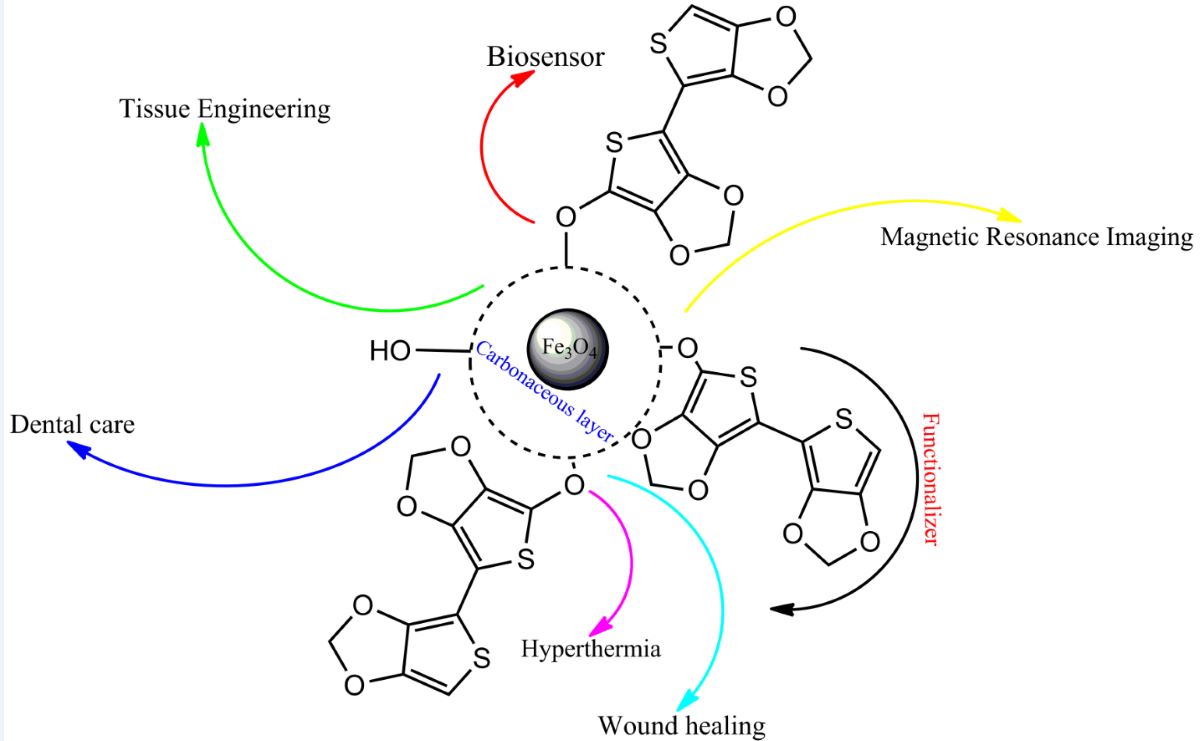
1. Introduction
In recent times, the realm of material science has witnessed a burgeoning interest in carbonaceous materials for their unique properties and diverse applications. Carbon as an elemental building block of life[
1], and among the most abundant element on the earth’s surface[
2], has gained huge attention for its versatility and adaptability. In the field of medicine, the emergence of carbonaceous materials has revolutionized the landscape of diagnostics, drug delivery, bio-marking, and tissue engineering. Carbonaceous materials, encompassing a broad array of structures such as carbon nanotubes, carbon dots, graphene, etc., have been harnessed for their exceptional properties which include biocompatibility, high surface area, and unique optical and electronic characteristics.[
3,
4,
5] The integration of carbonaceous materials in medical technologies opens up new frontier for precision medicine, enabling the development of innovative approaches that promise to enhance patient outcomes and the quality of healthcare. As a result of this, many researchers have been attracted to this interesting subject area.
Chella
et al., synthesized a composite carbonaceous material called MnFe
2O
4-graphene via solvothermal method for the investigation of its adsorption and antimicrobial properties. The results showed that the combination of graphene carbonaceous material and MnFe
2O
4 has a good adsorption efficiency for toxic heavy metal ions and high antimicrobial effects.[
6] In a related work involving a catalyzed reaction under visible light; Feng et al., synthesized an activated carbon through a simple calcination technique and used it to make a composite with g-C
3N
4 in the form of “activated carbon/g-C
3N
4”. Their result showed that the composite showed higher catalytic performance compare to g-C
3N
4 without carbonaceous material incorporated in it, indicating that the activated carbon material is responsible for the enhanced catalytic activity of the synthesized material.[
7] Magnetic biochar was equally found effective for toxic heavy metal removal as synthesized by Reddy and Lee.[
8]
The purpose of this review is to provide a comprehensive overview of the synthesis and characterization of carbonaceous materials with a specific focus on their application in the field of medicine. This review aims to throw light into the various synthesis techniques, such as chemical vapor deposition (CVD) and chemical reduction methods, in addition to characterization techniques employed to assess the structural, morphological, and surface properties of the materials. Additionally, it explores the critical aspect of biocompatibility and safety evaluation, shedding light on the intricate balance between harnessing the unique properties of carbonaceous materials and ensuring their safe application in medical field. This has a vital importance since the significance of this review lies in its ability to offer insights into the past, present, and future of carbonaceous materials in medicine. It equally explores their historical development, current medical applications, and emerging trends. Furthermore, we will discuss the challenges and limitations that researchers and medical practitioners face when working with carbonaceous materials, providing a foundation for the development and future directions in the use of different carbonaceous materials in the field of medicine.
1.1. The History of Carbonaceous Materials (CMs)
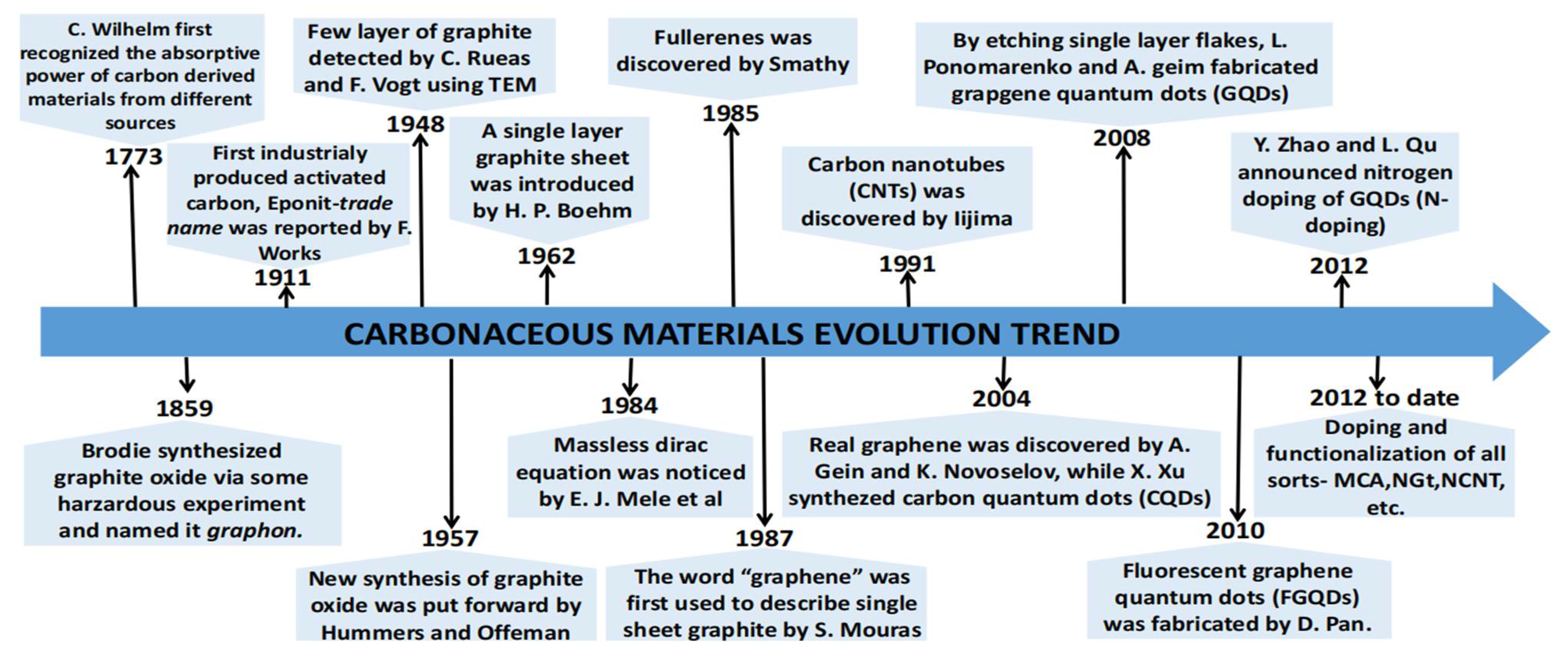
Carbon has been widely researched since the 19th century. The development of carbonaceous materials to date is shown in
Figure 1. In the year 1773, Car Wilhelm, a Pomerania Chemist from Baltic coast of Europe under Swedish control, first recognized the absorptive power of carbon-derived materials from different sources[
9], this led to commercial production of activated carbon under the trade name
Eponit by Fanto in Austria[
10]. About 1948, Ruess and Vogt[
11] successfully detected a few layers of graphite via transmission electron microscopy (TEM). In 1957, Hummers and Offeman[
12] developed a new, safe and efficient method of preparing graphite oxide when he synthesized the compound via the usual poisonous experiment[
13]. The idea of a single layer graphite sheet as described by Boehm[
14] in 1962 and by some theoretical physicists[
15], having a massless Dirac equation in 1984 was considered incredible in that era. As carbon materials evolve, the fullerene having a typical football-shape was discovered by Smally[
16] in 1985, which was a laudable breakthrough in the scientific world, this fetched him a Nobel Prize award in 1996. This was the first carbon allotropy which inspired scientists around the world in exploiting carbon materials. The word ‘graphene’ was introduced in 1987 by Mouras[
17] to represent single sheets of graphite. By the turn of 1911, another carbon allotropy called carbon nanotubes (CNTs) was later discovered by Lijima[
18]. More than a decade later, a monolayer graphene was developed in 2004 by Geim and Novoslov[
19] in the Manchester University using a Scotch tape technique. Their work paved way to many exciting scientific research into the two dimensional material, which could offer many novel applications. A Nobel Prize was awarded to Geim and Novoslov in 2010 for their experimental work on graphene. To date, the different graphitic forms, such as 0D fullerene, 1D carbon nanotubes, 2D graphene and 3D graphite, magnetic carbonaceous materials (MCs), N-doped graphite (NGt), N-doped graphene (NG), and N-doped carbon nanotube (NCNT)[
20] had successfully been discovered.
1.1.1. Development of Carbonaceous Materials in Medicine
The historical development of carbonaceous materials in medicine is a fascinating journey that has evolved over several decades. While the use of carbon-based materials in medicine dates to ancient times, the deliberate exploration and development for medical applications gained momentum in the 20
th century. One of the simplest and earliest forms of carbonaceous materials engaged for medicinal purposes for centuries is charcoal[
21]. It was utilized for its adsorptive properties, often to treat digestive tract ailments by adsorbing toxins and gases. This practice date back to ancient times as far back as ancient Greeks and Egypts[
22]. In the writings of the ancient Greek physician Hippocrates, often referred to as the father of modern medicine, he described the use of charcoal as a remedy for odors, wounds, and ulcers[
23]. Another ancient reference to the use of charcoal dated back to around 1500 BC found in the Ebers Paryrus, an ancient Egyptian medical text, where the use of charcoal to treat a variety of medical conditions, including infections and other gastrointestinal ailments was reported[
21]. The late 19th century marked the discovery and synthesis of novel carbon-based materials, such as carbon nanotubes and fullerenes (buckyballs). These materials exhibited remarkable properties, including high surface area, electrical conductivity, and biocompatibility[
24]. Researchers began exploring the potential of carbon nanotubes in drug delivery systems, scaffolds for tissue engineering, and as sensing devices. Early to mid-20th century saw the initial applications of activated carbon for medical purposes. Activated carbon was employed to address poisonings and overdoses by adsorbing toxins in the digestive system[
25,
26] .The development of activated carbon filters for gas masks during World War I marked a significant milestone in protecting soldiers from chemical/gas weapons [
27,
28]. Activated carbon was first used in medicine in the 1930s and 1940s to treat poisoning and drug overdoses [
25,
26]. This was possible because of the high porosity of activated carbon e.g. sugarcane bagasse-based activated carbon(SCBAC) as presented in the SEM image[
29] in
Figure 2. Another landmark development recorded in the 20
th century was the development of carbon-based implants for use in orthopedic surgery. These implants composed of materials such as carbon-carbon. Composite and carbon-fiber reinforced polymer, have been effectively used to repair injured bones or joints[
30].
The late twentieth and early twenty-first century saw a surge in research on carbonaceous materials in medicine, particularly in the field of nanomedicine. This led to the emergence of Graphene, another carbon allotrope with unique properties for various applications. Also discovered are Carbon quantum dots and Nanodots which gained attention as fluorescent imaging agents in medical diagnostics[
31,
32]. Carbonaceous materials played a significant role in the development of biosensors for detecting biomolecules and pathogens with high sensitivity and specificity[
33,
34]. The concept of theranostics (combination of therapy and diagnostics) using multifunctional nanoparticles, became a reality with the integration of carbonaceous materials[
35]. The current and future prospects of carbonaceous materials, such as carbon nanotubes, nanodiamonds and graphene, are in their being explored for targeted drug delivery, personalized medicine, and the development of novel cancer therapies[
36,
37,
38]. Carbon-based materials are integral to the development of wearable and implantable medical devices, offering enhanced biocompatibility and improved performance[
39]. The historical evolution of carbonaceous materials in medicine shows a remarkable move from conventional uses of charcoal to the cutting-edge applications of carbon nanotubes and graphene in nanomedicine. As our comprehension of these materials continues to expand, so does their potential to transform the field of medicine by enabling precise diagnostics, innovative therapies, and improved patient care. Ongoing research and development in this area hold the promise of addressing some of the most challenging medical issues prevalent today.
1.2. Synthesis and Characterization of Carbonaceous Materials for Medical Applications
Carbonaceous materials encompass a wide range of carbon-based materials exhibiting various structures, each with unique properties and characteristics. Understanding the synthesis, properties and characteristics of these carbonaceous materials is fundamental to their integration into medical applications. These materials have a wide range of properties and applications resulting from the diversity of carbon allotropes and the ability to form complex molecular structures[
40]. Understanding the different types of CMs and how they differ from each other, as well as their various structures would further enhance their specialized applications in the field of medicine[
41].
