1. Introduction
Recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC) has a poor prognosis of 3–6 months if untreated [
1,
2]. Although cytotoxic anticancer drugs and molecularly targeted agents can prolong life expectancy by several months, long-term survival is not expected [
3]. However, immune checkpoint inhibitors (ICIs) are capable of producing long-term survival [
4,
5]. Patients with R/M HNSCC who have undergone ICI treatment have a 2-year overall survival (OS) of 16.9%[
6] and 5-year OS of 19.2% [
7]. However, very few patients have achieved such benefit [
6,
7], and due to this, there remains no consensus if ICI therapy should be discontinued in such patients and if so, the appropriate time to discontinue [
8]. Long-term response to ICI is observed in patients with various types of cancers [
9,
10], and the timing of ICI discontinuation has been discussed in previous studies [
9,
10]. Although the discontinuation times in long-term responders have been suggested, such as 2 years in lung cancer [
9] and 6 months to 2 years in malignant melanoma [
10], this debate remains unresolved in other cancers.
Disease progression can be controlled by continuing treatment, but with the risk of treatment-related toxicity, subsequent loss of quality of life, and healthcare economic issues. In contrast, discontinuation of treatment may be accompanied by disease progression. Both scenarios are distressing for both patients and clinicians. ICI has been employed in clinical practice for more than 7 years; it is thus time to draw conclusions about the timing of active discontinuation in long-term responders. The purpose of this study was to clarify the timing of active discontinuation in long-term responders to nivolumab in R/M HNSCC by examining the following three points: (1) Whether the progression-free survival (PFS) curve plateaus, and if so, when? (2) Will long-term nivolumab treatment increase the frequency of immune-related adverse events (irAEs)? (3) What is the prognosis, duration of treatment, and treatment-free interval (TFI) of patients who discontinued treatment for reasons other than the physician’s ability to actively declare the end of treatment (irAE or their own reasons). The aim of this study is to use these data to suggest the optimal timing to actively discontinue nivolumab therapy.
2. Materials and Methods
2.1. Patients
We conducted a multicenter, retrospective study at Kyushu University Hospital (Fukuoka, Japan) and affiliated institutions. Our study included R/M HNSCC cases treated with nivolumab between April 2017 and December 2023. The observation period was defined as until death or a cut-off date (March 2024), and the median observation period was 11.5 months (range 0.3–82.9). Nivolumab was administered on a schedule of 240 mg/body weight once every 2 weeks or 480 mg/body weight once every 4 weeks. Antitumor effects were evaluated on computed tomography at intervals of 8–12 weeks. Nivolumab was continued until progressive disease (PD) occurred, severe irAE incidence, or patients’ decision to discontinue. The decision to discontinue ICI owing to the incidence of irAEs was made according to each center’s regulations, which were based on the same criteria. Severe irAEs that precluded re-institution of ICI were interstitial pneumonia, neuropathy, encephalitis, myocarditis (any grade), and hepatic and renal dysfunction (grade ≥ 2 and unresponsive to prednisone therapy). Patients who developed irAEs had a period of temporary withdrawal but resumed treatment.
The study was approved by the Institutional Review Board of Kyushu University (IRB No 22011-01) and each participating institution. All patients provided consent to participate in this study. The procedures followed were in accordance with the principles of the Declaration of Helsinki.
2.2. Definitions
PFS was defined as the interval from nivolumab administration until PD, and OS was defined as the interval from nivolumab administration until death. The Decision group comprised patients who voluntarily discontinued treatment, the Toxicity group comprised patients who had to discontinue treatment owing to severe irAEs, and the PD group comprised patients who exhibited PD almost simultaneously with discontinuation of treatment owing to irAEs. Treatment-free interval (TFI) was defined as the interval from the last nivolumab administration until the date of confirmed disease-free survival.
Combined Positive Score was calculated as the number of programmed death–ligand 1 (PD-L1) positive cells (tumor cells, macrophages, lymphocytes)/total number of tumor cells × 100 using the 22C3 PharmDx assay (Dako platform, Agilent Technologies, Santa Clara, CA).
2.3. Statistical Analysis
Statistical Package for Social Sciences version 22.0 (IBM Japan, Ltd., Tokyo, Japan) was used for statistical analysis. OS and PFS were estimated using the Kaplan–Meier method. Differences between groups were calculated using the log-rank test with Fisher’s exact test for categorical variables and Kruskal–Wallis test for continuous variables. A p value < 0.05 indicated statistical significance.
