Submitted:
25 June 2024
Posted:
26 June 2024
You are already at the latest version
Abstract
Keywords:
Introduction
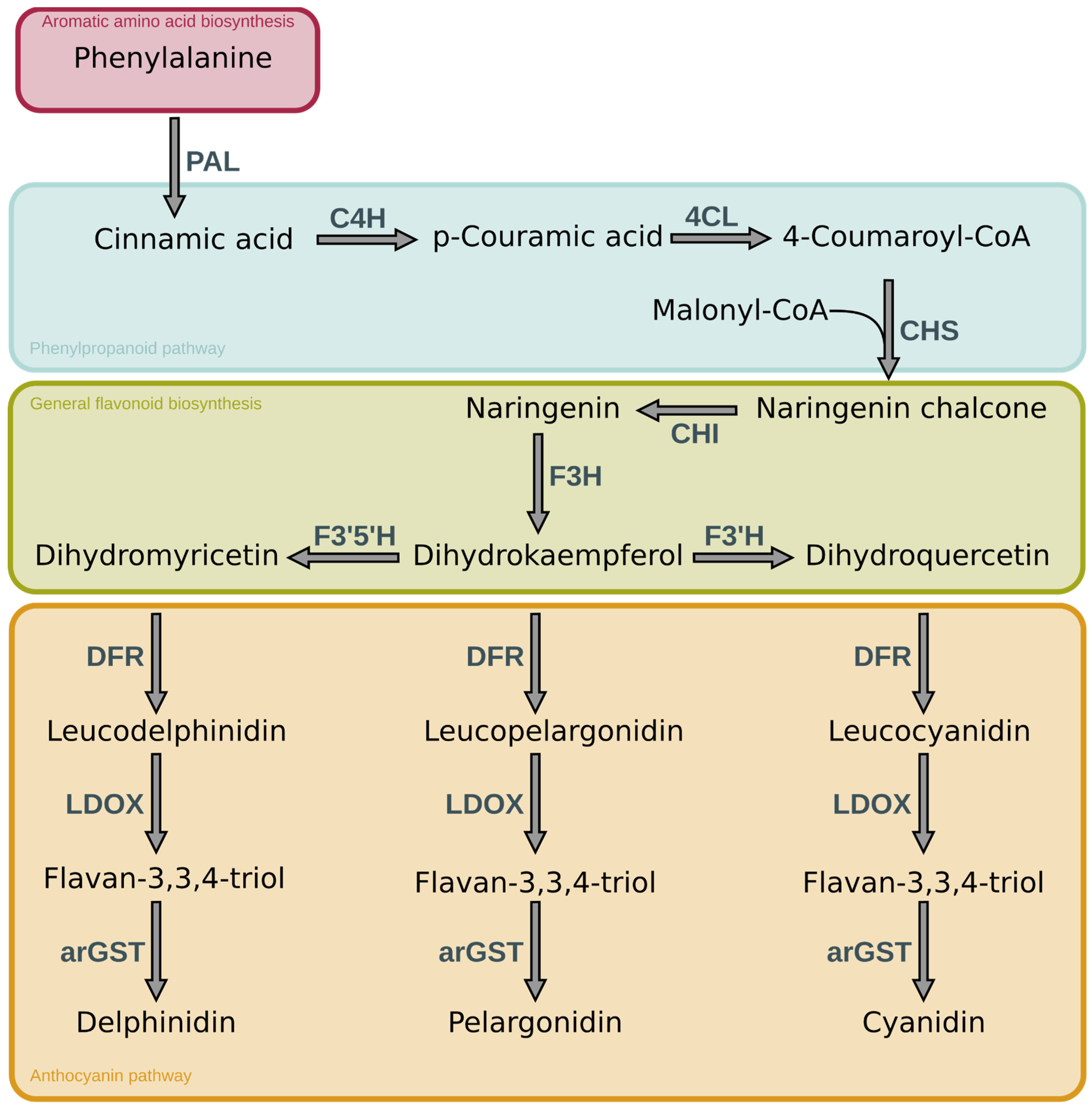
Structure and Biosynthesis of Anthocyanins
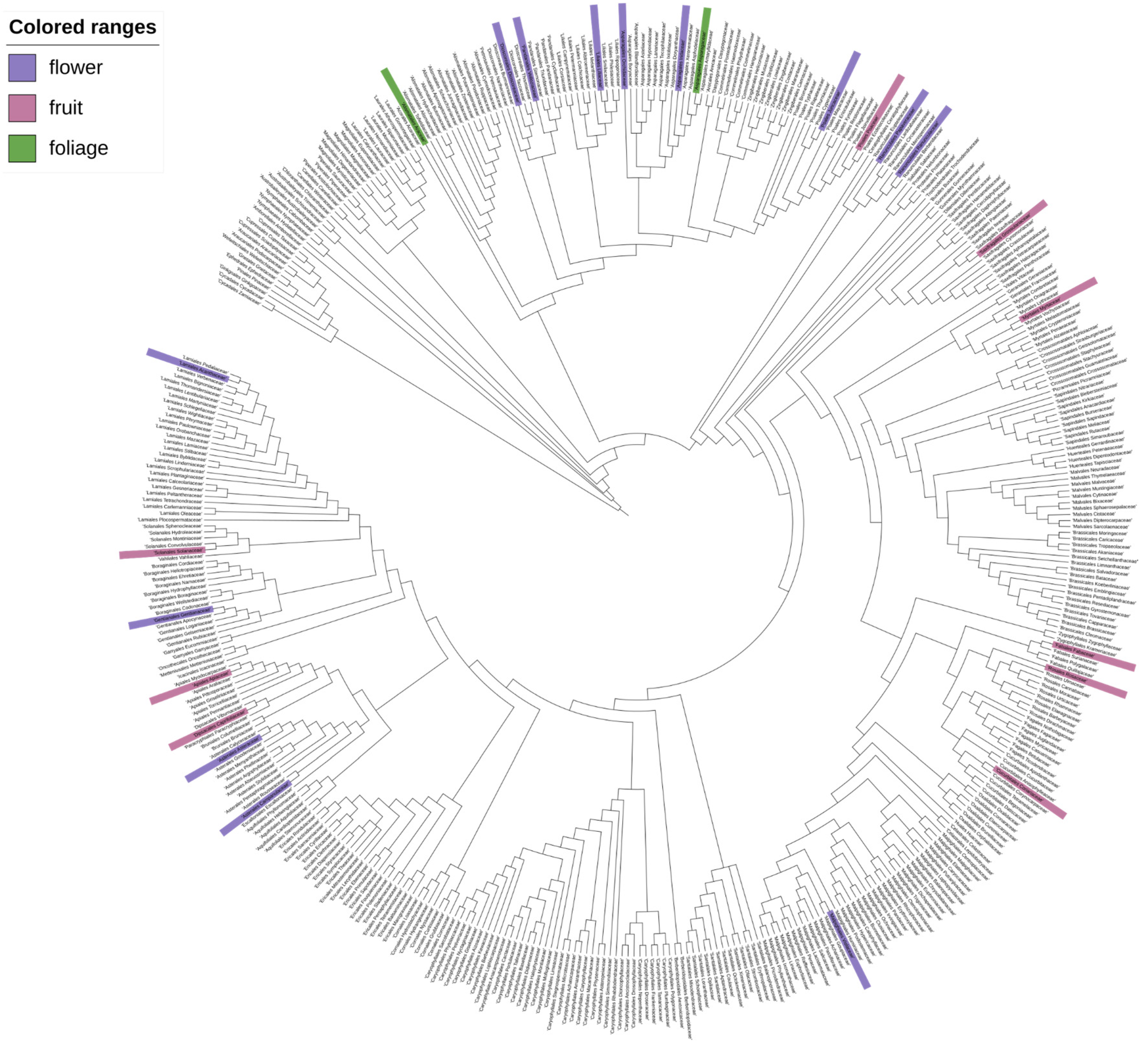
Dark Pigmentation in Plants - Where Does It Occur?
What Is Causing Dark Pigmentation?
How Is the Formation of Dark Pigmentation Regulated?
