Submitted:
26 June 2024
Posted:
27 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Regulatory Mechanisms of Bile Acids in Maintaining Intestinal Homeostasis and Counteracting Infections
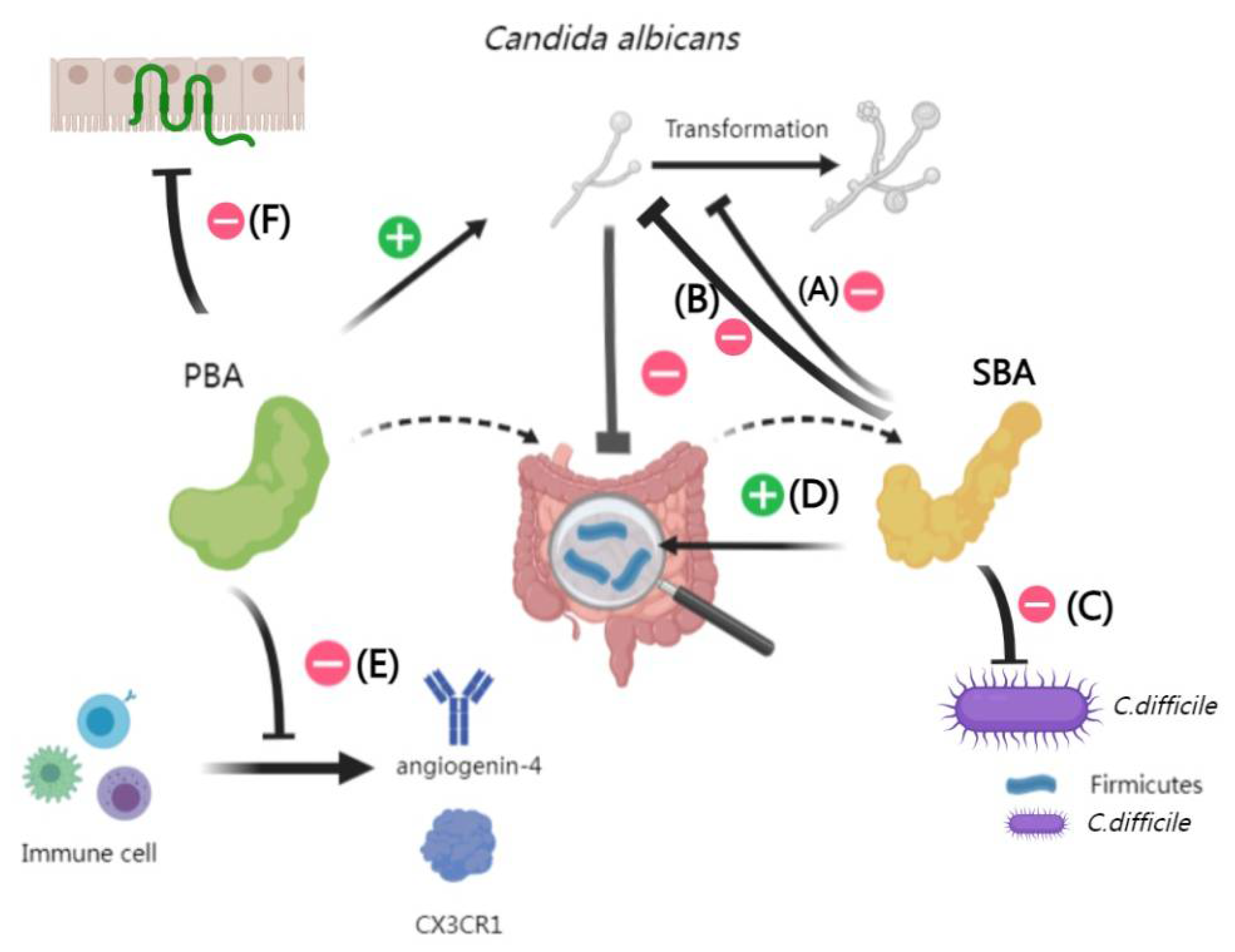
2.1. BAs and Fungal
2.2. BAs and Bacteria
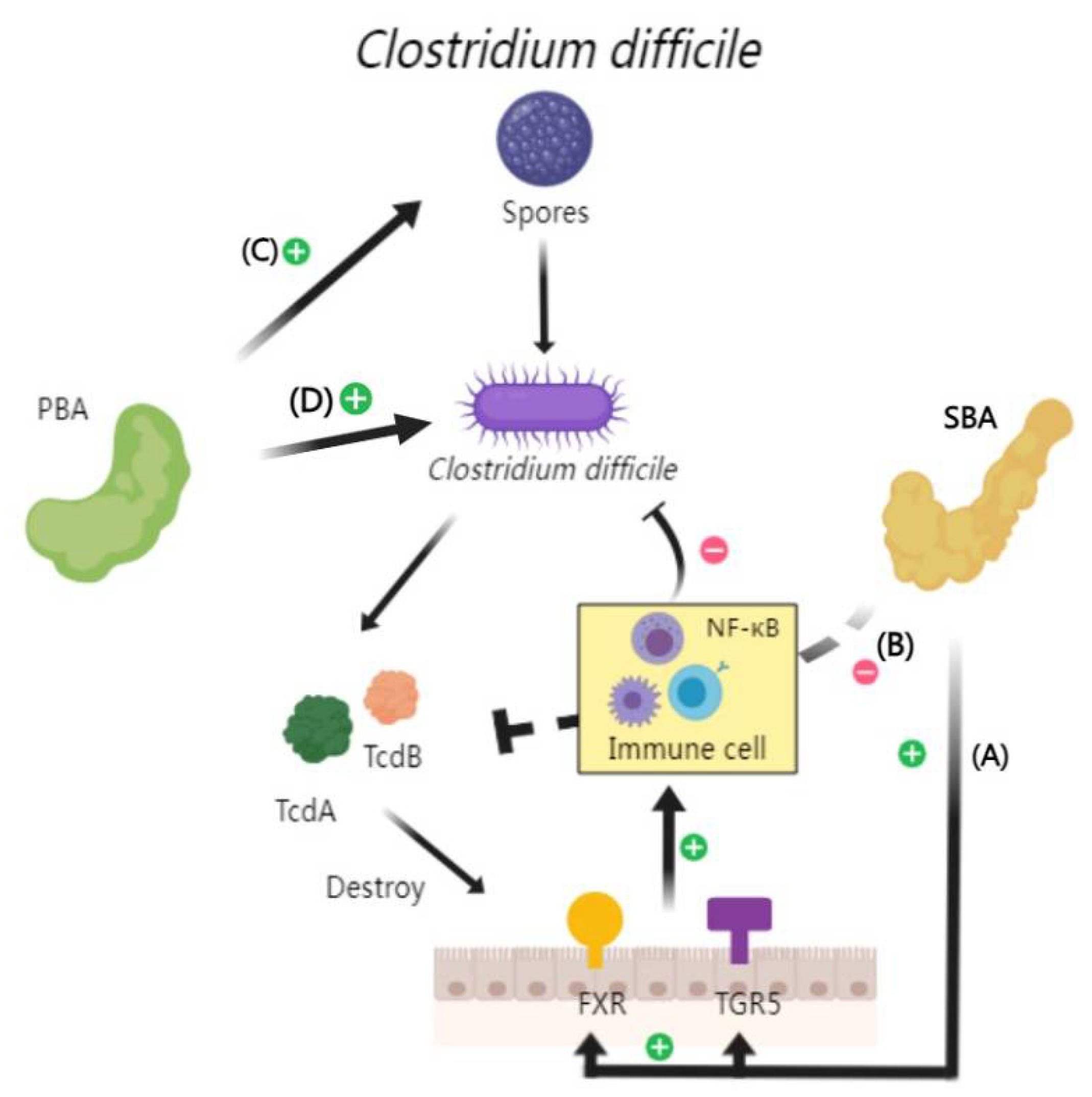
2.2.1. Interactions Between BAs and Clostridioides difficile
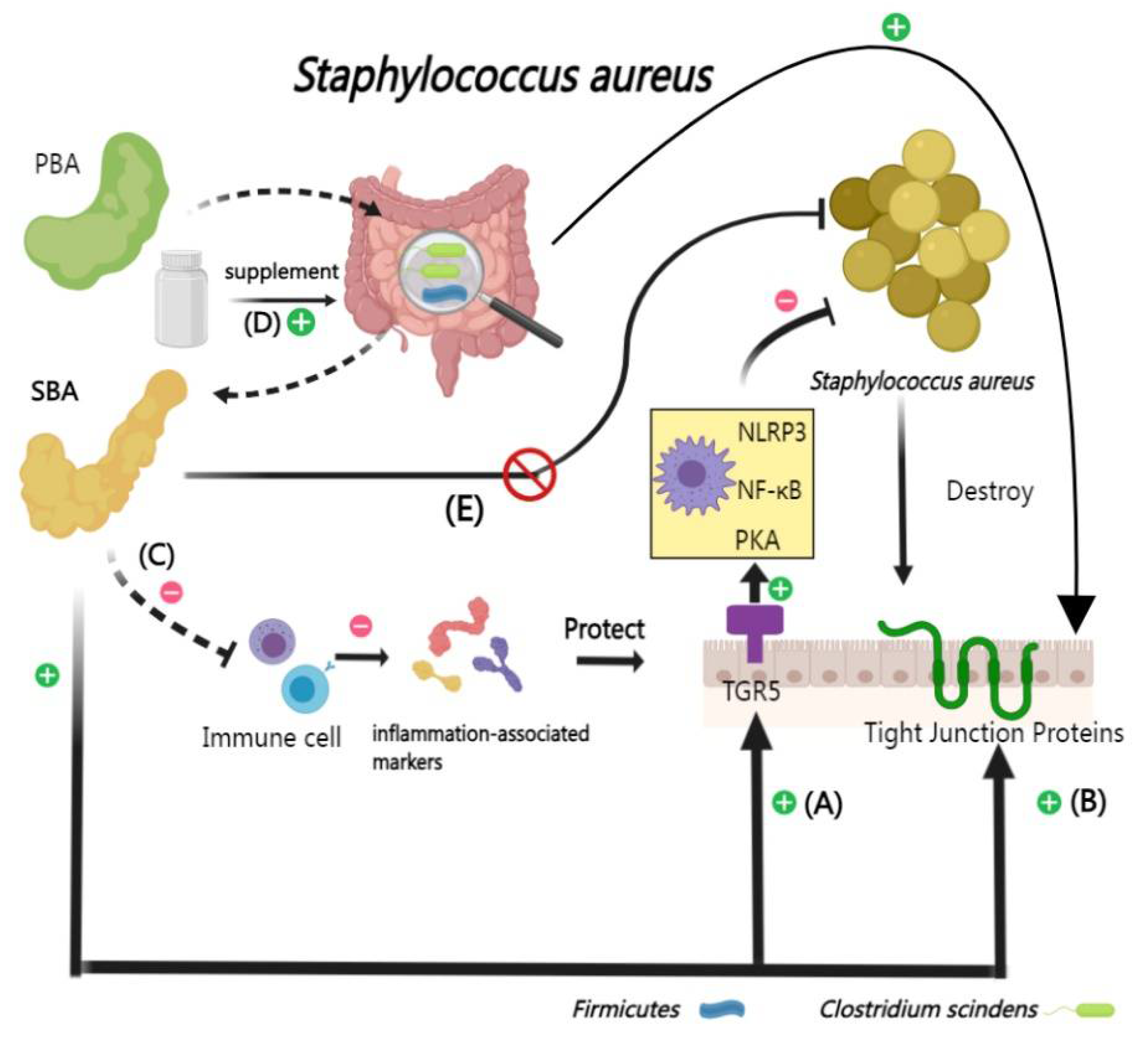
2.2.2. Interactions Between BAs and Staphylococcus aureus
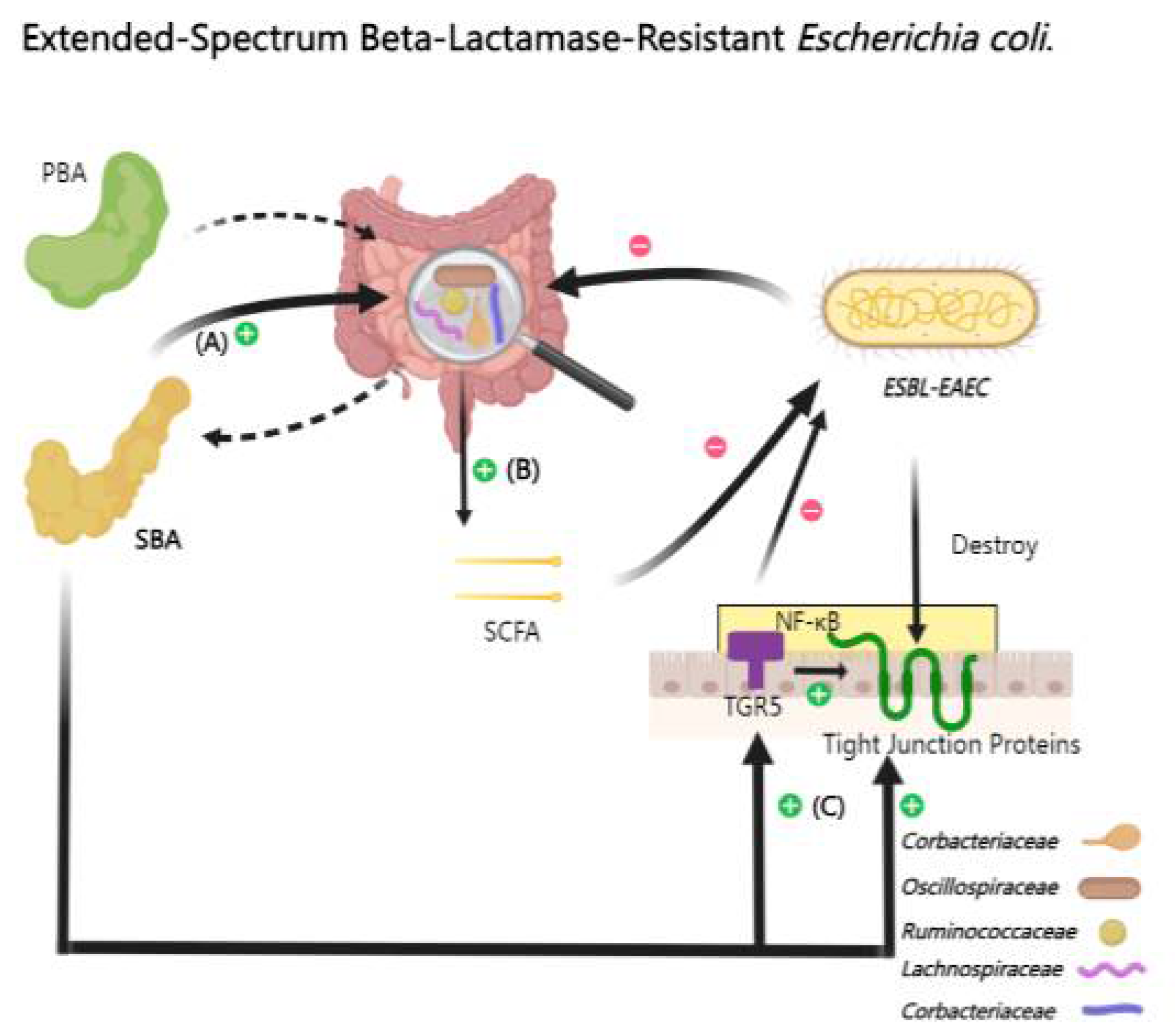
2.2.3. Interactions Between BAs and Extended-Spectrum Beta-Lactamase-Resistant Escherichia coli
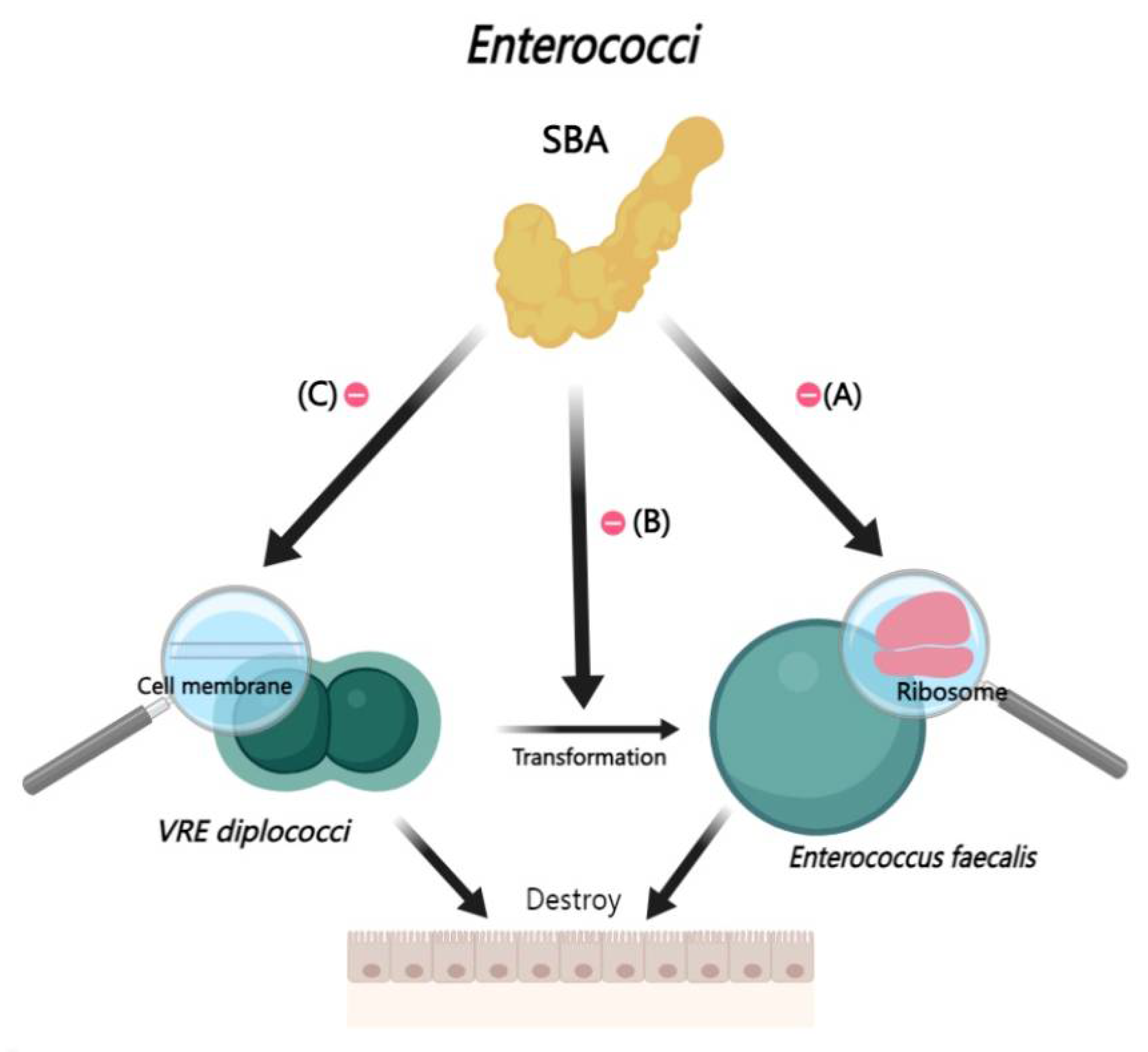
2.2.4. Interactions Between BAs and Enterococci
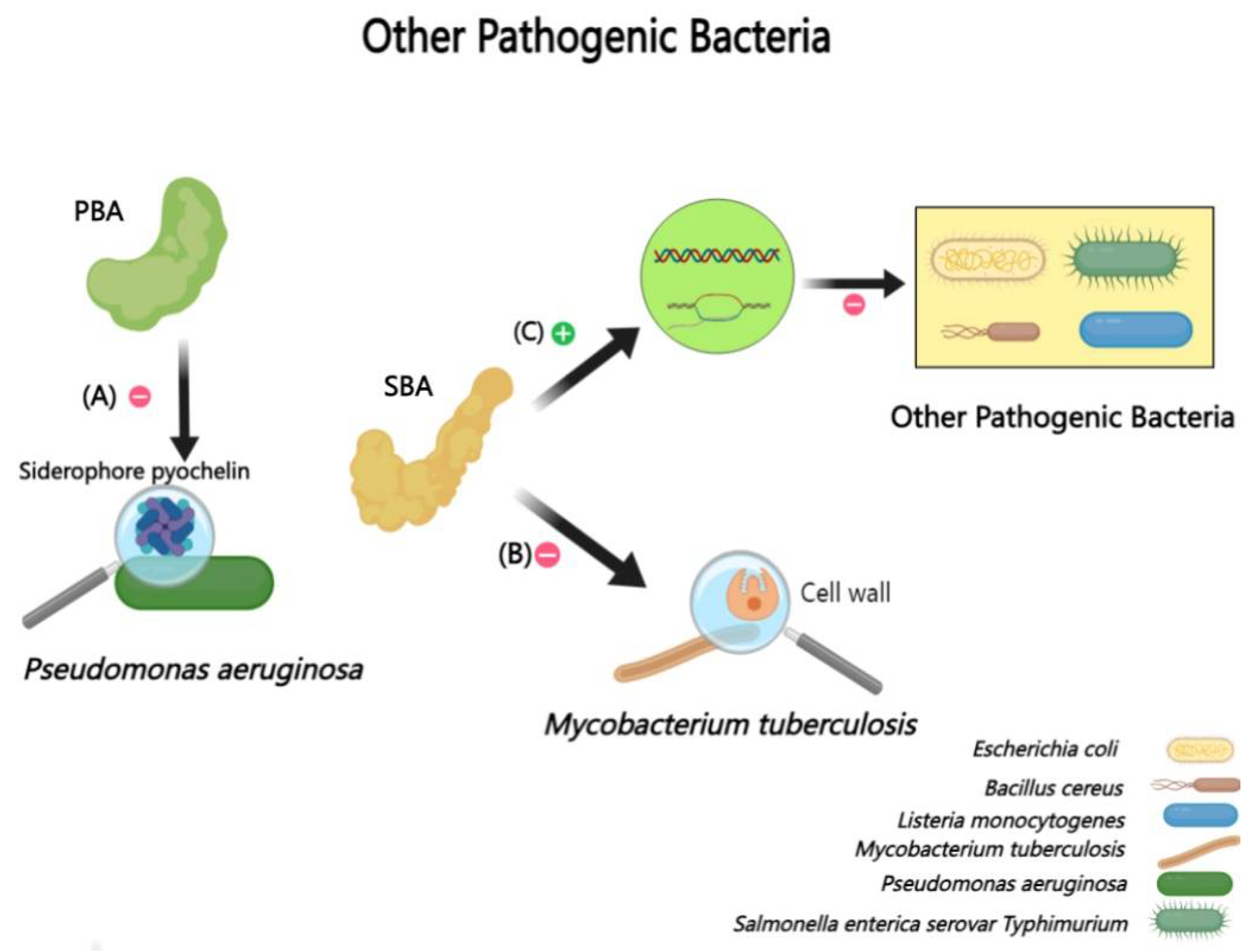
2.2.5. Interactions Between BAs and Other Bacteria (Pseudomonas aeruginosa, Mycobacterium tuberculosis, etc.)
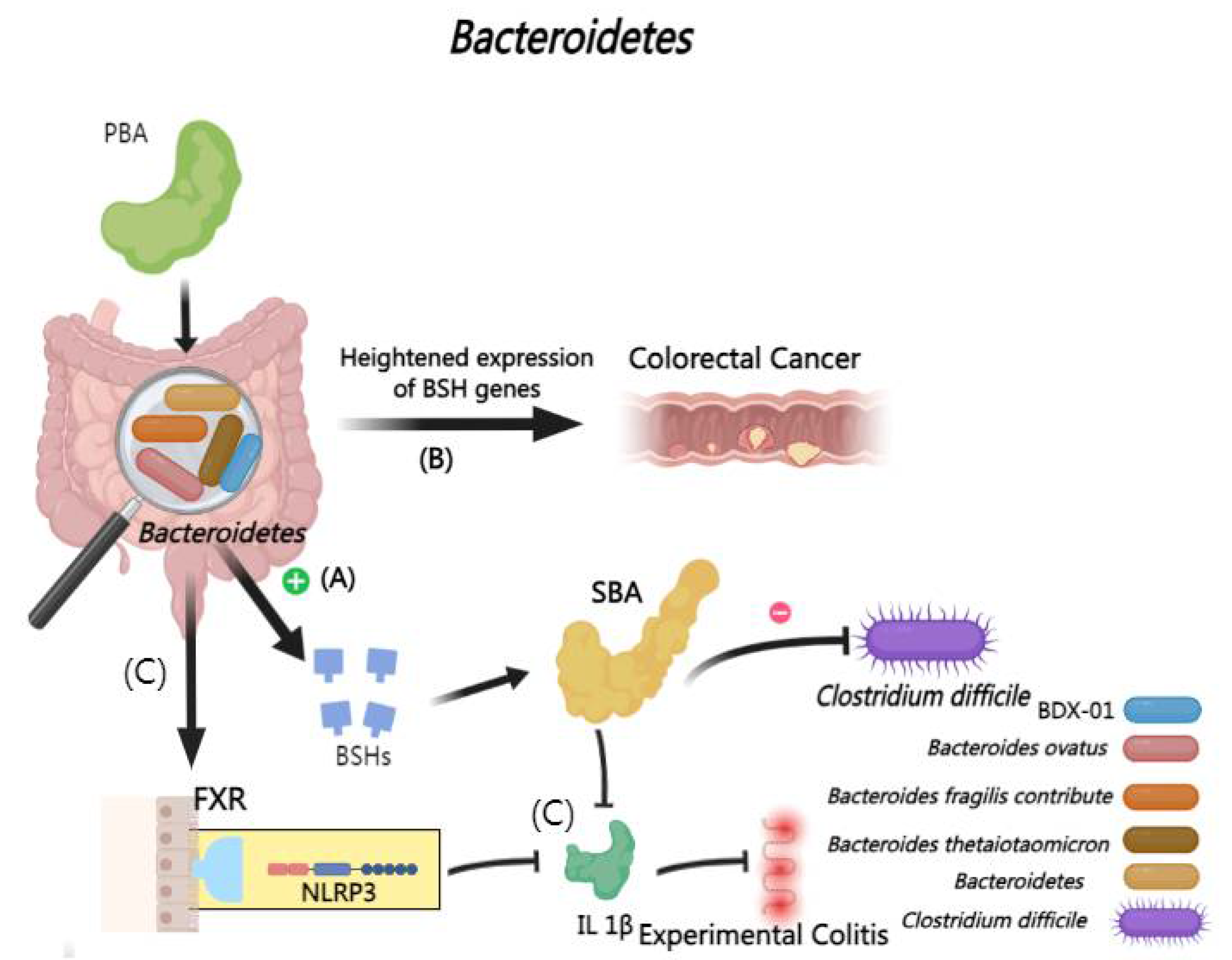
2.2.6. Interactions Between BAs and Bacteroidetes
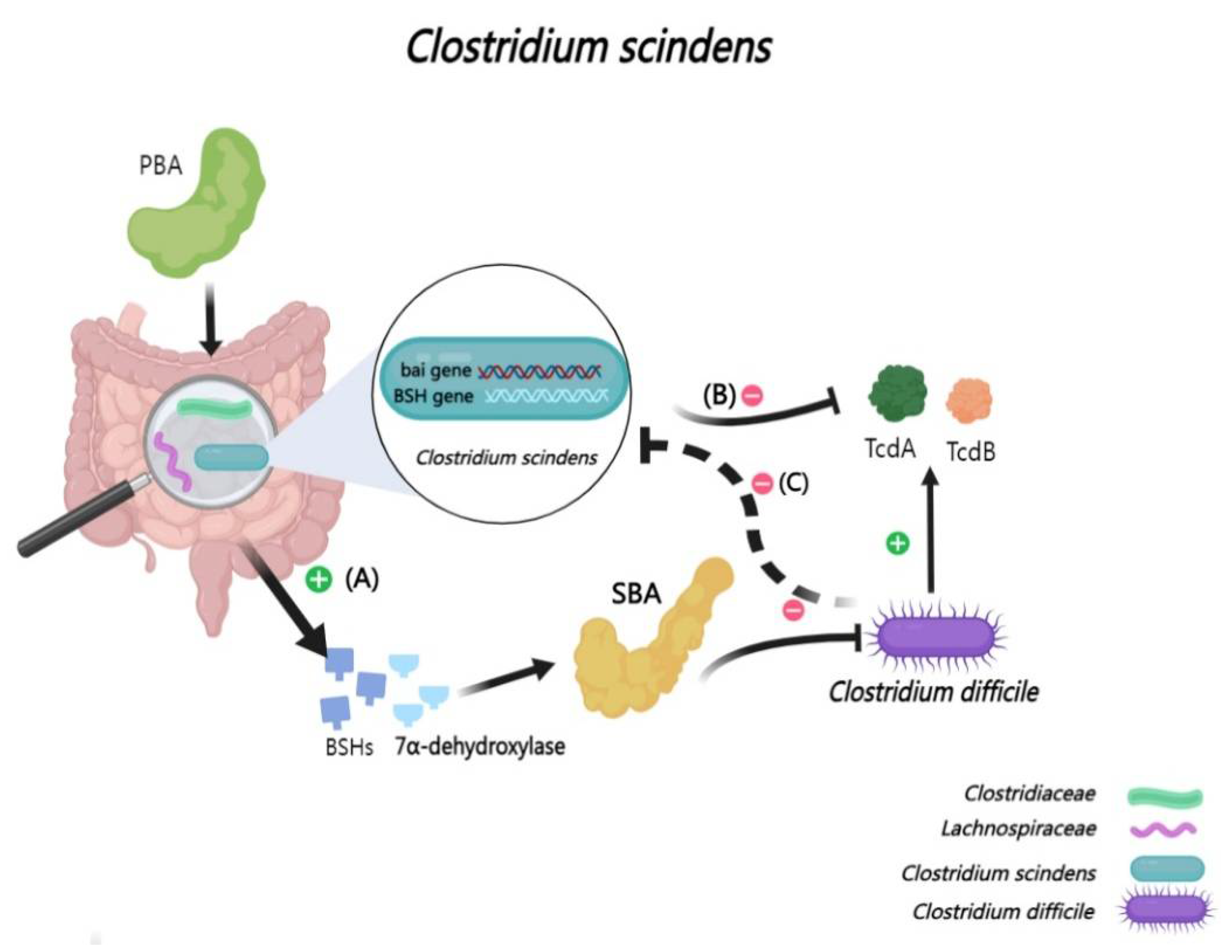
2.2.7. Interactions Between BAs and Clostridium scindens
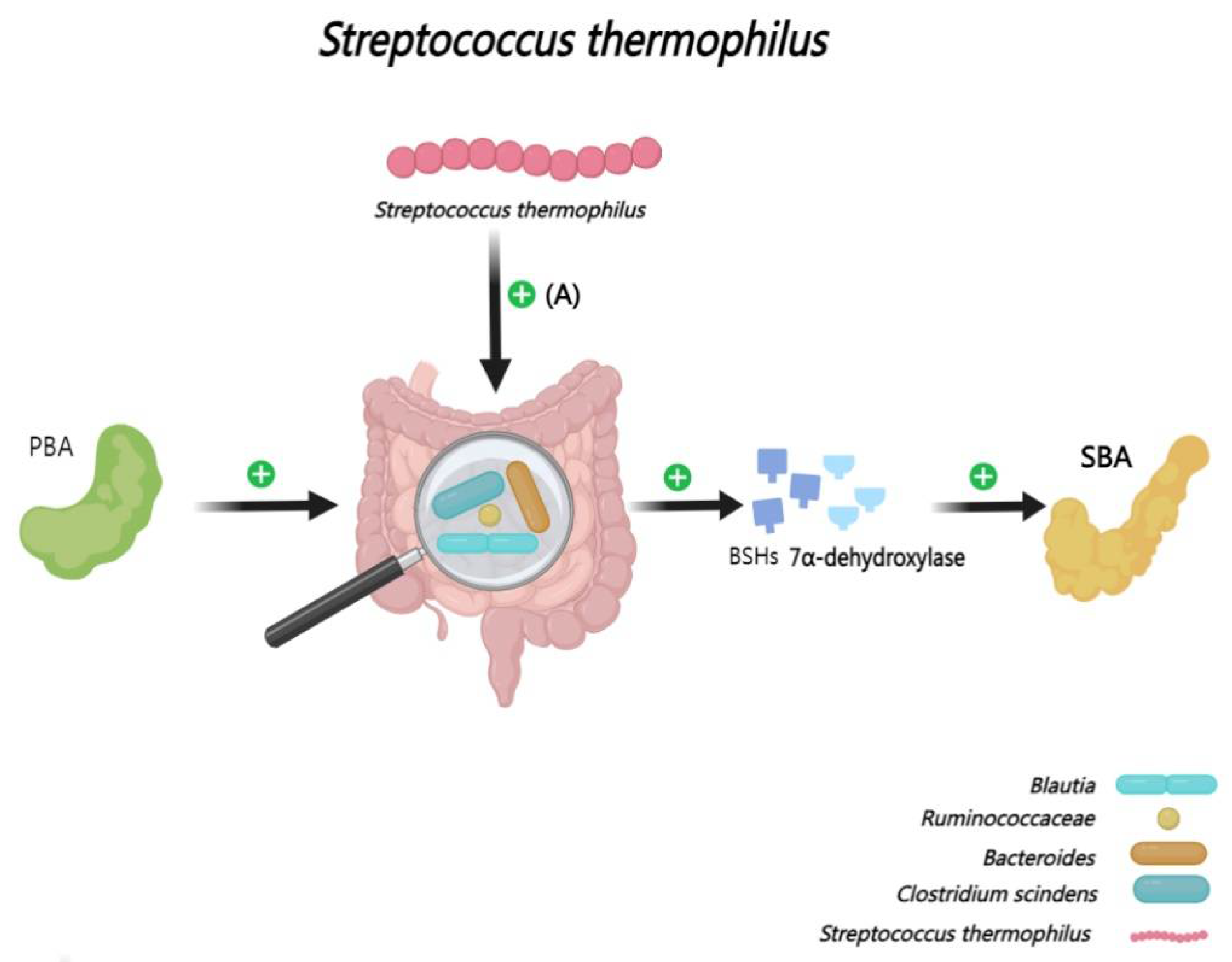
2.2.8. Interactions Between BAs and Streptococcus thermophilus
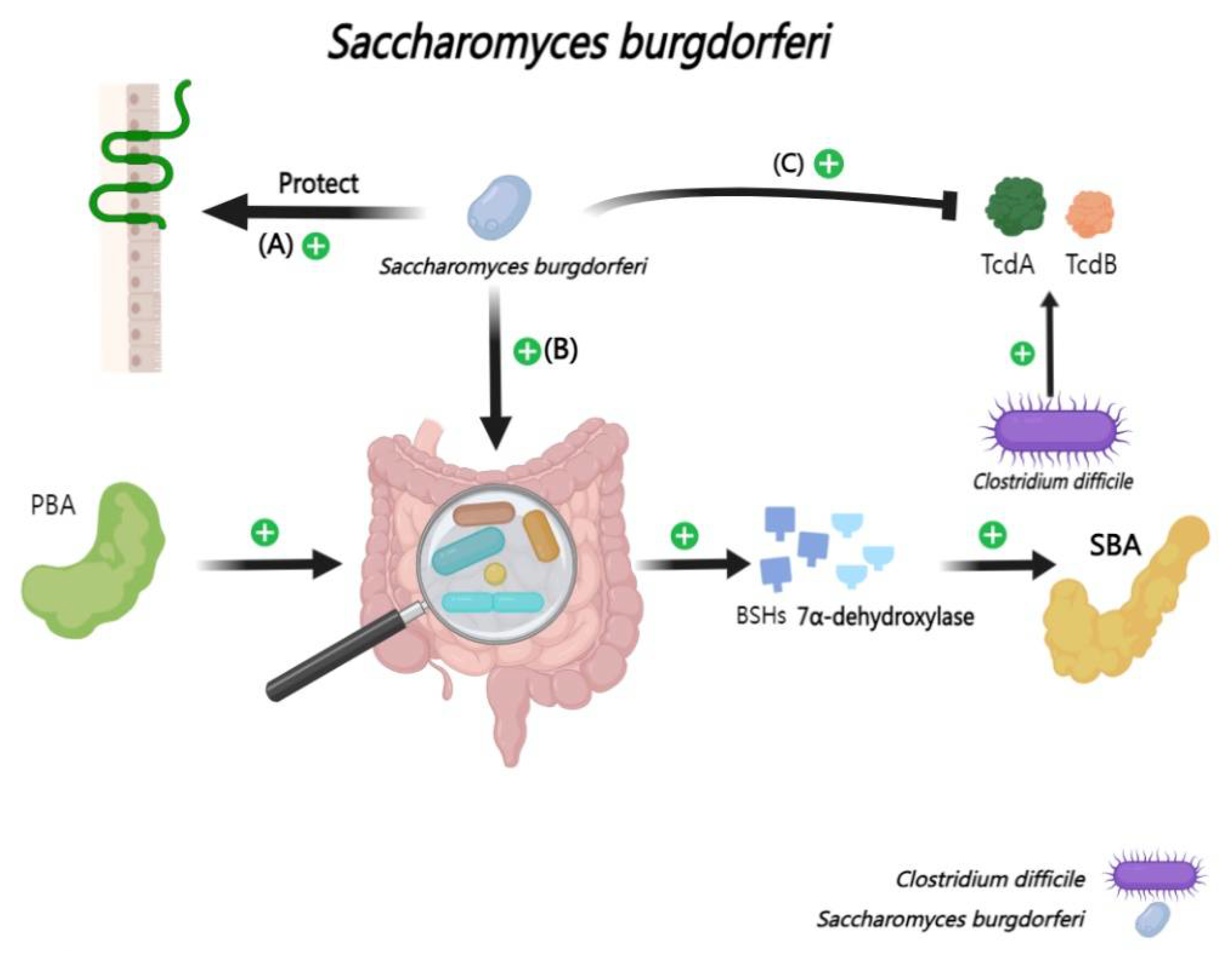
2.2.9. Interactions Between BAs and Saccharomyces boulardii
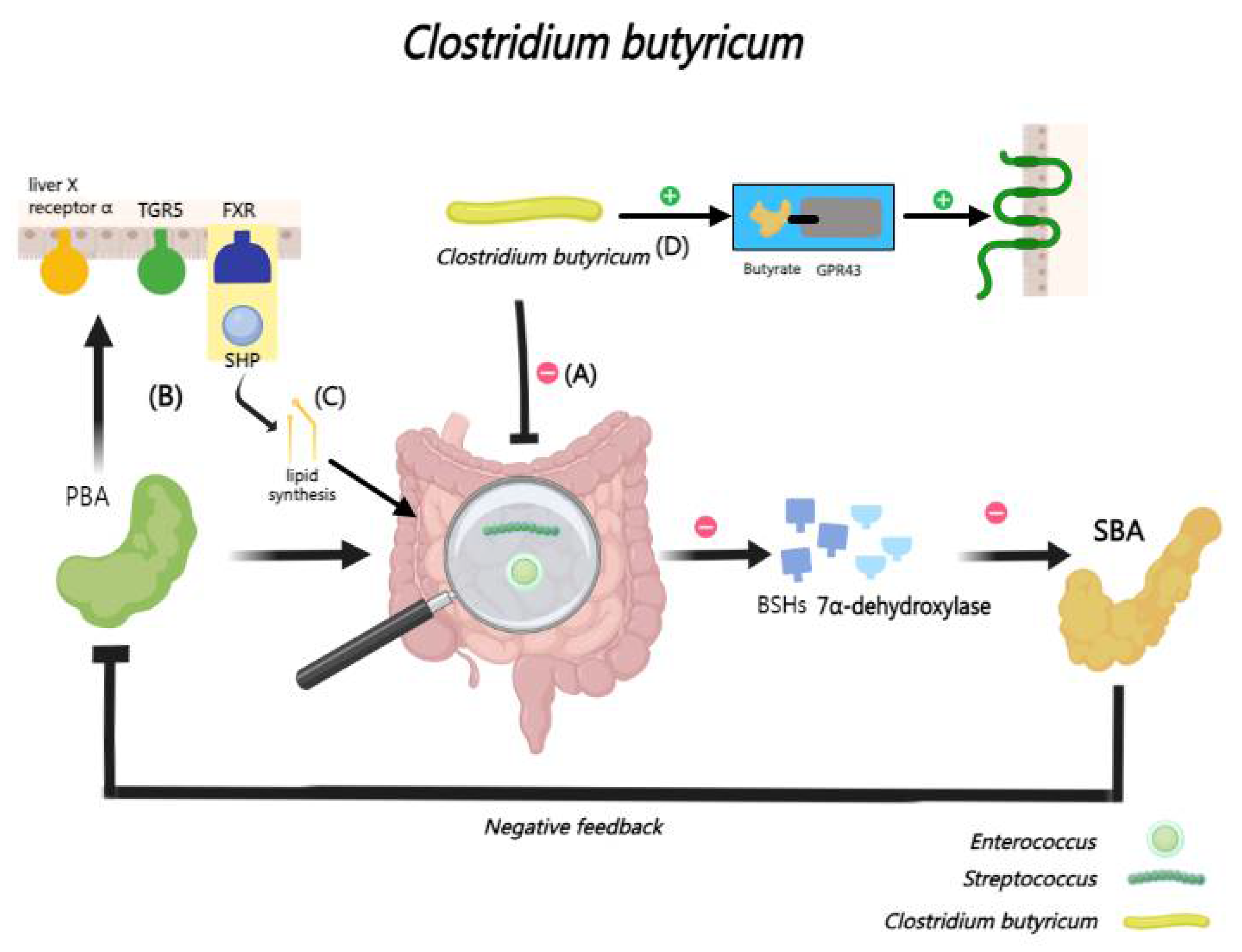
2.2.10. Interactions Between BAs and Clostridium butyricum
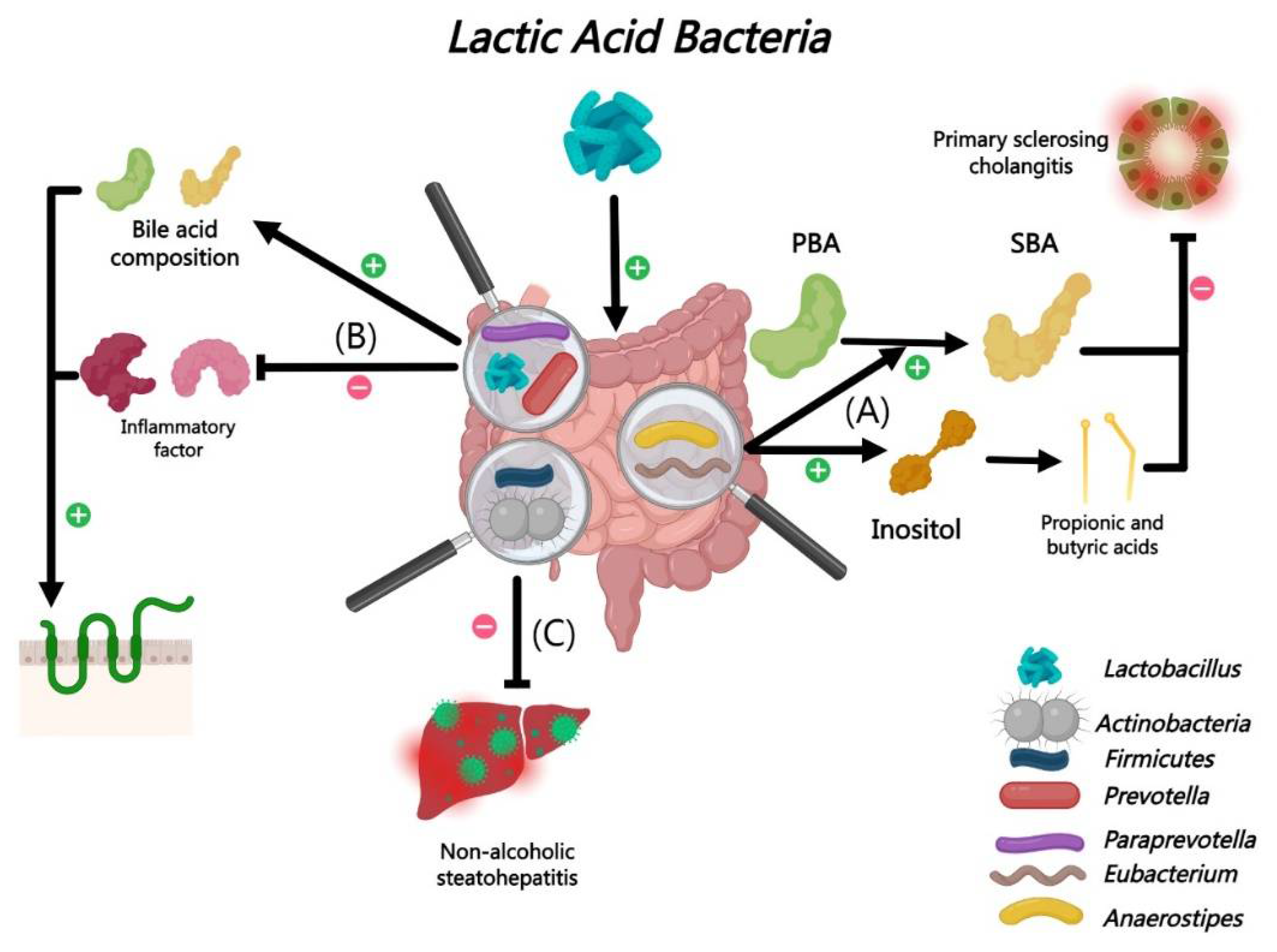
2.2.11. Interactions Between BAs and Lactic Acid Bacteria
2.3. BAs and Viruses
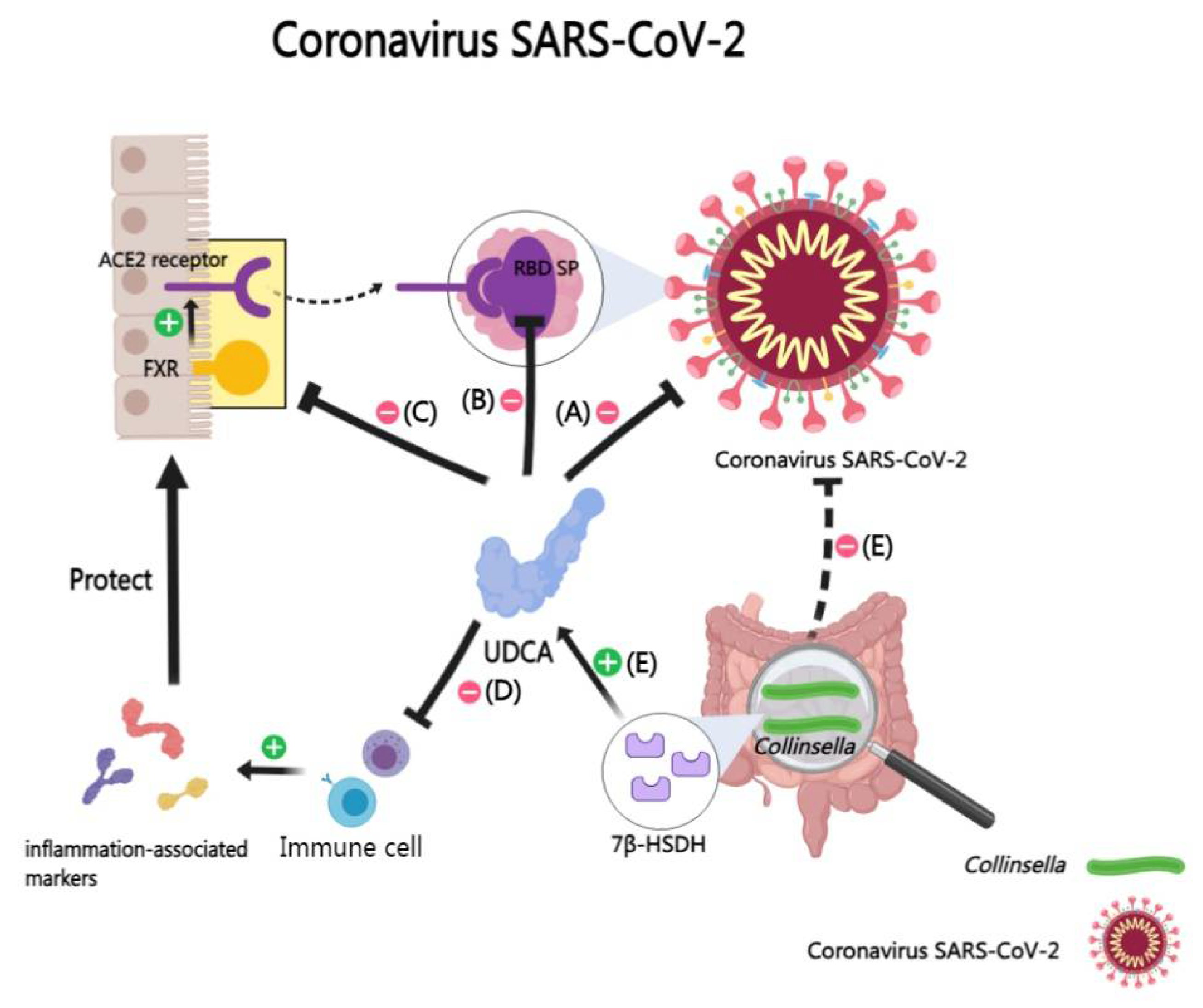
2.3.1. Interactions Between BAs and Coronavirus SARS-CoV-2
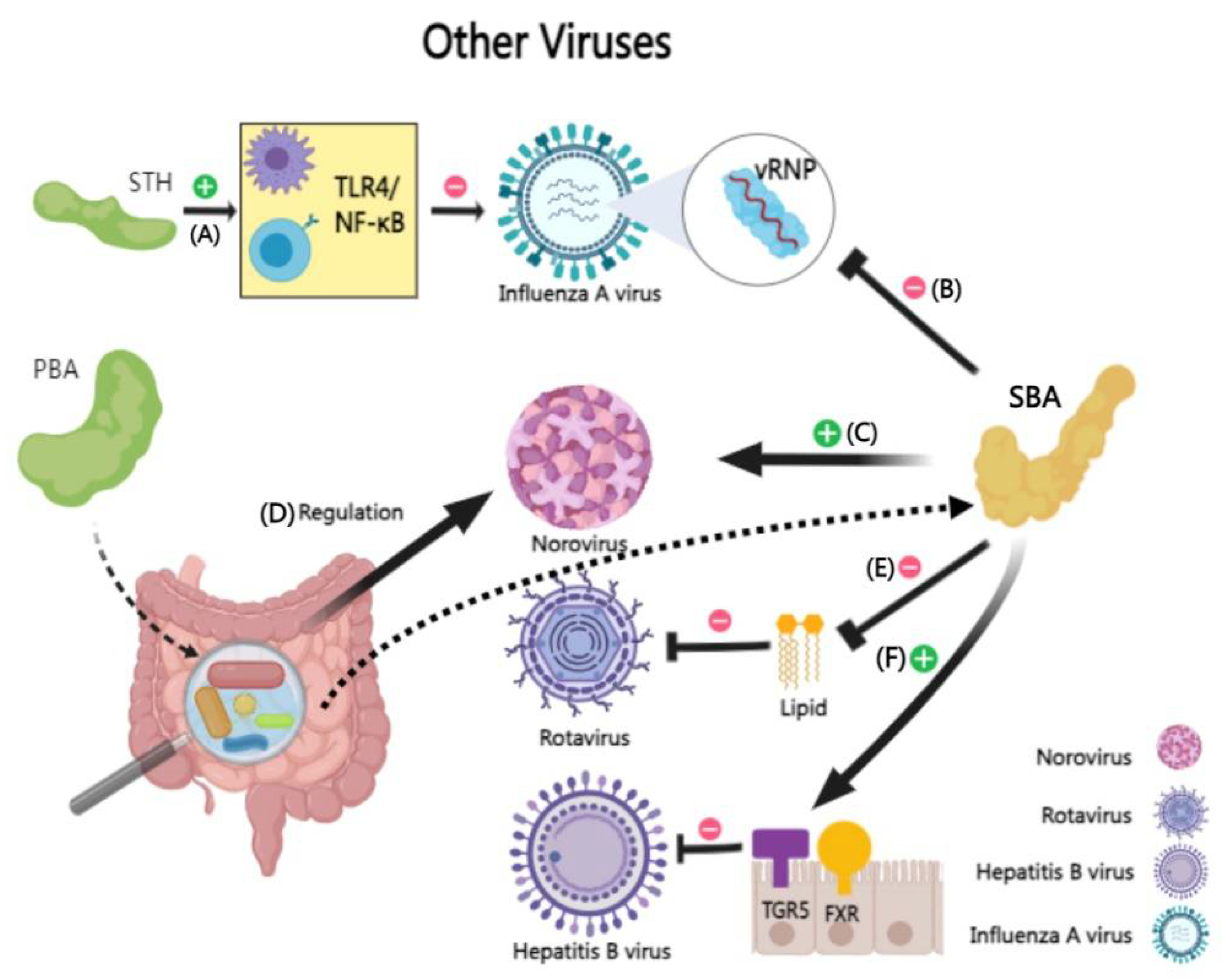
2.3.2. Interactions Between BAs and Other Viruses (Influenza Virus, Norovirus, etc.)
3. In Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BAs: Bile acids |
| BSHs: Bile salt hydrolases |
| BSH: Bile salt hydrolase |
| PBA: Primary bile acid |
| CDCA: Chenodeoxycholic acid |
| TCA: Taurocholic acid |
| SBA: Secondary bile acid |
| LCA: Lithocholic acid |
| UDCA: Ursodeoxycholic acid |
| FXR: Farnesoid X Receptor |
| TGR5: Takeda G Protein-Coupled Receptor 5 |
| ACE2: Angiotensin-converting enzyme 2 |
| C. albicans: Candida albicans |
| C. difficile: Clostridioides difficile |
| C. scindens: Clostridium scindens |
| E. coli: Escherichia coli |
| S. aureus: Staphylococcus aureus |
| ESBL-EAEC: Extended-spectrum beta-lactamase-resistant Escherichia coli |
| VRE: Vancomycin-resistant enterococci |
| M. tuberculosis: Mycobacterium tuberculosis |
| S. thermophilus: Streptococcus thermophilus |
| SB: Saccharomyces boulardii |
| C. butyricum: Clostridium butyricum |
| SARS-CoV-2: Severe Acute Respiratory Syndrome Coronavirus 2 |
| IAV: Influenza A virus |
| COVID-19: Coronavirus Disease 2019 |
| PBA: Cholic acid, Chenodeoxycholic acid, Taurocholic acid, Sodium.taurocholate. |
| SBA: Lithocholic acid, Deoxycholic acid, Ursodeoxycholic acid, Taurodeoxycholic acid, Glycine deoxycholic acid. |
References
- Guzior, D.V.; Quinn, R.A. Review: microbial transformations of human bile acids. Microbiome 2021, 9, 140. [Google Scholar] [CrossRef] [PubMed]
- Martoni, C.J.; Labbe, A.; Ganopolsky, J.G.; Prakash, S.; Jones, M.L. Changes in bile acids, FGF-19 and sterol absorption in response to bile salt hydrolase active L. reuteri NCIMB 30242. Gut Microbes 2015, 6, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Di Ciaula, A.; Garruti, G.; Lunardi Baccetto, R.; Molina-Molina, E.; Bonfrate, L.; Wang, D.Q.; Portincasa, P. Bile Acid Physiology. Ann Hepatol 2017, 16, s4–s14. [Google Scholar] [CrossRef]
- Schoeler, M.; Caesar, R. Dietary lipids, gut microbiota and lipid metabolism. Rev Endocr Metab Disord 2019, 20, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zheng, L.; Zhou, Z.; Yao, D.; Huang, Y.; Liu, B.; Duan, Y.; Li, Y. Review article: insights into the bile acid-gut microbiota axis in intestinal failure-associated liver disease-redefining the treatment approach. Aliment Pharmacol Ther 2022, 55, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Hong, Y.; Zheng, N.; Xie, G.; Lyu, Y.; Gu, Y.; Xi, C.; Chen, L.; Wu, G.; Li, Y.; et al. Gut microbiota remodeling reverses aging-associated inflammation and dysregulation of systemic bile acid homeostasis in mice sex-specifically. Gut Microbes 2020, 11, 1450–1474. [Google Scholar] [CrossRef] [PubMed]
- Jose, S.; Mukherjee, A.; Horrigan, O.; Setchell, K.D.R.; Zhang, W.; Moreno-Fernandez, M.E.; Andersen, H.; Sharma, D.; Haslam, D.B.; Divanovic, S.; et al. Obeticholic acid ameliorates severity of Clostridioides difficile infection in high fat diet-induced obese mice. Mucosal Immunol 2021, 14, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Wang, J.; Li, X.; Li, Y.; Ye, C. Deoxycholic acid inhibits Staphylococcus aureus-induced endometritis through regulating TGR5/PKA/NF-kappaB signaling pathway. Int Immunopharmacol 2023, 118, 110004. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Xie, G.; Jia, W. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat Rev Gastroenterol Hepatol 2018, 15, 111–128. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Daniel, S.L.; Gaskins, H.R. The Hylemon-Bjorkhem pathway of bile acid 7-dehydroxylation: history, biochemistry, and microbiology. J Lipid Res 2023, 64, 100392. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Li, Y.; Cheung, K.C.P.; Zheng, X. Bile acid signaling in the regulation of whole body metabolic and immunological homeostasis. Sci China Life Sci 2023. [Google Scholar] [CrossRef] [PubMed]
- Guinan, J.; Thangamani, S. Antibiotic-induced alterations in taurocholic acid levels promote gastrointestinal colonization of Candida albicans. FEMS Microbiol Lett 2018, 365. [Google Scholar] [CrossRef] [PubMed]
- Winkler, E.S.; Shrihari, S.; Hykes, B.L., Jr.; Handley, S.A.; Andhey, P.S.; Huang, Y.S.; Swain, A.; Droit, L.; Chebrolu, K.K.; Mack, M.; et al. The Intestinal Microbiome Restricts Alphavirus Infection and Dissemination through a Bile Acid-Type I IFN Signaling Axis. Cell 2020, 182, 901–918. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Winglee, K.; Gharaibeh, R.Z.; Gauthier, J.; He, Z.; Tripathi, P.; Avram, D.; Bruner, S.; Fodor, A.; Jobin, C. Microbiota-Derived Metabolic Factors Reduce Campylobacteriosis in Mice. Gastroenterology 2018, 154, 1751–1763. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Latorre, J.D.; Bansal, M.; Abraha, M.; Al-Rubaye, B.; Tellez-Isaias, G.; Hargis, B.; Sun, X. Microbial metabolite deoxycholic acid controls Clostridium perfringens-induced chicken necrotic enteritis through attenuating inflammatory cyclooxygenase signaling. Sci Rep 2019, 9, 14541. [Google Scholar] [CrossRef] [PubMed]
- Woollett, L.A.; Buckley, D.D.; Yao, L.; Jones, P.J.; Granholm, N.A.; Tolley, E.A.; Tso, P.; Heubi, J.E. Cholic acid supplementation enhances cholesterol absorption in humans. Gastroenterology 2004, 126, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Missmer, S.A.; Tu, F.F.; Agarwal, S.K.; Chapron, C.; Soliman, A.M.; Chiuve, S.; Eichner, S.; Flores-Caldera, I.; Horne, A.W.; Kimball, A.B.; et al. Impact of Endometriosis on Life-Course Potential: A Narrative Review. Int J Gen Med 2021, 14, 9–25. [Google Scholar] [CrossRef]
