1. Introduction
Cataract, the leading cause of visual impairment globally [
1,
2], is characterized by clouding of the crystalline lens, potentially leading to blindness if left untreated [
3]. Various factors, including ageing, genetics, medical conditions, eye trauma, and ultraviolet radiation exposure, contribute to cataract development [
4,
5,
6].
Surgical intervention, specifically phacoemulsification [
7,
8], is the primary treatment, involving the use of ultrasound to remove the clouded lens and implant an artificial intraocular lens (IOL). Cataract surgery is safe and effective, typically performed on an outpatient basis, with a short recovery period, resulting in improved vision and significant improvement in quality of life post-surgery [
9,
10,
11]. Both conventional extracapsular surgery and manual small incision operations are cost-effective alternatives to phacoemulsification [
12,
13,
14,
15]. However, phacoemulsification is considered the standard surgical procedure recommended within the clinical guidelines of the Kazakhstan Ophthalmic Society [
16].
Optical coherence tomography (OCT) can enhance the management of cataracts [
17]. OCT is a non-invasive imaging technique that provides high-resolution cross-sectional images of the eye [
18,
19,
20], allowing for detailed visualization and assessment of the anterior and posterior ocular structures [
21,
22]. In cataract management, OCT can aid in preoperative planning by providing precise measurements of the anterior chamber depth, lens thickness, and axial length. These measurements are essential for selecting the appropriate IOL power and calculating the desired refractive outcome [
23]. Additionally, OCT can identify any coexisting vitreomacular pathologies [
24,
25] and enable retinal layer thickness evaluation to detect glaucomatous degeneration of ganglion cell axons [
26,
27], which may impact postoperative visual outcomes and can be treated during the surgical appointment made for cataract surgery.
Despite its potential advantages, the initial financial investment required for acquiring OCT equipment may pose a challenge for low- and middle-income countries, including Kazakhstan’s public and private hospitals, especially in remote regions. The patterns of clinical practice in Kazakhstan provided an opportunity to directly compare outcomes and costs of cataract surgery with and without OCT. We hypothesised that pre-operative OCT would lead to a greater treated eye visual acuity improvement and reduce the cost of ophthalmology services by enabling multiple surgeries in one appointment. As a major public health concern, cataract is expected to rise in Kazakhstan [
28] due to the ageing of Kazakhstan’s population [
29] and environmental factors including air and water pollution caused by the growth of transport highways and waste generation [
30,
31].
The primary objective of the present study was to evaluate the clinical and economic impact of integrating OCT into the pre-surgical stage of cataract management.
2. Materials and Methods
2.1. Intervention and Design
We compared the improvement in visual acuity following cataract surgery in two groups of patients treated by the same ophthalmologist (MI). One group had pre-operative OCT and the other did not. In addition, we evaluated the cost-effectiveness of two surgical strategies: 1) one surgical appointment combining cataract surgery along with any other eye surgeries (pre-surgical OCT required); 2) multiple surgical appointments, one for cataract surgery and separate appointments for any other surgeries (pre-surgical OCT not required before cataract surgery). The comparisons of clinical outcomes and costs were made between two ophthalmology surgical centers in Almaty, Kazakhstan.
2.2. Participants and Group Selection Strategy
Inclusion criteria were phacoemulsification treatment for cataract with the implantation of a monofocal intraocular lens (IOL) delivered between January and December 2022. Exclusion criteria were pre-existing ocular pathologies that may affect the outcomes of cataract surgery, previous ocular surgeries or trauma, pre-existing conditions that may confound the assessment of visual acuity improvement, systemic diseases or conditions that may affect ocular health.
A total of 124 patients were prospectively recruited. The sample size was determined based on the availability of eligible patients within the study period and the capacity of the two clinics. The distribution of participants between the two groups was influenced by the clinical practice patterns and the logistical considerations of the surgical centers. The surgeries were performed at two clinics: Private Clinic EyeDoctor and State City Clinic No. 13, both located in Almaty, Kazakhstan. If a patient underwent surgery for both eyes during the study period, only the first eye surgery was included in the analysis.
Group allocation was based on the surgical strategy employed. The OCT group (n=75) consisted of patients who had pre-operative OCT as they were undergoing a combined surgical appointment for cataract surgery and any other necessary eye surgeries. The Control group (n=49) comprised patients who did not have pre-operative OCT.