The Role of Transcription Factors in Dark Pigmentation
Solanum Lycopersicum a Model to Understand the Regulation of Anthocyanin Accumulation in Dark Phenotypes
Genetic Modification Resulting in a Dark Phenotype
Stability of Anthocyanin Pigmentation
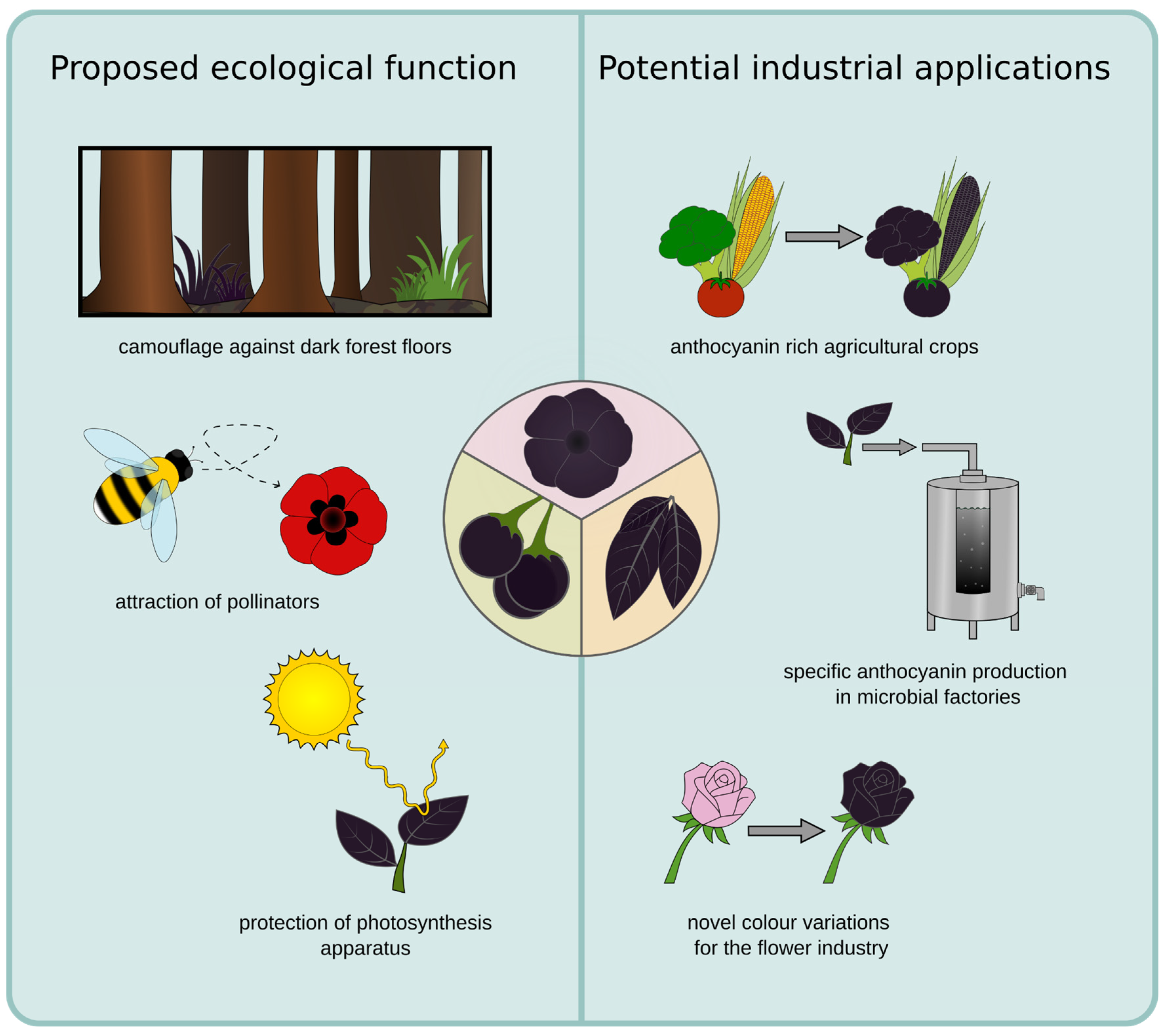
What Are the Proposed Ecological Functions and Potential Industrial Applications of Dark Pigmentation?