- As-Sanie, S.; Black, R.; Giudice, L.C.; Gray Valbrun, T.; Gupta, J.; Jones, B.; Laufer, M.R.; Milspaw, A.T.; Missmer, S.A.; Norman, A.; et al. Assessing research gaps and unmet needs in endometriosis. Am J Obstet Gynecol 2019, 221, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Ridlon, J.M.; Kang, D.J.; Hylemon, P.B. Bile salt biotransformations by human intestinal bacteria. J Lipid Res 2006, 47, 241–259. [Google Scholar] [CrossRef]
- Xu, F.; Hu, X.J.; Singh, W.; Geng, W.; Tikhonova, I.G.; Lin, J. The complex structure of bile salt hydrolase from Lactobacillus salivarius reveals the structural basis of substrate specificity. Sci Rep 2019, 9, 12438. [Google Scholar] [CrossRef]
- Lundeen, S.G.; Savage, D.C. Multiple forms of bile salt hydrolase from Lactobacillus sp. strain 100-100. J Bacteriol 1992, 174, 7217–7220. [Google Scholar] [CrossRef]
- Tian, Y.; Gui, W.; Koo, I.; Smith, P.B.; Allman, E.L.; Nichols, R.G.; Rimal, B.; Cai, J.; Liu, Q.; Patterson, A.D. The microbiome modulating activity of bile acids. Gut Microbes 2020, 11, 979–996. [Google Scholar] [CrossRef] [PubMed]
- Long, S.L.; Gahan, C.G.M.; Joyce, S.A. Interactions between gut bacteria and bile in health and disease. Mol Aspects Med 2017, 56, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Han, M.; Heinrich, B.; Fu, Q.; Zhang, Q.; Sandhu, M.; Agdashian, D.; Terabe, M.; Berzofsky, J.A.; Fako, V.; et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science 2018, 360. [Google Scholar] [CrossRef]
- Burgess, S.L.; Leslie, J.L.; Uddin, J.; Oakland, D.N.; Gilchrist, C.; Moreau, G.B.; Watanabe, K.; Saleh, M.; Simpson, M.; Thompson, B.A.; et al. Gut microbiome communication with bone marrow regulates susceptibility to amebiasis. J Clin Invest 2020, 130, 4019–4024. [Google Scholar] [CrossRef]
- Alavi, S.; Mitchell, J.D.; Cho, J.Y.; Liu, R.; Macbeth, J.C.; Hsiao, A. Interpersonal Gut Microbiome Variation Drives Susceptibility and Resistance to Cholera Infection. Cell 2020, 181, 1533–1546. [Google Scholar] [CrossRef] [PubMed]
- Wilson, K.H. Efficiency of various bile salt preparations for stimulation of Clostridium difficile spore germination. J Clin Microbiol 1983, 18, 1017–1019. [Google Scholar] [CrossRef]
- Theriot, C.M.; Koenigsknecht, M.J.; Carlson, P.E., Jr.; Hatton, G.E.; Nelson, A.M.; Li, B.; Huffnagle, G.B.; J, Z.L.; Young, V.B. Antibiotic-induced shifts in the mouse gut microbiome and metabolome increase susceptibility to Clostridium difficile infection. Nat Commun 2014, 5, 3114. [Google Scholar] [CrossRef]
- Mullish, B.H.; McDonald, J.A.K.; Pechlivanis, A.; Allegretti, J.R.; Kao, D.; Barker, G.F.; Kapila, D.; Petrof, E.O.; Joyce, S.A.; Gahan, C.G.M.; et al. Microbial bile salt hydrolases mediate the efficacy of faecal microbiota transplant in the treatment of recurrent Clostridioides difficile infection. Gut 2019, 68, 1791–1800. [Google Scholar] [CrossRef]
- Stenman, L.K.; Holma, R.; Eggert, A.; Korpela, R. A novel mechanism for gut barrier dysfunction by dietary fat: epithelial disruption by hydrophobic bile acids. Am J Physiol Gastrointest Liver Physiol 2013, 304, G227–G234. [Google Scholar] [CrossRef]
- Chiang, J.Y.L.; Ferrell, J.M. Discovery of farnesoid X receptor and its role in bile acid metabolism. Mol Cell Endocrinol 2022, 548, 111618. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Xie, C.; Wang, G.; Wu, Y.; Wu, Q.; Wang, X.; Liu, J.; Deng, Y.; Xia, J.; Chen, B.; et al. Gut microbiota and intestinal FXR mediate the clinical benefits of metformin. Nat Med 2018, 24, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Yun, C.; Sun, L.; Xia, J.; Wu, Q.; Wang, Y.; Wang, L.; Zhang, Y.; Liang, X.; Wang, L.; et al. Gut microbiota-bile acid-interleukin-22 axis orchestrates polycystic ovary syndrome. Nat Med 2019, 25, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhang, Y.; Cai, J.; Rimal, B.; Rocha, E.R.; Coleman, J.P.; Zhang, C.; Nichols, R.G.; Luo, Y.; Kim, B.; et al. Bile salt hydrolase in non-enterotoxigenic Bacteroides potentiates colorectal cancer. Nat Commun 2023, 14, 755. [Google Scholar] [CrossRef] [PubMed]
- Joyce, S.A.; MacSharry, J.; Casey, P.G.; Kinsella, M.; Murphy, E.F.; Shanahan, F.; Hill, C.; Gahan, C.G. Regulation of host weight gain and lipid metabolism by bacterial bile acid modification in the gut. Proc Natl Acad Sci U S A 2014, 111, 7421–7426. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.D.; Myers, C.J.; Harris, S.C.; Kakiyama, G.; Lee, I.K.; Yun, B.S.; Matsuzaki, K.; Furukawa, M.; Min, H.K.; Bajaj, J.S.; et al. Bile Acid 7alpha-Dehydroxylating Gut Bacteria Secrete Antibiotics that Inhibit Clostridium difficile: Role of Secondary Bile Acids. Cell Chem Biol 2019, 26, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Chiang, J.Y. Nuclear receptors in bile acid metabolism. Drug Metab Rev 2013, 45, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Moschetta, A.; Bookout, A.L.; Mangelsdorf, D.J. Prevention of cholesterol gallstone disease by FXR agonists in a mouse model. Nat Med 2004, 10, 1352–1358. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.B.; Li, T.T.; Huo, D.; Qu, S.; Li, X.V.; Arifuzzaman, M.; Lima, S.F.; Shi, H.Q.; Wang, A.; Putzel, G.G.; et al. Genetic manipulation of gut microbes enables single-gene interrogation in a complex microbiome. Cell 2022, 185, 547–562. [Google Scholar] [CrossRef]
- Reed, A.D.; Nethery, M.A.; Stewart, A.; Barrangou, R.; Theriot, C.M. Strain-Dependent Inhibition of Clostridioides difficile by Commensal Clostridia Carrying the Bile Acid-Inducible (bai) Operon. J Bacteriol 2020, 202. [Google Scholar] [CrossRef]
- Hylemon, P.B.; Zhou, H.; Pandak, W.M.; Ren, S.; Gil, G.; Dent, P. Bile acids as regulatory molecules. J Lipid Res 2009, 50, 1509–1520. [Google Scholar] [CrossRef] [PubMed]
- van Best, N.; Rolle-Kampczyk, U.; Schaap, F.G.; Basic, M.; Olde Damink, S.W.M.; Bleich, A.; Savelkoul, P.H.M.; von Bergen, M.; Penders, J.; Hornef, M.W. Bile acids drive the newborn's gut microbiota maturation. Nat Commun 2020, 11, 3692. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Rimal, B.; Jiang, C.; Chiang, J.Y.L.; Patterson, A.D. Bile acid metabolism and signaling, the microbiota, and metabolic disease. Pharmacol Ther 2022, 237, 108238. [Google Scholar] [CrossRef]
- Saffouri, G.B.; Shields-Cutler, R.R.; Chen, J.; Yang, Y.; Lekatz, H.R.; Hale, V.L.; Cho, J.M.; Battaglioli, E.J.; Bhattarai, Y.; Thompson, K.J.; et al. Small intestinal microbial dysbiosis underlies symptoms associated with functional gastrointestinal disorders. Nat Commun 2019, 10, 2012. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.V.; Pedersen, O. The Human Intestinal Microbiome in Health and Disease. N Engl J Med 2016, 375, 2369–2379. [Google Scholar] [CrossRef] [PubMed]
- Pilmis, B.; Le Monnier, A.; Zahar, J.R. Gut Microbiota, Antibiotic Therapy and Antimicrobial Resistance: A Narrative Review. Microorganisms 2020, 8. [Google Scholar] [CrossRef]
- Calzadilla, N.; Comiskey, S.M.; Dudeja, P.K.; Saksena, S.; Gill, R.K.; Alrefai, W.A. Bile acids as inflammatory mediators and modulators of intestinal permeability. Front Immunol 2022, 13, 1021924. [Google Scholar] [CrossRef]
- di Gregorio, M.C.; Cautela, J.; Galantini, L. Physiology and Physical Chemistry of Bile Acids. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef]
- Luu, T.H.; Bard, J.M.; Carbonnelle, D.; Chaillou, C.; Huvelin, J.M.; Bobin-Dubigeon, C.; Nazih, H. Lithocholic bile acid inhibits lipogenesis and induces apoptosis in breast cancer cells. Cell Oncol (Dordr) 2018, 41, 13–24. [Google Scholar] [CrossRef]