2.3. Eye Examination
Visual acuity (VA) was measured using the uniformly illuminated (100 lux) Sivtsev table, which consists of rows of Cyrillic letters, with each row representing a specific VA level. The viewing distance for VA testing was 6 meters and results were recorded as a logarithm of the minimum angle of resolution (logMAR). Comprehensive eye and vision examinations also included subjective refraction for the assessment of the best corrected VA, intraocular pressure (IOP) measurement, slit lamp biomicroscopy, indirect ophthalmoscopy, ultrasonography, and ocular biometry measurement with the calculation of intraocular lens power using the IOL Master 700 (Zeiss). Also, medical histories were collected to identify concomitant ocular and systemic diseases. Concomitant eye diseases (glaucoma, vitreomacular traction syndrome and macular hole) were diagnosed pre-surgery for the OCT group, whereas for the Control group, they were detected during the operation and/or post-surgery.
Optical coherence tomography scanning of the posterior eye was conducted for OCT group in the Private Clinic EyeDoctor, whereas the Control group did not receive retinal OCT imaging due to the absence of the device in the State City Clinic No. 13. OCT outcomes used in this study were peripapillary retinal nerve fibre layer (RNFL) thickness and macular neuronal retinal thickness: the region between the inner limiting membrane (ILM) and retinal pigment epithelium (RPE). OCT images were also used for the evaluation of the vitreous and retinal profiles for the detection of vitreomacular traction syndrome (VMTS), epiretinal membrane (ERM) and macular holes (MH)
2.4. Statistical Analysis
Our primary outcome was VA in the operated eye. Pre- and post-surgical VA was compared with factors of time (pre vs post) and group (OCT vs no OCT) using an analysis of variance (ANOVA). Secondary outcomes included demographic characteristics: between-group age was assessed for normality and an independent samples t-test was used to compare groups. Gender, treated eye (right or left) and concomitant diseases (glaucoma, vitreomacular traction syndrome, macular hole) were compared using the χ2 test of independence. Inserted IOL power (<18D, 18-19D, 19.1-20D, 20.1-21D, >21.1D) was compared using the Kruskal-Wallis H test for distribution comparison. A significance level of p < 0.05 was set for all tests. The financial cost of pre-OCT and no OCT procedures was calculated using the equation below (1). Data were organized in MS Excel and analysed using Python.
