Submitted:
26 June 2024
Posted:
28 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Stress Origin in Perovskite Films
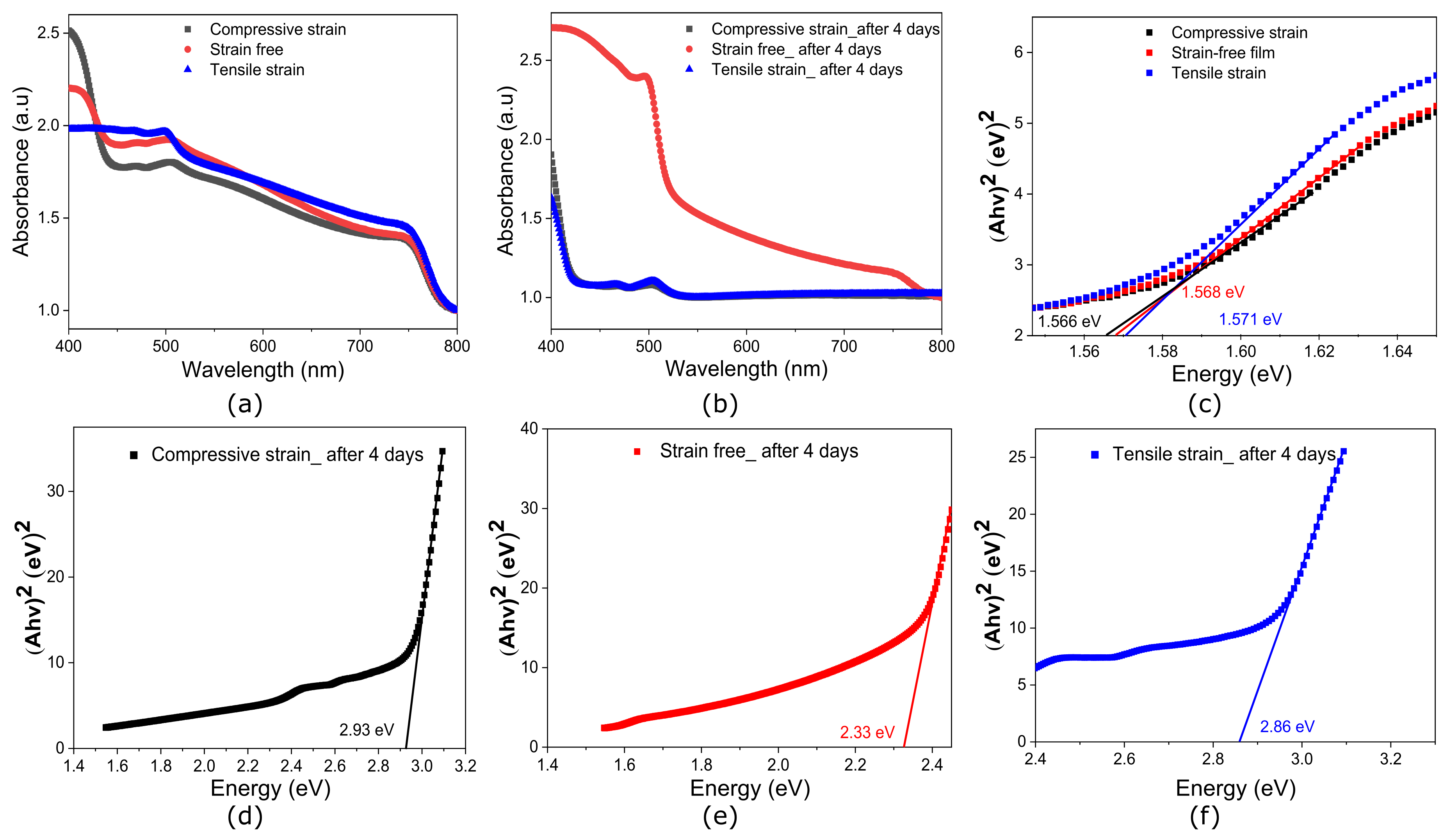