Conclusion and Future Perspectives
Supplementary Materials
Abbreviations
| PAL | phenylalanine ammonia-lyase |
| C4H | cinnamic acid 4-hydroxylase |
| 4CL | 4-coumarate-CoA ligase |
| CHS | chalcone synthase |
| CHI | chalcone isomerase |
| F3H | flavanone 3-hydroxylase |
| F3’H | flavonoid 3’-hydroxylase |
| F3’5’H | flavonoid 3’,5’-hydroxylase |
| DFR | dihydroflavonol 4-reductase |
| LDOX | leucoanthocyanidin dioxygenase |
| arGST | anthocyanin-related Glutathione S-transferase |
| CIE | Commission internationale de l’éclairage |
| MYB | Myeloblastosis |
| bHLH | basic helix-loop-helix |
References
- Alappat, B., & Alappat, J. (2020). Anthocyanin Pigments: Beyond Aesthetics. Molecules, 25(23), Article 23. [CrossRef]
- Albert, N. W., Iorizzo, M., Mengist, M. F., Montanari, S., Zalapa, J., Maule, A., Edger, P. P., Yocca, A. E., Platts, A. E., Pucker, B., & Espley, R. V. (2023). Vaccinium as a comparative system for understanding of complex flavonoid accumulation profiles and regulation in fruit. Plant Physiology, 192(3), 1696–1710. [CrossRef]
- Amr, A., & Al-Tamimi, E. (2007). Stability of the crude extracts of Ranunculus asiaticus anthocyanins and their use as food colourants. International Journal of Food Science & Technology, 42(8), 985–991. [CrossRef]
- Appelhagen, I., Wulff-Vester, A. K., Wendell, M., Hvoslef-Eide, A.-K., Russell, J., Oertel, A., Martens, S., Mock, H.-P., Martin, C., & Matros, A. (2018). Colour bio-factories: Towards scale-up production of anthocyanins in plant cell cultures. Metabolic Engineering, 48, 218–232. [CrossRef]
- Aramwit, P., Bang, N., & Srichana, T. (2010). The properties and stability of anthocyanins in mulberry fruits. Food Research International, 43(4), 1093–1097. [CrossRef]
- Awika, J. M., Rooney, L. W., & Waniska, R. D. (2005). Anthocyanins from black sorghum and their antioxidant properties. Food Chemistry, 90(1), 293–301. [CrossRef]
- Baguette, M., Bertrand, J. A. M., Stevens, V. M., & Schatz, B. (2020). Why are there so many bee-orchid species? Adaptive radiation by intra-specific competition for mnesic pollinators. Biological Reviews, 95(6), 1630–1663. [CrossRef]
- Baudry, A., Heim, M. A., Dubreucq, B., Caboche, M., Weisshaar, B., & Lepiniec, L. (2004). TT2, TT8, and TTG1 synergistically specify the expression of BANYULS and proanthocyanidin biosynthesis in Arabidopsis thaliana. The Plant Journal: For Cell and Molecular Biology, 39(3), 366–380. [CrossRef]
- Belwal, T., Nabavi, S. F., Nabavi, S. M., & Habtemariam, S. (2017). Dietary Anthocyanins and Insulin Resistance: When Food Becomes a Medicine. Nutrients, 9(10), Article 10. [CrossRef]
- Blando, F., Berland, H., Maiorano, G., Durante, M., Mazzucato, A., Picarella, M. E., Nicoletti, I., Gerardi, C., Mita, G., & Andersen, Ø. M. (2019). Nutraceutical Characterization of Anthocyanin-Rich Fruits Produced by “Sun Black” Tomato Line. Frontiers in Nutrition, 6. [CrossRef]
- Bradshaw, E., Rudall, P. J., Devey, D. S., Thomas, M. M., Glover, B. J., & Bateman, R. M. (2010). Comparative labellum micromorphology of the sexually deceptive temperate orchid genus Ophrys: Diverse epidermal cell types and multiple origins of structural colour. Botanical Journal of the Linnean Society, 162(3), 504–540. [CrossRef]
- Breitkopf, H., Onstein, R. E., Cafasso, D., Schlüter, P. M., & Cozzolino, S. (2015). Multiple shifts to different pollinators fuelled rapid diversification in sexually deceptive Ophrys orchids. New Phytologist, 207(2), 377–389. [CrossRef]
- Büdel, A. (1959). The microclimate of flowers blooming near the ground. https://scholar.google.com/scholar_lookup?journal=Z.+Bienenforsch.&title=The+microclimate+of+flowers+blooming+near+the+ground&author=A.+B%C3%BCdel&volume=4&publication_year=1959&pages=131-140&.