where CS – cataract surgery and CP – concomitant pathology.
2.5. Ethical Consideration
The study received approval from the Local Ethics Committee of Al-Farabi Kazakh National University (Code of Approval IRB-A470, 09.06.2022) and adhered to the principles outlined in the Declaration of Helsinki. Prior to their participation, all participants provided written and informed consent, ensuring their understanding and voluntary involvement in the study.
3. Results
3.1. Demographic Characteristics
Table 1 presents the study participant demographics and baseline clinical characteristics. Pre-existing concomitant diseases were diagnosed before surgery for the OCT group (25.3%), while for the Control group, they were identified during and after cataract surgery (28.6%). Notably, glaucoma was detected in 20-24.4% of cases, and 4% of patients in both groups had vitreomacular traction syndrome. Only one patient in the OCT group was diagnosed with a macular hole in the pre-surgical stage and subsequently underwent complex cataract and vitreoretinal (macular peeling) surgery. Statistical analysis revealed no significant differences in the prevalence of concomitant diseases between the two groups. Additionally, there were no significant differences in the calculated power of the intraocular lenses between the study groups.
3.2. Clinical Outcomes
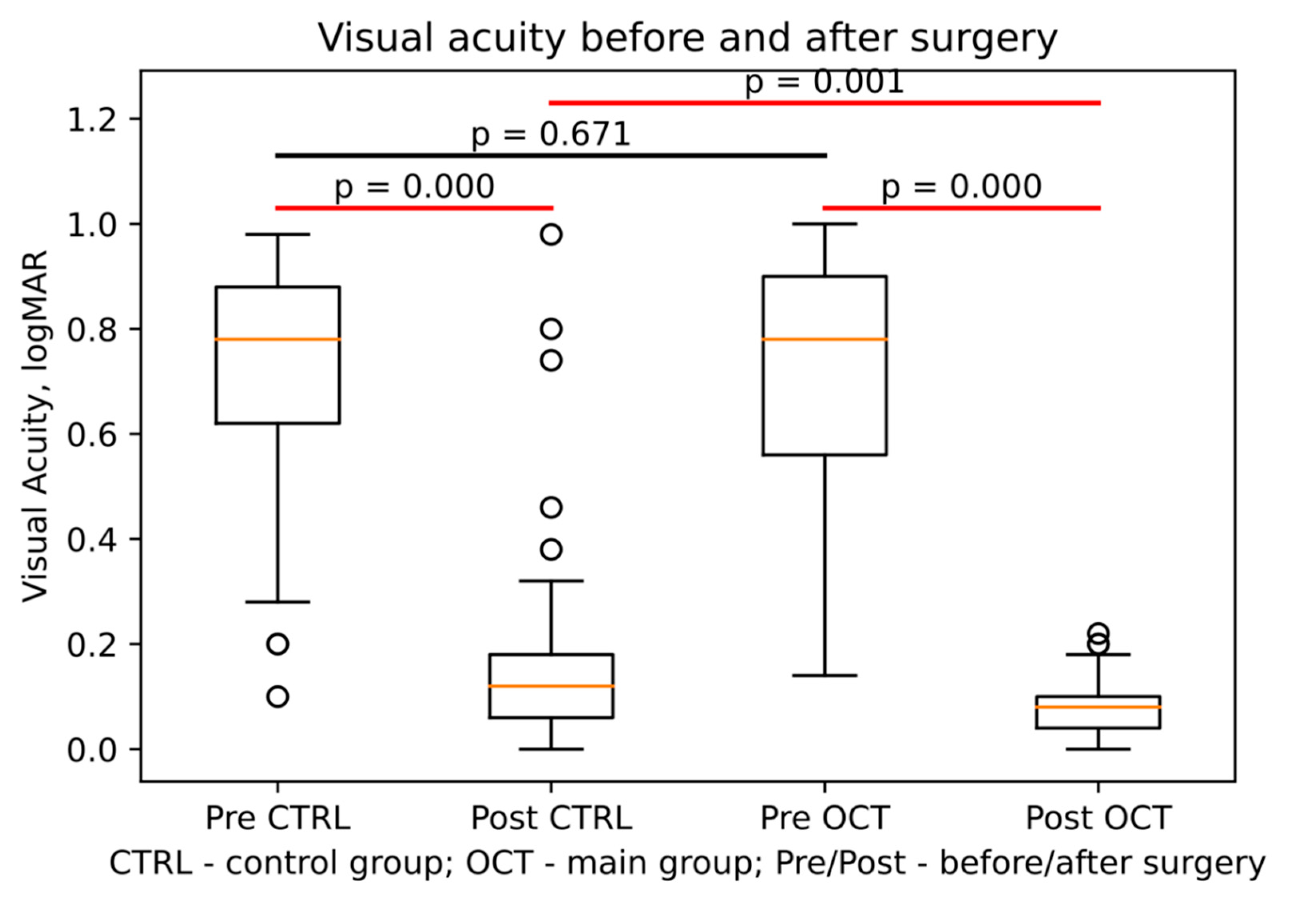
Table 2 shows the VA range (logMAR) before and 7 days after the surgery, the number of patients within each range, and the corresponding percentages for each group. The OCT group experienced a significantly greater improvement in VA (-0.647±0.232) compared to the no OCT control group (-0.543±0.244) with a significant interaction between time and group with F(df) = 217.2, p < 0.001 (
Figure 1).
3.3. Cost Analysis
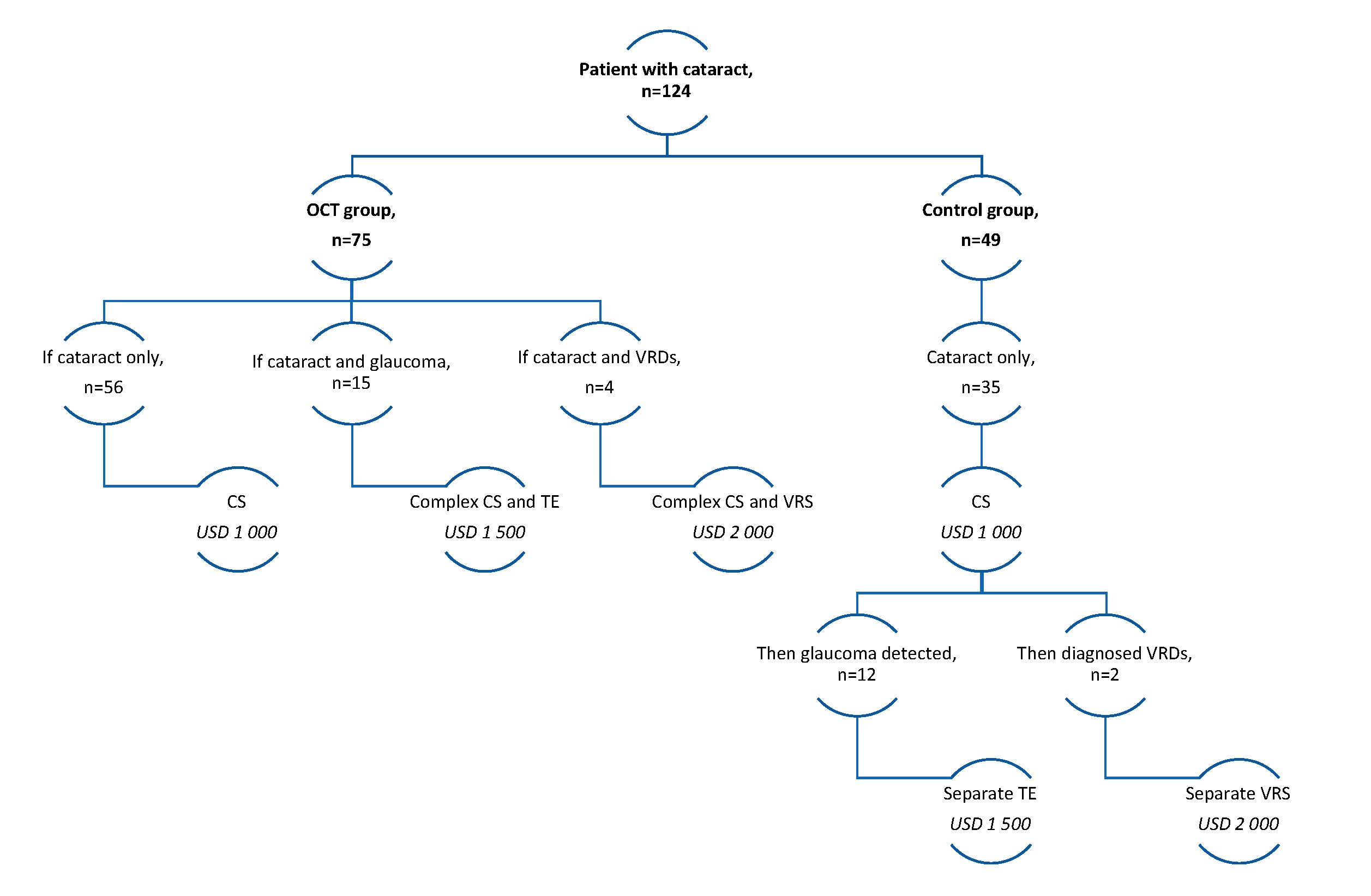
Figure 2 illustrates the cost of cataract management for each site (OCT vs no-OCT). In the Control group, 24.4% (n = 12) of patients who had glaucoma could have undergone filter surgery (trabeculectomy) at the same surgical visit if their condition had been diagnosed earlier. Additionally, 20% of patients with normal intraocular pressure (IOP) but a thin retinal nerve fibre layer (RNFL) and a cup-disc ratio greater than 0.5 (as observed through OCT en-face rendering, colour, and red-filter imaging) could have received complex surgery with trabeculectomy. It is worth noting that microinvasive surgery was not included in the clinical protocol for cataract management in Kazakhstan.