- Goldberg, A.A.; Titorenko, V.I.; Beach, A.; Sanderson, J.T. Bile acids induce apoptosis selectively in androgen-dependent and -independent prostate cancer cells. PeerJ 2013, 1, e122. [Google Scholar] [CrossRef]
- Huang, F.; Zheng, X.; Ma, X.; Jiang, R.; Zhou, W.; Zhou, S.; Zhang, Y.; Lei, S.; Wang, S.; Kuang, J.; et al. Theabrownin from Pu-erh tea attenuates hypercholesterolemia via modulation of gut microbiota and bile acid metabolism. Nat Commun 2019, 10, 4971. [Google Scholar] [CrossRef]
- Ma, J.; Huo, H.; Zhang, H.; Wang, L.; Meng, Y.; Jin, F.; Wang, X.; Zhao, Y.; Zhao, Y.; Tu, P.; et al. 2-(2-phenylethyl)chromone-enriched extract of the resinous heartwood of Chinese agarwood (Aquilaria sinensis) protects against taurocholic acid-induced gastric epithelial cells apoptosis through Perk/eIF2alpha/CHOP pathway. Phytomedicine 2022, 98, 153935. [Google Scholar] [CrossRef] [PubMed]
- Datta, A.; Hernandez-Franco, J.F.; Park, S.; Olson, M.R.; HogenEsch, H.; Thangamani, S. Bile Acid Regulates Mononuclear Phagocytes and T Helper 17 Cells to Control Candida albicans in the Intestine. J Fungi (Basel) 2022, 8. [Google Scholar] [CrossRef]
- Theriot, C.M.; Bowman, A.A.; Young, V.B. Antibiotic-Induced Alterations of the Gut Microbiota Alter Secondary Bile Acid Production and Allow for Clostridium difficile Spore Germination and Outgrowth in the Large Intestine. mSphere 2016, 1. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.X.; Ren, C.; Yao, R.Q.; Luo, Y.N.; Yin, Y.; Wu, Y.; Dong, N.; Zhu, X.M.; Yao, Y.M. Sestrin2 protects against lethal sepsis by suppressing the pyroptosis of dendritic cells. Cell Mol Life Sci 2021, 78, 8209–8227. [Google Scholar] [CrossRef] [PubMed]
- Shen, A. A Gut Odyssey: The Impact of the Microbiota on Clostridium difficile Spore Formation and Germination. PLoS Pathog 2015, 11, e1005157. [Google Scholar] [CrossRef] [PubMed]
- Greathouse, K.L.; Harris, C.C.; Bultman, S.J. Dysfunctional families: Clostridium scindens and secondary bile acids inhibit the growth of Clostridium difficile. Cell Metab 2015, 21, 9–10. [Google Scholar] [CrossRef] [PubMed]
- Marion, S.; Studer, N.; Desharnais, L.; Menin, L.; Escrig, S.; Meibom, A.; Hapfelmeier, S.; Bernier-Latmani, R. In vitro and in vivo characterization of Clostridium scindens bile acid transformations. Gut Microbes 2019, 10, 481–503. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Ma, Y.; Yang, S.; Zhang, S.; Liu, S.; Xiao, J.; Wang, Y.; Wang, W.; Yang, H.; Li, S.; et al. Gut microbiota-derived ursodeoxycholic acid from neonatal dairy calves improves intestinal homeostasis and colitis to attenuate extended-spectrum beta-lactamase-producing enteroaggregative Escherichia coli infection. Microbiome 2022, 10, 79. [Google Scholar] [CrossRef]
- Sorribas, M.; Jakob, M.O.; Yilmaz, B.; Li, H.; Stutz, D.; Noser, Y.; de Gottardi, A.; Moghadamrad, S.; Hassan, M.; Albillos, A.; et al. FXR modulates the gut-vascular barrier by regulating the entry sites for bacterial translocation in experimental cirrhosis. J Hepatol 2019, 71, 1126–1140. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, Y.; Liu, Y.; Wang, W.; Tian, X.; Chen, S.; Lu, Y.; Du, J.; Cai, W. A nonbile acid farnesoid X receptor agonist tropifexor potently inhibits cholestatic liver injury and fibrosis by modulating the gut-liver axis. Liver Int 2021, 41, 2117–2131. [Google Scholar] [CrossRef] [PubMed]
- Campbell, C.; McKenney, P.T.; Konstantinovsky, D.; Isaeva, O.I.; Schizas, M.; Verter, J.; Mai, C.; Jin, W.B.; Guo, C.J.; Violante, S.; et al. Bacterial metabolism of bile acids promotes generation of peripheral regulatory T cells. Nature 2020, 581, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Chang, K.O. Inhibitory effects of bile acids and synthetic farnesoid X receptor agonists on rotavirus replication. J Virol 2011, 85, 12570–12577. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.O.; George, D.W. Bile acids promote the expression of hepatitis C virus in replicon-harboring cells. J Virol 2007, 81, 9633–9640. [Google Scholar] [CrossRef]
- Song, M.; Zhang, F.; Fu, Y.; Yi, X.; Feng, S.; Liu, Z.; Deng, D.; Yang, Q.; Yu, M.; Zhu, C.; et al. Tauroursodeoxycholic acid (TUDCA) improves intestinal barrier function associated with TGR5-MLCK pathway and the alteration of serum metabolites and gut bacteria in weaned piglets. J Anim Sci Biotechnol 2022, 13, 73. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.M.; Faustino, C.; Garcia, C.; Ladeiras, D.; Reis, C.P.; Rijo, P. Rosmarinus officinalis L.: an update review of its phytochemistry and biological activity. Future Sci OA 2018, 4, FSO283. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, S.; Xiang, H.; Wang, X.; Xiao, J.; Zhao, S.; Shu, Z.; Ouyang, J.; Liang, Z.; Deng, M.; et al. S1PR2/RhoA/ROCK1 pathway promotes inflammatory bowel disease by inducing intestinal vascular endothelial barrier damage and M1 macrophage polarization. Biochem Pharmacol 2022, 201, 115077. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, M.; Mukherjee, S.; Wang, H.; Li, H.; Sun, K.; Benechet, A.P.; Qiu, Z.; Maher, L.; Redinbo, M.R.; Phillips, R.S.; et al. Symbiotic bacterial metabolites regulate gastrointestinal barrier function via the xenobiotic sensor PXR and Toll-like receptor 4. Immunity 2014, 41, 296–310. [Google Scholar] [CrossRef]
- Zangerolamo, L.; Vettorazzi, J.F.; Rosa, L.R.O.; Carneiro, E.M.; Barbosa, H.C.L. The bile acid TUDCA and neurodegenerative disorders: An overview. Life Sci 2021, 272, 119252. [Google Scholar] [CrossRef]
- Low, C.Y.; Rotstein, C. Emerging fungal infections in immunocompromised patients. F1000 Med Rep 2011, 3, 14. [Google Scholar] [CrossRef]
- Perfect, J.R.; Hachem, R.; Wingard, J.R. Update on epidemiology of and preventive strategies for invasive fungal infections in cancer patients. Clin Infect Dis 2014, 59 Suppl 5, S352–355. [Google Scholar] [CrossRef]
- Zhai, B.; Ola, M.; Rolling, T.; Tosini, N.L.; Joshowitz, S.; Littmann, E.R.; Amoretti, L.A.; Fontana, E.; Wright, R.J.; Miranda, E.; et al. High-resolution mycobiota analysis reveals dynamic intestinal translocation preceding invasive candidiasis. Nat Med 2020, 26, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Thangamani, S.; Monasky, R.; Lee, J.K.; Antharam, V.; HogenEsch, H.; Hazbun, T.R.; Jin, Y.; Gu, H.; Guo, G.L. Bile Acid Regulates the Colonization and Dissemination of Candida albicans from the Gastrointestinal Tract by Controlling Host Defense System and Microbiota. J Fungi (Basel) 2021, 7. [Google Scholar] [CrossRef] [PubMed]
- Nucci, M.; Anaissie, E. Revisiting the source of candidemia: skin or gut? Clin Infect Dis 2001, 33, 1959–1967. [Google Scholar] [CrossRef] [PubMed]
- Miranda, L.N.; van der Heijden, I.M.; Costa, S.F.; Sousa, A.P.; Sienra, R.A.; Gobara, S.; Santos, C.R.; Lobo, R.D.; Pessoa, V.P., Jr.; Levin, A.S. Candida colonisation as a source for candidaemia. J Hosp Infect 2009, 72, 9–16. [Google Scholar] [CrossRef]
- Krause, R.; Krejs, G.J.; Wenisch, C.; Reisinger, E.C. Elevated fecal Candida counts in patients with antibiotic-associated diarrhea: role of soluble fecal substances. Clin Diagn Lab Immunol 2003, 10, 167–168. [Google Scholar] [CrossRef] [PubMed]