1.3. Various Synthesis Approach of Carbonaceous Materials
1.3.1. Chemical Vapor Deposition (CVD)
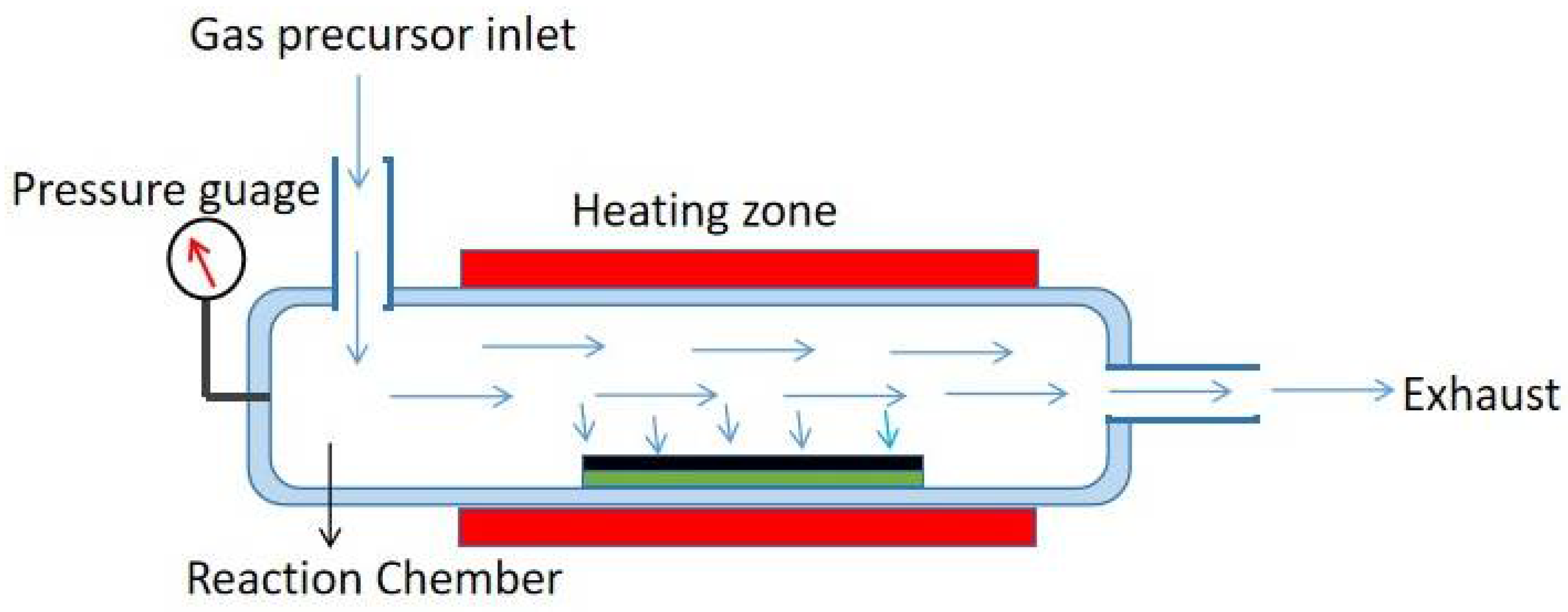
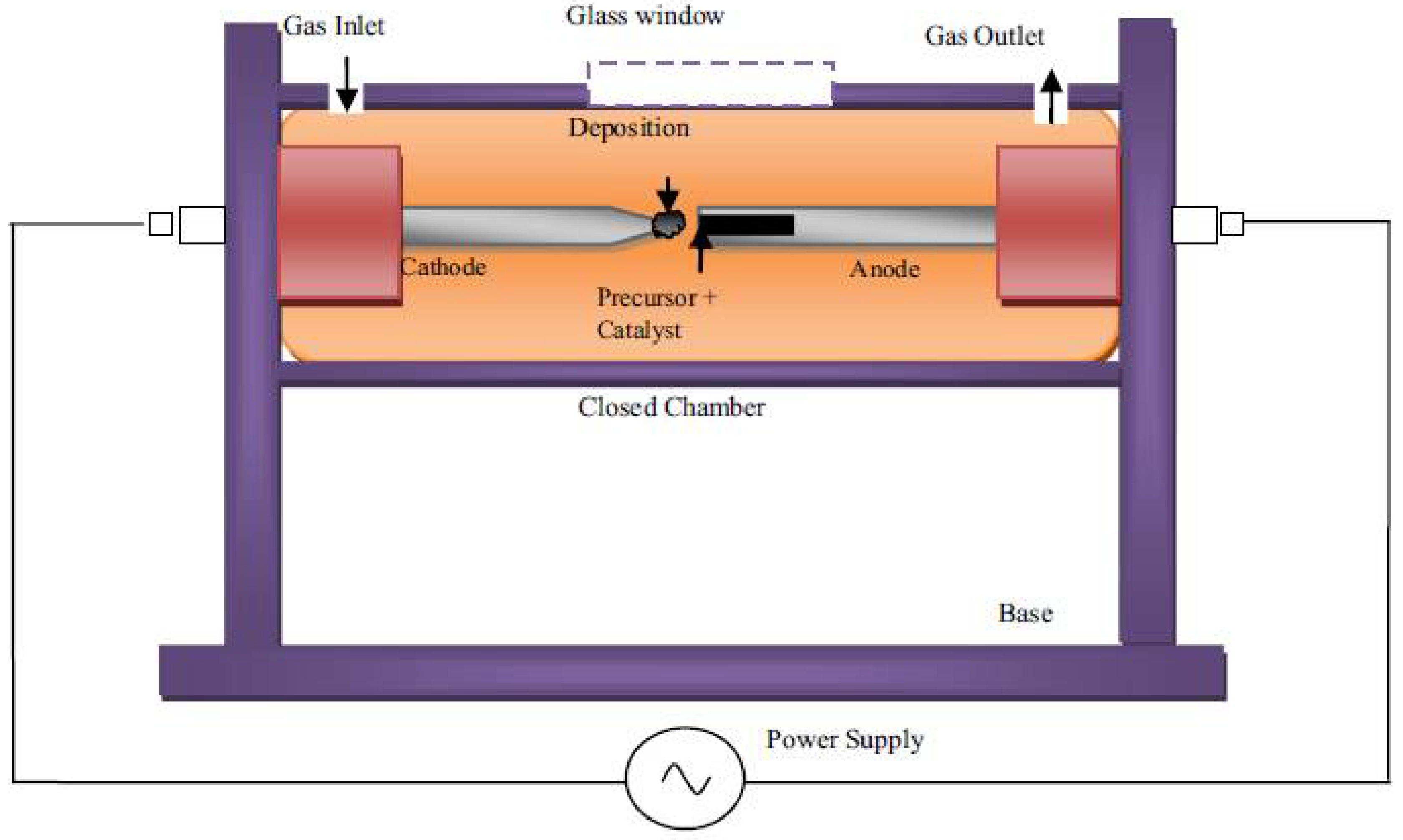
Chemical Vapor Deposition (CVD) is a versatile approach for producing a wide range of carbon-based materials, including carbon nanotubes (CNTs), graphene, carbon fibers, and other carbon nanostructures.
Figure 3 showed a schematic illustration of CVD method. This approach involves the deposition of carbon atoms on the surface of a substrate from a gaseous precursor. It allows for precise control over material structure, composition, and morphology. This usually occurs at high temperature in the presence of a catalyst. CVD is widely used for the controlled growth of carbon nanotubes (CNTs) and graphene[
42]. Precursor selection CVD produces carbonaceous materials by decomposing gaseous precursors onto a substrate. These precursors can be hydrocarbons like methane, ethylene, or acetylene, as well as other carbon-containing gases like carbon monoxide. The properties of the resulting carbon material are determined by the precursor used[
43]. The principles of CVD for carbonaceous materials synthesis largely hinges on substrate choice. The substrate on which the carbonaceous material grows has a significant impact on the structure and qualities of the resulting material. Silicon, quartz, metals, and different carbon substrates are examples of common substrates[
44]. Reaction conditions such as controlling temperature, pressure, gas flow rates, and other parameters are critical to obtaining the appropriate development of carbonaceous materials. Different reaction circumstances can cause differences in the structure, shape, and characteristics of synthesized materials[
45,
46].
Saipriya Sahu
et al, 2024, synthesized and investigated hydrogen storage capacity of carbon nano-onion by thermal CVD for the purpose of energy storage[
47]. Li and co-workers [
48] engaged direct growth on metal surfaces approach to grow graphene directly on metal surfaces like copper or nickel. This method typically involves the decomposition of hydrocarbons such as methane or ethylene at high temperatures. However, catalysed-assissted growth method used by Renal
et. al 2008, which involves using catalysts such as transition metals to facilitate graphene growth, lead to controlled growth and higher quality graphene[
49]. Researchers have explored methods to grow graphene in patterned regions, allowing for precise control over the material's spatial distribution[
50]. One of the earliest CVD methods for CNT synthesis involves arc discharge between two graphite electrodes in an inert atmosphere, this method can produce multi-walled carbon nanotubes[
51]. Recent Advances in CVD Synthesis of Carbonaceous Materials are works done by Gao, L.
et al; 2010[
52], they created an ambient pressure chemical vapor deposition (CVD) system for the rapid synthesis of high-quality graphene sheets on Cu foils. This CVD method allows for the affordable and high-throughput creation of high-quality graphene sheets, for the need of large-area graphene growth which is required for the development and production of electronic devices Li et al.[
53] demonstrated successfully the growing of large-area graphene or few-layer graphene films on metal substrate such as Ni and Cu. However, despite significant progress, challenges remain in achieving large-scale production, enhancing the uniformity, and controlling the properties of carbonaceous materials synthesized through CVD. Future research directions include exploring new precursors, creating advanced catalysts, and optimizing growth conditions to enhance the scalability, efficiency, and quality of carbonaceous materials synthesized via CVD method.
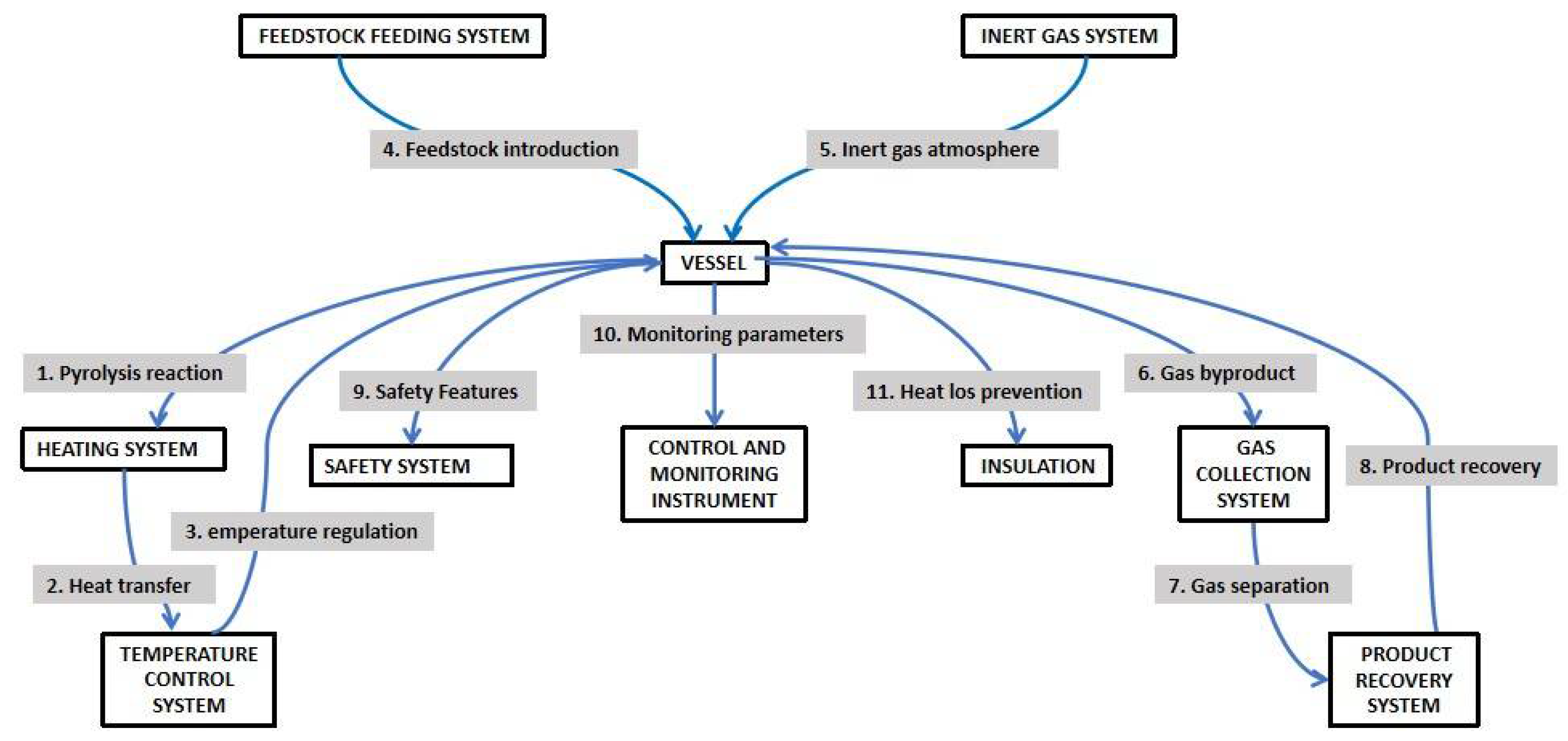
1.3.2. Chemical Reduction Methods
Carbonaceous materials can be synthesized via a variety of chemical reduction methods, including hydrothermal synthesis, solvothermal synthesis, and chemical vapor deposition (CVD) as shown in
Figure 4. It is a popular technique in research labs for synthesizing nanoparticles [
54,
55,
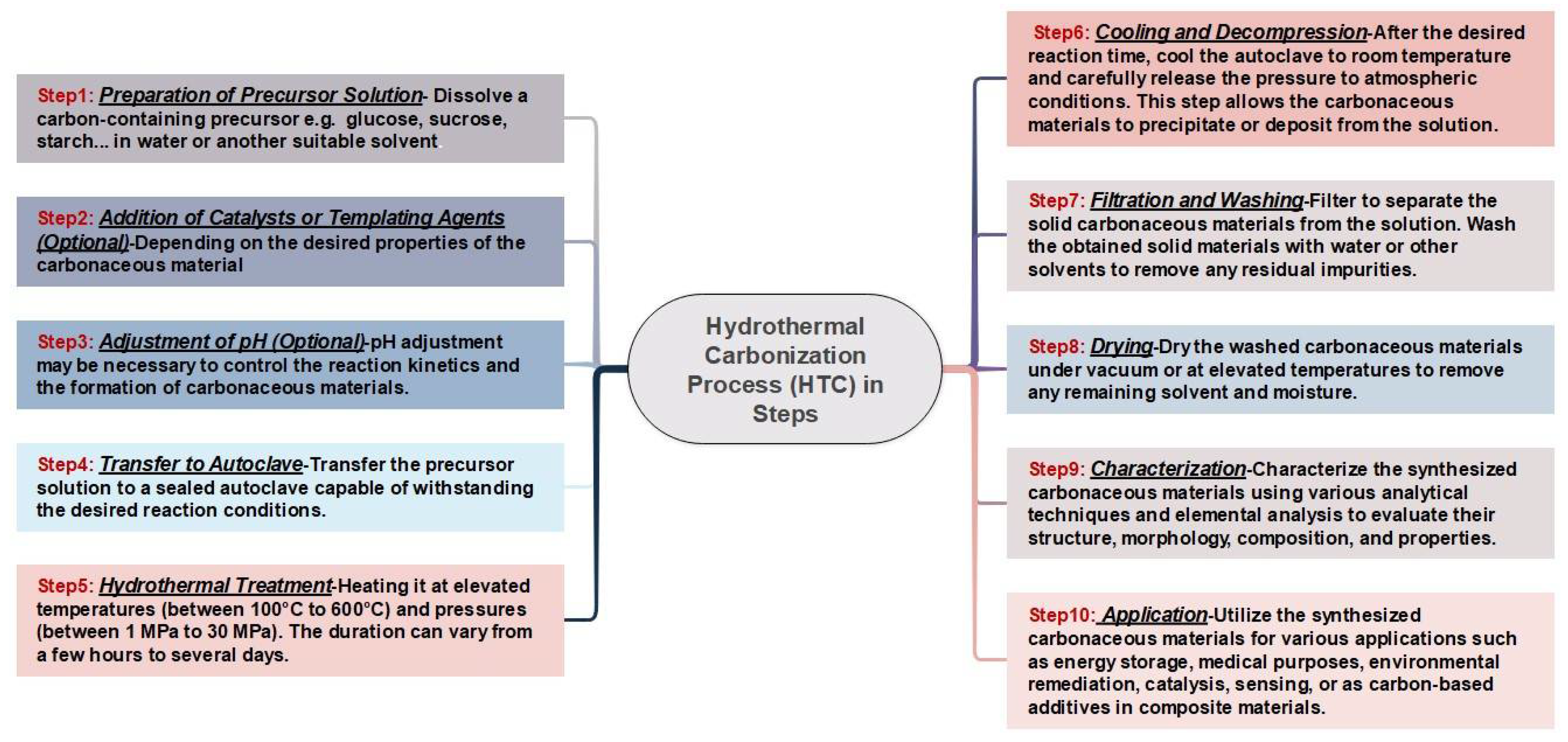
56].Because of its affordability, simplicity of usage, and capacity to regulate the nanoparticles' structural characteristics, this approach is recommended. To regulate the synthesis process, several elements are used, including solvents, reducing agents, stabilizers, and metal precursors. The final properties of the nanoparticles can be modified by varying the synthesis conditions and techniques. Hydrothermal synthesis is the reaction of precursors in a high-pressure, aqueous environment at high temperatures.
This technology allows for the creation of a variety of carbonaceous materials with precise morphology and characteristics. Temperature, pressure, and reaction time are all important factors in determining the properties of the final product[
57,
58,
59]. For a cleaner hydrothermal process, Bressi and co-workers [
57] demonstrated green synthesis and formation mechanism of Carbon Dots CDs from biomass, focusing on innovative one-pot hydrothermal and microwave processes. The reaction mechanisms hydrothermal carbonization HTC includes hydrolysis, dehydration, decarboxylation, aromatization, and re-condensation, which are simultaneous and cooperative steps. Similar to pyrolysis, raw materials, residence time, and reaction temperature all have a significant impact on the characteristics of hydrochar produced by the HTC process[
60].
On the other hand, Solvothermal synthesis involves the use of organic solvents instead of water, enabling the controlled synthesis of carbonaceous compounds at specific pressure and temperature levels as shown in
Figure 5. Benefits of this approach include more control over the kinetics of the reaction and the capacity to modify the final materials' qualities[
61].
1.3.3. Sol-Gel Processes
The sol-gel method is a versatile and frequently used approach for synthesizing a variety of materials, including carbonaceous ones. In the context of carbonaceous materials, the sol-gel process entails converting a precursor solution (sol) into a gel and then into a carbon-containing solid. This process provides exact control over the composition, structure, and characteristics of the resultant carbon material[
62].The sol-gel process is considered to be an excellent way to change substrate surfaces. The most significant advantage of the sol-gel technology is the ability to produce large surface areas and stable surfaces[
63]. A Sol-Gel process involves sol preparation, gel formation, drying, carbonization, and post treatment (optional). In the sol-gel synthesis of carbonaceous materials, the initial step is to prepare a solution containing the carbon precursors. These precursors can include organic chemicals like formaldehyde, resorcinol, and other carbon-containing substances. [
64] examined the use of biomass-derived lignin as a renewable precursor for the sol-gel synthesis of carbon compounds, establishing the viability of lignin-based precursors for sustainable carbon material production. Once prepared, the sol polymerizes or cross-links the precursor molecules to form a gel. This gelation phase results in the production of a three-dimensional network structure, which acts as the foundation for the next carbonization stage[
65]. Recent research has focused on understanding the gelation kinetics and mechanisms to customize the gel structure to the required material qualities. Konuk and workers [
66] examined how many factors, including pH and solvent content, affected the gelation process during the carbon aerogel sol-gel synthesis, offering insights into how to best optimize gel formation for improved material performance.