Similar considerations apply to patients with vitreomacular traction syndrome. The probability of this syndrome occurring was 4%, with three patients in the OCT group and two patients in the Control group affected. For patients in the OCT group, complex one-time cataract and vitreoretinal surgery was recommended. Conversely, patients in the Control group with vitreomacular traction syndrome were required to wait for separate visits for vitreoretinal surgery to minimize the risk of complications.
To determine the cost of one surgical appointment (separate surgeries) rather than multiple appointments (complex surgeries), probability coefficients were computed to assess the occurrence of concomitant pathologies for both groups. These coefficients are presented in
Table 3. The cost calculations (Equation 2) for separate and complex surgeries are presented in
Figure 2.
where CP – concomitant pathology, which differs depending on whether it is separate or complex, 0.76 is the probability of Cataract surgery without concomitant pathology.
Despite not factoring into the cost analysis, the management of the OCT group (Equation 3) was found to be more cost-effective than that of the Control group (Equation 4).
Taking into account the aforementioned factors and excluding other potential concomitant pathologies that could be detected using OCT, it can be inferred that the utilisation of OCT in cataract management results in lower costs (US $1190, or KZT 530000) compared to managing cataracts without this device (US $1380, or KZT 615000).
4. Discussion
This study aimed to evaluate the clinical and economic impact of integrating OCT in the pre-surgical stage of cataract management. While traditional methods like fundus photography and biomicroscopy detect ocular complications such as diabetes-related conditions, full-thickness macular holes, and vein occlusions, OCT offers additional capabilities, identifying epiretinal membranes [
32], vitreomacular traction [
33], age-related macular degeneration [
34], central serous chorioretinopathy [
35], retinoschisis [
36], and other pathologies.
Within the cohort, we calculated the probability coefficients for pre-existing conditions such as glaucoma (0.2-0.244) and vitreomacular traction syndrome (0.04-0.041). Both groups exhibited similar rates of these conditions, indicating no significant differences in the distribution of concomitant diseases, demographics (age and gender), calculated IOL power, or the treated eye (right vs. left). This suggests that these variables did not influence patient allocation and did not confound the comparison between groups.