3. Results
3.1. Baseline Patient Characteristics
At the March 2024 time point, 19 (7.7%) patients were in the ongoing group and 227 (92.3%) in the discontinuation group (9 [3.7%], Decision; 12 [4.9%] Toxicity; and 206 [83.7%] PD group). Patient characteristics are described in
Table 1. No significant differences were observed between the groups in sex, age, performance status, primary site, platinum sensitivity/resistance, or PD-L1 expression.
3.2. Clinical Outcomes Analysis
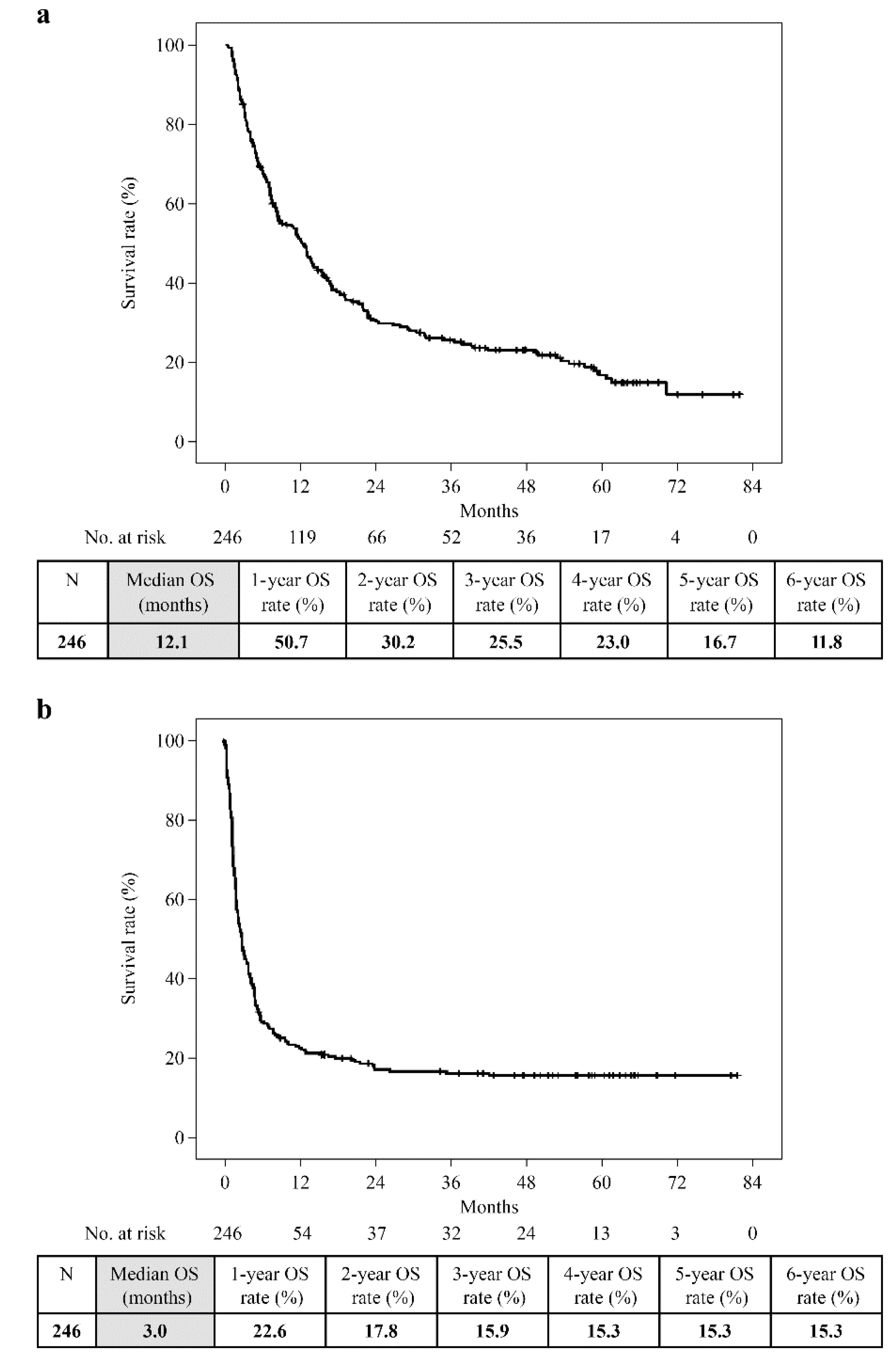
Overall, the 6-year OS was 11.8% (median, 12.1 months), and the 6-year PFS was 15.3% (median, 3.0 months) (