After gel formation, the solvent is removed from the gel by drying. Various drying processes, such as freeze-drying, air drying, or supercritical drying, can be used to modify the pore structure and surface properties of the final carbon material[
67]. Xu, Y.
et al. (2019)[
68] studied the influence of various drying methods on the porosity and electrochemical properties of carbon aerogels synthesized using the sol-gel process, emphasizing the importance of drying factors in enhancing material performance. The dried gel is then exposed to a controlled high-temperature treatment in an inert atmosphere to transform the organic components into carbon. This is the carbonization process, breakdown and removal of volatile species takes place resulting in the formation of a carbonaceous structure with desired properties[
69]. The influence of carbonization temperature and duration on the structural evolution and electrochemical properties of carbon materials synthesized via sol-gel method, have provided useful insights into the relationship between carbonization conditions and material performance[
70]. In some cases, post-treatment steps such as activation, functionalization, or doping may be carried out to further modify the properties of the carbon material according to requirement(s). demonstrated the post-synthesis alteration of carbonaceous materials synthesized using the sol-gel process through nitrogen doping and surface functionalization, resulting in increased catalytic activity for oxygen reduction reactions in fuel cells [
71].
1.3.4. Arc Discharge
The arc discharge is mostly utilized to produce carbon nanotubes (CNT) as shown in
Figure 6. It involves creating a high-current electric arc across graphite electrodes in an inert environment, which results in the creation of carbon nanotubes[
72].The principle of arc discharge technique is of the fact that when a high-current electric arc is formed between two graphite electrodes in an inert gas (usually argon or helium),the arc's extreme heat and pressure evaporates the graphite electrodes, causing the carbon atoms to condense as carbon nanotubes[
72]. It is well established that parameters such as arc voltage, arc current, electrode separation, gas pressure, and duration of the arc discharge significantly determine the yield, quality, and morphology of the synthesized carbon nanotubes[
73,
74]. Catalysts (such as transition metals like iron, cobalt, or nickel) on the electrode surface can catalyze the growth of carbon nanotubes, manipulating their chirality, diameter, and alignment [
75]. Post-synthesis treatments such as annealing, acid purification, and functionalization are finally engaged to remove impurities, improve the purity, and tailor the properties of the synthesized carbon nanotubes to the specific requirement. [
76] hypothesize that purification of the crystalline structure of dry-spun carbon nanotube yarns increase the electron carrier density by doping.
1.3.5. Pyrolysis
Pyrolysis (
Figure 7) is a well-established process of producing carbonaceous materials by heating organic precursors to high temperatures in the absence of oxygen. It is a thermochemical reaction that degrades organic molecules by splitting up their chemical bonds. Over the years, research in this field has concentrated on optimizing process parameters, investigating new precursor materials, and understanding the underlying principles of pyrolysis[
78,
79]. For the optimization of process parameters, research has been focused on the heating rate, residence time, pyrolysis temperature and precursor materials. For example, studies have examined the effect of temperature scaling profiles on creating carbon nanomaterials with regulated structures[
80].In the bid for alternative precursors, including biomass, polymers and waste materials. The studies aim to develop cost-effective and sustainable routes for carbon material synthesis. This has paved way for the exploration of novel precursors for synthesis of carbonaceous materials for various applications. For example, Predeanu, G.
et al. [
81] recycled certain lignocellulosic waste (walnut shells, kernels of peach, apricot, and olive) to design advanced carbon material precursors to be used for obtaining nano-powders with high applicative potential in pollution abatement. In the recent time, researchers are focusing on tailoring the characteristics of carbonaceous materials generated through pyrolysis for specific purposes such as environmental remediation, energy storage and catalysis. Strategies being utilized includes nano structuring, doping, and surface functionalization[
82].
1.3.6. Electrochemical Deposition
Electrochemical deposition, commonly known as electrodeposition, is an effective synthesis technique that employs an electric field and a redox reaction. It can process a wide range of materials, including polymers, metals, and ceramics[
83].The advantages of electrochemical deposition include reduced cost and enhanced interfacial bonding between the coating material and the substrate prior to heat treatment or sintering[
84]. This technique can be performed under galvanostatic (an electrode is maintained at a constant current in an electrolyte) or potentiostatic (involves changing current density while keeping the potential constant) circumstances. Recent research focused on utilizing electrochemical deposition techniques to enhance the control and tunability of carbonaceous material production. By adjusting parameters such as current density, electrolyte composition, voltage, and deposition time, researchers have been able to tailor the properties of the resulting materials better precision[
85,
86,
87]. The patented work of Zhou and Chow [
88] showed that the electrochemical deposition conditions have a strong influence on the growth process of the carbon nanotubes. Graphene nanosheet synthesized by electrochemical deposition lead to the enhancement in the mechanical properties of the Ni matrix[
89].Doping and functionalization are also achieved through electrochemical deposition to enhance the materials' properties, such as conductivity, catalytic activity, and surface chemistry, leading to improved performance in specific applications. One of the recent works is the efficient uranium electrochemical deposition with a functional phytic acid-doped polyaniline/graphite sheet electrode by adsorption-electrodeposition strategy[
90]. The issue of scale-up and commercialization of electrochemical deposition process for carbonaceous material synthesis to meet industrial production demands are also being exploited recently. Wang and authors [
91] demonstrated scalability through continuous preparation of high performance flexible asymmetric supercapacitors based on carbon cloth by a quick, low-cost, simple and scalable one-step electrochemical process within minutes without any other additives.
1.3.7. Template-Assisted Synthesis
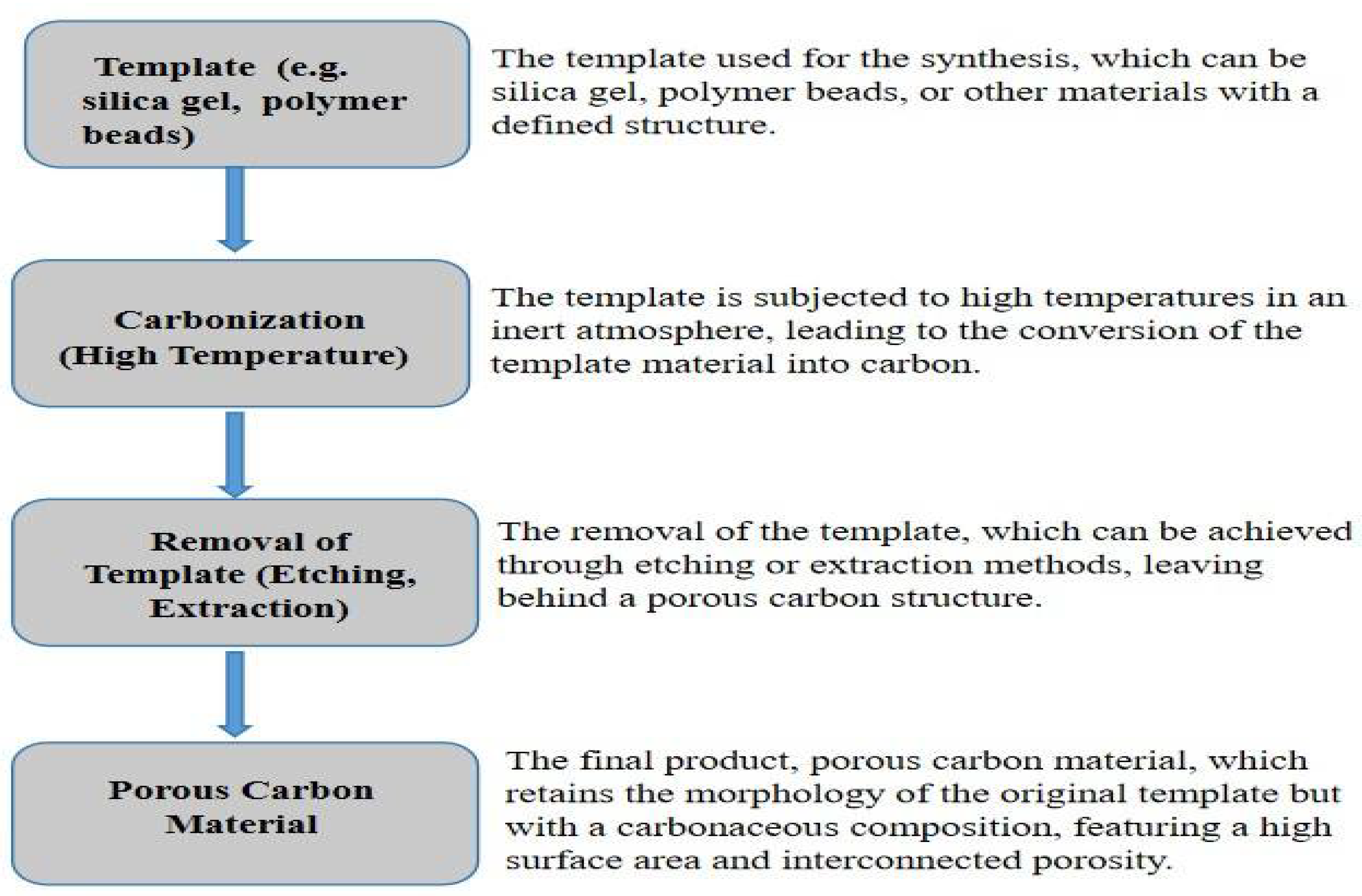
Template-assisted synthesis of carbonaceous materials as shown in
Figure 8 uses pre-existing structures or templates to direct the generation and morphology of carbon-based materials. This method provides fine control over the structural features and functions of the resultant materials, making it an adaptable solution for a variety of applications[
92].Template-assisted synthesis method was used by [
93] to fabricate biomass-derived porous carbon with a controllable structure using alginate as precursor. Templates-assisted approach though expensive, enables for the fabrication of high surface area and porous carbon materials, which are useful for energy storage, catalysis, and adsorption as demonstrated by [
94] were reported as a facile way to prepare porous carbons from metal-organic gel (MOG) template, an extended MOF structure. They surprisingly, discovered that the carbon products inherited the very porous nature of MOF and combined it with the gel's integrated character, resulting in hierarchical porous architectures with ultrahigh surface areas and enormous pore volumes.
1.3.8. Microwave-Assisted Synthesis
Microwave-assisted synthesis (MAS) of carbonaceous materials is a technique in which microwaves are utilized to begin and drive chemical processes in the manufacture of carbon-based compounds[
95].MAS has been used in the production of nanoparticles because it combines the advantages of speed and uniform heating of the precursor materials, leading to shorter reaction times and higher energy efficiency compared to conventional heating methods[
96,
97]. Microwave irradiation has a penetrating feature, which allows for the homogenous heating of the reaction solution[
98].In the study of Brazil and other author; a domestic microwave oven was adopted. With no radiation leak, being a safe, simple, and fast method for activated carbon production. Activated carbon samples from coffee grounds, olive stones, and Kraft lignin were produced by chemical activation (H
3PO
4 as activating agent), resulting in surface areas of 550, 1125, and 1170 m
2g
−1 and yields of 19, 21, and 23%, respectively[
99]. Controlled morphology and properties of carbonaceous materials by adjusting the reaction parameters such as microwave power, irradiation time, and precursor composition have also been reported[
97,
100]. Microwave irradiation promotes rapid and uniform reactions, leading to higher purity and yield of carbonaceous materials compared to traditional methods, the reduced reaction time also minimizes side reactions and impurities, resulting in higher product quality[
101,
102]. MAS of carbonaceous materials often employs renewable and biomass-derived precursors, making it a sustainable and environmentally acceptable method[
102]. Furthermore, the lower energy usage and quicker reaction times add to the green synthesis feature.
1.3.9. Laser Ablation
Laser ablation synthesis (LAS) of carbonaceous materials is a robust and promising method for producing tailored carbon-based nanostructures. It involves using high-energy laser pulses to vaporize a target substrate, usually graphite or a carbon-containing compound, in a controlled setting. The resulting plume of vaporized material undergoes fast cooling and condensation, resulting in the creation of carbon nanoparticles or thin films[
103,
104].Use of LAS was first reported in 1995 by Guo, T.
et. al when they engaged direct laser vaporization of transition-metal/graphite composite rods to produce single-walled carbon nanotubes (SWT) in the condensing vapor in a heated flow tube[
105]. Synthesis of reduced graphite oxide (rGO) nanosheets which constituted, approximately, 73% of the nanostructures in the sample through Laser ablation has also been reported[
106]. Notable advantage of LAS is production of high purity carbonaceous materials, as it involves minimal contamination from catalysts or other impurities, it is simple and clean[
107]. It also offers precise control over the morphology, size, and crystallization of the synthesized carbon nanostructures, allowing for tailored properties suited for various applications[
106,
108,
109,
110]. Despite these advantages, the current production rate of this technique remains limited, with typical rates in the milligram per hour range, recent research is focusing on scalability for commercial production[
111].
2. Characterization of Carbonaceous Materials for Medical Applications
The Materials and Methods should be described with sufficient details to allow others to replicate and build on the published results. To improve its overall performance and fully realize the medical applications of carbonaceous materials, accurate information about the microstructure and chemical behavior must be known.[
112] Material characterization provides information regarding the microstructure and functional groups characteristic of a particular product, thus; confirming its formation and reaction mechanisms[
113,
114]. The structural and chemical nature of carbonaceous materials such as carbon nanotubes, graphene, activated carbon and biochar can be elucidated via characterization using analytical methods which include; X-ray diffraction (XRD), Fourier Transfer Infrared (FT-IR), scanning electron microscopy (SEM), elemental analyses, optical microscopy, field emission scanning electron microscopy (FE-SEM), transmission electron microscopy (TEM),high performance liquid chromatography (HPLC) and inverse gas chromatography (IGC).
2.1. X-Ray Diffraction (XRD) Analysis of Carbonaceous Materials
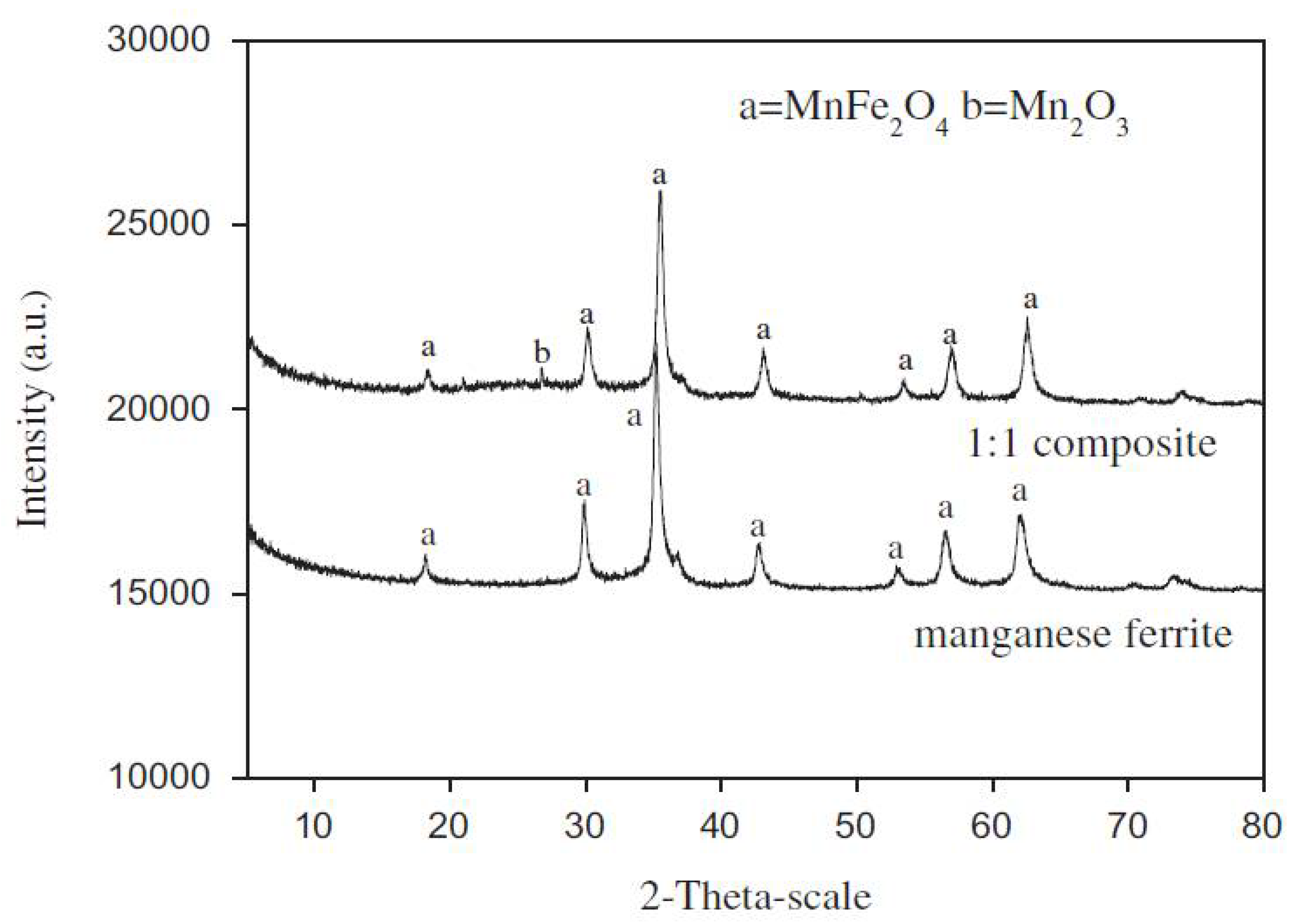
XRD defines the crystal structure, providing information on the different planes. If the sample is amorphous it displays a mere broad peak which contains no crystal structure information[
115]. However, crystalline samples can be indexed using XRD analysis and the result of the peaks compared or matched with the standard peaks of the compound as contained in the JCPDS data card. For instance, the XRD pattern of manganese ferrite-graphene (MnFe
2O
4-G) was characterized by Chella
et al. showing the diffraction peaks at 2θ values of 18.03°, 30.18°, 35.46°, 43.02°, 53.81°, 56.89°, 62.55°, 70.20° and 73.34° which correspond to (1 1 1), (2 2 0), (3 1 1), (4 0 0), (4 2 2), (3 3 3), (4 4 0),(5 3 3) and (6 2 2) crystal planes of MnFe
2O
4-G in accordance with the standard JCPDS no. 74-2403 with which the data was matched[
6]. The absence of 11.08° confirms the reduction of graphene oxide to graphene nanosheets as reported by the authors. Shao
et al. demonstrated the characterization of a composite of MnFe
2O
4/activated carbon in the ratio of 1:1 used for the removal of tetracycline antibiotic[
116]. The results shows that the composite displayed XRD crystalline peaks at 2θ = 18.1°, 30.2°,35.1°, 43.0°, 53.5°, 56.8° and 62.5° which were indexed to the crystal plane of spinel ferrite (111), (220), (311), (400), (422), (511),and (440), respectively. Thus, the composite XRD pattern follows the cubic spinel of manganese ferrite (MnFe
2O
4) which matched with the JCPDS data base as shown in
Figure 9. This analysis shows the presence of MnFe
2O
4 in the composite with no evidence of activated carbon. Similar effects were observed in the XRD pattern of activated carbon/CoFe
2O
4 composites prepared by Ai
et al. no characteristic peaks of impurities are detected in the XRD pattern, mostly the spinel phase is identified.[
117] Therefore, care should be taken in characterizing such composites in XRD, as further analysis would be needed to define the carbon component of the system because there is no difference between the XRD pattern of the composite and the pure manganese ferrite (see
Figure 9).