- Butelli, E., Bulling, K., Hill, L., & Martin, C. (2021). Beyond Purple Tomatoes: Combined Strategies Targeting Anthocyanins to Generate Crimson, Magenta, and Indigo Fruit. Horticulturae, 7(9), Article 9. [CrossRef]
- Butelli, E., Titta, L., Giorgio, M., Mock, H.-P., Matros, A., Peterek, S., Schijlen, E. G. W. M., Hall, R. D., Bovy, A. G., Luo, J., & Martin, C. (2008). Enrichment of tomato fruit with health-promoting anthocyanins by expression of select transcription factors. Nature Biotechnology, 26(11), 1301–1308. [CrossRef]
- Cao, X., Qiu, Z., Wang, X., Van Giang, T., Liu, X., Wang, J., Wang, X., Gao, J., Guo, Y., Du, Y., Wang, G., & Huang, Z. (2017). A putative R3 MYB repressor is the candidate gene underlying atroviolacium, a locus for anthocyanin pigmentation in tomato fruit. Journal of Experimental Botany, 68(21–22), 5745–5758. [CrossRef]
- Chalker-Scott, L. (1999). Environmental Significance of Anthocyanins in Plant Stress Responses. Photochemistry and Photobiology, 70(1), 1–9. [CrossRef]
- Christie, P. J., Alfenito, M. R., & Walbot, V. (1994). Impact of low-temperature stress on general phenylpropanoid and anthocyanin pathways: Enhancement of transcript abundance and anthocyanin pigmentation in maize seedlings. Planta, 194(4), 541–549. [CrossRef]
- Colanero, S., Tagliani, A., Perata, P., & Gonzali, S. (2020). Alternative Splicing in the Anthocyanin Fruit Gene Encoding an R2R3 MYB Transcription Factor Affects Anthocyanin Biosynthesis in Tomato Fruits. Plant Communications, 1(1), 100006. [CrossRef]
- Davies, K. M., Albert, N. W., Schwinn, K. E., Davies, K. M., Albert, N. W., & Schwinn, K. E. (2012). From landing lights to mimicry: The molecular regulation of flower colouration and mechanisms for pigmentation patterning. Functional Plant Biology, 39(8), 619–638. [CrossRef]
- de Jager, M. L., Willis-Jones, E., Critchley, S., & Glover, B. J. (2017). The impact of floral spot and ring markings on pollinator foraging dynamics. Evolutionary Ecology, 31(2), 193–204. [CrossRef]
- de Rosso, V. V., & Mercadante, A. Z. (2007). Evaluation of colour and stability of anthocyanins from tropical fruits in an isotonic soft drink system. Innovative Food Science & Emerging Technologies, 8(3), 347–352. [CrossRef]
- Deguchi, A., Ohno, S., Hosokawa, M., Tatsuzawa, F., & Doi, M. (2013). Endogenous post-transcriptional gene silencing of flavone synthase resulting in high accumulation of anthocyanins in black dahlia cultivars. Planta, 237(5), 1325–1335. [CrossRef]
- Deguchi, A., Tatsuzawa, F., Hosokawa, M., Doi, M., & Ohno, S. (2016). Quantitative Evaluation of the Contribution of Four Major Anthocyanins to Black Flower Coloring of Dahlia Petals. The Horticulture Journal, advpub, MI–121. [CrossRef]
- Devic, M., Guilleminot, J., Debeaujon, I., Bechtold, N., Bensaude, E., Koornneef, M., Pelletier, G., & Delseny, M. (1999). The BANYULS gene encodes a DFR-like protein and is a marker of early seed coat development. The Plant Journal: For Cell and Molecular Biology, 19(4), 387–398. [CrossRef]
- Díaz-García, M. C., Castellar, M. R., Obón, J. M., Obón, C., Alcaraz, F., & Rivera, D. (2015). Production of an anthocyanin-rich food colourant from Thymus moroderi and its application in foods. Journal of the Science of Food and Agriculture, 95(6), 1283–1293. [CrossRef]
- Dong, Y., Wu, X., Han, L., Bian, J., He, C., El-Omar, E., Gong, L., & Wang, M. (2022). The Potential Roles of Dietary Anthocyanins in Inhibiting Vascular Endothelial Cell Senescence and Preventing Cardiovascular Diseases. Nutrients, 14(14), Article 14. [CrossRef]
- Duan, Q., Goodale, E., & Quan, R. (2014). Bird fruit preferences match the frequency of fruit colours in tropical Asia. Scientific Reports, 4(1), 5627. [CrossRef]
- Dubos, C., Stracke, R., Grotewold, E., Weisshaar, B., Martin, C., & Lepiniec, L. (2010). MYB transcription factors in Arabidopsis. Trends in Plant Science, 15(10), 573–581. [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). (2012). Scientific Opinion on the re-evaluation of vegetable carbon (E 153) as a food additive. EFSA Journal, 10(4), 2592. [CrossRef]