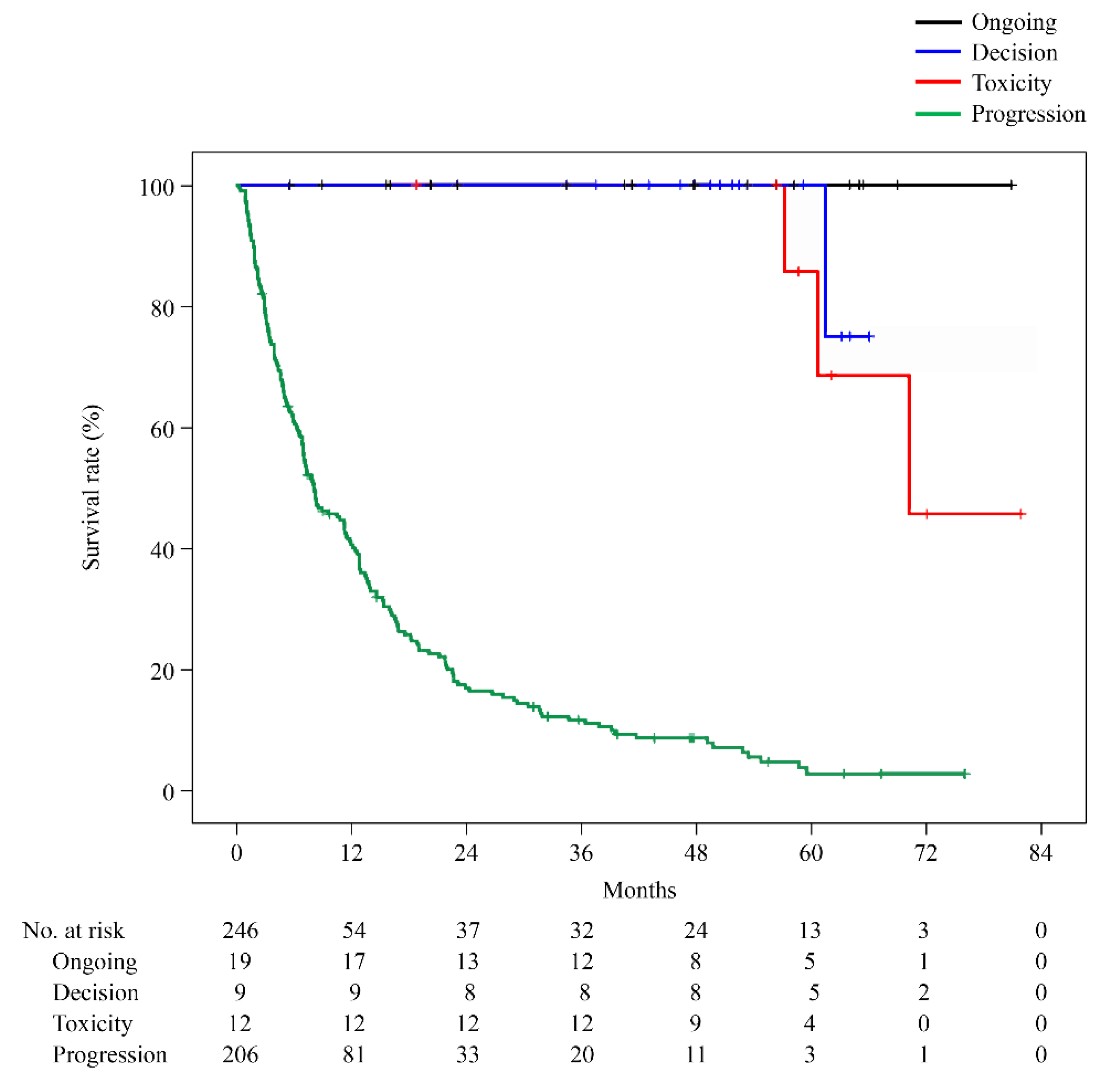
Figure 1). PFS remained unchanged at 3 years. The 6-year OS of the Ongoing, Decision, and PD groups was 100%, 45.7%, and 2.9%, respectively. The longest observation period in the Toxicity group was 5 years and 6 months, and the OS was 75.0%. Only the PD group showed a significantly worse prognosis (PD vs Ongoing: p = 0.000, PD vs Decision: p = 0.000, PD vs Toxicity: p = 0.000). However, no significant difference was observed between the Ongoing, Decision, and Toxicity groups (Ongoing vs Decision: p = 0.144, Ongoing vs Toxicity: p = 0.264, Decision vs Toxicity: p = 0.675;
Figure 2).