2.2. Fourier-Transform infrared (FTIR) Analysis of Carbonaceous Materials
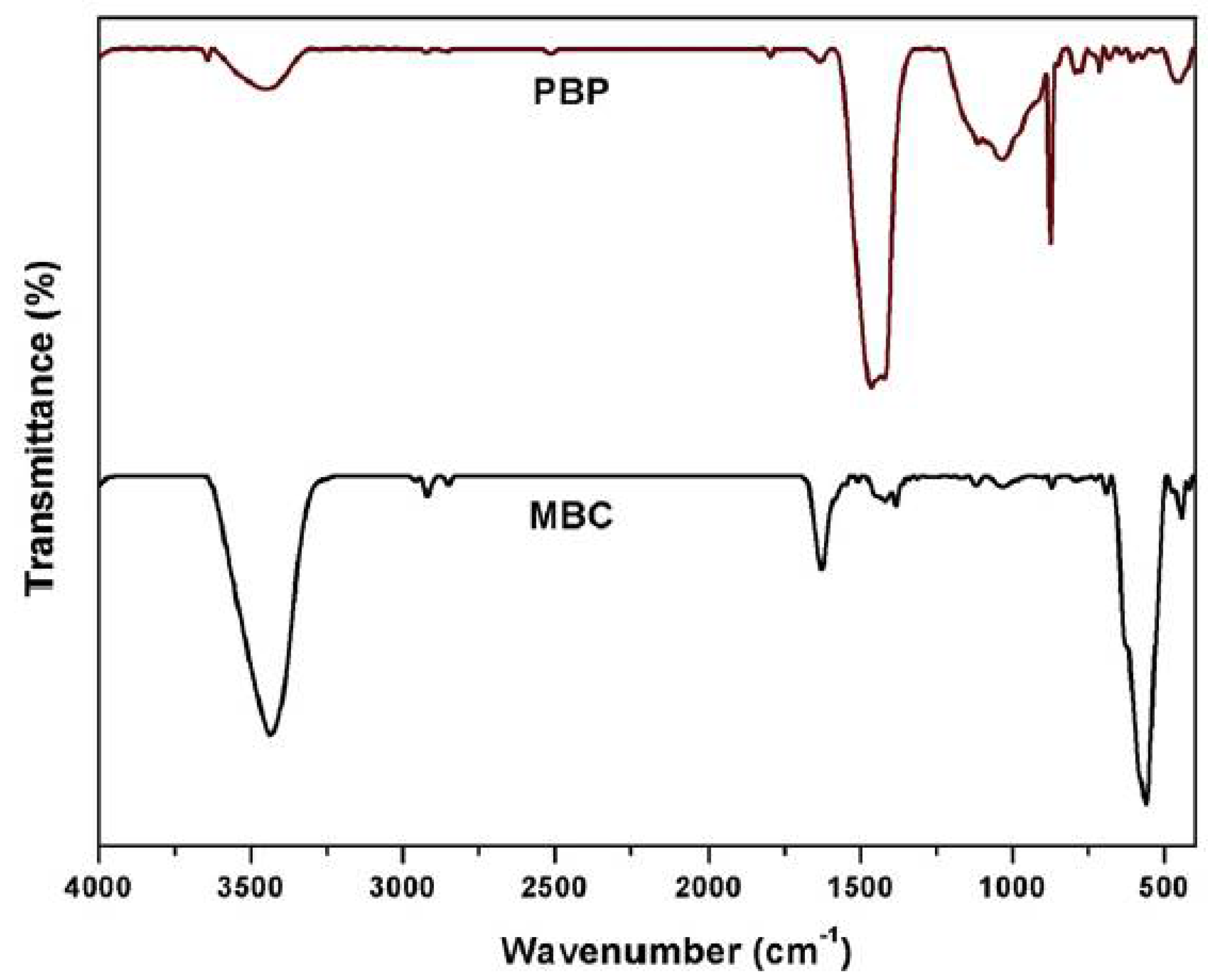
Fourier-transform infrared spectroscopy (FTIR) is a good follow-up to XRD techniques especially for carbonaceous materials. This technique is used to obtain an infrared spectrum for the absorption or emission of different materials which maybe solid, liquid, or gas. Thus, this method is very good for the characterization of carbonaceous products. An FTIR spectrometer simultaneously collects high-resolution spectral data over a wide spectral range. The principle of FTIR technique is to measure how much light a sample absorbs at each wavelength characteristic of the functional groups of the material being measured. Several researchers have utilized FTIR for the characterization of different carbonaceous materials. Reddy and Lee characterized the functional groups of pine bark powder (PBP) and magnetic biochar composite (MBC) via FTIR[
8] the spectra is shown in
Figure 10. There is a clear difference in the peaks of the two materials via FTIR characterization. The functional groups related to carbon materials are available as expected at bands around 3400 cm
−1, 2928 cm
−1 and 1631 cm
−1 for stretching vibrations of OH, CH
2 and CO, respectively[
118]. Similar characterization for other carbonaceous material such as graphene oxide (GO) nanosheets and activated carbon were studied by Muniyalakshmi
et al.[
119] and Feng
et al.[
7] respectively, in which related functional groups were found characterizing the presence of the two carbon materials however, intensities and broadening of the peaks are different due to concentrations and impurities[
120].
2.3. Raman Spectroscopy of Carbonaceous Materials
Raman spectroscopy is a closely related characterization to FTIR and can be used solely or as a complementary analysis. Raman spectroscopy is a spectroscopic technique typically used to determine vibrational modes of molecules, although rotational and other low-frequency interactions maybe observed. Raman spectroscopy is commonly used in chemistry to provide a structural fingerprint by which molecules can be identified. This technique relies on inelastic scattering of photons, known as Raman scatter. For Raman spectroscopy, laser is used as the light source which interacts with molecular vibrations causing a shift in the energy of the incident ray. The shift in energy gives information about the vibrational modes in the system thus characterizing them[
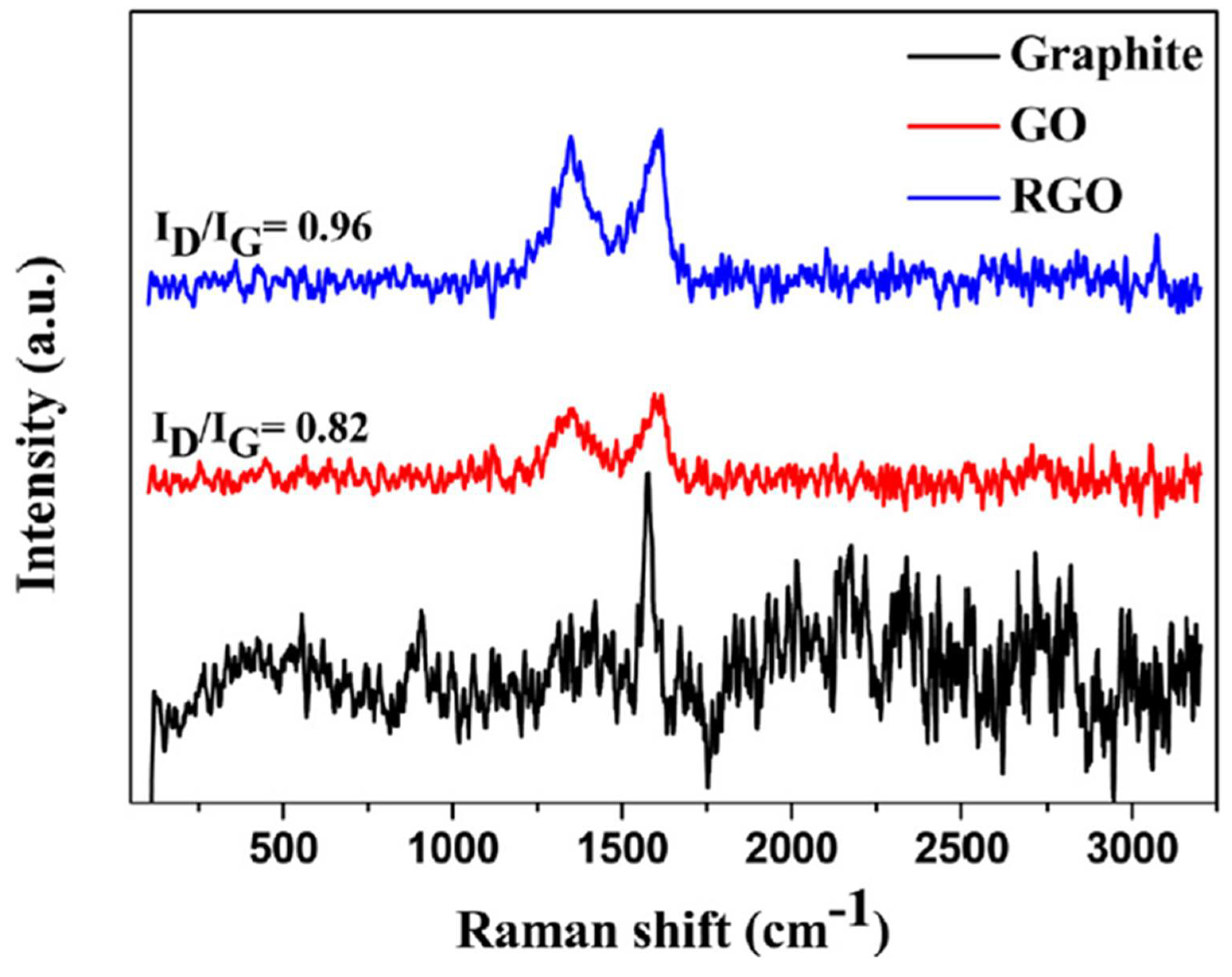
121]. Thus Infrared spectroscopy typically yields similar and yet complementary information. Several researchers have utilized this technique in characterizing new carbonaceous materials. Zhang
et al. demonstrated the use of Raman spectroscopy to differentiate pristine graphite from graphene oxide and reduced graphene oxide both formed from it[
120]. According to
Figure 11, the strong band found around 1582 cm
-1 is known as the G-band, its intensity increases with the increasing numbers of layers and a second band around 1350 cm
-1 called the D-band which is correlated to defect. It appears as a strong band when the number of graphene layers in a sample is large[
122]. The ratio of the intensities of the G and D bands, IG/ID is related to the in-plane crystallite size of GO and RGO samples whereas the intensities of the 2D and G bands is sensitive to hole or electron doping. In-plane vibration is another property detectable by Raman spectroscopy for characterization of carbonaceous materials. In a related study Maria M. Titirici
et al. discussed a typical in-plane vibrations as a good characteristic of activated amorphous carbons[
123]. Similar result was recorded by other authors on GO.[
119]. For in-depth analysis other characterization methods are usually added.
2.4. Scanning Electron Microscopy (SEM) of Carbonaceous Materials
Scanning Electron Microscopy and other characterizations via imaging are highly useful for the characterization of carbonaceous materials[
124]. Scanning electron microscopy (SEM) is a versatile method for the investigation of surface morphology of various materials[
125]. Depending on its electron source, SEM can be classified into thermal and field emission type. In both cases, images are obtained by scanning with a very narrow beam of electrons through a sample, at different magnifications. The electrons interact with the atoms of the material, producing various signals that contain information about the surface topography and composition of such sample. When a high electron absorption capacity or nonconducting materials are investigated, sputtering or covering the samples with thin films of metals such as gold or platinum is done to achieve high quality resolutions[
126]. This technique enables the surfaces and pore structures of materials to be characterized.
Figure 12 shows the SEM micrographs of different synthesized materials with a clear contrast in their morphology, see characteristic spherical shape for MnFe
2O
4 (
Figure 12a), cylindrical shape for g-C3N4 (
Figure 12b), layered structure for GO-nanosheet (
Figure 12c) and bulk massive nature of MBC (
Figure 12d) as studied by different authors characterizing the various nanostructures.[
6,
7,
8,
119]. In addition to imaging, colour mapping and elemental analyses can equally be carried out in the presence of EDX detector coupled to SEM instruments which helps to deepen the analyses with detailed information[
6,
127].
2.5. X-Ray Photoelectron Spectroscopy (XPS) of Carbonaceous Materials
X-ray photoelectron spectroscopy (XPS) is yet another surface-sensitive qualitative and quantitative spectroscopic technique that works with the principle of photoelectric effect. It is used for identification and quantification of the elemental composition within a material or coated surface, as well as their chemical state, bond nature and overall electronic structure. This technique can be used to profile the cross-sectional elemental composition when paired with ion-beam etching. All elements can be detected via XPS except hydrogen and helium. The detection limit goes in parts per thousand range, but parts per million (ppm) are equally achievable with long collection times and concentration. Because the energy of an X-ray with particular wavelength is known and the kinetic energies of the emitted electrons are measured, the binding energy of each of the emitted electrons can thus be determined[
128]. XPS is routinely used to analyze inorganic compounds such as ceramics, metal alloys, polymers, catalysts, glasses, paints, papers, inks, woods, teeth, bones, medical implants, bio-materials, coatings, and many other materials. The different carbon environments can be identified. For instance, Maria M. Titirici
et al characterized Carbonaceous material using XPS[
123].
2.6. Other Imaging and Related Techniques for Carbonaceous Materials
Other imaging techniques such as optical microscopy, atomic force microscopy and transmission electron microscopy are utilized in the characterization of carbonaceous material. Optical microscope, also referred to as light microscope, is a type of analytical instrument that mainly uses visible light and a system of lenses to produce magnified images of a small object. A range of objective lenses with different magnifications are usually provided, mounted on a turret, allowing them to be rotated into place and providing an ability to zoom in or out. The maximum magnification power of optical microscopes is typically limited to around 1000x because of the limited resolving power of visible light. However, the use of optical microscope in the characterization of carbonaceous material is well known.[
129,
130]. Atomic force microscopy (AFM) is another type of scanning probe microscopy (SPM), with demonstrated resolution in the order of fractions of a nanometer, more than 1000 times better than the optical diffraction limit. The characterization is achieved by moving the mechanical probe to feel or touch the surface. Piezoelectric elements that facilitate tiny but accurate and precise movements on command enable precise scanning which can provide information on topography, surface roughness and film thickness[
131] Several studies has employed AFM for characterizing a number of carbonaceous materials. In the case of layered carbon material such as graphene and graphene oxide the focus is on probing the thickness. This approach was utilized to characterize and provide information on the number of graphene layers present in a sample. By differential height measurements carried out at the folded edge, they obtained a graphene layer of 4 Å, which is close to the thickness of a monolayer (3.4 Å) thus confirming that the graphene is a single layer[
132].