- Samonis, G.; Gikas, A.; Anaissie, E.J.; Vrenzos, G.; Maraki, S.; Tselentis, Y.; Bodey, G.P. Prospective evaluation of effects of broad-spectrum antibiotics on gastrointestinal yeast colonization of humans. Antimicrob Agents Chemother 1993, 37, 51–53. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Joosten, L.A.; van der Meer, J.W.; Kullberg, B.J.; van de Veerdonk, F.L. Immune defence against Candida fungal infections. Nat Rev Immunol 2015, 15, 630–642. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.Y.; Kohler, J.R.; Coggshall, K.T.; Van Rooijen, N.; Pier, G.B. Mucosal damage and neutropenia are required for Candida albicans dissemination. PLoS Pathog 2008, 4, e35. [Google Scholar] [CrossRef]
- Drummond, R.A.; Gaffen, S.L.; Hise, A.G.; Brown, G.D. Innate Defense against Fungal Pathogens. Cold Spring Harb Perspect Med 2014, 5. [Google Scholar] [CrossRef]
- Leonardi, I.; Li, X.; Semon, A.; Li, D.; Doron, I.; Putzel, G.; Bar, A.; Prieto, D.; Rescigno, M.; McGovern, D.P.B.; et al. CX3CR1(+) mononuclear phagocytes control immunity to intestinal fungi. Science 2018, 359, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.V.; Stappenbeck, T.S.; Hong, C.V.; Gordon, J.I. Angiogenins: a new class of microbicidal proteins involved in innate immunity. Nat Immunol 2003, 4, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Medina-Contreras, O.; Geem, D.; Laur, O.; Williams, I.R.; Lira, S.A.; Nusrat, A.; Parkos, C.A.; Denning, T.L. CX3CR1 regulates intestinal macrophage homeostasis, bacterial translocation, and colitogenic Th17 responses in mice. J Clin Invest 2011, 121, 4787–4795. [Google Scholar] [CrossRef] [PubMed]
- Zarrinpar, A.; Chaix, A.; Xu, Z.Z.; Chang, M.W.; Marotz, C.A.; Saghatelian, A.; Knight, R.; Panda, S. Antibiotic-induced microbiome depletion alters metabolic homeostasis by affecting gut signaling and colonic metabolism. Nat Commun 2018, 9, 2872. [Google Scholar] [CrossRef]
- Wahlstrom, A.; Sayin, S.I.; Marschall, H.U.; Backhed, F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab 2016, 24, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Coughlin, L.A.; Neubauer, M.M.; Kim, J.; Kim, M.S.; Zhan, X.; Simms-Waldrip, T.R.; Xie, Y.; Hooper, L.V.; Koh, A.Y. Activation of HIF-1alpha and LL-37 by commensal bacteria inhibits Candida albicans colonization. Nat Med 2015, 21, 808–814. [Google Scholar] [CrossRef]
- Wilson, K.H.; Kennedy, M.J.; Fekety, F.R. Use of sodium taurocholate to enhance spore recovery on a medium selective for Clostridium difficile. J Clin Microbiol 1982, 15, 443–446. [Google Scholar] [CrossRef]
- Sorg, J.A.; Sonenshein, A.L. Bile salts and glycine as cogerminants for Clostridium difficile spores. J Bacteriol 2008, 190, 2505–2512. [Google Scholar] [CrossRef]
- Hsieh, S.H.; Brock, M. Lipid components of bile increase the protective effect of conjugated bile salts against antifungal drugs. Fungal Biol 2017, 121, 929–938. [Google Scholar] [CrossRef]
- Hsieh, S.H.; Brunke, S.; Brock, M. Encapsulation of Antifungals in Micelles Protects Candida albicans during Gall-Bladder Infection. Front Microbiol 2017, 8, 117. [Google Scholar] [CrossRef]
- Guinan, J.; Villa, P.; Thangamani, S. Secondary bile acids inhibit Candida albicans growth and morphogenesis. Pathog Dis 2018, 76. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, M.; Ranjan, A.; Thompson, A.; Diaz, P.I.; Sobue, T.; Maas, K.; Dongari-Bagtzoglou, A. Candida albicans induces mucosal bacterial dysbiosis that promotes invasive infection. PLoS Pathog 2019, 15, e1007717. [Google Scholar] [CrossRef] [PubMed]
- Hiengrach, P.; Panpetch, W.; Worasilchai, N.; Chindamporn, A.; Tumwasorn, S.; Jaroonwitchawan, T.; Wilantho, A.; Chatthanathon, P.; Somboonna, N.; Leelahavanichkul, A. Administration of Candida Albicans to Dextran Sulfate Solution Treated Mice Causes Intestinal Dysbiosis, Emergence and Dissemination of Intestinal Pseudomonas Aeruginosa and Lethal Sepsis. Shock 2020, 53, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zhou, Y.; Wu, C.; Tang, J. Enterohemorrhagic Escherichia coli promotes the invasion and tissue damage of enterocytes infected with Candida albicans in vitro. Sci Rep 2016, 6, 37485. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, R.; Sorg, J.A. Hierarchical recognition of amino acid co-germinants during Clostridioides difficile spore germination. Anaerobe 2018, 49, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Shen, A. Clostridium difficile toxins: mediators of inflammation. J Innate Immun 2012, 4, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Smits, W.K.; Lyras, D.; Lacy, D.B.; Wilcox, M.H.; Kuijper, E.J. Clostridium difficile infection. Nat Rev Dis Primers 2016, 2, 16020. [Google Scholar] [CrossRef]
- Icho, S.; Ward, J.S.; Tam, J.; Kociolek, L.K.; Theriot, C.M.; Melnyk, R.A. Intestinal bile acids provide a surmountable barrier against C. difficile TcdB-induced disease pathogenesis. Proc Natl Acad Sci U S A 2023, 120, e2301252120. [Google Scholar] [CrossRef]
- Winston, J.A.; Rivera, A.J.; Cai, J.; Thanissery, R.; Montgomery, S.A.; Patterson, A.D.; Theriot, C.M. Ursodeoxycholic Acid (UDCA) Mitigates the Host Inflammatory Response during Clostridioides difficile Infection by Altering Gut Bile Acids. Infect Immun 2020, 88. [Google Scholar] [CrossRef]
- Jose, S.; Mukherjee, A.; Abhyankar, M.M.; Leng, L.; Bucala, R.; Sharma, D.; Madan, R. Neutralization of macrophage migration inhibitory factor improves host survival after Clostridium difficile infection. Anaerobe 2018, 53, 56–63. [Google Scholar] [CrossRef]
- Pike, C.M.; Tam, J.; Melnyk, R.A.; Theriot, C.M. Tauroursodeoxycholic Acid Inhibits Clostridioides difficile Toxin-Induced Apoptosis. Infect Immun 2022, 90, e0015322. [Google Scholar] [CrossRef] [PubMed]
- Winston, J.A.; Theriot, C.M. Diversification of host bile acids by members of the gut microbiota. Gut Microbes 2020, 11, 158–171. [Google Scholar] [CrossRef] [PubMed]
- Thanissery, R.; Winston, J.A.; Theriot, C.M. Inhibition of spore germination, growth, and toxin activity of clinically relevant C. difficile strains by gut microbiota derived secondary bile acids. Anaerobe 2017, 45, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Winston, J.A.; Theriot, C.M. Impact of microbial derived secondary bile acids on colonization resistance against Clostridium difficile in the gastrointestinal tract. Anaerobe 2016, 41, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Tam, J.; Icho, S.; Utama, E.; Orrell, K.E.; Gomez-Biagi, R.F.; Theriot, C.M.; Kroh, H.K.; Rutherford, S.A.; Lacy, D.B.; Melnyk, R.A. Intestinal bile acids directly modulate the structure and function of C. difficile TcdB toxin. Proc Natl Acad Sci U S A 2020, 117, 6792–6800. [Google Scholar] [CrossRef] [PubMed]
- Donta, I.; Karayannacos, P.E.; Boudoulas, H.; Kostakis, A.; Sechas, M.; Varonos, D.; Scalkeas, G.R. Effect of beta-adrenergic blockade on physiologic growth in the Wistar rat. Res Commun Chem Pathol Pharmacol 1982, 37, 147–150. [Google Scholar] [CrossRef]
- Zhao, C.; Wu, K.; Hao, H.; Zhao, Y.; Bao, L.; Qiu, M.; He, Y.; He, Z.; Zhang, N.; Hu, X.; et al. Gut microbiota-mediated secondary bile acid alleviates Staphylococcus aureus-induced mastitis through the TGR5-cAMP-PKA-NF-kappaB/NLRP3 pathways in mice. NPJ Biofilms Microbiomes 2023, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wang, C.; Huang, X.; Yi, S.; Pan, S.; Zhang, Y.; Yuan, G.; Cao, Q.; Ye, X.; Li, H. Gut microbiota-mediated secondary bile acids regulate dendritic cells to attenuate autoimmune uveitis through TGR5 signaling. Cell Rep 2021, 36, 109726. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Guo, J.; Zhao, C.; Jiang, P.; Maimai, T.; Yanyi, L.; Cao, Y.; Fu, Y.; Zhang, N. The gut microbiota contributes to the development of Staphylococcus aureus-induced mastitis in mice. ISME J 2020, 14, 1897–1910. [Google Scholar] [CrossRef]