3.3. Frequency of irAEs
Overall, the incidence of irAEs was 29.7% (73/246), and the probability of new irAEs occurring beyond 2 years was 1.2% (3/246); 95.5% of irAEs occurred within 2 years of treatment initiation. The incidence of irAEs in the Ongoing group was 42.1% (8/19), and the probability of new irAEs occurring after 2 years was 0.0% (0/19). In the Decision, Toxicity, and PD groups, these rates were 44.4% (4/9) and 11.1% (1/9), 100% (12/12) and 8.3% (1/12), and 23.8% (49/206) and 0.5% (1/206), respectively (
Table 2).
3.4. Duration of Nivolumab Treatment and TFI
Overall, the median duration of nivolumab treatment was 2.9 months (range 0.03–81.9): Ongoing, 41.8 months (5.6–81.9); Decision, 36.8 months (4.0–70.1); Toxicity, 18.9 months (0.03–50.3); and PD, 2.0 months (0.03–42.9).
TFI in the Decision group was 15.1 months (0.6–61.6) and 30.6 months (2.8–64.8) in the Toxicity group. For 52.9% (109/206) of PD cases, second-line treatments such as paclitaxel, cetuximab, or TS-1 were administered, and 47.1% (97/206) were switched to best supportive care (
Table 2).
4. Discussion
To our knowledge, this is the first report proposing a timeframe for active discontinuation based on TFI and PFS for R/MHNSCC patients treated with nivolumab.
The introduction of ICIs, including nivolumab, has brought dramatic changes to R/M HNSCC treatment [
11,
12]. In the past, patients who could not be treated with surgery or radiation therapy were treated with cytotoxic anticancer agents or molecularly targeted agents, but the duration of efficacy was mostly in months rather than years [
3]. As such, clinicians faced the challenge of achieving and maintaining antitumor effects. ICI treatment results in long-term sustained complete response (CR) and is continued on a yearly basis, although for a small percentage of R/M HNSCC patients [
6,
7]. Therefore, it has become necessary to examine the appropriate timing of ICI discontinuation in cases of persistent CR. Currently, the widely accepted concept in R/M HNSCC is to administer ICI until disease progression or unacceptable toxicity such as irAEs [
8]. No guidelines exist regarding the timing of ICI discontinuation in persistent CR cases. Reportedly, patients have sustained responses for up to 59.2 months [
13], and no restrictions are implemented on long-term use of nivolumab in real-world clinical practice. The decision to discontinue in long-term responder cases, except in cases of adverse events, is currently at the discretion of patients.
To offer discontinuation to long-term ICI responders with maintained CR, it is necessary to examine this decision from three perspectives. First, irAEs are known to occur initially and after several months to a year [
14]. Thus, if irAEs increase with long-term administration, ICI treatment should be discontinued. However, a study of irAEs after ICI treatment in 50,347 patients reported that 98%–99% of irAEs appeared within 18 months, with no subsequent increase in frequency [
14]. The same was true in our study, with only 1.2% of cases developing irAEs after 2 years. It therefore did not seem reasonable to limit the duration of continuous administration because of the risk of developing irAEs.
Second, the relapse rate and time to relapse after discontinuation. In lung cancer patients treated with ICI for >18 months, treatment was discontinued in 50% of cases (including 22% who discontinued owing to toxicity) and progression was subsequently observed in 33% of patients with a median time to progression of 10.0 months [
15]. In malignant melanoma, the progression-free survival rate was 78.4% after an additional 2 years of follow-up for patients who completed 2 years of ICI treatment [
16]. In a report involving several types of solid tumors, 15% of patients discontinued ICI (approximately 5% owing to CR and 3% owing to irAEs); 12% of those who discontinued owing to CR relapsed [
17]. Therefore, even in patients with stable disease after long-term treatment, 10%–30% of patients relapse after discontinuation. At this stage, discontinuation of treatment in patients who have achieved CR or after long-term ICI treatment must be carefully and individually considered, whether in malignant melanoma [
16] or lung cancer [
18]. To date, we have not systematically discontinued nivolumab in any of our patients. However, in daily practice, TFIs occasionally occur owing to adverse events or unplanned personal circumstances. We found that TFI for patients who discontinued treatment owing to irAEs was prolonged for a median of 30 months, confirming that TFI is effective in suppressing disease progression in the long term after ICI discontinuation, even in a small percentage of patients with severe irAEs. The Decision group also achieved a median TFI of 15 months, confirming the low relapse rate and efficacy in maintaining CR after discontinuation of ICI in R/M HNSCC. Inferring from the favorable results of unplanned TFI, we believe it would be feasible to proactively consider planned discontinuation for long-term ICI responders in R/MHNSCC.