Using a cost calculation model that factored in the management of separate and complex surgeries and the probability of concomitant pathologies, we found that pre-surgical OCT was more cost-effective than management without it. The OCT group had a lower overall cost (KZT 530,000) compared to the Control group (KZT 615,000), primarily due to early detection and appropriate treatment of concomitant pathologies in a single surgical visit. This indicates better resource allocation and cost savings, particularly relevant for low- and middle-income countries.
Clinically, OCT integration led to significantly greater improvements in visual acuity post-surgery compared to the Control group. Enhanced pre-surgical assessment of vitreoretinal pathologies allowed for more precise surgical planning and better postoperative outcomes, which is crucial for overall quality of life [
37,
38].
However, this study has limitations. We focused only on vitreomacular traction syndrome and glaucoma, and a larger cohort might reveal different probabilities for other vitreoretinal conditions [
39,
40,
41]. Additionally, we did not study advanced-technology IOL implantations [
40], such as multifocal lenses [
42], which might affect the variables analysed. Specifically, multifocal lenses could improve post-op VA at multiple distances compared to monofocal lenses, although they are more expensive and might affect the cost-effectiveness of cataract management. Future research should investigate long-term post-surgical complications like pseudophakic cystoid macular oedema [
43] and diabetic retinopathy [
44], as they could alter postoperative strategies [
45,
46].
Our findings regarding the cost-effectiveness of OCT align with previous studies in high-income countries. For instance, Chang et al. (2008) reported significant cost savings with OCT compared to fluorescein angiograms, with the equipment investment recovered within four months [
47]. Balancing financial investment in diagnostic equipment with potential diagnostic errors and missed pathologies could improve cataract management strategies and quality-adjusted life years [
48,
49].
Though no device currently provides a realistic post-cataract surgery vision expectation, OCT minimizes ocular complications and treatment expenses [
49]. Advances in ultrahigh ultrafast OCT systems, adaptive optics [
50], multimodal OCT [
51], and machine learning algorithms for image analysis [
52] enhance OCT’s diagnostic capabilities. Implementing pre-surgical OCT improves cataract patient management by expanding diagnostic options and reducing the risk of underdiagnosing vitreomacular diseases. These outcomes are particularly beneficial for low and middle-income countries like Kazakhstan.
5. Conclusions
Despite the challenges associated with the initial investment in OCT equipment [
53], especially in remote regions with limited resources, this study demonstrates the significant clinical and economic benefits of OCT in cataract management. Long-term cost savings and improved patient outcomes [
54,
55] underscore the importance of integrating OCT into pre-surgical protocols.
Author Contributions
Conceptualization, M.I. and M.K.; methodology, M.K.; software, M.K.; validation, M.I., G.B. and A.B.; formal analysis, M.K.; investigation, M.I.; resources, M.I.; data curation, G.B.; writing—original draft preparation, M.I. and M.K.; writing—review and editing, B.T.; visualization, M.K.; supervision, B.T.; project administration, M.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. BT and MK were supported by the InnoHK initiative and the Hong Kong Special Administrative Region Government.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Local Ethics Committee of Al-Farabi Kazakh National University (Code of Approval IRB-A470, 09.06.2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Acknowledgments