Studies revealed that transmission electron microscope which has the higher resolution model now available called high-resolution transmission electron microscope is an imaging-instrument that allows for direct imaging of the atomic structure of samples usually used at the confirmatory stage of the analysis. It is a powerful tool for the investigation of nanomaterials such as semiconductors, nanoparticles, and carbonaceous materials on the atomic scale. At present, the highest point of resolution realized with this instrument is around 0.5 angstroms (0.050 nm). At such small scales, individual atoms of a crystal and defects can be resolved. Information such as shape, size, and distribution of particles, including their crystal structure can be obtained. Detection of a layer of carbonaceous material can be observed; for instance, a folded graphene sheet is locally parallel to the electron beam of TEM and for single-layer graphene, a fold exhibits one dark line[
133]. High-resolution transmission electron microscope (HR-TEM) will give a very high atomic resolution of the microstructural images of the synthesized products and thus is highly used in today’s study of carbonaceous materials[
134]. Additional analysis depending on the research focus include inverse gas chromatography (IGC) which is a powerful and sensitive technique that can be used to characterize the surface-physicochemical properties of carbonaceous materials[
135]. The main application of IGC is to measure the surface energy of solids such as fibers, particulates, and films. Surface energy is the amount of energy required to create a unit area of a solid surface; this is similar to surface tension of a liquid. It is possible to ascertain both the dispersive component of the surface energy and acid-base properties via IGC[
136,
137].Thermogravimetric or thermal gravimetric analysis (TGA) is another very important way of characterizing carbon materials for medical and other applications[
138]. This method of thermal analysis focuses on the measurement of the sample’s change in mass with time and temperature. This measurement provides information about physical phenomena, such as phase transitions, absorption, adsorption and desorption; as well as chemical phenomena including chemisorptions, thermal decomposition, and solid-gas reactions. Other methods such as Braun-Emmet-Teller (BET) analysis which utilizes the multilayer adsorption framework helps to understand the adsorption pattern as well as particle size distribution. The surface area of carbonaceous materials in critical to the adsorption potentials of the material. BET is useful in the curation of surface area, pore volume and sizes of materials. In fact, it is essential to understand the adsorption of guest molecules on carbon-based materials for both theoretical and practical reasons.
3. Application of Carbonaceous Materials in Medical Sciences
3.1. Cancer Cell Amelioration (Hyperthermia)
Cancer is the leading cause of morbidity and mortality worldwide: about 8.8 million deaths in 2015 were cancer related; the most common types are lungs, liver, colorectal, stomach and breast cancers[
139]. The outset of cancer starts with the presence of foreign cells in the body that grow in pseudo form, spreading to other area of the body, contaminating and degrading body elements at an alarming rate. There is no defined cure for cancer; preventive and palliative medicines are used as management procedures to manage cancer cases[
140,
141]. Hyperthermia is the application of heat to denature tumor and cancer cells. It involves the application of an external alternating magnetic field to magnetic nanoparticles to create a re-orientation of magnetic moment thus converting magnetic energy to heat energy which is then applied to denature cancerous cells in the body[
142,
143]. Hyperthermia procedure takes advantage of the lower sensitivity of the body cell to heat compared to the infectious cell; at temperatures within 41°C – 46°C cancer causing cells are easily denatured[
144]. Heating mechanism of magnetic materials is based on Brown relaxation (heat due to friction arising from total particles oscillations), Neel relaxation (heat due to rotation of the magnetic moment with each field oscillation) and hysteresis loss (heat loss in reversing the magnetization of the material)[
145,
146] Specific absorption rate (SAR) and power loss are the primary quantifications used to describe localized hyperthermia resulting from the application of an alternating magnetic field and it’s determined analytically from the integration of the hysteresis loop[
147]. Appreciable SAR value is focal to the adaptation of magnetic materials in biological medium. High SAR value would translate to lower concentration of material required to achieve optimum temperature range for full therapeutic efficacy[
148].
Hyperthermia have been applied to different types of cancer; such as melanoma[
149], brain cancer[
150], prostate cancer[
151], and breast cancer[
152]. Moorthy and co-workers engineered a magnetic multifunctional nanocomosite of primary silica coating and secondary coatings of amine-polyglycidol and fluorescein isothiocynate (FTC) [
153]. The fluorophore impacted bio-imaging properties while the SiO
2 created biocompatibility for the organic moiety. The composite response time to climax a temperature of 45 °C was 4.5 min while the specific absorption rate (SAR) was 112.8 Wg
-1. This was enough to kill the cancer cells. Cole
et al. [
154]compared the properties of cross-linked aminated starch magnetic oxide composite;aminosilane based polyethylene glycol(PEG), and starch-based polyethyleneglycol. They concluded that theaminosilane composite gave the highest magnetization saturation (Ms) with value of 106.8emu/g. However, polyethylene glycol (PEG) magnetic composite performed best showing sustained size stability in cell culture medium at 37 °C. A mono-dispersed magnetic phosphorylated methoxy poly(ethleneglycol) (mPEG) synthesized via solution phase thermal decomposition with high SAR value of 930 Wg
-1 was reported by [
155]. Khot and co-workers compared influences of induction heating on bare and dextran coated MgFe
2O
4 [
156].It was found that 5mgmL
-1 of dextran coated MgFe
2O
4 degraded against mice fibroplast cell line by 86%. 1mgmL
-1 of water soluble La
0.7Sr
0.3MnO
3(LSMO) magnetic nanoparticle coated with oleic acid, showed 92% degradation of human negroid cervix epitheloid carcinoma after 48 h [
157]. Iron oxide (magnetite and maghemite) was functionalized in the presence of three carboxylic acid ligands; tiopronin(N-(2-mercaptopropionyl)-glycine, oxamic and succinic acid. It was discovered that 50 mg/ml of Tiopronin coated material gave the best heating response to an alternating AC magnetic field resulting in SAR value of 1179 Wg
-1. [
158]. Eva
et al. have also reported a bacterially synthesized ferrite nanoparticles coated with
geobactersulfureducens coating; with SAR value of 253 Wg
-1[
159].
3.2. Magnetic Resonance Imaging (MRI)
Magnetic resonance imaging (MRI) is a non-invasive imaging technique applied in biological field to produce high resolution images of internal organs; the science of the operation is based on nuclear magnetic resonance and radio frequency pulses. On application of radio frequency, the protons oscillate allowing net magnetisation by response to the applied magnetic field. When the field is off the molecules return to their original state by emitting energy in the form of photons called the relaxation process[
160]. Magnetic Resonance Imaging (MRI) relies on the ability of a bio-compactible substrate to differentiate between benign and malign cell in body tissues. The contrast efficacy of the material for MRI can be assessed by determining their R
1 and R
2relaxivities. Magnetic resonance contrast agents act by selectively reducing T
1 (and T
2) relaxation times of tissue water through spin interaction between electron spins of the metal-containing contrast agent and water protons in tissue; the dominant effect is on T
2 [
161]. In principle, T
1 increases linearly with contrast agent concentration; the slope of this dependence is known as the relaxivity, typically reported in units of mmol
-1sec
-1. This is a measure of how potent the agent is for catalysing relaxation[
161]. Wang
et al. engineered a super-paramagnetic composite with dual functionality as magnetic resonance and optical tracer [
162]. The photosensitizer, a tetra-substituted carboxyl aluminum phthalocyanine (AlC
4Pc); exhibited excellent photo-oxidation efficiency, and a T
2 value of 194.66 mM
-1S
-1. In the work of Fang and co-workers [
163]; Gadolium(III) doped carbon dots was prepared via a solvothermal process. The pH sensitive smart material exhibited bright red fluorescence at 620 nm and had a relaxivity value of 7.0 mM
-1s
-1. In another study [
164]; FeCo of size 10nm with graphitic carbon shell decorated with poly(ethylene glycol) was investigated for photothermal and magnetothermal properties. It was reported that the nanomaterial provided signal intensity that is sixfold and fifteen-fold higher than the signal intensity of superparamagnetic iron oxide tracers VivoTrax and Feraheme respectively. The properties of FeCo are viable for cancer imaging and hyperthermia therapy.
3.3. Biosensors in Medicine
Biosensors are responsive analytical devices designed using a biological material and a signal detector. The detector converts the surface reaction into electric and optic signal which are decoded and quantified by an appropriate portable electronic device[
165]. Electrochemical biosensors measure electrical impulses and are applicable to substrate that has oxidative-reductive properties[
166,
167]. Since the chemical reaction occurs at the surface of the electrodes and the analyte; the surface properties of the electrode are fundamental to the sensitivity of the detector. The properties of the biocomponent would determine the selectivity or specificity of the biosensor[
168] Electrochemical based techniques are desirous in medicine because of their high selectivity, low cost, portability, automation and ease of use [
169,
170]. In recent years, magnetic sensors have found wide acceptance and usage in biomedical application because of their smart and eco-friendly properties. The doping of biosensor with magnetic materials enhances the sensitivity, selectivity and stability of the electrode[
171]. A novel magnetic functionalized electrochemical sensor was designed by Sun
et al.[
171] for the direct electrochemistry and electrocatalysis of lobetyolin. The sensor was reproducible, long-term stable and highly selective for the detection of lobetyolin in
Codonopsispilosula with a recovery rate in the range of 96.12 – 102.66%. Chiral recognition is a vital biological assay in differentiation of toxic and useful enantiomers in biological science and disease control. Shi
et al. [
172]propounded a magnetic electrochemical chiral sensor for the enantiorecognition of Tyrosine. The enantiorecognition efficiency was improved by the magnetic field and showed a wide linear range, low detection limit and high stability for L-Tyr than D-Tyr. An electrochemical method for the quick detection of ciprofloxacin; a ubiquitous antibiotics in pharmaceutical formulation and biological fluids has been formulated[
173]. The fabricated magnetic multiwalled carbon nanotubes selectively responded to ciprofloxacin; quantified detection limit and linear detection range were 0.0017 µmol L
-1 and (3S
b/m) of 0.005-0.85 µmol L
-1 respectively. Jeong and co-authors designed an integrated magneto-electrochemical sensor for exosome analysis[
174]. The miniature point-of-care electronic equipment was cell-specific and sensitive and allowed for the simultaneous analysis of multiple protein markers from ovarian cancer samples.
3.4. Dental Care Formulation
Activated carbon constituted oral and dental care formulation is a growing scientific hybrid in dental healthcare. Activated carbon has the unique ability to extract poisonous materials from the teeth and mouth[
175]. The charcoal binds to the toxin via weak molecular forces through its surface functional groups, which can be easily washed during brushing. Activated charcoal toothpaste and brushes are potent against microbes and absorption of gas causing malador[
176,
177]. The presence of AC in toothpaste complements the retention of essential mineral elements in the mouth[
178,
179]. Results have showed that there are significant differences in the morphological structure of the enamel surface after brushing with activated charcoal[
180]. The authors also inferred that toothpaste constituted with AC have potentials for whitening effect that can affect the colour and the surface roughness of the teeth. A comparative study on dentin abrasivity and cleaning efficacy of novel/alternative toothpaste laden with diamond particle, active carbon and sea salt or organic oil was conducted[
181]. One hundred and thirty-two (132) samples were utilized to investigate both abrasivity and cleaning efficacy. The results revealed that Lavera Neutral Zahngel (sea salt 9.2 µm) and Elmex Kariesschutz group (hydrated silica 6.0 µm) exhibited the highest abrasivity. Similarly, the oil constituted toothpaste showed the lowest abrasivity and cleaning efficacy. Conclusively, authors reported that the addition of diamond powder or active carbon to toothpaste could offer high cleaning efficacy with low dentin abrasivity.
3.5. Tissue Engineering
Tissue engineering involves the regeneration of construction of tissues and organs that are biologically compactible from carbon rich materials that can be used to replace a damage cell[
182]. Tissue engineering procedures normally requires three basic tools which are cell, scaffold, and growth factor[
183,
184]. The cell is used in creating matrices or building block for the new tissue, while the scaffold provides the appropriate environment for the cell’s synergy. The function of growth factors is to enhance tissue regeneration from the cells. Tissue engineering is useful in skeletal system repair, cardiovascular system engineering, nerve and spinal cord system repair, auricular cartilage reconstruction and skin repair. Serafin and authors [
185] prepared a printable alginate/gelatin hydrogel reinforced with carbon nanofibers as electrically conductive scaffolds for tissue engineering. The hydrogel exhibited electroactivity with conductivity reaching 4.1 x 10
-4 ± 2 x 10
-5 S/cm. The cell cytotoxicity studies revealed that the hybrid system was biocompatible and can enhance cellular attachment and proliferation. Samadian and team [
186] has also fabricated an osteoconductive electrospun carbon nanofibers (CNFs) decorated with hydroxyapatite (HA) crystals for use in the bone tissue engineering in animal model. The
in vitro studies revealed that nanocomposite was biocompatible. CNFs/HA enhanced
in vivo bone formation in a defective rat’s femur. The progress of the study was captured and monitored using Computed Tomography (CT) scan image and historical evaluation.
3.6. Human System Detoxification
Activated charcoal is a universal antidote for most poisons[
187,
188]. Activated charcoal is usually produced through the process of carbonization of materials rich in carbon. Activation is carried out using solvents as simple as water to mineral acids. Activated charcoal has a surface area about 1000m
2/g and can adsorb many drugs[
189].The adsorption potential of activated charcoal depends on the drug or toxin, quality and dose of the charcoal, pH of the medium in which it acts, and gastric contents[
190,
191]. Activated charcoal directly adsorbs drugs or toxic substances thereby interfering with absorption by the digestive tract. In addition, activated charcoal possibly interferes with gastro-enteral recirculation of toxins from systemic circulation into the gastrointestinal mucosa and contents by binding to conjugated drug/toxin or de-conjugated drug/toxin with subsequent elimination through the intestinal contents[
192,
193]. Carbonaceous materials have been found useful for the elimination of diclofenac and oxytetracycline from synthetic urine by furfuryl alcohol derived mesoporous carbon[
93]. The high specific surface area of the mesoporous materials at 1022.61 m
2/g promoted the fast sorption of the organic moieties. Based on kinetic studies, equilibrium adsorption was attained after 120 min; the sorption process followed the pseudo-second order kinetics and fitted well with the Sips isotherm model. The maximum adsorption capacities recorded were 411.8 mg/g and 465.9 mg/g for diclofenac and oxytetracycline respectively. Adsorption mechanism revealed that the uptake of the drugs was via π-π interaction, hydrogen bonding and electrostatic interactions. Lupascu and co-authors [
194].
3.7. Wound Healing
Activated charcoal dressings have the capacity to adsorb microbes and toxins on wound surface, thereby promoting healing[
195]. The infection of wound can prolong healing time; the presence of endotoxins in wounds have been shown as a causative agent for delayed wound healing[
196,
197]. Recently[
198] utilized activated carbon as an absorptive material for the elimination of bacteria toxins. In the study, AC was incorporated into Kenaf Nanocrystalline Celluloses (NCC)-Chitosan (CS) hydrogel and use to eliminate bacteria toxins in wound. The results revealed that the hydrogel exhibited low cytotoxicity towards human fibroblast and keratinocytes cells. The hydrogel adsorbed 85% of endotoxins when treated with a concentration of 0.1 EU/mL of AC constituted hydrogel. An iron-doped carbon dots (Fe-CDs) of approximately 3nm in size was reported as a viable composite for wound healing [
199]. The authors revealed that Fe impacted CDs with photoenhanced peroxidase (POD)-like activity, which lead to the generation of heat and reactive oxygen species (ROS) for the extinction of Gram-positive and Gram-negative bacteria. In a similar study [
199]; Ce-doped carbon dots (Ce-CNDs) fabricated via hydrothermal method, was utilized in wound healing. The multifunctional nanocomposite of less than 4nm can be utilized in cell imaging, antibacterial and would healing. The study revealed that Ce-CNDs combined the antioxidant property of Ce and biocompatibility of CNDs, exhibiting both photoluminescent and photostability properties. In another study, Shakiba and co-authors[
200]; synthesized a carboxylated multiwalled carbon nanotube embedded in a polyamide nanofibrous composite for controlled drug release and wound healing applications. The results showed that the incorporation of polyamide enhanced wettability and biodegradability. Furthermore, the nanofiber showed high antibacterial activity by inhibiting the growth of
Staphylococcus aureus and
Escherichia coli bacteria.
5. Conclusions
As medical science advances, the integration of carbonaceous materials into medical devices, drug delivery systems, imaging agents, biosensors, and tissue engineering scaffolds promises to redefine the boundaries of what is achievable in healthcare. By facilitating early disease detection, targeted/precision drug delivery, and personalized therapies, carbonaceous materials have the potential to improve patient outcomes and transform the way we approach healthcare. Collaborative synergy between scientists, researchers, and healthcare professionals is therefore imperative to promote the harnessing of extraordinary properties of carbonaceous materials for the betterment of human health. More studies are essential to advance the synthesis, characterization, and application of these materials, ultimately paving the way for cutting-edge medical technologies that will shape the future of healthcare. To find appropriate application in medicine and healthcare, Carbonaceous materials (CMs) are required to possess enhanced structural, optical, chemical, and electrical properties that will boost their functionalities. These materials have shown great versatility because they can be chemically combined with other carbon-based materials and with a range of different elements to form strong covalent bonds. As a result, this tends to make them exhibit excellent characteristics such as high tensile strength, high density, and high hardness. The existence of both inorganic semiconducting properties and organic π-π stacking characteristics have made this possible.Magnetic materials are novel platforms that are much desirable in nanomedicine because of their smart properties such as multifunctionality, low-cost, stability, enhanced sensitivity, better selectivity, biocompatibility, and ergonomic system design. Considering the high cost and the unavailability of complex machine; coupled with lost man hours associated with emerging life-threatening diseases and sickness. There is an urgent need to develop curative point-of-care facilities to mitigate against the outset of diseases. There is also need for the design and development of more environment friendly and multifunctional hyphenated magnetic materials for use in the biological and medical field. The influences of magnetic field and any inherent toxicity associated with the use of magnetic materials must also be investigated and properly profiled for informed decision making.