- Ellis, A. G., Brockington, S. F., de Jager, M. L., Mellers, G., Walker, R. H., & Glover, B. J. (2014). Floral trait variation and integration as a function of sexual deception in Gorteria diffusa. Philosophical Transactions of the Royal Society B: Biological Sciences, 369(1649), 20130563. [CrossRef]
- Enaru, B., Drețcanu, G., Pop, T. D., Stǎnilǎ, A., & Diaconeasa, Z. (2021). Anthocyanins: Factors Affecting Their Stability and Degradation. Antioxidants, 10(12), Article 12. [CrossRef]
- Fattorini, R., Khojayori, F. N., Mellers, G., Moyroud, E., Herrero, E., Kellenberger, R. T., Walker, R., Wang, Q., Hill, L., & Glover, B. J. (2024). Complex petal spot formation in the Beetle Daisy (Gorteria diffusa) relies on spot-specific accumulation of malonylated anthocyanin regulated by paralogous GdMYBSG6 transcription factors. New Phytologist, n/a(n/a). [CrossRef]
- Fenger, J.-A., Sigurdson, G. T., Robbins, R. J., Collins, T. M., Giusti, M. M., & Dangles, O. (2021). Acylated Anthocyanins from Red Cabbage and Purple Sweet Potato Can Bind Metal Ions and Produce Stable Blue Colors. International Journal of Molecular Sciences, 22(9), Article 9. [CrossRef]
- Francis, F. J., & Markakis, P. C. (1989). Food colorants: Anthocyanins. Critical Reviews in Food Science & Nutrition. [CrossRef]
- Ghareaghajlou, N., Hallaj-Nezhadi, S., & Ghasempour, Z. (2021). Red cabbage anthocyanins: Stability, extraction, biological activities and applications in food systems. Food Chemistry, 365, 130482. [CrossRef]
- Ghosh, S., Sarkar, T., Das, A., & Chakraborty, R. (2022). Natural colorants from plant pigments and their encapsulation: An emerging window for the food industry. LWT, 153, 112527. [CrossRef]
- Glagoleva, A. Y., Shoeva, O. Y., & Khlestkina, E. K. (2020). Melanin Pigment in Plants: Current Knowledge and Future Perspectives. Frontiers in Plant Science, 11. [CrossRef]
- Gonzalez, A., Brown, M., Hatlestad, G., Akhavan, N., Smith, T., Hembd, A., Moore, J., Montes, D., Mosley, T., Resendez, J., Nguyen, H., Wilson, L., Campbell, A., Sudarshan, D., & Lloyd, A. (2016). TTG2 controls the developmental regulation of seed coat tannins in Arabidopsis by regulating vacuolar transport steps in the proanthocyanidin pathway. Developmental Biology, 419(1), 54–63. [CrossRef]
- Gonzalez, A., Zhao, M., Leavitt, J. M., & Lloyd, A. M. (2008). Regulation of the anthocyanin biosynthetic pathway by the TTG1/bHLH/Myb transcriptional complex in Arabidopsis seedlings. The Plant Journal, 53(5), 814–827. [CrossRef]
- Goto, T., Takase, S., & Kondo, T. (1978). PMR spectra of natural acylated anthocyanins determination of stereostructure of awobanin, shisonin and violanin. Tetrahedron Letters, 19(27), 2413–2416. [CrossRef]
- Gould, K. S. (2004). Nature’s Swiss Army Knife: The Diverse Protective Roles of Anthocyanins in Leaves. Journal of Biomedicine and Biotechnology, 2004(5), 314–320. [CrossRef]
- Gould, K. S., McKelvie, J., & Markham, K. R. (2002). Do anthocyanins function as antioxidants in leaves? Imaging of H2O2 in red and green leaves after mechanical injury. Plant, Cell & Environment, 25(10), 1261–1269. [CrossRef]
- Gould, K. S., Neill, S. O., & Vogelmann, T. C. (2002). A unified explanation for anthocyanins in leaves? In Advances in Botanical Research (Bd. 37, S. 167–192). Academic Press. [CrossRef]
- Hatier, J.-H. B., Clearwater, M. J., & Gould, K. S. (2013). The Functional Significance of Black-Pigmented Leaves: Photosynthesis, Photoprotection and Productivity in Ophiopogon planiscapus ‘Nigrescens’. PLOS ONE, 8(6), e67850. [CrossRef]
- Hilber, I., Blum, F., Schmidt, H.-P., & Bucheli, T. D. (2022). Current analytical methods to quantify PAHs in activated carbon and vegetable carbon (E153) are not fit for purpose. Environmental Pollution, 309, 119599. [CrossRef]
- Houghton, A., Appelhagen, I., & Martin, C. (2021). Natural Blues: Structure Meets Function in Anthocyanins. Plants, 10(4), Article 4. [CrossRef]
- Hsu, C.-C., Su, C.-J., Jeng, M.-F., Chen, W.-H., & Chen, H.-H. (2019). A HORT1 Retrotransposon Insertion in the PeMYB11 Promoter Causes Harlequin/Black Flowers in Phalaenopsis Orchids. Plant Physiology, 180(3), 1535–1548. [CrossRef]