We thank all patients and their families for participating in this study. We thank all ophthalmologists and nurses from Private Clinic EyeDoctor and State City Clinic No. 13 (both in Almaty, Kazakhstan) for their assistance in the collection of the data. We thank Yuliya Semenova (Nazarbayev University School of Medicine) and Natalya Glushkova (Al-Farabi Kazakh National University) for their assistance in manuscript writing and their invaluable consultations.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Bourne, R.R.; Stevens, G.A.; White, R.A.; Smith, J.L.; Flaxman, S.R.; Price, H.; Jonas, J.B.; Keeffe, J.; Leasher, J.; Naidoo, K; et al. Causes of vision loss worldwide, 1990-2010: a systematic analysis. Lancet Glob. Health. 2013, 1, e339–e349. [Google Scholar] [CrossRef] [PubMed]
- Burton, M.J.; Ramke, J.; Marques, A.P.; Bourne, R.R.A.; Congdon, N.; Jones, I.; Ah Tong, B.A.M.; Arunga, S.; Bachani, D.; Bascaran, C.; et al. The Lancet Global Health Commission on Global Eye Health: vision beyond 2020. Lancet Glob. Health. 2021, 9, e489–e551. [Google Scholar] [CrossRef] [PubMed]
- Javitt, J.C.; Wang, F.; West, S.K. Blindness due to cataract: epidemiology and prevention. Annu. Rev. Public Health. 1996, 17, 159–177. [Google Scholar] [CrossRef]
- Asbell, P.A.; Dualan, I.; Mindel, J.; Brocks, D.; Ahmad, M.; Epstein, S. Age-related cataract. Lancet. 2005, 365, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Robman, L.; Taylor, H. External factors in the development of cataract. Eye (Lond). 2005, 19, 1074–1082. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Lee, H.; Kim, H. Association between Exposure to Ambient Air Pollution and Age-Related Cataract: A Nationwide Population-Based Retrospective Cohort Study. Int. J. Environ. Res. Public Health. 2020, 17, 9231. [Google Scholar] [CrossRef] [PubMed]
- Kohnen, T.; Baumeister, M.; Kook, D.; Klaproth, O.K.; Ohrloff, C. Cataract surgery with implantation of an artificial lens. Dtsch. Arztebl. Int. 2009, 106, 695–702. [Google Scholar] [CrossRef]
- Lapp, T.; Wacker, K.; Heinz, C.; Maier, P.; Eberwein, P.; Reinhard, T. Cataract Surgery-Indications, Techniques, and Intraocular Lens Selection. Dtsch. Arztebl. Int. 2023, 120, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Yorston, D. High-volume surgery in developing countries. Eye (Lond). 2005, 19, 1083–1089. [Google Scholar] [CrossRef]
- Heemraz, B.S.; Lee, C.N.; Hysi, P.G.; Jones, C.A.; Hammond, C.J.; Mahroo, O.A. Changes in quality of life shortly after routine cataract surgery. Can. J. Ophthalmol. 2016, 51, 282–287. [Google Scholar] [CrossRef]
- Han, X.; Zhang, J.; Liu, Z; Tan, X. ; Jin, G.; He, M.; Luo, L.; Liu, Y. Real-world visual outcomes of cataract surgery based on population-based studies: a systematic review. Br. J. Ophthalmol. 2023, 107, 1056–1065. [Google Scholar] [CrossRef]
- Asimakis, P.; Coster, D.J.; Lewis, D.J. Cost effectiveness of cataract surgery. A comparison of conventional extracapsular surgery and phacoemulsification at Flinders Medical Centre. Aust. N. Z. J. Ophthalmol. 1996, 24, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Manaf, M.R.; Aljunid, S.M.; Annuar, F.H.; Leong, C.K.; Mansor, N. Cost-effectiveness analysis of cataract surgery with intraocular lens implantation: extracapsular cataract extraction versus phacoemulsification. Med. J. Indones. 2007, 16, 25. [Google Scholar] [CrossRef]
- Jongsareejit, A.; Wiriyaluppa, C.; Kongsap, P.; Phumipan, S. Cost-effectiveness analysis of manual small incision cataract surgery (MSICS) and phacoemulsification (PE). J. Med. Assoc. Thai. 2012, 95, 212–220. [Google Scholar] [PubMed]
- Khan, A.; Amitava, A.K.; Rizvi, S.A.; Siddiqui, Z.; Kumari, N.; Grover, S. Cost-effectiveness analysis should continually assess competing health care options especially in high volume environments like cataract surgery. Indian J. Ophthalmol. 2015, 63, 496–500. [Google Scholar] [PubMed]
- Clinical protocols of the Ministry of Health of the Republic of Kazakhstan – 2017. Available online: https://diseases.medelement.com/disease/катаракта-2017/15346 (accessed on 05.04.2024).