Author Contributions
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. The following statements should be used “Conceptualization, X.X. and Y.Y.; methodology, X.X.; software, X.X.; validation, X.X., Y.Y. and Z.Z.; formal analysis, X.X.; investigation, X.X.; resources, X.X.; data curation, X.X.; writing—original draft preparation, X.X.; writing—review and editing, X.X.; visualization, X.X.; supervision, X.X.; project administration, X.X.; funding acquisition, Y.Y. All authors have read and agreed to the published version of the manuscript.” Please turn to the CRediT taxonomy for the term explanation. Authorship must be limited to those who have contributed substantially to the work reported.
Funding
This research received no external funding.
Acknowledgments
In this section, you can acknowledge any support given which is not covered by the author contribution or funding sections. This may include administrative and technical support, or donations in kind (e.g., materials used for experiments).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Rimmer, P.B.; Shorttle, O. Origin of life’s building blocks in carbon-and nitrogen-rich surface hydrothermal vents. Life 2019, 9, 12. [Google Scholar] [CrossRef] [PubMed]
-
Editors, N.R.F.w.t.U.o.S.S.C.I.S.T.G.o.; Authors; Messel, H. Abridged Science for High School Students: An Integrated Four-year Course in Physics, Chemistry; Nuclear Research Foundation, University of Sydney: 1966.
- Kim, C.-H.; Lee, S.-Y.; Rhee, K.Y.; Park, S.-J. Carbon-based composites in biomedical applications: A comprehensive review of properties, applications, and future directions. Adv Compos Mater 2024, 7, 55. [CrossRef]
- Hu, Z.; Srinivasan, M.P.; Ni, Y. Preparation of mesoporous high-surface-area activated carbon. Adv Mater 2000, 12, 62–65. [CrossRef]
- Kruss, S.; Hilmer, A.J.; Zhang, J.; Reuel, N.F.; Mu, B.; Strano, M.S. Carbon nanotubes as optical biomedical sensors. Adv Drug Deliv Rev 2013, 65, 1933–1950. [CrossRef] [PubMed]
- Chella, S.; Kollu, P.; Komarala, E.V.P.; Doshi, S.; Saranya, M.; Felix, S.; Ramachandran, R.; Saravanan, P.; Koneru, V.L.; Venugopal, V. Solvothermal synthesis of MnFe2O4-graphene composite—Investigation of its adsorption and antimicrobial properties. Appl Surf Sci 2015, 327, 27–36. [CrossRef]
- Feng, P.; Cui, K.; Hai, Z.; Wang, J.; Wang, L. Facile synthesis of activated carbon loaded g-C3N4 composite with enhanced photocatalytic performance under visible light. Diam Relat Mater 2023, 136, 109921. [CrossRef]
- Reddy, D.H.K.; Lee, S.-M. Magnetic biochar composite: facile synthesis, characterization, and application for heavy metal removal. Coll Surf A Colloid Surf A Physicochem Eng Asp 2014, 454, 96–103. [CrossRef]
- Derbyshire, F.; Jagtoyen, M.; Thwaites, M. Activated carbons-production and applications. Porosity in carbons 1995, 252.
- Leimkuehler, E.P. Production, characterization, and applications of activated carbon, University of Missouri-Columbia, 2010.
- Ruess, G.; Vogt, F. Höchstlamellarer Kohlenstoff aus Graphitoxyhydroxyd. Über den Ort der aktiven Eigenschaften am Kohlenstoffkristall. Monatshefte für Chemie und verwandte Teile anderer Wissenschaften 1948, 78, 222–242.
- Hummers Jr, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339–1339. [CrossRef]
- Brodie, B.C. XIII. On the atomic weight of graphite. Philosophical transactions of the Royal Society of London 1859, 249–259.
- Boehm, H.-P.; Clauss, A.; Fischer, G.; Hofmann, U. Das adsorptionsverhalten sehr dünner kohlenstoff-folien. Zeitschrift für anorganische und allgemeine Chemie 1962, 316, 119–127. [CrossRef]
- DiVincenzo, D.; Mele, E. Self-consistent effective-mass theory for intralayer screening in graphite intercalation compounds. Phys Rev 1984, 29, 1685. [CrossRef]
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163.
- Mouras, S.; Hamm, A.; Djurado, D.; Cousseins, J.-C. Synthesis of first stage graphite intercalation compounds with fluorides. Revue de chimie minérale 1987, 24, 572–582.
- Lijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.-e.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669.
- Dinadayalane, T.; Lazare, J.; Alzaaqi, N.F.; Herath, D.; Hill, B.; Campbell, A.E. Structures, properties, and applications of nitrogen-doped graphene. In Theoretical and Computational Chemistry, Elsevier: 2022; Vol. 21, pp. 211-248.
- Metwaly, A.M.; Ghoneim, M.M.; Eissa, I.H.; Elsehemy, I.A.; Mostafa, A.E.; Hegazy, M.M.; Afifi, W.M.; Dou, D. Traditional ancient Egyptian medicine: A review. Saudi J Biol Sci 2021, 28, 5823–5832.
- Marketos, S.G.; Androutsos, G. Charcoal: from antiquity to artificial kidney. J. Nephrol 2004, 17, 453–456.
- Al Jumaan, M.A. The Role of Activated Charcoal in Prehospital Care. Medical Archives 2023, 77, 64. [CrossRef]
- Dresselhaus, M.S.; Dresselhaus, G.; Eklund, P.C. Science of fullerenes and carbon nanotubes: their properties and applications; Elsevier, 1996.
- Soonmin, H.; Akram, M.; Rashid, A.; Laila, U.; Zainab, R. Uses of activated carbon in medicine area: Short review. EPRA -(IJRD) 2022, 7, 34–39.
- Juurlink, D.N. Activated charcoal for acute overdose: a reappraisal. Br. J. Clin. Pharmacol. 2016, 81, 482–487. [CrossRef]
- Jacoby, M.; Building, A. Better Gas Mask. C&EN 2014, 92, 34–38.
- Editors, H.c. Second Battle of Ypres begins. https://www.history.com/this-day-in-history/second-battle-of-ypres-begins, 5 Nov 2009.
- Mohamed, E.F.; El-Hashemy, M.A.; Abdel-Latif, N.M.; Shetaya, W.H. Production of sugarcane bagasse-based activated carbon for formaldehyde gas removal from potted plants exposure chamber. J. Air Waste Manag. Assoc, 2015; 65, 1413–1420.
- Navarro, M.; Michiardi, A.; Castano, O.; Planell, J. Biomaterials in orthopaedics. J. R. Soc. Interface. 2008, 5, 1137–1158.
- Alas, M.O.; Alkas, F.B.; Aktas Sukuroglu, A.; Genc Alturk, R.; Battal, D. Fluorescent carbon dots are the new quantum dots: an overview of their potential in emerging technologies and nanosafety. J. Mater. Sci. 2020, 55, 15074–15105.
- El-Shafey, A.M. Carbon dots: Discovery, structure, fluorescent properties, and applications. Green Process. Synth. 2021, 10, 134–156. [CrossRef]
- Dai, B.; Zhou, R.; Ping, J.; Ying, Y.; Xie, L. Recent advances in carbon nanotube-based biosensors for biomolecular detection. TrAC 2022, 154, 116658.
- Mokhtarzadeh, A.; Eivazzadeh-Keihan, R.; Pashazadeh, P.; Hejazi, M.; Gharaatifar, N.; Hasanzadeh, M.; Baradaran, B.; de la Guardia, M. Nanomaterial-based biosensors for detection of pathogenic virus. TrAC 2017, 97, 445–457.
- Kościk, I.; Jankowski, D.; Jagusiak, A. Carbon nanomaterials for theranostic use. C 2021, 8, 3.
- Zhang, W.; Zhang, Z.; Zhang, Y. The application of carbon nanotubes in target drug delivery systems for cancer therapies. Nanoscale Res. Lett. 2011, 6, 1–22.
- Ho, D.; Wang, C.-H.K.; Chow, E.K.-H. Nanodiamonds: The intersection of nanotechnology, drug development, and personalized medicine. Sci. Adv. 2015, 1, e1500439. [CrossRef]
- Mohan, H.; Fagan, A.; Giordani, S. Carbon Nanomaterials (CNMs) in Cancer Therapy: A Database of CNM-Based Nanocarrier Systems. Pharmaceutics 2023, 15, 1545. [CrossRef] [PubMed]
- Hassan, S.; Nadeem, A.Y.; Qaiser, H.; Kashif, A.S.; Ahmed, A.; Khan, K.; Altaf, A. A review of carbon-based materials and their coating techniques for biomedical implants applications. Carbon Letters 2023, 33, 1171–1188. [CrossRef]
- Nasir, S.; Hussein, M.Z.; Zainal, Z.; Yusof, N.A. Carbon-based nanomaterials/allotropes: A glimpse of their synthesis, properties, and some applications. Materials 2018, 11, 295. [CrossRef] [PubMed]
- Reza, M.S.; Afroze, S.; Kuterbekov, K.; Kabyshev, A.; Zh. Bekmyrza, K.; Haque, M.N.; Islam, S.N.; Hossain, M.A.; Hassan, M.; Roy, H. Advanced applications of carbonaceous materials in sustainable water treatment, energy storage, and CO2 capture: a comprehensive review. Sustainability 2023, 15, 8815.
- Kong, J.; Soh, H.T.; Cassell, A.M.; Quate, C.F.; Dai, H. Synthesis of individual single-walled carbon nanotubes on patterned silicon wafers. Nature 1998, 395, 878–881. [CrossRef]
- Bhagabati, P.; Rahaman, M.; Bhandari, S.; Roy, I.; Dey, A.; Gupta, P.; Ansari, M.; Dutta, A.; Chattopadhyay, D. Synthesis/preparation of carbon materials. Carbon-Containing Polymer Composites 2019, 1-64.
- Shah, K.A.; Tali, B.A. Synthesis of carbon nanotubes by catalytic chemical vapour deposition: A review on carbon sources, catalysts and substrates. Mater. Sci. Semicond. Process. 2016, 41, 67–82. [CrossRef]
- Magrez, A.; Seo, J.W.; Smajda, R.; Mionić, M.; Forró, L. Catalytic CVD synthesis of carbon nanotubes: towards high yield and low temperature growth. Materials 2010, 3, 4871–4891. [CrossRef] [PubMed]
- Yahya, N.; Koziol, K.; Boskovic, B.O; Yahya, N. Synthesis of carbon nanostructures by CVD method. Carbon and Oxide Nanostructures: Synthesis, Characterisation and Applications 2011, 23-49.
- Sahu, S.; Khan, M.S.; Gupta, N.; Chennakesavulu, K.; Sasikumar, C. The hydrogen storage capacity of carbon nano-onions fabricated by thermal chemical vapour deposition. Int. J. Hydrogen Energy. 2024, 52, 1371–1383. [CrossRef]
- Li, X.; Cai, W.; An, J.; Kim, S.; Nah, J.; Yang, D.; Piner, R.; Velamakanni, A.; Jung, I.; Tutuc, E. Large-area synthesis of high-quality and uniform graphene films on copper foils. Science 2009, 324, 1312–1314. [CrossRef]
- Reina, A.; Jia, X.; Ho, J.; Nezich, D.; Son, H.; Bulovic, V.; Dresselhaus, M.S.; Kong, J. Large area, few-layer graphene films on arbitrary substrates by chemical vapor deposition. Nano letters 2009, 9, 30–35. [CrossRef]
- Ago, H.; Tanaka, I.; Orofeo, C.M.; Tsuji, M.; Ikeda, K.i. Patterned growth of graphene over epitaxial catalyst. Small 2010, 6, 1226–1233. [CrossRef] [PubMed]
- Wang, X.-D.; Vinodgopal, K.; Dai, G.-P. Synthesis of carbon nanotubes by catalytic chemical vapor deposition. Perspective of carbon nanotubes 2019, 1–19.
- Gao, L.; Ren, W.; Zhao, J.; Ma, L.-P.; Chen, Z.; Cheng, H.-M. Efficient growth of high-quality graphene films on Cu foils by ambient pressure chemical vapor deposition. Appl. Phys. Lett. 2010, 97.
- Li, X.; Cai, W.; Colombo, L.; Ruoff, R.S. Evolution of graphene growth on Ni and Cu by carbon isotope labeling. Nano letters 2009, 9, 4268–4272. [CrossRef] [PubMed]
- Martínez-Castañon, G.-A.; Nino-Martinez, N.; Martinez-Gutierrez, F.; Martínez-Mendoza, J.; Ruiz, F. Synthesis and antibacterial activity of silver nanoparticles with different sizes. J. Nanoparticle Res. 2008, 10, 1343–1348. [CrossRef]
- Jana, N.R.; Gearheart, L.; Murphy, C.J. Wet chemical synthesis of high aspect ratio cylindrical gold nanorods. The Journal of Physical Chemistry B 2001, 105, 4065–4067. [CrossRef]
- Pillai, Z.S.; Kamat, P.V. What factors control the size and shape of silver nanoparticles in the citrate ion reduction method? J. Phys. Chem. B 2004, 108, 945–951. [CrossRef]
- Bressi, V.; Balu, A.M.; Iannazzo, D.; Espro, C. Recent advances in the synthesis of carbon dots from renewable biomass by high-efficient hydrothermal and microwave green approaches. Curr. Opin. Green Sustain. Chem. 2023, 40, 100742. [CrossRef]
- Zhang, X.; Cao, T.; Zhang, G.; Liu, Q.; Kong, G.; Wang, K.; Jiang, Y.; Zhang, X.; Han, L. Sustainable hydrothermal carbon for advanced electrochemical energy storage. J. Mater. Chem. A. 2024.
- Wang, Y.; Hu, Y.-J.; Hao, X.; Peng, P.; Shi, J.-Y.; Peng, F.; Sun, R.-C. Hydrothermal synthesis and applications of advanced carbonaceous materials from biomass: a review. Adv. Compos. Hybrid Mater. 2020, 3, 267–284. [CrossRef]
- Nasrollahzadeh, M.; Nezafat, Z.; Shafiei, N. Lignin chemistry and valorization. Biopolymer-Based Metal Nanoparticle Chemistry for Sustainable Applications; Elsevier: Amsterdam, The Netherlands 2021, 145-183.
- Pathak, M.; Jeong, S.M.; Rout, C.S. Spinel NiCo2O4 based hybrid materials for supercapacitors: Recent developments and future perspectives. J. Energy Storage 2023, 73, 108881. [CrossRef]
- Bokov, D.; Turki Jalil, A.; Chupradit, S.; Suksatan, W.; Javed Ansari, M.; Shewael, I.H.; Valiev, G.H.; Kianfar, E. Nanomaterial by sol-gel method: synthesis and application. Adv. Mater. Sci. Eng. 2021, 2021, 1–21.
- Yilmaz, E.; Soylak, M. Functionalized nanomaterials for sample preparation methods. In Handbook of Nanomaterials in analytical chemistry, Elsevier: 2020; pp. 375-413.
- Singh, K.; Meena, R.S.; Kumar, S.; Dhyani, S.; Sheoran, S.; Singh, H.M.; Pathak, V.V.; Khalid, Z.; Singh, A.; Chopra, K. India's renewable energy research and policies to phase down coal: Success after Paris agreement and possibilities post-Glasgow Climate Pact. Biomass Bioenergy 2023, 177, 106944.
- Yang, H.; Zhu, M.; Li, Y. Sol–gel research in China: a brief history and recent research trends in synthesis of sol–gel derived materials and their applications. J. Sol-Gel Sci. Technol. 2023, 106, 406–421. [CrossRef]
- Konuk, O.P.; Alsuhile, A.A.; Yousefzadeh, H.; Ulker, Z.; Bozbag, S.E.; García-González, C.A.; Smirnova, I.; Erkey, C. The effect of synthesis conditions and process parameters on aerogel properties. Front. chem. 2023, 11.
- Dervin, S.; Pillai, S.C. An introduction to sol-gel processing for aerogels. Sol-gel materials for energy, environment and electronic applications. 2017, 1-22.
- Xu, Y.; Liu, Z.; Ren, B.; Wang, S.; Zhang, L. Facile Preparation of Carbon Aerogels with Different Drying Methods. In Proceedings of IOP Conference Series: Materials Science and Engineering; p. 012101.
- Lin, C.; Ritter, J.A. Carbonization and activation of sol–gel derived carbon xerogels. Carbon 2000, 38, 849–861. [CrossRef]
- Théry, A.; Béguin, F.; Kocon, L.; Lillo-Rodenas, M.; Linares-Solano, A.; Rouzaud, J.-N. Influence of carbonisation temperature on the structural and electrochemical properties of carbon aerogels. In Proceedings of Carbon Conference 2004.
- Lipeng, Z.; Jianbing, N.; Liming, D.; Zhenhai, X. Effect of Microstructure of Nitrogen-Doped Graphene on Oxygen Reduction Activity in Fuel Cells. 2012.
- Chen, F.; Yan, T.-H.; Bashir, S.; Liu, J.L. Synthesis of nanomaterials using top-down methods. Advanced Nanomaterials and Their Applications in Renewable Energy 2022, 37–60.