Third, the optimal time for planned discontinuation. The duration of ICI administration is associated with the relapse rate after treatment discontinuation. In a study of solid tumor cases, discontinuation of ICI treatment within 12 months was associated with a higher risk of recurrence than discontinuation after 12 months [
17]. Similarly, in lung cancer patients treated with pembrolizumab, the median PFS of 24.7 vs 9.4 months was better in the continuous treatment group than in the group that discontinued treatment at 1 year; discontinuation at 1 year should be carefully considered [
19]. Additionally, long-term data of lung cancer patients also reported that 2 years of continuous ICI treatment significantly reduced the risk of disease progression [
20]. Other lung cancer reports also suggest that an optimal ICI discontinuation period should be considered because a certain percentage of patients relapse even when the duration of treatment is extended 1–2 years, and deaths due to recurrence occur even after long-term ICI treatment [
21]. Six months of treatment is sufficient for patients with CR in malignant melanoma; however, in other cases, treatment should be continued for at least 2 years and possibly indefinitely [
10]. In our study, the appropriate time of discontinuation was considered when the PFS curve plateaued. The PFS curve almost plateaued 2 years after the start of treatment and plateaued completely at 3 years, which we considered to be the recommended timing for discontinuation. Additionally, since the median time in the Decision group was 36.8 months, we believe that 3 years coincides with a period when patients can achieve a certain level of reassurance. Therefore, we propose that completing treatment in 3 years is the most likely timeframe when physicians and patients can reach an agreement.
A limitation of this study is the small sample size of patients in the Decision group. Additionally, the median TFI of the Decision group patients was only 15 months; therefore, we could verify whether re-increase does not occur even in the long term. Further studies including follow-up of these patients and accumulation of cases are warranted to resolve this limitation, and we plan to investigate this in the future.
5. Conclusions
The prognostic impact of ICI discontinuation in R/M HNSCC patients treated with ICI who progressed to long-term responders is unknown. We found a few patients, albeit limited, who had unplanned discontinuation of nivolumab and gained TFI for > 1 year. Regarding the minimum optimal duration of treatment that would not be considered overtreatment for this subset of patients, the data show that PFS completely plateaued at 3 years, the median time patients wished to discontinue treatment was approximately 3 years, and the incidence of irAEs reduced after 2 years. Considering these three perspectives, we propose completion of treatment in 3 years.
Author Contributions
Conceptualization, writing, formal analysis, M.Matsuo; validation, M.Y.; investigation, M.S., S.M.; data curation, K.H., R.K.; review and editing, M.Masuda; supervision, T.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of KYUSHU UNIVERSITY (protocol code 22011-01 and date of approval March 29, 2023).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data sets analyzed in this study cannot be openly shared to protect the privacy of the study participants but are available from the corresponding author upon reasonable request.