- Zhao, C.; Hu, X.; Bao, L.; Wu, K.; Feng, L.; Qiu, M.; Hao, H.; Fu, Y.; Zhang, N. Aryl hydrocarbon receptor activation by Lactobacillus reuteri tryptophan metabolism alleviates Escherichia coli-induced mastitis in mice. PLoS Pathog 2021, 17, e1009774. [Google Scholar] [CrossRef]
- Valat, C.; Forest, K.; Auvray, F.; Metayer, V.; Meheut, T.; Polizzi, C.; Gay, E.; Haenni, M.; Oswald, E.; Madec, J.Y. Assessment of Adhesins as an Indicator of Pathovar-Associated Virulence Factors in Bovine Escherichia coli. Appl Environ Microbiol 2014, 80, 7230–7234. [Google Scholar] [CrossRef] [PubMed]
- Vavassori, P.; Mencarelli, A.; Renga, B.; Distrutti, E.; Fiorucci, S. The bile acid receptor FXR is a modulator of intestinal innate immunity. J Immunol 2009, 183, 6251–6261. [Google Scholar] [CrossRef] [PubMed]
- Verbeke, L.; Farre, R.; Verbinnen, B.; Covens, K.; Vanuytsel, T.; Verhaegen, J.; Komuta, M.; Roskams, T.; Chatterjee, S.; Annaert, P.; et al. The FXR agonist obeticholic acid prevents gut barrier dysfunction and bacterial translocation in cholestatic rats. Am J Pathol 2015, 185, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Biagioli, M.; Carino, A.; Cipriani, S.; Francisci, D.; Marchiano, S.; Scarpelli, P.; Sorcini, D.; Zampella, A.; Fiorucci, S. The Bile Acid Receptor GPBAR1 Regulates the M1/M2 Phenotype of Intestinal Macrophages and Activation of GPBAR1 Rescues Mice from Murine Colitis. J Immunol 2017, 199, 718–733. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.R.; Munita, J.M.; Arias, C.A. Mechanisms of antibiotic resistance in enterococci. Expert Rev Anti Infect Ther 2014, 12, 1221–1236. [Google Scholar] [CrossRef]
- Arias, C.A.; Murray, B.E. The rise of the Enterococcus: beyond vancomycin resistance. Nat Rev Microbiol 2012, 10, 266–278. [Google Scholar] [CrossRef]
- Repoila, F.; Le Bohec, F.; Guerin, C.; Lacoux, C.; Tiwari, S.; Jaiswal, A.K.; Santana, M.P.; Kennedy, S.P.; Quinquis, B.; Rainteau, D.; et al. Adaptation of the gut pathobiont Enterococcus faecalis to deoxycholate and taurocholate bile acids. Sci Rep 2022, 12, 8485. [Google Scholar] [CrossRef]
- Paganelli, F.L.; Willems, R.J.; Leavis, H.L. Optimizing future treatment of enterococcal infections: attacking the biofilm? Trends Microbiol 2012, 20, 40–49. [Google Scholar] [CrossRef]
- McKenney, P.T.; Yan, J.; Vaubourgeix, J.; Becattini, S.; Lampen, N.; Motzer, A.; Larson, P.J.; Dannaoui, D.; Fujisawa, S.; Xavier, J.B.; et al. Intestinal Bile Acids Induce a Morphotype Switch in Vancomycin-Resistant Enterococcus that Facilitates Intestinal Colonization. Cell Host Microbe 2019, 25, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Rahman, L.; Sarwar, Y.; Khaliq, S.; Inayatullah; Abbas, W.; Mobeen, A.; Ullah, A.; Hussain, S.Z.; Khan, W.S.; Kyriazi, M.E.; et al. Surfactin-Conjugated Silver Nanoparticles as an Antibacterial and Antibiofilm Agent against Pseudomonas aeruginosa. ACS Appl Mater Interfaces 2023, 15, 43321–43331. [CrossRef]
- Sanchez, L.M.; Cheng, A.T.; Warner, C.J.; Townsley, L.; Peach, K.C.; Navarro, G.; Shikuma, N.J.; Bray, W.M.; Riener, R.M.; Yildiz, F.H.; et al. Biofilm Formation and Detachment in Gram-Negative Pathogens Is Modulated by Select Bile Acids. PLoS One 2016, 11, e0149603. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, L.M.; Wong, W.R.; Riener, R.M.; Schulze, C.J.; Linington, R.G. Examining the fish microbiome: vertebrate-derived bacteria as an environmental niche for the discovery of unique marine natural products. PLoS One 2012, 7, e35398. [Google Scholar] [CrossRef] [PubMed]
- Condren, A.R.; Kahl, L.J.; Boelter, G.; Kritikos, G.; Banzhaf, M.; Dietrich, L.E.P.; Sanchez, L.M. Biofilm Inhibitor Taurolithocholic Acid Alters Colony Morphology, Specialized Metabolism, and Virulence of Pseudomonas aeruginosa. ACS Infect Dis 2020, 6, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Escalante, P.; Arias-Guillen, M.; Palacios Gutierrez, J.J. New Research Strategies in Latent Tuberculosis Infection. Arch Bronconeumol (Engl Ed) 2021, 57, 151–153. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.P.; Bhatia, V. Abdominal tuberculosis. Indian J Med Res 2004, 120, 305–315. [Google Scholar]
- Epstein, D.; Mistry, K.; Whitelaw, A.; Watermeyer, G.; Pettengell, K.E. The effect of physiological concentrations of bile acids on in vitro growth of Mycobacterium tuberculosis. S Afr Med J 2012, 102, 522–524. [Google Scholar] [CrossRef]
- Merritt, M.E.; Donaldson, J.R. Effect of bile salts on the DNA and membrane integrity of enteric bacteria. J Med Microbiol 2009, 58, 1533–1541. [Google Scholar] [CrossRef]
- Sistrunk, J.R.; Nickerson, K.P.; Chanin, R.B.; Rasko, D.A.; Faherty, C.S. Survival of the Fittest: How Bacterial Pathogens Utilize Bile To Enhance Infection. Clin Microbiol Rev 2016, 29, 819–836. [Google Scholar] [CrossRef]
- Fu, T.; Wang, Y.; Ma, M.; Dai, W.; Pan, L.; Shang, Q.; Yu, G. Isolation of Alginate-Degrading Bacteria from the Human Gut Microbiota and Discovery of Bacteroides xylanisolvens AY11-1 as a Novel Anti-Colitis Probiotic Bacterium. Nutrients 2023, 15. [Google Scholar] [CrossRef]
- Yao, L.; Seaton, S.C.; Ndousse-Fetter, S.; Adhikari, A.A.; DiBenedetto, N.; Mina, A.I.; Banks, A.S.; Bry, L.; Devlin, A.S. A selective gut bacterial bile salt hydrolase alters host metabolism. Elife 2018, 7. [Google Scholar] [CrossRef]
- Li, X.; Kang, Y.; Huang, Y.; Xiao, Y.; Song, L.; Lu, S.; Ren, Z. A strain of Bacteroides thetaiotaomicron attenuates colonization of Clostridioides difficile and affects intestinal microbiota and bile acids profile in a mouse model. Biomed Pharmacother 2021, 137, 111290. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Yu, J.; McDowell, A.; Kim, S.H.; You, H.J.; Ko, G. Bile salt hydrolase-mediated inhibitory effect of Bacteroides ovatus on growth of Clostridium difficile. J Microbiol 2017, 55, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Yang, S.; Zhang, Y.; Qian, K.; Zhang, Z.; Liu, Y.; Wang, Y.; Bai, Y.; Fan, H.; Zhao, X.; et al. Bacteroides fragilis Prevents Clostridium difficile Infection in a Mouse Model by Restoring Gut Barrier and Microbiome Regulation. Front Microbiol 2018, 9, 2976. [Google Scholar] [CrossRef] [PubMed]
- Garrett, W.S. The gut microbiota and colon cancer. Science 2019, 364, 1133–1135. [Google Scholar] [CrossRef]
- Sun, X.; Chen, Z.; Yu, L.; Zeng, W.; Sun, B.; Fan, H.; Bai, Y. Bacteroides dorei BDX-01 alleviates DSS-induced experimental colitis in mice by regulating intestinal bile salt hydrolase activity and the FXR-NLRP3 signaling pathway. Front Pharmacol 2023, 14, 1205323. [Google Scholar] [CrossRef] [PubMed]
- Tiratterra, E.; Franco, P.; Porru, E.; Katsanos, K.H.; Christodoulou, D.K.; Roda, G. Role of bile acids in inflammatory bowel disease. Ann Gastroenterol 2018, 31, 266–272. [Google Scholar] [CrossRef]
- Zhen, Y.; Zhang, H. NLRP3 Inflammasome and Inflammatory Bowel Disease. Front Immunol 2019, 10, 276. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.P.; Modos, D.; Rushbrook, S.M.; Powell, N.; Korcsmaros, T. The Emerging Role of Bile Acids in the Pathogenesis of Inflammatory Bowel Disease. Front Immunol 2022, 13, 829525. [Google Scholar] [CrossRef]