- Jia, L., Clegg, M. T., & Jiang, T. (2004). Evolutionary dynamics of the DNA-binding domains in putative R2R3-MYB genes identified from rice subspecies indica and japonica genomes. Plant Physiology, 134(2), 575–585. [CrossRef]
- Jia, N., Kong, B. H., & Liu, Q. (2013). Influence of Light on the Anthocyanins Stability and Antioxidant Activity of Black Currant (Ribes nigrum L.) Extract. Applied Mechanics and Materials, 448–453, 1119–1122. [CrossRef]
- Johnson, S. D., & Midgley, J. J. (1997). Fly pollination of Gorteria diffusa (Asteraceae), and a possible mimetic function for dark spots on the capitulum. American Journal of Botany, 84(4), 429–436. [CrossRef]
- Jokioja, J., Yang, B., & Linderborg, K. M. (2021). Acylated anthocyanins: A review on their bioavailability and effects on postprandial carbohydrate metabolism and inflammation. Comprehensive Reviews in Food Science and Food Safety, 20(6), 5570–5615. [CrossRef]
- Kearsley, M. W., & Rodriguez, N. (1981). The stability and use of natural colours in foods: Anthocyanin, β-carotene and riboflavin. International Journal of Food Science & Technology, 16(4), 421–431. [CrossRef]
- Lacey, E. P., Lovin, M. E., Richter, S. J., & Herington, D. A. (2010). Floral Reflectance, Color, and Thermoregulation: What Really Explains Geographic Variation in Thermal Acclimation Ability of Ectotherms? The American Naturalist, 175(3), 335–349. [CrossRef]
- Landi, M., Tattini, M., & Gould, K. S. (2015). Multiple functional roles of anthocyanins in plant-environment interactions. Environmental and Experimental Botany, 119, 4–17. [CrossRef]
- Lee, Y.-M., Yoon, Y., Yoon, H., Park, H.-M., Song, S., & Yeum, K.-J. (2017). Dietary Anthocyanins against Obesity and Inflammation. Nutrients, 9(10), Article 10. [CrossRef]
- Li, A., Xiao, R., He, S., An, X., He, Y., Wang, C., Yin, S., Wang, B., Shi, X., & He, J. (2019). Research Advances of Purple Sweet Potato Anthocyanins: Extraction, Identification, Stability, Bioactivity, Application, and Biotransformation. Molecules, 24(21), 3816. [CrossRef]
- Li, H.-T., Luo, Y., Gan, L., Ma, P.-F., Gao, L.-M., Yang, J.-B., Cai, J., Gitzendanner, M. A., Fritsch, P. W., Zhang, T., Jin, J.-J., Zeng, C.-X., Wang, H., Yu, W.-B., Zhang, R., van der Bank, M., Olmstead, R. G., Hollingsworth, P. M., Chase, M. W., … Li, D.-Z. (2021). Plastid phylogenomic insights into relationships of all flowering plant families. BMC Biology, 19(1), 232. [CrossRef]
- Lightbourn, G. J., Griesbach, R. J., Novotny, J. A., Clevidence, B. A., Rao, D. D., & Stommel, J. R. (2008). Effects of Anthocyanin and Carotenoid Combinations on Foliage and Immature Fruit Color of Capsicum annuum L. Journal of Heredity, 99(2), 105–111. [CrossRef]
- Liu, Y., Li, M., Li, T., Chen, Y., Zhang, L., Zhao, G., Zhuang, J., Zhao, W., Gao, L., & Xia, T. (2020). Airborne fungus-induced biosynthesis of anthocyanins in Arabidopsis thaliana via jasmonic acid and salicylic acid signaling. Plant Science, 300, 110635. [CrossRef]
- Liu, Y., Liu, Y., Tao, C., Liu, M., Pan, Y., & Lv, Z. (2018). Effect of temperature and pH on stability of anthocyanin obtained from blueberry. Journal of Food Measurement and Characterization, 12(3), 1744–1753. [CrossRef]
- Lloyd, A., Brockman, A., Aguirre, L., Campbell, A., Bean, A., Cantero, A., & Gonzalez, A. (2017). Advances in the MYB–bHLH–WD Repeat (MBW) Pigment Regulatory Model: Addition of a WRKY Factor and Co-option of an Anthocyanin MYB for Betalain Regulation. Plant and Cell Physiology, 58(9), 1431–1441. [CrossRef]
- Luo, J., Nishiyama, Y., Fuell, C., Taguchi, G., Elliott, K., Hill, L., Tanaka, Y., Kitayama, M., Yamazaki, M., Bailey, P., Parr, A., Michael, A. J., Saito, K., & Martin, C. (2007). Convergent evolution in the BAHD family of acyl transferases: Identification and characterization of anthocyanin acyl transferases from Arabidopsis thaliana. The Plant Journal, 50(4), 678–695. [CrossRef]
- Maleknia, S. D., & Downard, K. M. (2016). New Anthocyanins from Black Elderberry of Inhibitory Potential Revealed by Mass Spectrometry. The Natural Products Journal, 6(2), 94–102.