- Nguyen, P.; Chopra, V. Applications of optical coherence tomography in cataract surgery. Curr Opin Ophthalmol. 2013, 24, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, J.G.; Pitris, C.; Boppart, S.A.; Brezinski, M.E. Optical coherence tomography: an emerging technology for biomedical imaging and optical biopsy. Neoplasia 2000, 2, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Drexler, W.; Morgner, U.; Ghanta, R.K.; Kärtner, F.X.; Schuman, J.S.; Fujimoto, J.G. Ultrahigh-resolution ophthalmic optical coherence tomography. Nat. Med. 2001, 7, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Kulmaganbetov, M.; Bevan, R.J.; Anantrasirichai, N.; Achim, A.; Erchova, I.; White, N.; Albon, J.; Morgan, J.E. Textural Feature Analysis of Optical Coherence Tomography Phantoms. Electronics 2022, 11, 669. [Google Scholar] [CrossRef]
- Chen, S.; Liu, X.; Wang, N.; Wang, X.; Xiong, Q.; Bo, E.; Yu, X.; Chen, S.; Liu, L. Visualizing Micro-anatomical Structures of the Posterior Cornea with Micro-optical Coherence Tomography. Sci. Rep. 2017, 7, 10752. [Google Scholar] [CrossRef]
- Ang, M.; Baskaran, M.; Werkmeister, R.M.; Chua, J.; Schmidl, D.; Aranha Dos Santos, V.; Garhöfer, G.; Mehta, J.S.; Schmetterer, L. Anterior segment optical coherence tomography. Prog. Retin. Eye Res. 2018, 66, 132–156. [Google Scholar] [CrossRef] [PubMed]
- Hipólito-Fernandes, D.; Luís, M.E.; Serras-Pereira, R.; Gil, P.; Maduro, V.; Feijão, J.; Alves, N. Anterior chamber depth, lens thickness and intraocular lens calculation formula accuracy: nine formulas comparison. Br. J. Ophthalmol. 2022, 106, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, J.R.; Puliafito, C.A.; Hee, M.R.; Duker, J.S.; Reichel, E.; Coker, J.G.; Schuman, J.S.; Swanson, E.A.; Fujimoto, J.G. Characterization of epiretinal membranes using optical coherence tomography. Ophthalmology 1996, 103, 2142–2151. [Google Scholar] [CrossRef] [PubMed]
- Trichonas, G.; Kaiser, P.K. Optical coherence tomography imaging of macular oedema. Br. J. Ophthalmol. 2014, 98, ii24–ii29. [Google Scholar] [CrossRef] [PubMed]
- Frenkel, S.; Morgan, J.; Blumenthal, E. Histological measurement of retinal nerve fibre layer thickness. Eye 2005, 19, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Unterlauft, J.D.; Rehak, M.; Böhm, M.R.R.; Rauscher, F.G. Analyzing the impact of glaucoma on the macular architecture using spectral-domain optical coherence tomography. PLoS One 2018, 13, e0209610. [Google Scholar] [CrossRef] [PubMed]
- Kabylbekova, A.; Meirmanov, S.; Aringazina, A.; Orazbekov, L.; Auyezova, A. Clinical characteristics of congenital and developmental cataract in Kazakhstan. Indian J Ophthalmol. 2022, 70, 4325–4330. [Google Scholar] [CrossRef] [PubMed]
- Sidorenko AV, Eshmanova AK, Abikulova AK. Population aging in Kazakhstan. Problems and opportunities [Russian]. Adv. Gerontol. 2017, 30, 505–515. [Google Scholar]
- Nugumanova, L.; Frey, M.; Yemelina, N.; Yugay, S. Environmental problems and policies in Kazakhstan: Air pollution, waste and water. IOS Working Papers 2017, 366. [Google Scholar]
- Alimbaev, T.; Mazhitova, Zh.; Omarova, B.; Kamzayev, B.; Atanakova, K. Ecological problems of modern central Kazakhstan: challenges and possible solutions. E3S Web Conf 2020, 157, 03018. [Google Scholar] [CrossRef]
- Stevenson, W.; Prospero Ponce, C.M.; Agarwal, D.R.; Gelman, R.; Christoforidis, J.B. Epiretinal membrane: optical coherence tomography-based diagnosis and classification. Clin. Ophthalmol. 2016, 10, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Cereda, M.; Caimi, A.; Bottoni, F.; Staurenghi, G. Optical coherence tomography in eyes with vitreomacular traction. Ophthalmology 2013, 120, e46–e47. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, Y.; Akbari, M.; Moghadam, R.S.; Medghalchi, A.; Dourandeesh, M.; Bromandpoor, F. Macular Optical Coherence Tomography before Cataract Surgery. J. Curr. Ophthalmol. 2021, 33, 317–322. [Google Scholar] [PubMed]
- Iida, T.; Hagimura, N.; Sato, T.; Kishi, S. Evaluation of central serous chorioretinopathy with optical coherence tomography. Am. J. Ophthalmol. 2000, 129, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Ip, M.; Garza-Karren, C.; Duker, J.S.; Reichel, E.; Swartz, J.C.; Amirikia, A.; Puliafito, C.A. Differentiation of degenerative retinoschisis from retinal detachment using optical coherence tomography. Ophthalmology 1999, 106, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.W.; Wong, J.C.; Chan, K.S.; Wong, W.K.; Tam, K.C.; Chau, P.S. Evaluation of quality of life in patients with cataract in Hong Kong. J. Cataract Refract. Surg. 2003, 29, 1753–1760. [Google Scholar] [CrossRef] [PubMed]