Acknowledgments
We would like to thank Editage (
www.editage.jp) for English language editing.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Shah, D.; Hoffman, G. R. Outcome of head and neck cancer patients who did not receive curative-intent treatment. J. Oral Maxillofac. Surg. 2017, 75, 2456–2464. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, L. P.; Carvalho, A. L. Natural history of untreated head and neck cancer. Eur. J. Cancer 2000, 36, 1032–1037. [Google Scholar] [CrossRef] [PubMed]
- Vermorken, J. B.; Mesia, R.; Rivera, F.; Remenar, E.; Kawecki, A.; Rottey, S.; Erfan, J.; Zabolotnyy, D.; Kienzer, H.-R.; Cupissol, D.; Peyrade, F.; Benasso, M.; Vynnychenko, I.; De Raucourt, D.; Bokemeyer, C.; Schueler, A.; Amellal, N.; Hitt, R. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N. Engl. J. Med. 2008, 359, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Ferris, R. L.; Blumenschein, G. Jr.; Fayette, J.; Guigay, J.; Colevas, A. D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E. E. , Even, C.; Worden, F.; Saba, N. F.; Iglesias Docampo, L. C.; Haddad, R.; Rordorf, T.; Kiyota, N.; Tahara, M.; Monga, M.; Lynch, M.; Geese, W.J.; Kopit, J.; Shaw, J.W.; Gillison, M. L. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef] [PubMed]
- Burtness, B.; Harrington, K. J.; Greil, R.; Soulières, D.; Tahara, M.; de Castro, G.; Psyrri, A.; Basté, N.; Neupane, P.; Bratland, Å.; Fuereder, T.; Hughes, B. G. M.; Mesía, R.; Ngamphaiboon, N.; Rordorf, T.; Wan Ishak, W. Z.; Hong, R.-L.; González Mendoza, R.; Roy, A.; Zhang, Y.; Gumuscu, B.; Cheng, J. D.; Jin, F.; Rischin, D.; KEYNOTE-048 Investigators. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomized, open-label, phase 3 study. Lancet 2019, 394, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- Ferris, R. L.; Blumenschein, G. Jr.; Fayette, J.; Guigay, J.; Colevas, A. D.; Licitra, L.; Harrington, K. J.; Kasper, S.; Vokes, E. E.; Even, C.; Worden, F.; Saba, N. F.; Docampo, L. C. I.; Haddad, R.; Rordorf, T.; Kiyota, N.; Tahara, M.; Lynch, M.; Jayaprakash, V.; Li, L.; Gillison, M. L. Nivolumab vs investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol. 2018, 81, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, M.; Yasumatsu, R.; Masuda, M.; Yamauchi, M.; Wakasaki, T.; Hashimoto, K.; Jiromaru, R.; Manako, T.; Nakagawa, T. Five-year follow-up of patients with head and neck cancer treated with nivolumab and long-term responders for over two years. In Vivo 2022, 36, 1881–1886. [Google Scholar] [CrossRef]
- Stöth, M.; Meyer, T.; Gehrke, T.; Hagen, R.; Scheich, M.; Hackenberg, S.; Scherzad, A. Discontinuation of anti-programmed cell death protein 1 immune checkpoint inhibition after complete remission in head and neck squamous cell carcinoma: A case report and literature review. Oncol. Lett. 2023, 26, 489. [Google Scholar] [CrossRef]
- Brahmer, J. R.; Lee, J.-S.; Ciuleanu, T.-E.; Caro, R. B.; Nishio, M.; Urban, L.; Audigier-Valette, C.; Lupinacci, L.; Sangha, R.; Pluzanski, A.; Burgers, J.; Mahave, M.; Ahmed, S.; Schoenfeld, A. J.; Paz-Ares, L. G.; Reck, M.; Borghaei, H.; O’Byrne, K. J.; Gupta, R. G.; Bushong, J.; Li, L.; Blum, S. I.; Eccles, L. J.; Ramalingam, S. S. Five-year survival outcomes with nivolumab plus ipilimumab versus chemotherapy as first-line treatment for metastatic non–small-cell lung cancer in checkmate 227. J. Clin. Oncol. 2022, 41, 1200–1212. [Google Scholar] [CrossRef]
- De Risi, I.; Sciacovelli, A. M.; Guida, M. Checkpoint inhibitors immunotherapy in metastatic melanoma: when to stop treatment? Biomedicines 2022, 10, 2424. [Google Scholar] [CrossRef]
- Johnson, D. E.; Burtness, B.; Leemans, C. R.; Lui, V. W. Y.; Bauman, J. E.; Grandis, J. R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef]