- Madhurima, V.; Kumari, K.; Jain, P. Synthesis and study of carbon nanomaterials through arc discharge technique for efficient adsorption of organic dyes. Diam. Relat. Mater. 2024, 141, 110538. [CrossRef]
- De Heer, W.A.; Bacsa, W.; Chatelain, A.; Gerfin, T.; Humphrey-Baker, R.; Forro, L.; Ugarte, D. Aligned carbon nanotube films: production and optical and electronic properties. Science 1995, 268, 845–847. [CrossRef]
- Huang, Z.; Wang, D.; Wen, J.; Sennett, M.; Gibson, H.; Ren, Z. Effect of nickel, iron and cobalt on growth of aligned carbon nanotubes. Appl. Phys. A 2002, 74, 387–391. [CrossRef]
- Watanabe, T.; Itoh, A.; Watanabe, T.; Kizaki, T.; Inaguma, M.; Hosoi, A.; Kawada, H. Post-synthesis treatment improves the electrical properties of dry-spun carbon nanotube yarns. Carbon 2021, 185, 314–323. [CrossRef]
- Arora, N.; Sharma, N.N. Arc discharge synthesis of carbon nanotubes: Comprehensive review. Diam. Relat. Mater. 2014,; 50, 135–150.
- Devi, M.; Rawat, S.; Sharma, S. A comprehensive review of the pyrolysis process: From carbon nanomaterial synthesis to waste treatment. Oxford Open Materials Science 2021, 1, itab014.
- Asif, F.C.; Saha, G.C. Graphene-like carbon structure synthesis from biomass pyrolysis: A critical review on feedstock–process–properties relationship. C 2023, 9, 31.
- Lataf, A.; Jozefczak, M.; Vandecasteele, B.; Viaene, J.; Schreurs, S.; Carleer, R.; Yperman, J.; Marchal, W.; Cuypers, A.; Vandamme, D. The effect of pyrolysis temperature and feedstock on biochar agronomic properties. J. Anal. Appl. Pyrolysis 2022, 168, 105728.
- Predeanu, G.; Slăvescu, V.; Drăgoescu, M.F.; Bălănescu, N.M.; Fiti, A.; Meghea, A.; Samoila, P.; Harabagiu, V.; Ignat, M.; Manea-Saghin, A.-M. Green synthesis of advanced carbon materials used as precursors for adsorbents applied in wastewater treatment. Materials 2023, 16, 1036. [CrossRef]
- Ng, C.H.; Mistoh, M.A.; Teo, S.H.; Galassi, A.; Taufiq-Yap, Y.H.; Siambun, N.J.; Foo, J.; Sipaut, C.S.; Seay, J.; Janaun, J. The roles of carbonaceous wastes for catalysis, energy, and environmental remediation. Catal. Commun. 2024, 106845.
- Shang, S.; Zeng, W. Conductive nanofibres and nanocoatings for smart textiles. In Multidisciplinary know-how for smart-textiles developers, Elsevier: 2013; pp. 92-128.
- Augello, C.; Liu, H. Surface modification of magnesium by functional polymer coatings for neural applications. In Surface Modification of Magnesium and Its Alloys for Biomedical Applications, Elsevier: 2015; pp. 335-353.
- Suzuki, Y.; Takeda, T.; Goto, T. Direct electrochemical formation of carbonaceous material from CO2 in LiCl-KCl melt. Electrochim. Acta 2023, 456, 142464. [CrossRef]
- Zhou, D.; Anoshkina, E.V.; Chow, L.; Chai, G. Synthesis of carbon nanotubes by electrochemical deposition at room temperature. Carbon 2006, 44, 1013–1016. [CrossRef]
- Arul, P.; Gowthaman, N.; John, S.A.; Tominaga, M. Tunable electrochemical synthesis of 3D nucleated microparticles like Cu-BTC MOF-carbon nanotubes composite: Enzyme free ultrasensitive determination of glucose in a complex biological fluid. Electrochim. Acta 2020, 354, 136673. [CrossRef]
- Zhou, D.; Chow, L. Electrochemical deposition of carbon nanoparticles from organic solutions. Google Patents: 2004.
- Ren, Z.; Meng, N.; Shehzad, K.; Xu, Y.; Qu, S.; Yu, B.; Luo, J. Mechanical properties of nickel-graphene composites synthesized by electrochemical deposition. Nanotechnology 2015, 26, 065706. [CrossRef]
- Huang, M.; Xie, L.; Wang, Y.; He, H.; Yu, H.; Cui, J.; Feng, X.; Lou, Z.; Xiong, Y. Efficient uranium electrochemical deposition with a functional phytic Acid-Doped Polyaniline/Graphite sheet electrode by Adsorption-electrodeposition strategy. Chem. Eng. J. 2023, 457, 141221. [CrossRef]
- Wang, Z.; Du, J.; Zhang, M.; Yu, J.; Liu, H.; Chai, X.; Yang, B.; Zhu, C.; Xu, J. Continuous preparation of high performance flexible asymmetric supercapacitor with a very fast, low-cost, simple and scalable electrochemical co-deposition method. J. Power Sources 2019, 437, 226827. [CrossRef]
- Pavlenko, V.; Żółtowska, S.; Haruna, A.; Zahid, M.; Mansurov, Z.; Supiyeva, Z.; Galal, A.; Ozoemena, K.; Abbas, Q.; Jesionowski, T. A comprehensive review of template-assisted porous carbons: Modern preparation methods and advanced applications. Mater. Sci. Eng.: R: Rep. 2022, 149, 100682. [CrossRef]
- Xu, X.; He, Z.; Tang, H.; Sun, Y.; Zhang, S.; Shi, D.; Ji, F. Removal of diclofenac and oxytetracycline from synthetic urine by furfuryl alcohol-derived mesoporous carbon. Chemosphere 2022, 288, 132317. [CrossRef]
- Xia, W.; Qiu, B.; Xia, D.; Zou, R. Facile preparation of hierarchically porous carbons from metal-organic gels and their application in energy storage. Scientific reports 2013, 3, 1935. [CrossRef] [PubMed]
- Dong, S.; Song, Y.; Fang, Y.; Zhu, K.; Ye, K.; Gao, Y.; Yan, J.; Wang, G.; Cao, D. Microwave-assisted synthesis of carbon dots modified graphene for full carbon-based potassium ion capacitors. Carbon 2021, 178, 1–9. [CrossRef]
- Sathish, S.; Nirmala, R.; Kim, H.Y.; Navamathavan, R. Deriving activated carbon using microwave combustion technique and its energy storage applications: a topical review. Carbon Lett. 2022, 32, 1151–1171. [CrossRef]
- Xia, X.; Cheng, C.-F.; Zhu, Y.; Vogt, B.D. Ultrafast microwave-assisted synthesis of highly nitrogen-doped ordered mesoporous carbon. Microporous Mesoporous Mater. 2021, 310, 110639. [CrossRef]
- Onwudiwe, D.C. Microwave-assisted synthesis of PbS nanostructures. Heliyon 2019, 5.
- Brazil, T.R.; Gonçalves, M.; dos Anjos, E.G.R.; de Oliveira Junior, M.S.; Rezende, M.C. Microwave-assisted production of activated carbon in an adapted domestic oven from lignocellulosic waste. Biomass Convers. Biorefin. 2022, 1-14.
- Zhou, Y.; Yan, W.; Yu, X.; Chen, T.; Wang, S.; Zhao, W. Boron and nitrogen co-doped porous carbon for supercapacitors: A comparison between a microwave-assisted and a conventional hydrothermal process. J. Energy Storage 2020, 32, 101706. [CrossRef]
- Baghbanzadeh, M.; Carbone, L.; Cozzoli, P.D.; Kappe, C.O. Microwave-assisted synthesis of colloidal inorganic nanocrystals. Angew. Chem. Int. Ed. 2011, 50, 11312–11359. [CrossRef]
- Albuquerque, H.M.; Pinto, D.C.; Silva, A.M. Microwave irradiation: Alternative heating process for the synthesis of biologically applicable chromones, quinolones, and their precursors. Molecules 2021, 26, 6293. [CrossRef]
- Larosi, M.B.; García, J.d.V.; Rodríguez, A.R. Laser synthesis of nanomaterials. MDPI: 2022; Vol. 12, p 2903.
- Myungjoon, K.; Saho, O.; Taesung, K.; Hidenori, H.; Takafumi, S. Synthesis of nanoparticles by laser ablation: a review. KONA Powder Part. J. 2017, 34, 80–90. [CrossRef]
- Guo, T.; Nikolaev, P.; Thess, A.; Colbert, D.T.; Smalley, R.E. Catalytic growth of single-walled manotubes by laser vaporization. Chem. Phys. Lett. 1995, 243, 49–54. [CrossRef]
- Ganash, E.A.; Al-Jabarti, G.A.; Altuwirqi, R.M. The synthesis of carbon-based nanomaterials by pulsed laser ablation in water. Mater. Res. Express 2019, 7, 015002.
- Yang, G. Laser ablation in liquids: Applications in the synthesis of nanocrystals. Prog. Mater. Sci. 2007, 52, 648–698. [CrossRef]
- Zhang, H.; Zhang, X.; Sun, X.; Ma, Y. Shape-controlled synthesis of nanocarbons through direct conversion of carbon dioxide. Sci. Rep. 2013, 3, 3534. [CrossRef] [PubMed]
- Al-Hamaoy, A.; Chikarakara, E.; Jawad, H.; Gupta, K.; Kumar, D.; Rao, M.R.; Krishnamurthy, S.; Morshed, M.; Fox, E.; Brougham, D. Liquid Phase–Pulsed Laser Ablation: A route to fabricate different carbon nanostructures. Appl. Surf. Sci. 2014, 302, 141–144. [CrossRef]
- Kazemizadeh, F.; Malekfar, R.; Parvin, P. Pulsed laser ablation synthesis of carbon nanoparticles in vacuum. J. Phys. Chem. Solids 2017, 104, 252–256. [CrossRef]
- Khairani, I.Y.; Mínguez-Vega, G.; Doñate-Buendía, C.; Gökce, B. Green nanoparticle synthesis at scale: a perspective on overcoming the limits of pulsed laser ablation in liquids for high-throughput production. Phys. Chem. Chem. Phys 2023, 25, 19380–19408. [CrossRef] [PubMed]
- Neves, V.; Heister, E.; Costa, S.; Tîlmaciu, C.; Flahaut, E.; Soula, B.; Coley, H.M.; Mcfadden, J.; Silva, S.R.P. Design of double-walled carbon nanotubes for biomedical applications. Nanotechnology 2012, 23, 365102.
- Zhang, F.; Xia, Y.; Xu, L.; Gu, N. Surface modification and microstructure of single-walled carbon nanotubes for dental resin-based composites. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 86, 90–97.
- González-García, P.; Centeno, T.; Urones-Garrote, E.; Ávila-Brande, D.; Otero-Díaz, L. Microstructure and surface properties of lignocellulosic-based activated carbons. Appl. Surf. Sci. 2013, 265, 731–737. [CrossRef]
- Chukwuike, V.; Sankar, S.S.; Kundu, S.; Barik, R. Capped and uncapped nickel tungstate (NiWO4) nanomaterials: A comparison study for anti-corrosion of copper metal in NaCl solution. Corros. Sci. 2019, 158, 108101. [CrossRef]
- Shao, L.; Ren, Z.; Zhang, G.; Chen, L. Facile synthesis, characterization of a MnFe2O4/activated carbon magnetic composite and its effectiveness in tetracycline removal. Mater. Chem. Phys. 2012, 135, 16–24. [CrossRef]
- Ai, L.; Huang, H.; Chen, Z.; Wei, X.; Jiang, J. Activated carbon/CoFe2O4 composites: facile synthesis, magnetic performance and their potential application for the removal of malachite green from water. Chem. Eng. J. 2010, 156, 243–249. [CrossRef]
- Argun, M.E.; Dursun, S.; Karatas, M. Removal of Cd (II), Pb (II), Cu (II) and Ni (II) from water using modified pine bark. Desalination 2009, 249, 519–527. [CrossRef]
- Muniyalakshmi, M.; Sethuraman, K.; Silambarasan, D. Synthesis and characterization of graphene oxide nanosheets. Mater. Today: Proc. 2020, 21, 408–410.
- Zhang, X.; Li, K.; Li, H.; Lu, J.; Fu, Q.; Chu, Y. Graphene nanosheets synthesis via chemical reduction of graphene oxide using sodium acetate trihydrate solution. Synth. Met. 2014, 193, 132–138. [CrossRef]
- Rao, A.M.; Richter, E.; Bandow, S.; Chase, B.; Eklund, P.; Williams, K.; Fang, S.; Subbaswamy, K.; Menon, M.; Thess, A. Diameter-selective Raman scattering from vibrational modes in carbon nanotubes. Science 1997, 275, 187–191. [CrossRef]
- Rao, C.; Subrahmanyam, K.; Ramakrishna Matte, H.; Maitra, U.; Moses, K.; Govindaraj, A. Graphene: synthesis, functionalization and properties. Int. J. Mod. Phys. B 2011, 25, 4107–4143.
- Titirici, M.M.; Thomas, A.; Yu, S.-H.; Müller, J.-O.; Antonietti, M. A direct synthesis of mesoporous carbons with bicontinuous pore morphology from crude plant material by hydrothermal carbonization. Chem. Mat. 2007, 19, 4205–4212. [CrossRef]
- Servant, A.; Jacobs, I.; Bussy, C.; Fabbro, C.; Da Ros, T.; Pach, E.; Ballesteros, B.; Prato, M.; Nicolay, K.; Kostarelos, K. Gadolinium-functionalised multi-walled carbon nanotubes as a T1 contrast agent for MRI cell labelling and tracking. Carbon 2016, 97, 126–133. [CrossRef]
- Liu, F.; Wu, J.; Chen, K.; Xue, D. Morphology study by using scanning electron microscopy. Microscopy: science, technology, applications and education 2010, 3, 1781–1792.
- Wang, Z.G.; Liu, K.G.; Jing, M.; Pang, B. SEM analysis of non-conducting materials under low vacuum conditions. Adv. Mater. Res. 2011, 152, 897–901.
- Hanifah, M.F.R.; Jaafar, J.; Othman, M.; Ismail, A.; Rahman, M.A.; Yusof, N.; Salleh, W.; Aziz, F. Facile synthesis of highly favorable graphene oxide: Effect of oxidation degree on the structural, morphological, thermal and electrochemical properties. Materialia 2019, 6, 100344. [CrossRef]
- Chambers, S.A. Elastic scattering and interference of backscattered primary, Auger and X-ray photoelectrons at high kinetic energy: principles and applications. Surf. Sci. Rep. 1992, 16, 261–331. [CrossRef]
- Beny-Bassez, C.; Rouzaud, J. Characterization of carbonaceous materials by correlated electron and optical microscopy and Raman microspectroscopy. Scanning electron microscopy 1985, 1, 119–132.
- Crelling, J.; Bensley, D. Characterization of carbon materials using quantitative optical microscopy. Preprints of Papers, American Chemical Society, Division of Fuel Chemistry 1995, 40.
- Peng, X.; Barber, Z.; Clyne, T. Surface roughness of diamond-like carbon films prepared using various techniques. Surf. Coat. Technol. 2001, 138, 23–32. [CrossRef]
- Usachov, D.; Dobrotvorskii, A.; Varykhalov, A.; Rader, O.; Gudat, W.; Shikin, A.; Adamchuk, V. Experimental and theoretical study of the morphology of commensurate and incommensurate graphene layers on Ni single-crystal surfaces. Phys. Rev. B 2008, 78, 085403. [CrossRef]
- Robertson, A.W.; Warner, J.H. Atomic resolution imaging of graphene by transmission electron microscopy. Nanoscale 2013, 5, 4079–4093. [CrossRef]
- Trudeau, M.; Laul, D.; Veillette, R.; Serventi, A.; Mauger, A.; Julien, C.; Zaghib, K. In situ high-resolution transmission electron microscopy synthesis observation of nanostructured carbon coated LiFePO4. J. Power Sources. 2011, 196, 7383–7394. [CrossRef]
- Menzel, R.; Bismarck, A.; Shaffer, M.S. Deconvolution of the structural and chemical surface properties of carbon nanotubes by inverse gas chromatography. Carbon 2012, 50, 3416–3421. [CrossRef]
- Legras, A.; Kondor, A.; Alcock, M.; Heitzmann, M.; Truss, R. Inverse gas chromatography for natural fibre characterisation: dispersive and acid-base distribution profiles of the surface energy. Cellulose 2017, 24, 4691–4700. [CrossRef]
- Belgacem, M.N.; Gandini, A. Inverse gas chromatography as a tool to characterize dispersive and acid-base properties of the surface of fibers and powders. Surfactant science series 1999, 41-124.
- Farivar, F.; Yap, P.L.; Karunagaran, R.U.; Losic, D. Thermogravimetric analysis (TGA) of graphene materials: effect of particle size of graphene, graphene oxide and graphite on thermal parameters. C 2021, 7, 41.
- WHO, W.H.O. Ambient air pollution: A global assessment of exposure and burden of disease. 2016.
- Wassersug, R.J.; Fox, I.N. The molecule that makes prostate cancer easy to find shows why it will be so difficult to cure. JOMH 2021, 17, 1–3.
- Upadhyay, A. Cancer: An unknown territory; rethinking before going ahead. Genes & diseases 2021, 8, 655–661.