- Keane, P.A.; Sadda, S.R. Predicting visual outcomes for macular disease using optical coherence tomography. Saudi J. Ophthalmol. 2011, 25, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhang, Z.; Wang, J.; Meng, X.; Chen, T.; Wu, Z. Macular assessment of preoperative optical coherence tomography in ageing Chinese undergoing routine cataract surgery. Sci. Rep. 2018, 8, 5103. [Google Scholar] [CrossRef] [PubMed]
- Klein, B.R.; Brown, E.N.; Casden, R.S. Preoperative macular spectral-domain optical coherence tomography in patients considering advanced-technology intraocular lenses for cataract surgery. J. Cataract Refract. Surg. 2016, 42, 537–541. [Google Scholar] [CrossRef]
- McKeague, M.; Sharma, P.; Ho, A.C. Evaluation of the macula prior to cataract surgery. Curr. Opin. Ophthalmol. 2018, 29, 4–8. [Google Scholar] [CrossRef]
- Hinnig, R.B.; Martins, L.F.S.; Penha, F.M. Spectral domain oct for screening of macular diseases prior to multifocal intraocular lens implantation. Int. J. Retina Vitreous 2022, 8, 77. [Google Scholar] [CrossRef] [PubMed]
- Henderson, B.A.; Kim, J.Y.; Ament, C.S.; Ferrufino-Ponce, Z.K.; Grabowska, A.; Cremers, S.L. Clinical pseudophakic cystoid macular edema. Risk factors for development and duration after treatment. J. Cataract Refract. Surg. 2007, 33, 1550–1558. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.; Mitchell, P.; de Loryn, T.; Rochtchina, E.; Cugati, S.; Wang, J.J. Development and progression of diabetic retinopathy 12 months after phacoemulsification cataract surgery. Ophthalmology 2009, 116, 1510–1514. [Google Scholar] [CrossRef] [PubMed]
- Curković, T.; Vukojević, N.; Bućan, K. Treatment of pseudophakic cystoid macular oedema. Coll. Antropol. 2005, 29, 103–105. [Google Scholar] [PubMed]
- Ghasemi Falavarjani, K.; Parvaresh, M.M.; Modarres, M.; Hashemi, M.; Samiy, N. Intravitreal bevacizumab for pseudophakic cystoid macular edema; a systematic review. J Ophthalmic Vis Res. 2012, 7, 235–239. [Google Scholar]
- Chang, D.L.; Subramanian, M.L.; Legutko, P.A.; Daly, M.K. Cost-Benefit Analysis on the Use of Optical Coherence Tomography versus Fluorescein Angiogram in the Diagnosis of Macular Diseases at the VA Boston Healthcare System, Jamaica Plain Campus. Invest. Ophthalmol. Vis. Sci. 2008, 49, 1860. [Google Scholar]
- Leung, E.H.; Gibbons, A.; Koch, D.D. Cost-Effectiveness of Preoperative OCT in Cataract Evaluation for Multifocal Intraocular Lens. Ophthalmology 2020, 127, 859–865. [Google Scholar] [CrossRef]
- Goldhardt, R.; Rosen, B.S. Optical Coherence Tomography: Critical Tool to Manage Expectations after Cataract Extraction. Curr Ophthalmol. Rep. 2020, 8, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Pircher, M.; Zawadzki, R.J. Review of adaptive optics OCT (AO-OCT): principles and applications for retinal imaging [Invited]. Biomed. Opt. Express. 2017, 8, 2536–2562. [Google Scholar] [CrossRef]
- Shirazi, M.F.; Andilla, J.; Lefaudeux, N.; Valdes, C.; Schwarzhans, F.; Durand, M.; Ntatsis, K.; De Jesus, D.A.; Sanchez Brea, L.; Gocho, K.; et al. Multi-modal and multi-scale clinical retinal imaging system with pupil and retinal tracking. Sci. Rep. 2022, 12, 9577. [Google Scholar] [CrossRef]
- Kulmaganbetov, M.; Albon, J.; White, N.; Morgan, J.E. Texture analysis of OCT phantoms. In Biophotonics Congress: Biomedical Optics; Optica Publishing Group: Washington, DC, USA, 2020; p. JTu3A.23. [Google Scholar]
- Swanson, E.A.; Fujimoto, J.G. The ecosystem that powered the translation of OCT from fundamental research to clinical and commercial impact [Invited]. Biomed Opt Express. 2017, 8, 1638–1664. [Google Scholar] [CrossRef] [PubMed]
- Jindal, A.; Ctori, I.; Fidalgo, B.; Dabasia, P.; Balaskas, K.; Lawrenson, J.G. Impact of optical coherence tomography on diagnostic decision-making by UK community optometrists: a clinical vignette study. Ophthalmic Physiol. Opt. 2019, 39, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Olson, J.; Sharp, P.; Goatman, K.; Prescott, G.; Scotland, G.; Fleming, A.; Philip, S.; Santiago, C.; Borooah, S.; Broadbent, D.; et al. Improving the economic value of photographic screening for optical coherence tomography-detectable macular oedema: a prospective, multicentre, UK study. Health Technol. Assess. 2013, 17, 1–142. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).