- Markham, K. R., Bloor, S. J., Nicholson, R., Rivera, R., Shemluck, M., Kevan, P. G., & Michener, C. (2004). Black Flower Coloration in Wild Lisianthius nigrescens: Its Chemistry and Ecological Consequences. Zeitschrift Für Naturforschung C, 59(9–10), 625–630. [CrossRef]
- Mazzucato, A., Willems, D., Bernini, R., Picarella, M. E., Santangelo, E., Ruiu, F., Tilesi, F., & Soressi, G. P. (2013). Novel phenotypes related to the breeding of purple-fruited tomatoes and effect of peel extracts on human cancer cell proliferation. Plant Physiology and Biochemistry, 72, 125–133. [CrossRef]
- Menconi, J., Perata, P., & Gonzali, S. (2023). Novel R2R3 MYB transcription factors regulate anthocyanin synthesis in Aubergine tomato plants. BMC Plant Biology, 23(1), 148. [CrossRef]
- Mes, P. J., Boches, P., Myers, J. R., & Durst, R. (2008). Characterization of Tomatoes Expressing Anthocyanin in the Fruit. Journal of the American Society for Horticultural Science, 133(2), 262–269. [CrossRef]
- Mori, K., Goto-Yamamoto, N., Kitayama, M., & Hashizume, K. (2007). Loss of anthocyanins in red-wine grape under high temperature. Journal of Experimental Botany, 58(8), 1935–1945. [CrossRef]
- Murre, C., Bain, G., van Dijk, M. A., Engel, I., Furnari, B. A., Massari, M. E., Matthews, J. R., Quong, M. W., Rivera, R. R., & Stuiver, M. H. (1994). Structure and function of helix-loop-helix proteins. Biochimica Et Biophysica Acta, 1218(2), 129–135. [CrossRef]
- Naing, A. H., Park, K. I., Ai, T. N., Chung, M. Y., Han, J. S., Kang, Y.-W., Lim, K. B., & Kim, C. K. (2017). Overexpression of snapdragon Delila (Del) gene in tobacco enhances anthocyanin accumulation and abiotic stress tolerance. BMC Plant Biology, 17, 65. [CrossRef]
- Noda, N., Aida, R., Kishimoto, S., Ishiguro, K., Fukuchi-Mizutani, M., Tanaka, Y., & Ohmiya, A. (2013). Genetic Engineering of Novel Bluer-Colored Chrysanthemums Produced by Accumulation of Delphinidin-Based Anthocyanins. Plant and Cell Physiology, 54(10), 1684–1695. [CrossRef]
- Noda, Y., Kneyuki, T., Igarashi, K., Mori, A., & Packer, L. (2000). Antioxidant activity of nasunin, an anthocyanin in eggplant peels. Toxicology, 148(2), 119–123. [CrossRef]
- Norgate, M., Boyd-Gerny, S., Simonov, V., Rosa, M. G. P., Heard, T. A., & Dyer, A. G. (2010). Ambient Temperature Influences Australian Native Stingless Bee (Trigona carbonaria) Preference for Warm Nectar. PLOS ONE, 5(8), e12000. [CrossRef]
- Okitsu, N., Noda, N., Chandler, S., & Tanaka, Y. (2018). Flower Color and Its Engineering by Genetic Modification. In J. Van Huylenbroeck (Hrsg.), Ornamental Crops (S. 29–62). Springer International Publishing. [CrossRef]
- Peng, M., Hudson, D., Schofield, A., Tsao, R., Yang, R., Gu, H., Bi, Y.-M., & Rothstein, Steven. J. (2008). Adaptation of Arabidopsis to nitrogen limitation involves induction of anthocyanin synthesis which is controlled by the NLA gene. Journal of Experimental Botany, 59(11), 2933–2944. [CrossRef]
- Ramsay, N. A., & Glover, B. J. (2005). MYB-bHLH-WD40 protein complex and the evolution of cellular diversity. Trends in Plant Science, 10(2), 63–70. [CrossRef]
- Rick, C., Cisneros, P., Chetelat, R., & DeVerna, J. (1994). Abg—A gene on chromosome 10 for purple fruit derived from S. lycopersicoides.
- Rosinski, J. A., & Atchley, W. R. (1998). Molecular evolution of the Myb family of transcription factors: Evidence for polyphyletic origin. Journal of Molecular Evolution, 46(1), 74–83. [CrossRef]
- Rowan, D. D., Cao, M., Lin-Wang, K., Cooney, J. M., Jensen, D. J., Austin, P. T., Hunt, M. B., Norling, C., Hellens, R. P., Schaffer, R. J., & Allan, A. C. (2009). Environmental regulation of leaf colour in red 35S:PAP1 Arabidopsis thaliana. New Phytologist, 182(1), 102–115. [CrossRef]
- Sapir, M., Oren-Shamir, M., Ovadia, R., Reuveni, M., Evenor, D., Tadmor, Y., Nahon, S., Shlomo, H., Chen, L., Meir, A., & Levin, I. (2008). Molecular Aspects of Anthocyanin fruit Tomato in Relation to high pigment-1. Journal of Heredity, 99(3), 292–303. [CrossRef]
- Sasaki, K., & Takahashi, T. (2002). A flavonoid from Brassica rapa flower as the UV-absorbing nectar guide. Phytochemistry, 61(3), 339–343. [CrossRef]
- Schaefer, H. M., Levey, D. J., Schaefer, V., & Avery, M. L. (2006). The role of chromatic and achromatic signals for fruit detection by birds. Behavioral Ecology, 17(5), 784–789. [CrossRef]
- Schwinn, K., Miosic, S., Davies, K., Thill, J., Gotame, T. P., Stich, K., & Halbwirth, H. (2014). The B-ring hydroxylation pattern of anthocyanins can be determined through activity of the flavonoid 3′-hydroxylase on leucoanthocyanidins. Planta, 240(5), 1003–1010. [CrossRef]
- Smith, T. F., Gaitatzes, C., Saxena, K., & Neer, E. J. (1999). The WD repeat: A common architecture for diverse functions. Trends in Biochemical Sciences, 24(5), 181–185. [CrossRef]
- Sun, C., Deng, L., Du, M., Zhao, J., Chen, Q., Huang, T., Jiang, H., Li, C.-B., & Li, C. (2020). A Transcriptional Network Promotes Anthocyanin Biosynthesis in Tomato Flesh. Molecular Plant, 13(1), 42–58. [CrossRef]
- Thill, J., Miosic, S., Ahmed, R., Schlangen, K., Muster, G., Stich, K., & Halbwirth, H. (2012). „Le Rouge et le Noir“: A decline in flavone formation correlates with the rare color of black dahlia (Dahlia variabilis hort.) flowers. BMC Plant Biology, 12(1), 225. [CrossRef]
- Thomas, M. M., Rudall, P. J., Ellis, A. G., Savolainen, V., & Glover, B. J. (2009). Development of a complex floral trait: The pollinator-attracting petal spots of the beetle daisy, Gorteria diffusa (Asteraceae). American Journal of Botany, 96(12), 2184–2196. [CrossRef]
- Tikhomirov, B., Shamurin, V., & Shtepa, V. (1960). The temperature of arctic plants. https://scholar.google.com/scholar_lookup?journal=Russ.+Acad.+Sci.&title=The+temperature+of+arctic+plants&author=B.+Tikhomirov&author=V.+Shamurin&author=V.+Shtepa&volume=3&publication_year=1960&pages=429-442&.