- Chang, D.S.; Lasley, F.D.; Das, I.J.; Mendonca, M.S.; Dynlacht, J.R.; Chang, D.S.; Lasley, F.D.; Das, I.J.; Mendonca, M.S.; Dynlacht, J.R. Hyperthermia. Basic Radiotherapy Physics and Biology 2021, 331–336.
- Szwed, M.; Marczak, A. Application of Nanoparticles for Magnetic Hyperthermia for Cancer Treatment—The Current State of Knowledge. Cancers 2024, 16, 1156. [CrossRef] [PubMed]
- Suleman, M.; Riaz, S.; Jalil, R. A mathematical modeling approach toward magnetic fluid hyperthermia of cancer and unfolding heating mechanism. J. Therm. Anal. Calorim. 2021, 146, 1193–1219. [CrossRef]
- Osaci, M.; Cacciola, M. Influence of the magnetic nanoparticle coating on the magnetic relaxation time. Beilstein J. Nanotechnol. 2020, 11, 1207–1216. [CrossRef]
- Anand, M. Magnetic relaxation in two-dimensional assembly of dipolar interacting nanoparticles. J. Magn. Magn. Mater. 2022, 552, 169201. [CrossRef]
- Barrera, C.A.; Francavilla, M.L.; Serai, S.D.; Edgar, J.C.; Jaimes, C.; Gee, M.S.; Roberts, T.P.; Otero, H.J.; Adzick, N.S.; Victoria, T. Specific absorption rate and specific energy dose: comparison of 1.5-T versus 3.0-T fetal MRI. Radiology 2020, 295, 664–674. [CrossRef]
- Das, P.; Salvioni, L.; Malatesta, M.; Vurro, F.; Mannucci, S.; Gerosa, M.; Rizzuto, M.A.; Tullio, C.; Degrassi, A.; Colombo, M. Colloidal polymer-coated Zn-doped iron oxide nanoparticles with high relaxivity and specific absorption rate for efficient magnetic resonance imaging and magnetic hyperthermia. J. Colloid Interface Sci. 2020, 579, 186–194.
- Huang, P.-C.; Chaney, E.J.; Aksamitiene, E.; Barkalifa, R.; Spillman Jr, D.R.; Bogan, B.J.; Boppart, S.A. Biomechanical sensing of in vivo magnetic nanoparticle hyperthermia-treated melanoma using magnetomotive optical coherence elastography. Theranostics 2021, 11, 5620. [CrossRef] [PubMed]
- Liu, F.; Wu, H.; Peng, B.; Zhang, S.; Ma, J.; Deng, G.; Zou, P.; Liu, J.; Chen, A.T.; Li, D. Vessel-targeting nanoclovers enable noninvasive delivery of magnetic hyperthermia–chemotherapy combination for brain cancer treatment. Nano Letters 2021, 21, 8111–8118. [CrossRef] [PubMed]
- Le Guevelou, J.; Chirila, M.E.; Achard, V.; Guillemin, P.C.; Lorton, O.; Uiterwijk, J.W.; Dipasquale, G.; Salomir, R.; Zilli, T. Combined hyperthermia and radiotherapy for prostate cancer: a systematic review. Int. J. Hyperth. 2022, 39, 547-556.
- Hatamie, S.; Balasi, Z.M.; Ahadian, M.M.; Mortezazadeh, T.; Shams, F.; Hosseinzadeh, S. Hyperthermia of breast cancer tumor using graphene oxide-cobalt ferrite magnetic nanoparticles in mice. J. Drug Deliv. Sci. Technol. 2021, 65, 102680. [CrossRef]
- Moorthy, M.S.; Oh, Y.; Bharathiraja, S.; Manivasagan, P.; Rajarathinam, T.; Jang, B.; Phan, T.T.V.; Jang, H.; Oh, J. Synthesis of amine-polyglycidol functionalised Fe3O4@SiO2 nanocomposites for magnetic hyperthermia, pH-responsive drug delivery, and boimaging applications. RSC Adv. 2016, 6, 110444–110453.
- Cole, A.J.; David, A.E.; Wang, J.; Galbán, C.J.; Hill, H.L.; Yang, V.C. Polyethylene glycol modified, cross-linked starch-coated iron oxide nanoparticles for enhanced magnetic tumor targeting. Biomaterials 2011, 32, 2183–2193. [CrossRef] [PubMed]
- Liu, X.L.; Fan, H.M.; Yi, J.B.; Yang, Y.; Choo, E.S.G.; Xue, J.M.; Di Fan, D.; Ding, J. Optimization of surface coating on Fe3O4 nanoparticles for high performance magnetic hyperthermia agents. J. Mater. Chem. 2012, 22, 8235–8244. [CrossRef]
- Khot, V.; Salunkhe, A.; Thorat, N.; Ningthoujam, R.; Pawar, S. Induction heating studies of dextran coated MgFe2O4 nanoparticles for magnetic hyperthermia. Dalton Trans. 2013, 42, 1249–1258. [CrossRef]
- Thorat, N.; Patil, R.; Khot, V.; Salunkhe, A.; Prasad, A.; Barick, K.; Ningthoujam, R.; Pawar, S. Highly water-dispersible surface-functionalized LSMO nanoparticles for magnetic fluid hyperthermia application. New J. Chem. 2013, 37, 2733–2742. [CrossRef]
- Thomas, L.A.; Dekker, L.; Kallumadil, M.; Southern, P.; Wilson, M.; Nair, S.P.; Pankhurst, Q.A.; Parkin, I.P. Carboxylic acid-stabilised iron oxide nanoparticles for use in magnetic hyperthermia. J. Mater. Chem. 2009, 19, 6529–6535. [CrossRef]
- Céspedes, E.; Byrne, J.M.; Farrow, N.; Moise, S.; Coker, V.S.; Bencsik, M.; Lloyd, J.R.; Telling, N.D. Bacterially synthesized ferrite nanoparticles for magnetic hyperthermia applications. Nanoscale 2014, 6, 12958–12970. [CrossRef] [PubMed]
- Nayak, K.S.; Lim, Y.; Campbell-Washburn, A.E.; Steeden, J. Real-time magnetic resonance imaging. J. Magn. Reson. Imaging 2022, 55, 81–99. [CrossRef] [PubMed]
- Serai, S.D. Basics of magnetic resonance imaging and quantitative parameters T1, T2, T2*, T1rho and diffusion-weighted imaging. Pediatr. Radiol. 2022, 52, 217–227. [CrossRef] [PubMed]
- Wang, F.; Chen, X.; Zhao, Z.; Tang, S.; Huang, X.; Lin, C.; Cai, C.; Zheng, N. Synthesis of magnetic, fluorescent and mesoporous core-shell-structured nanoparticles for imaging, targeting and photodynamic therapy. J. Mater. Chem 2011, 21, 11244–11252. [CrossRef]
- Fang, Y.; Zhou, L.; Zhao, J.; Zhang, Y.; Yang, M.; Yi, C. Facile synthesis of pH-responsive gadolinium (III)-doped carbon nanodots with red fluorescence and magnetic resonance properties for dual-readout logic gate operations. Carbon 2020, 166, 265–272. [CrossRef]
- Song, G.; Kenney, M.; Chen, Y.-S.; Zheng, X.; Deng, Y.; Chen, Z.; Wang, S.X.; Gambhir, S.S.; Dai, H.; Rao, J. Carbon-coated FeCo nanoparticles as sensitive magnetic-particle-imaging tracers with photothermal and magnetothermal properties. Nat. Biomed. Eng. 2020, 4, 325–334. [CrossRef] [PubMed]
- Karawdeniya, B.I.; Damry, A.M.; Murugappan, K.; Manjunath, S.; Bandara, Y.N.D.; Jackson, C.J.; Tricoli, A.; Neshev, D. Surface functionalization and texturing of optical metasurfaces for sensing applications. Chem. Rev. 2022, 122, 14990–15030.
- Pan, C.; Wei, H.; Han, Z.; Wu, F.; Mao, L. Enzymatic electrochemical biosensors for in situ neurochemical measurement. Curr. Opin. Electrochem. 2020, 19, 162–167. [CrossRef]
- Huang, X.; Zhu, Y.; Kianfar, E. Nano biosensors: properties, applications and electrochemical techniques. J. Mater. Res. Technol. 2021, 12, 1649–1672. [CrossRef]
- Bucur, B.; Purcarea, C.; Andreescu, S.; Vasilescu, A. Addressing the selectivity of enzyme biosensors: solutions and perspectives. Sensors 2021, 21, 3038. [CrossRef] [PubMed]
- Liu, D.; Wang, J.; Wu, L.; Huang, Y.; Zhang, Y.; Zhu, M.; Wang, Y.; Zhu, Z.; Yang, C. Trends in miniaturized biosensors for point-of-care testing. TrAC 2020, 122, 115701.
- Tu, J.; Torrente-Rodríguez, R.M.; Wang, M.; Gao, W. The era of digital health: A review of portable and wearable affinity biosensors. Adv. Funct. Mater. 2020, 30, 1906713. [CrossRef]
- Sun, B.; Gou, X.; Bai, R.; Abdelmoaty, A.A.A.; Ma, Y.; Zheng, X.; Hu, F. Direct electrochemistry and electrocatalysis of lobetyolin via magnetic functionalized reduced graphene oxide film fabricated electrochemical sensor. Mater. Sci. Eng. C 2017, 74, 515–524. [CrossRef] [PubMed]
- Shi, X.; Wang, Y.; Peng, C.; Zhang, Z.; Chen, J.; Zhou, X.; Jiang, H. Enantiorecognition of tyrosine based on a novel magnetic electrochemical chiral sensor. Electrochim. Acta 2017, 241, 386–394. [CrossRef]
- Bagheri, H.; Khoshsafar, H.; Amidi, S.; Ardakani, Y.H. Fabrication of an electrochemical sensor based on magnetic multi-walled carbon nanotubes for the determination of ciprofloxacin. Anal. Methods 2016, 8, 3383–3390. [CrossRef]
- Jeong, S.; Park, J.; Pathania, D.; Castro, C.M.; Weissleder, R.; Lee, H. Integrated magneto–electrochemical sensor for exosome analysis. ACS nano 2016, 10, 1802–1809. [CrossRef]
- Thakur, A.; Ganeshpurkar, A.; Jaiswal, A. Charcoal in dentistry. Natural Oral Care in Dental Therapy 2020, 197-209.
- Aziz, A.; Ayesha, A.; Sevianti, E.; Makalalag, G.V.; Noordiansyah, M.A.; Wicaksono, M.P.; Khairunnisa, S.; Yuniarsih, N. Effectiveness of Toothpaste from Activated Charcoal as Teeth Whitening: A Systematic Literature Review. Eureka Herba Indonesia 2023, 4, 263–267. [CrossRef]
-
Balasubramanian, P.; Selvam, S.M. Valorization of biomass to activated carbon for wound dressing applications: Recent trends and future challenges. Bioresour. Technol. 2023, 101562.
- Roberts, E.; Mason, S. Oral Care–A Mouthful of Chemistry. 2020.
- Rajendiran, M.; Trivedi, H.M.; Chen, D.; Gajendrareddy, P.; Chen, L. Recent development of active ingredients in mouthwashes and toothpastes for periodontal diseases. Molecules 2021, 26, 2001. [CrossRef]
- Huaman-Sarmiento, E.; Mayta-Tovalino, F.; Munive-Degregori, A.A.; Mendoza, R.; Barja-Ore, J.; Mauricio-Vilchez, C. Uses and applications of activated charcoal in the manufacture of toothpastes and oral rinses: A narrative review. J. Int. Oral Health 2023, 15, 237–241. [CrossRef]
- Hamza, B.; Tanner, M.L.; Attin, T.; Wegehaupt, F.J. Dentin abrasivity and cleaning efficacy of novel/alternative toothpastes. Oral Health Prev. Dent. 2020, 18, 713–718.
- Heydari, Z.; Najimi, M.; Mirzaei, H.; Shpichka, A.; Ruoss, M.; Farzaneh, Z.; Montazeri, L.; Piryaei, A.; Timashev, P.; Gramignoli, R. Tissue engineering in liver regenerative medicine: insights into novel translational technologies. Cells 2020, 9, 304. [CrossRef]
- Qasim, M.; Chae, D.S.; Lee, N.Y. Bioengineering strategies for bone and cartilage tissue regeneration using growth factors and stem cells. J. Biomed. Mater. Res. A 2020, 108, 394–411. [CrossRef] [PubMed]
- Zurina, I.M.; Presniakova, V.S.; Butnaru, D.V.; Svistunov, A.A.; Timashev, P.S.; Rochev, Y.A. Tissue engineering using a combined cell sheet technology and scaffolding approach. Acta Biomater. 2020, 113, 63–83. [CrossRef] [PubMed]
- Serafin, A.; Murphy, C.; Rubio, M.C.; Collins, M.N. Printable alginate/gelatin hydrogel reinforced with carbon nanofibers as electrically conductive scaffolds for tissue engineering. Mater. Sci. Eng. C 2021, 122, 111927. [CrossRef] [PubMed]
- Samadian, H.; Mobasheri, H.; Azami, M.; Faridi-Majidi, R. Osteoconductive and electroactive carbon nanofibers/hydroxyapatite nanocomposite tailored for bone tissue engineering: in vitro and in vivo studies. Sci. Rep. 2020, 10, 14853. [CrossRef]
- Ghosh, P.; Peters, J. Impulsive differential equation model in methanol poisoning detoxification: Efficacy of activated charcoal antidote in combating methanol poisoning. J. Math. Chem. 2020, 58, 126–145. [CrossRef]
- Al-Jelaify, M.; AlHomidah, S. The individualized management approach for acute poisoning. Adv. Pharmacol. Pharm. Sci. 2021, 2021, 9926682.
- Sharma, G.; Sharma, S.; Kumar, A.; Lai, C.W.; Naushad, M.; Shehnaz; Iqbal, J.; Stadler, F.J. Activated carbon as superadsorbent and sustainable material for diverse applications. Adsorpt. Sci. Technol. 2022, 2022, 4184809. [CrossRef]
- Vinarov, Z.; Abdallah, M.; Agundez, J.A.; Allegaert, K.; Basit, A.W.; Braeckmans, M.; Ceulemans, J.; Corsetti, M.; Griffin, B.T.; Grimm, M. Impact of gastrointestinal tract variability on oral drug absorption and pharmacokinetics: An UNGAP review. Eur. J. Pharm. Sci. 2021, 162, 105812. [CrossRef] [PubMed]
- Nnadozie, E.C.; Ajibade, P.A. Adsorption, kinetic and mechanistic studies of Pb (II) and Cr (VI) ions using APTES functionalized magnetic biochar. Microporous Mesoporous Mater. 2020, 309, 110573. [CrossRef]
- Skov, K.; Graudal, N.A.; Jürgens, G. The effect of activated charcoal on drug exposure following intravenous administration: A meta-analysis. Basic Clin. Pharmacol. Toxicol. 2021, 128, 568–578. [CrossRef] [PubMed]
- Zhang, D.; Wei, C.; Hop, C.E.; Wright, M.R.; Hu, M.; Lai, Y.; Khojasteh, S.C.; Humphreys, W.G. Intestinal excretion, intestinal recirculation, and renal tubule reabsorption are underappreciated mechanisms that drive the distribution and pharmacokinetic behavior of small molecule drugs. J. Med. Chem. 2021, 64, 7045–7059.
- Lupaşcu, T.; Petuhov, O.; Ţîmbaliuc, N.; Cibotaru, S.; Rotaru, A. Adsorption capacity of vitamin B12 and creatinine on highly-mesoporous activated carbons obtained from lignocellulosic raw materials. Molecules 2020, 25, 3095. [CrossRef] [PubMed]
- Lim, B.-Y.; Azmi, F.; Ng, S.-F. LL37 Microspheres Loaded on Activated Carbon-chitosan Hydrogel: Anti-bacterial and Anti-toxin Wound Dressing for Chronic Wound Infections. AAPS PharmSciTech 2024, 25, 110. [CrossRef] [PubMed]
- Brown, G.C.; Heneka, M.T. The endotoxin hypothesis of Alzheimer’s disease. Molecular Neurodegeneration 2024, 19, 30. [CrossRef] [PubMed]
- Uberoi, A.; McCready-Vangi, A.; Grice, E.A. The wound microbiota: microbial mechanisms of impaired wound healing and infection. Nat. Rev. Microbiol. 2024, 1-15.
- Rameli, N.; Lim, B.-Y.; Leong, P.-Y.; Lim, C.-C.; Ng, S.-F. Chitosan-reinforced nanocrystalline cellulose hydrogels containing activated carbon as antitoxin wound dressing Macromol. Res. 2024, 1–12.
- Liu, Y.; Xu, B.; Lu, M.; Li, S.; Guo, J.; Chen, F.; Xiong, X.; Yin, Z.; Liu, H.; Zhou, D. Ultrasmall Fe-doped carbon dots nanozymes for photoenhanced antibacterial therapy and wound healing. Bioact. Mater. 2022, 12, 246–256.
- Shakiba, M.; Jahangiri, P.; Rahmani, E.; Hosseini, S.M.; Bigham, A.; Foroozandeh, A.; Tajiki, A.; Pourmadadi, M.; Nasiri, S.; Jouybar, S. Drug-loaded carbon nanotube incorporated in nanofibers: a multifunctional nanocomposite for smart chronic wound healing. ACS Appl. Polym. Mater. 2023, 5, 5662–5675.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).