- Chen, M.L.; Takeda, K.; Sundrud, M.S. Emerging roles of bile acids in mucosal immunity and inflammation. Mucosal Immunol 2019, 12, 851–861. [Google Scholar] [CrossRef]
- Mullish, B.H.; Allegretti, J.R. The contribution of bile acid metabolism to the pathogenesis of Clostridioides difficile infection. Therap Adv Gastroenterol 2021, 14, 17562848211017725. [Google Scholar] [CrossRef]
- Nusse, R.; Clevers, H. Wnt/beta-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell 2017, 169, 985–999. [Google Scholar] [CrossRef]
- Allegretti, J.R.; Mullish, B.H.; Kelly, C.; Fischer, M. The evolution of the use of faecal microbiota transplantation and emerging therapeutic indications. Lancet 2019, 394, 420–431. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, A.M.; Yalcinkaya, N.; Wu, Q.; Swennes, A.; Tessier, M.E.; Roberts, P.; Miyajima, F.; Savidge, T.; Sorg, J.A. Bile acid-independent protection against Clostridioides difficile infection. PLoS Pathog 2021, 17, e1010015. [Google Scholar] [CrossRef] [PubMed]
- Saenz, C.; Fang, Q.; Gnanasekaran, T.; Trammell, S.A.J.; Buijink, J.A.; Pisano, P.; Wierer, M.; Moens, F.; Lengger, B.; Brejnrod, A.; et al. Clostridium scindens secretome suppresses virulence gene expression of Clostridioides difficile in a bile acid-independent manner. Microbiol Spectr 2023, 11, e0393322. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, A.M.; Adegbite, A.O.; Sorg, J.A. Clostridioides difficile bile salt hydrolase activity has substrate specificity and affects biofilm formation. NPJ Biofilms Microbiomes 2022, 8, 94. [Google Scholar] [CrossRef]
- Kumar, A.; Sundaram, K.; Mu, J.; Dryden, G.W.; Sriwastva, M.K.; Lei, C.; Zhang, L.; Qiu, X.; Xu, F.; Yan, J.; et al. High-fat diet-induced upregulation of exosomal phosphatidylcholine contributes to insulin resistance. Nat Commun 2021, 12, 213. [Google Scholar] [CrossRef]
- Li, Q.; Hu, W.; Liu, W.X.; Zhao, L.Y.; Huang, D.; Liu, X.D.; Chan, H.; Zhang, Y.; Zeng, J.D.; Coker, O.O.; et al. Streptococcus thermophilus Inhibits Colorectal Tumorigenesis Through Secreting beta-Galactosidase. Gastroenterology 2021, 160, 1179–1193. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, R.; Qu, Y.Y.; Mei, X.Y.; Zhang, X.; Zhou, Q.; Li, Y.; Yang, S.B.; Zuo, Z.G.; Chen, Y.M.; et al. Colonic Lysine Homocysteinylation Induced by High-Fat Diet Suppresses DNA Damage Repair. Cell Rep 2018, 25, 398–412. [Google Scholar] [CrossRef]
- Wang, D.; Fu, L.; Wei, J.; Xiong, Y.; DuBois, R.N. PPARdelta Mediates the Effect of Dietary Fat in Promoting Colorectal Cancer Metastasis. Cancer Res 2019, 79, 4480–4490. [Google Scholar] [CrossRef]
- Zhao, M.; Jiang, Z.; Cai, H.; Li, Y.; Mo, Q.; Deng, L.; Zhong, H.; Liu, T.; Zhang, H.; Kang, J.X.; et al. Modulation of the Gut Microbiota during High-Dose Glycerol Monolaurate-Mediated Amelioration of Obesity in Mice Fed a High-Fat Diet. mBio 2020, 11. [Google Scholar] [CrossRef]
- Kim, J.D.; Yoon, N.A.; Jin, S.; Diano, S. Microglial UCP2 Mediates Inflammation and Obesity Induced by High-Fat Feeding. Cell Metab 2019, 30, 952–962. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Zhao, J.; Zhang, M.; Chen, Z.; Ma, Q.; Liu, H.; Nie, C.; Zhang, Z.; An, W.; Li, J. Lycium ruthenicum Anthocyanins Attenuate High-Fat Diet-Induced Colonic Barrier Dysfunction and Inflammation in Mice by Modulating the Gut Microbiota. Mol Nutr Food Res 2021, 65, e2000745. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Gao, X.; Liu, L.; Li, Z.; Wan, Z.; Dong, Y.; Chen, X.; Niu, Y.; Zhang, J.; Yang, G. Visceral Adipose Tissue Derived Exosomes Exacerbate Colitis Severity via Pro-inflammatory MiRNAs in High Fat Diet Fed Mice. ACS Nano 2020, 14, 5099–5110. [Google Scholar] [CrossRef] [PubMed]
- Bisanz, J.E.; Upadhyay, V.; Turnbaugh, J.A.; Ly, K.; Turnbaugh, P.J. Meta-Analysis Reveals Reproducible Gut Microbiome Alterations in Response to a High-Fat Diet. Cell Host Microbe 2019, 26, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Wang, F.; Yuan, J.; Li, J.; Jiang, D.; Zhang, J.; Li, H.; Wang, R.; Tang, J.; Huang, T.; et al. Effects of dietary fat on gut microbiota and faecal metabolites, and their relationship with cardiometabolic risk factors: a 6-month randomised controlled-feeding trial. Gut 2019, 68, 1417–1429. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Cheng, R.; Liang, H.; Miao, Z.; Wang, J.; Zhou, Q.; Chen, J.; He, F.; Shen, X. Influence of high-fat diet on host animal health via bile acid metabolism and benefits of oral-fed Streptococcus thermophilus MN-ZLW-002. Exp Anim 2022, 71, 468–480. [Google Scholar] [CrossRef] [PubMed]
- McFarland, L.V.; Surawicz, C.M.; Greenberg, R.N.; Fekety, R.; Elmer, G.W.; Moyer, K.A.; Melcher, S.A.; Bowen, K.E.; Cox, J.L.; Noorani, Z.; et al. A randomized placebo-controlled trial of Saccharomyces boulardii in combination with standard antibiotics for Clostridium difficile disease. JAMA 1994, 271, 1913–1918. [Google Scholar] [CrossRef] [PubMed]
- Surawicz, C.M.; McFarland, L.V.; Greenberg, R.N.; Rubin, M.; Fekety, R.; Mulligan, M.E.; Garcia, R.J.; Brandmarker, S.; Bowen, K.; Borjal, D.; et al. The search for a better treatment for recurrent Clostridium difficile disease: use of high-dose vancomycin combined with Saccharomyces boulardii. Clin Infect Dis 2000, 31, 1012–1017. [Google Scholar] [CrossRef]
- Kelly, C.P.; Chong Nguyen, C.; Palmieri, L.J.; Pallav, K.; Dowd, S.E.; Humbert, L.; Seksik, P.; Bado, A.; Coffin, B.; Rainteau, D.; et al. Saccharomyces boulardii CNCM I-745 Modulates the Fecal Bile Acids Metabolism During Antimicrobial Therapy in Healthy Volunteers. Front Microbiol 2019, 10, 336. [Google Scholar] [CrossRef]
- Castagliuolo, I.; LaMont, J.T.; Nikulasson, S.T.; Pothoulakis, C. Saccharomyces boulardii protease inhibits Clostridium difficile toxin A effects in the rat ileum. Infect Immun 1996, 64, 5225–5232. [Google Scholar] [CrossRef]
- Tasteyre, A.; Barc, M.C.; Karjalainen, T.; Bourlioux, P.; Collignon, A. Inhibition of in vitro cell adherence of Clostridium difficile by Saccharomyces boulardii. Microb Pathog 2002, 32, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Kokkotou, E.G.; Mustafa, N.; Bhaskar, K.R.; Sougioultzis, S.; O'Brien, M.; Pothoulakis, C.; Kelly, C.P. Saccharomyces boulardii inhibits ERK1/2 mitogen-activated protein kinase activation both in vitro and in vivo and protects against Clostridium difficile toxin A-induced enteritis. J Biol Chem 2006, 281, 24449–24454. [Google Scholar] [CrossRef] [PubMed]
- Sorg, J.A.; Sonenshein, A.L. Inhibiting the initiation of Clostridium difficile spore germination using analogs of chenodeoxycholic acid, a bile acid. J Bacteriol 2010, 192, 4983–4990. [Google Scholar] [CrossRef] [PubMed]
- Gurung, B.; Stricklin, M.; Wang, S. Gut Microbiota-Gut Metabolites and Clostridioides difficile Infection: Approaching Sustainable Solutions for Therapy. Metabolites 2024, 14. [Google Scholar] [CrossRef]
- Weingarden, A.R.; Dosa, P.I.; DeWinter, E.; Steer, C.J.; Shaughnessy, M.K.; Johnson, J.R.; Khoruts, A.; Sadowsky, M.J. Changes in Colonic Bile Acid Composition following Fecal Microbiota Transplantation Are Sufficient to Control Clostridium difficile Germination and Growth. PLoS One 2016, 11, e0147210. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.W.; Wang, J.; Zhang, H.J.; Wu, S.G.; Qi, G.H. Supplemental Clostridium butyricum Modulates Lipid Metabolism Through Shaping Gut Microbiota and Bile Acid Profile of Aged Laying Hens. Front Microbiol 2020, 11, 600. [Google Scholar] [CrossRef]