- Van Buren, J. P., Hrazdina, G., & Robinson, W. B. (1974). COLOR OF ANTHOCYANIN SOLUTIONS EXPRESSED IN LIGHTNESS AND CHROMATICITY TERMS. Effect of pH and Type of Anthocyanin. Journal of Food Science, 39(2), 325–328. [CrossRef]
- van der Kooi, C. J., & Stavenga, D. G. (2019). Vividly coloured poppy flowers due to dense pigmentation and strong scattering in thin petals. Journal of Comparative Physiology A, 205(3), 363–372. [CrossRef]
- Wang, X., Hansen, C., & Allen, K. (2013). Identification of Anthocyanins Isolated from Black Bean Canning Wastewater by Macroporous Resin Using Optimized Conditions. Food and Nutrition Sciences, 4(8), Article 8. [CrossRef]
- Weatherall, I. L., & Lee, W. G. (1991). Instrumental evaluation of some New Zealand fruit colours using CIELAB values. New Zealand Journal of Botany, 29(2), 197–205. [CrossRef]
- Winkel-Shirley, B. (2001). Flavonoid Biosynthesis. A Colorful Model for Genetics, Biochemistry, Cell Biology, and Biotechnology. Plant Physiology, 126(2), 485–493. [CrossRef]
- Zhang, Y., Butelli, E., & Martin, C. (2014). Engineering anthocyanin biosynthesis in plants. Current Opinion in Plant Biology, 19, 81–90. [CrossRef]
- Zhang, Y., Chu, G., Hu, Z., Gao, Q., Cui, B., Tian, S., Wang, B., & Chen, G. (2016). Genetically engineered anthocyanin pathway for high health-promoting pigment production in eggplant. Molecular Breeding, 36(5), 54. [CrossRef]
- Zhao, Y.-W., Wang, C.-K., Huang, X.-Y., & Hu, D.-G. (2021). Anthocyanin stability and degradation in plants. Plant Signaling & Behavior, 16(12), 1987767. [CrossRef]
- Zozio, S., Pallet, D., & Dornier, M. (2011). Evaluation of anthocyanin stability during storage of a coloured drink made from extracts of the Andean blackberry ( Rubus glaucus Benth.), açai ( Euterpe oleracea Mart.)and black carrot ( Daucus carota L.). Fruits, 66(3), 203–215. [CrossRef]


| Anthocyanin | R3′ | R5′ | chemical structure |
|---|---|---|---|
| Pelargonidin | −H | −H | 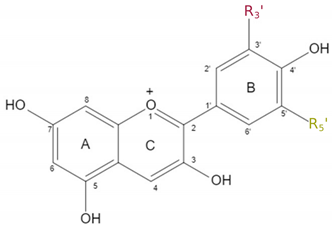 |
| Cyanidin | −OH | −H | |
| Delphinidin | −OH | −OH | |
| Peonidin | −OCH3 | −H | |
| Petunidin | −OH | −OCH3 | |
| Malvidin | −OCH3 | −OCH3 |
| Species | Anthocyanin | Reference |
|---|---|---|
|
Viola tricolor (pansy) |
delphinidin-5-O-glucoside-3-O-[4-p-coumaroylrhamnosyl(1-6)-glucoside] | (Goto et al., 1978) |
|
Lisianthius nigrescens (Flower of Death) |
delphinidin-3-O-rhamnol(1-6)galactoside | (Markham et al., 2004) |
| Dahlia variabilis (garden dahlia) | cyanidin-3-(6″-malonylglucoside)-5-glucoside | (Deguchi et al., 2016) |
| Capsicum annuum (pepper) | delphinidin-3-p-coumaroyl-rutinoside-5-glucoside | (Lightbourn et al., 2008) |
| Solanum melongena (Eggplant) | Delphinidin-3-(p-coumaroylrutinoside)-5-glucoside (nasunin) | (Y. Noda et al., 2000) |
| Solanum lycopersicum (Tomato) | petunidin-3-O-[6″-O-(4‴-O-E-p-coumaroyl-α-rhamnopyranosyl)-β-glucopyranoside]-5-O-β-glucopyranoside (petanin) malvidin-3-O-[6″-O-(4‴-O-E-p-coumaroyl-α-rhamnopyranosyl)-β-glucopyranoside]-5-O-β-glucopyranoside (negretein) |
(Blando et al., 2019) |
| black sorghum (sorghum) | Luteolinidin and apigeninidin * | (Awika et al., 2005) |
| Phaseolus vulgaris (Black Bean) | delphinidin 3-glucoside, petunidin 3- glucoside and maldvidin 3-glucoside | (Wang et al., 2013) |
| Sambucus nigra (black elderberry) | cyanidin-3-O-sambubioside, prenylated and other derivatives of cyanidin-3-acetylrutinoside | (Maleknia & Downard, 2016) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