- Abraham, P.; Kish, J. K.; Korytowsky, B.; Radtchenko, J.; Singh, P.; Shaw, J.; Feinberg, B. Real-world treatment patterns, cost of care and effectiveness of therapies for patients with squamous cell carcinoma of head and neck pre and post approval of immuno-oncology agents. J. Med. Econ. 2020, 23, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Taguchi, Y.; Kitabayashi, T.; Sato, N.; Kaya, H.; Abe, T.; Endo, T.; Suzuki, H.; Kawasaki, Y.; Yamada, T. Serum albumin as an independent predictor of long-term survival in patients with recurrent and metastatic head and neck squamous cell carcinoma treated with nivolumab. J. Clin. Med. 2024, 13, 2456. [Google Scholar] [CrossRef]
- Gougis, P.; Jochum, F.; Abbar, B.; Dumas, E.; Bihan, K.; Lebrun-Vignes, B.; Moslehi, J.; Spano, J.-P.; Laas, E.; Hotton, J.; Reyal, F.; Hamy, A.-S.; Salem, J.-E. Clinical spectrum and evolution of immune-checkpoint inhibitors toxicities over a decade-a worldwide perspective. EClinicalMedicine 2024, 70, 102536. [Google Scholar] [CrossRef]
- Bilger, G.; Girard, N.; Doubre, H.; Levra, M. G.; Giroux-Leprieur, E.; Giraud, F.; Decroisette, C.; Carton, M.; Massiani, M. A. Discontinuation of immune checkpoint inhibitor (ICI) above 18 months of treatment in real-life patients with advanced non-small cell lung cancer (NSCLC): INTEPI, a multicentric retrospective study. Cancer Immunol. Immunother. 2022, 71, 1719–1731. [Google Scholar] [CrossRef]
- Davies, M. A. Is it safe to stop anti-PD-1 immunotherapy in patients with metastatic melanoma who achieve a complete response? J. Clin. Oncol. 2020, 38(15), 1645–1647. [Google Scholar] [CrossRef] [PubMed]
- Gauci, M.-L.; Lanoy, E.; Champiat, S.; Caramella, C.; Ammari, S.; Aspeslagh, S.; Varga, A.; Baldini, C.; Bahleda, R.; Gazzah, A.; Michot, J.-M.; Postel-Vinay, S.; Angevin, E.; Ribrag, V.; Hollebecque, A.; Soria, J.-C.; Robert, C.; Massard, C.; Marabelle, A. Long-term survival in patients responding to anti-PD-1/PD-L1 therapy and disease outcome upon treatment discontinuation. Clin. Cancer Res. 2019, 25, 946–956. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, D. M.; Garon, E. B.; Chandler, J.; McCleod, M.; Hussein, M.; Jotte, R.; Horn, L.; Daniel, D. B.; Keogh, G.; Creelan, B.; Einhorn, L. H.; Baker, J.; Kasbari, S.; Nikolinakos, P.; Babu, S.; Couture, F.; Leighl, N. B.; Reynolds, C.; Blumenschein, G.; Gunuganti, V.; Li, A.; Aanur, N.; Spigel, D. R. Continuous versus 1-year fixed-duration nivolumab in previously treated advanced non–small-cell lung cancer: CheckMate 153. J. Clin. Oncol. 2020, 38, 3863–3873. [Google Scholar] [CrossRef]
- Ferdinandus, J.; Metzenmacher, M.; Kessler, L.; Umutlu, L.; Aigner, C.; Karl, K. O.; Grünwald, V.; Eberhardt, W. E. E.; Fendler, W. P.; Herrmann, K.; Faehling, M.; Christoph, D. C. Complete metabolic response in patients with advanced non-small cell lung cancer with prolonged response to immune checkpoint inhibitor therapy. J. Immunother. Cancer 2021, 9, e002262. [Google Scholar] [CrossRef]
- Kim, H.; Kim, D.-W.; Kim, M.; Lee, Y.; Ahn, H. K.; Cho, J. H.; Kim, I. H.; Lee, Y.-G.; Shin, S.-H.; Park, S. E.; Jung, J.; Kang, E. J.; Myung-Ju, A. Long-term outcomes in patients with advanced and/or metastatic non–small cell lung cancer who completed 2 years of immune checkpoint inhibitors or achieved a durable response after discontinuation without disease progression: Multicenter, real-world data (KCSG LU20-11). Cancer 2022, 128, 778–787. [Google Scholar] [CrossRef]
- Ito, S.; Asahina, H.; Honjo, O.; Tanaka, H.; Honda, R.; Oizumi, S.; Nakamura, K.; Takamura, K.; Hommura, F.; Kawai, Y.; Ito, K.; Sukoh, N.; Yokoo, K.; Morita, R.; Harada, T.; Takashina, T.; Goda, T.; Dosaka-Akita, H.; Isobe, H.; Hokkaido Lung Cancer Clinical Study Group Trial. Prognostic factors in patients with advanced non-small cell lung cancer after long-term anti-PD-1 therapy (HOT1902). Lung Cancer 2021, 156, 12–19. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).