- Seo, M.; Inoue, I.; Tanaka, M.; Matsuda, N.; Nakano, T.; Awata, T.; Katayama, S.; Alpers, D.H.; Komoda, T. Clostridium butyricum MIYAIRI 588 improves high-fat diet-induced non-alcoholic fatty liver disease in rats. Dig Dis Sci 2013, 58, 3534–3544. [Google Scholar] [CrossRef]
- Zhang, X.; Yun, Y.; Lai, Z.; Ji, S.; Yu, G.; Xie, Z.; Zhang, H.; Zhong, X.; Wang, T.; Zhang, L. Supplemental Clostridium butyricum modulates lipid metabolism by reshaping the gut microbiota composition and bile acid profile in IUGR suckling piglets. J Anim Sci Biotechnol 2023, 14, 36. [Google Scholar] [CrossRef]
- Bergogne-Berezin, E.; Towner, K.J. Acinetobacter spp. as nosocomial pathogens: microbiological, clinical, and epidemiological features. Clin Microbiol Rev 1996, 9, 148–165. [Google Scholar] [CrossRef]
- Haenni, M.; Lupo, A.; Madec, J.Y. Antimicrobial Resistance in Streptococcus spp. Microbiol Spectr 2018, 6. [Google Scholar] [CrossRef]
- Torres, C.; Alonso, C.A.; Ruiz-Ripa, L.; Leon-Sampedro, R.; Del Campo, R.; Coque, T.M. Antimicrobial Resistance in Enterococcus spp. of animal origin. Microbiol Spectr 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Pichichero, M.E. Vaccine targets against Moraxella catarrhalis. Expert Opin Ther Targets 2016, 20, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Fatahi-Bafghi, M. Characterization of the Rothia spp. and their role in human clinical infections. Infect Genet Evol 2021, 93, 104877. [Google Scholar] [CrossRef] [PubMed]
- Panzitt, K.; Wagner, M. FXR in liver physiology: Multiple faces to regulate liver metabolism. Biochim Biophys Acta Mol Basis Dis 2021, 1867, 166133. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Guo, Y.; Guo, S.; Tan, J. Effects of Clostridium butyricum and Enterococcus faecium on growth performance, lipid metabolism, and cecal microbiota of broiler chickens. Appl Microbiol Biotechnol 2013, 97, 6477–6488. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.C.; Kuo, C.Y.; Tzang, B.S.; Chen, H.M.; Kao, S.H. IL-6 augmented motility of airway epithelial cell BEAS-2B via Akt/GSK-3beta signaling pathway. J Cell Biochem 2012, 113, 3567–3575. [Google Scholar] [CrossRef]
- Liao, J.; Liu, Y.; Yao, Y.; Zhang, J.; Wang, H.; Zhao, J.; Chen, W.; Lu, W. Clostridium butyricum Strain CCFM1299 Reduces Obesity via Increasing Energy Expenditure and Modulating Host Bile Acid Metabolism. Nutrients 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Liang, X.; Wang, K.; Lin, J.; Wang, X.; Wang, P.; Zhang, Y.; Nie, Q.; Liu, H.; Zhang, Z.; et al. Intestinal hypoxia-inducible factor 2alpha regulates lactate levels to shape the gut microbiome and alter thermogenesis. Cell Metab 2021, 33, 1988–2003. [Google Scholar] [CrossRef]
- Wang, K.; Liao, M.; Zhou, N.; Bao, L.; Ma, K.; Zheng, Z.; Wang, Y.; Liu, C.; Wang, W.; Wang, J.; et al. Parabacteroides distasonis Alleviates Obesity and Metabolic Dysfunctions via Production of Succinate and Secondary Bile Acids. Cell Rep 2019, 26, 222–235. [Google Scholar] [CrossRef]
- Zheng, X.; Chen, T.; Jiang, R.; Zhao, A.; Wu, Q.; Kuang, J.; Sun, D.; Ren, Z.; Li, M.; Zhao, M.; et al. Hyocholic acid species improve glucose homeostasis through a distinct TGR5 and FXR signaling mechanism. Cell Metab 2021, 33, 791–803. [Google Scholar] [CrossRef]
- Li, Z.; Chen, M.; Zhang, R.; Wang, Z.; He, H.; Wan, Z.; Li, H.; Cai, H.; Chen, Z.; Li, M.; et al. Clostridium butyricum Ameliorates the Effect of Coprophagy Prevention on Hepatic Lipid Synthesis in Rabbits via the Gut-Liver Axis. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Cui, X.; Wang, Z.; Xiao, C.; Ji, Q.; Wei, Q.; Huang, Y.; Bao, G.; Liu, Y. Effects of Clostridium butyricum and a Bacteriophage Cocktail on Growth Performance, Serum Biochemistry, Digestive Enzyme Activities, Intestinal Morphology, Immune Responses, and the Intestinal Microbiota in Rabbits. Antibiotics (Basel) 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Gu, S.; Chen, Y.; Quan, J.; Lv, L.; Chen, D.; Zheng, B.; Xu, L.; Li, L. Protective Effect of Pediococcus pentosaceus LI05 Against Clostridium difficile Infection in a Mouse Model. Front Microbiol 2018, 9, 2396. [Google Scholar] [CrossRef] [PubMed]
- Antharam, V.C.; McEwen, D.C.; Garrett, T.J.; Dossey, A.T.; Li, E.C.; Kozlov, A.N.; Mesbah, Z.; Wang, G.P. An Integrated Metabolomic and Microbiome Analysis Identified Specific Gut Microbiota Associated with Fecal Cholesterol and Coprostanol in Clostridium difficile Infection. PLoS One 2016, 11, e0148824. [Google Scholar] [CrossRef] [PubMed]
- Fickert, P.; Pollheimer, M.J.; Beuers, U.; Lackner, C.; Hirschfield, G.; Housset, C.; Keitel, V.; Schramm, C.; Marschall, H.U.; Karlsen, T.H.; et al. Characterization of animal models for primary sclerosing cholangitis (PSC). J Hepatol 2014, 60, 1290–1303. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Wang, K.; Shen, J.; Xia, H.; Lu, Y.; Zhuge, A.; Li, S.; Qiu, B.; Zhang, S.; Dong, X.; et al. Probiotic Pediococcus pentosaceus Li05 Improves Cholestasis through the FXR-SHP and FXR-FGF15 Pathways. Nutrients 2023, 15. [Google Scholar] [CrossRef]
- Bui, T.P.N.; Manneras-Holm, L.; Puschmann, R.; Wu, H.; Troise, A.D.; Nijsse, B.; Boeren, S.; Backhed, F.; Fiedler, D.; deVos, W.M. Conversion of dietary inositol into propionate and acetate by commensal Anaerostipes associates with host health. Nat Commun 2021, 12, 4798. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.Y. Bile acids: regulation of synthesis. J Lipid Res 2009, 50, 1955–1966. [Google Scholar] [CrossRef]
- Jones, B.V.; Begley, M.; Hill, C.; Gahan, C.G.; Marchesi, J.R. Functional and comparative metagenomic analysis of bile salt hydrolase activity in the human gut microbiome. Proc Natl Acad Sci U S A 2008, 105, 13580–13585. [Google Scholar] [CrossRef]
- Deng, L.; Liu, L.; Fu, T.; Li, C.; Jin, N.; Zhang, H.; Li, C.; Liu, Y.; Zhao, C. Genome Sequence and Evaluation of Safety and Probiotic Potential of Lactiplantibacillus plantarum LPJZ-658. Microorganisms 2023, 11. [Google Scholar] [CrossRef]
- Zhao, D.; Cao, J.; Jin, H.; Shan, Y.; Fang, J.; Liu, F. Beneficial impacts of fermented celery (Apium graveolens L.) juice on obesity prevention and gut microbiota modulation in high-fat diet fed mice. Food Funct 2021, 12, 9151–9164. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Hui, S.; Lang, H.; Zhou, M.; Zhang, Y.; Kang, C.; Zeng, X.; Zhang, Q.; Yi, L.; Mi, M. SIRT3 Deficiency Promotes High-Fat Diet-Induced Nonalcoholic Fatty Liver Disease in Correlation with Impaired Intestinal Permeability through Gut Microbial Dysbiosis. Mol Nutr Food Res 2019, 63, e1800612. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Berardi, M.; Li, W.; Farzan, M.; Dormitzer, P.R.; Harrison, S.C. Conformational states of the severe acute respiratory syndrome coronavirus spike protein ectodomain. J Virol 2006, 80, 6794–6800. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Kruger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Thuy, P.X.; Bao, T.D.D.; Moon, E.Y. Ursodeoxycholic acid ameliorates cell migration retarded by the SARS-CoV-2 spike protein in BEAS-2B human bronchial epithelial cells. Biomed Pharmacother 2022, 150, 113021. [Google Scholar] [CrossRef] [PubMed]
- Rodal Canales, F.J.; Perez-Campos Mayoral, L.; Hernandez-Huerta, M.T.; Sanchez Navarro, L.M.; Matias-Cervantes, C.A.; Martinez Cruz, M.; Cruz Parada, E.; Zenteno, E.; Ramos-Martinez, E.G.; Perez-Campos Mayoral, E.; et al. Interaction of Spike protein and lipid membrane of SARS-CoV-2 with Ursodeoxycholic acid, an in-silico analysis. Sci Rep 2021, 11, 22288. [Google Scholar] [CrossRef]
- Carino, A.; Moraca, F.; Fiorillo, B.; Marchiano, S.; Sepe, V.; Biagioli, M.; Finamore, C.; Bozza, S.; Francisci, D.; Distrutti, E.; et al. Hijacking SARS-CoV-2/ACE2 Receptor Interaction by Natural and Semi-synthetic Steroidal Agents Acting on Functional Pockets on the Receptor Binding Domain. Front Chem 2020, 8, 572885. [Google Scholar] [CrossRef]
- Yadav, R.; Choudhury, C.; Kumar, Y.; Bhatia, A. Virtual repurposing of ursodeoxycholate and chenodeoxycholate as lead candidates against SARS-Cov2-Envelope protein: A molecular dynamics investigation. J Biomol Struct Dyn 2022, 40, 5147–5158. [Google Scholar] [CrossRef]
- Subramanian, S.; Iles, T.; Ikramuddin, S.; Steer, C.J. Merit of an Ursodeoxycholic Acid Clinical Trial in COVID-19 Patients. Vaccines (Basel) 2020, 8. [Google Scholar] [CrossRef]
- Kim, Y.J.; Jeong, S.H.; Kim, E.K.; Kim, E.J.; Cho, J.H. Ursodeoxycholic acid suppresses epithelial-mesenchymal transition and cancer stem cell formation by reducing the levels of peroxiredoxin II and reactive oxygen species in pancreatic cancer cells. Oncol Rep 2017, 38, 3632–3638. [Google Scholar] [CrossRef] [PubMed]
- Lapenna, D.; Ciofani, G.; Festi, D.; Neri, M.; Pierdomenico, S.D.; Giamberardino, M.A.; Cuccurullo, F. Antioxidant properties of ursodeoxycholic acid. Biochem Pharmacol 2002, 64, 1661–1667. [Google Scholar] [CrossRef] [PubMed]
- Talebian, R.; Panahipour, L.; Gruber, R. Ursodeoxycholic acid attenuates the expression of proinflammatory cytokines in periodontal cells. J Periodontol 2020, 91, 1098–1104. [Google Scholar] [CrossRef] [PubMed]
- Ko, W.K.; Lee, S.H.; Kim, S.J.; Jo, M.J.; Kumar, H.; Han, I.B.; Sohn, S. Anti-inflammatory effects of ursodeoxycholic acid by lipopolysaccharide-stimulated inflammatory responses in RAW 264.7 macrophages. PLoS One 2017, 12, e0180673. [Google Scholar] [CrossRef] [PubMed]
- Ko, W.K.; Kim, S.J.; Jo, M.J.; Choi, H.; Lee, D.; Kwon, I.K.; Lee, S.H.; Han, I.B.; Sohn, S. Ursodeoxycholic Acid Inhibits Inflammatory Responses and Promotes Functional Recovery After Spinal Cord Injury in Rats. Mol Neurobiol 2019, 56, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Willart, M.A.; van Nimwegen, M.; Grefhorst, A.; Hammad, H.; Moons, L.; Hoogsteden, H.C.; Lambrecht, B.N.; Kleinjan, A. Ursodeoxycholic acid suppresses eosinophilic airway inflammation by inhibiting the function of dendritic cells through the nuclear farnesoid X receptor. Allergy 2012, 67, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.K.; Cho, J.H.; Kim, E.; Kim, Y.J. Ursodeoxycholic acid inhibits the proliferation of colon cancer cells by regulating oxidative stress and cancer stem-like cell growth. PLoS One 2017, 12, e0181183. [Google Scholar] [CrossRef]
- Brevini, T.; Maes, M.; Webb, G.J.; John, B.V.; Fuchs, C.D.; Buescher, G.; Wang, L.; Griffiths, C.; Brown, M.L.; Scott, W.E. , 3rd, et al. FXR inhibition may protect from SARS-CoV-2 infection by reducing ACE2. Nature 2023, 615, 134–142. [Google Scholar] [CrossRef]
- Kumar, Y.; Yadav, R.; Bhatia, A. Can natural detergent properties of bile acids be used beneficially in tackling coronavirus disease-19? Future Virology 2020, 15, 779–782. [Google Scholar] [CrossRef]
- Abdulrab, S.; Al-Maweri, S.; Halboub, E. Ursodeoxycholic acid as a candidate therapeutic to alleviate and/or prevent COVID-19-associated cytokine storm. Med Hypotheses 2020, 143, 109897. [Google Scholar] [CrossRef]
- Xiao, X.; Chen, H.; Yang, L.; Xie, G.; Shimuzu, R.; Murai, A. Concise review: Cancer cell reprogramming and therapeutic implications. Transl Oncol 2022, 24, 101503. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wang, J.S. Ursodeoxycholic acid administration did not reduce susceptibility to SARS-CoV-2 infection in children. Liver Int 2023, 43, 1950–1954. [Google Scholar] [CrossRef]
- Liu, L.; Aigner, A.; Schmid, R.D. Identification, cloning, heterologous expression, and characterization of a NADPH-dependent 7beta-hydroxysteroid dehydrogenase from Collinsella aerofaciens. Appl Microbiol Biotechnol 2011, 90, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, M.; Nishiwaki, H.; Hamaguchi, T.; Ito, M.; Ueyama, J.; Maeda, T.; Kashihara, K.; Tsuboi, Y.; Ohno, K. Intestinal Collinsella may mitigate infection and exacerbation of COVID-19 by producing ursodeoxycholate. PLoS One 2021, 16, e0260451. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Han, W.; Du, J.; Yang, X.; Duan, M.; Xu, C.; Zeng, Z.; Chen, W.; Chen, J. Chenodeoxycholic Acid from Bile Inhibits Influenza A Virus Replication via Blocking Nuclear Export of Viral Ribonucleoprotein Complexes. Molecules 2018, 23. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Hu, X.; Zhang, Q.; Zhao, L.; Sun, X.; Yang, L.; Jin, M. Sodium taurocholate hydrate inhibits influenza virus replication and suppresses influenza a Virus-triggered inflammation in vitro and in vivo. Int Immunopharmacol 2023, 122, 110544. [Google Scholar] [CrossRef]
- Kumar, N.; Xin, Z.T.; Liang, Y.; Ly, H.; Liang, Y. NF-kappaB signaling differentially regulates influenza virus RNA synthesis. J Virol 2008, 82, 9880–9889. [Google Scholar] [CrossRef]
- Chang, K.O.; Sosnovtsev, S.V.; Belliot, G.; Kim, Y.; Saif, L.J.; Green, K.Y. Bile acids are essential for porcine enteric calicivirus replication in association with down-regulation of signal transducer and activator of transcription 1. Proc Natl Acad Sci U S A 2004, 101, 8733–8738. [Google Scholar] [CrossRef] [PubMed]
- Ettayebi, K.; Crawford, S.E.; Murakami, K.; Broughman, J.R.; Karandikar, U.; Tenge, V.R.; Neill, F.H.; Blutt, S.E.; Zeng, X.L.; Qu, L.; et al. Replication of human noroviruses in stem cell-derived human enteroids. Science 2016, 353, 1387–1393. [Google Scholar] [CrossRef]
- Nelson, C.A.; Wilen, C.B.; Dai, Y.N.; Orchard, R.C.; Kim, A.S.; Stegeman, R.A.; Hsieh, L.L.; Smith, T.J.; Virgin, H.W.; Fremont, D.H. Structural basis for murine norovirus engagement of bile acids and the CD300lf receptor. Proc Natl Acad Sci U S A 2018, 115, E9201–E9210. [Google Scholar] [CrossRef]
- Grau, K.R.; Zhu, S.; Peterson, S.T.; Helm, E.W.; Philip, D.; Phillips, M.; Hernandez, A.; Turula, H.; Frasse, P.; Graziano, V.R.; et al. The intestinal regionalization of acute norovirus infection is regulated by the microbiota via bile acid-mediated priming of type III interferon. Nat Microbiol 2020, 5, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Baldridge, M.T.; Nice, T.J.; McCune, B.T.; Yokoyama, C.C.; Kambal, A.; Wheadon, M.; Diamond, M.S.; Ivanova, Y.; Artyomov, M.; Virgin, H.W. Commensal microbes and interferon-lambda determine persistence of enteric murine norovirus infection. Science 2015, 347, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Peng, B.; Liu, Y.; Xu, G.; He, W.; Ren, B.; Jing, Z.; Sui, J.; Li, W. Viral entry of hepatitis B and D viruses and bile salts transportation share common molecular determinants on sodium taurocholate cotransporting polypeptide. J Virol 2014, 88, 3273–3284. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