1.2. Impact on Electrical, Optical Properties and Stability
1.3. Regulation of Strain in Perovskite Films
2. Experimental Section
3. Results and Discussion
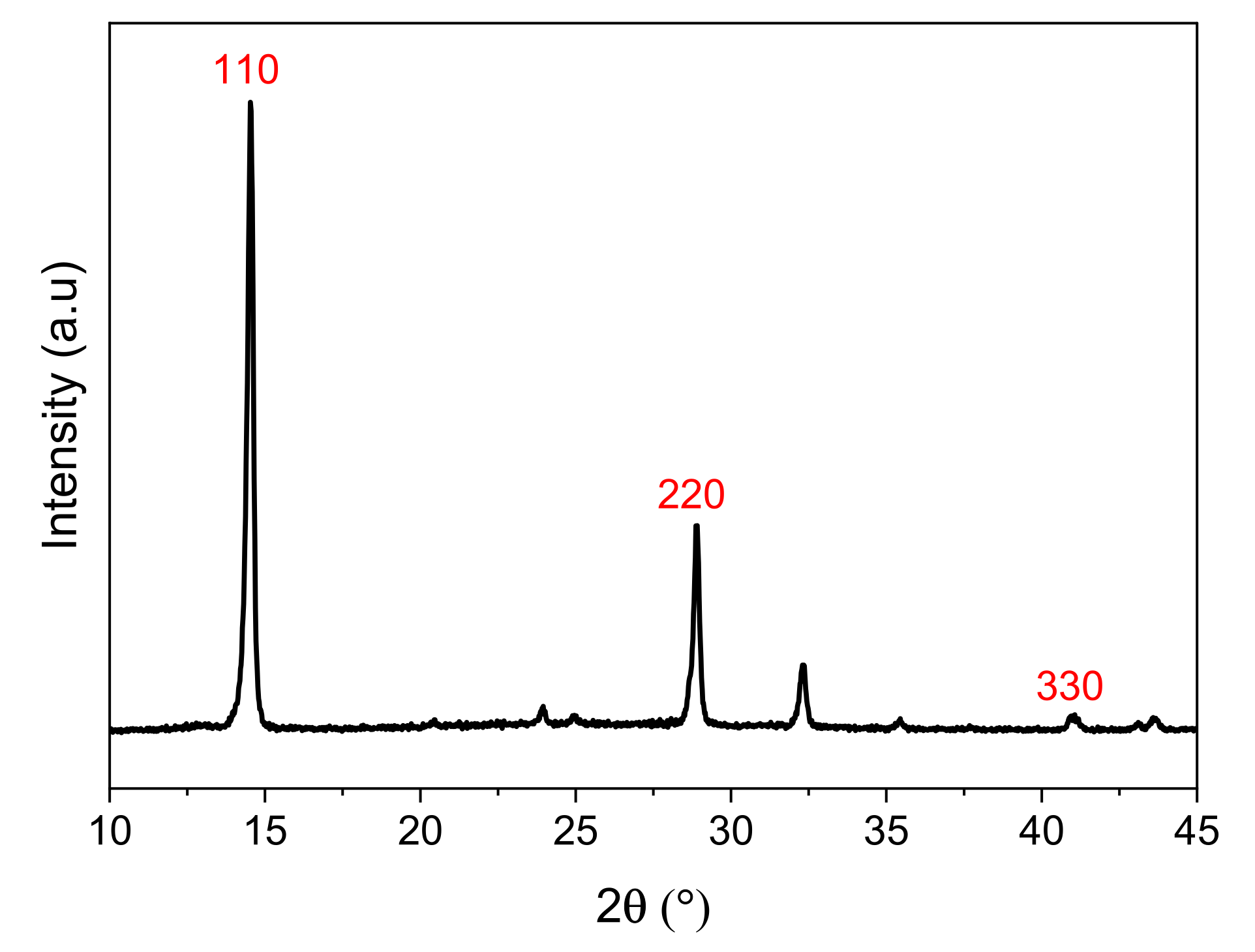
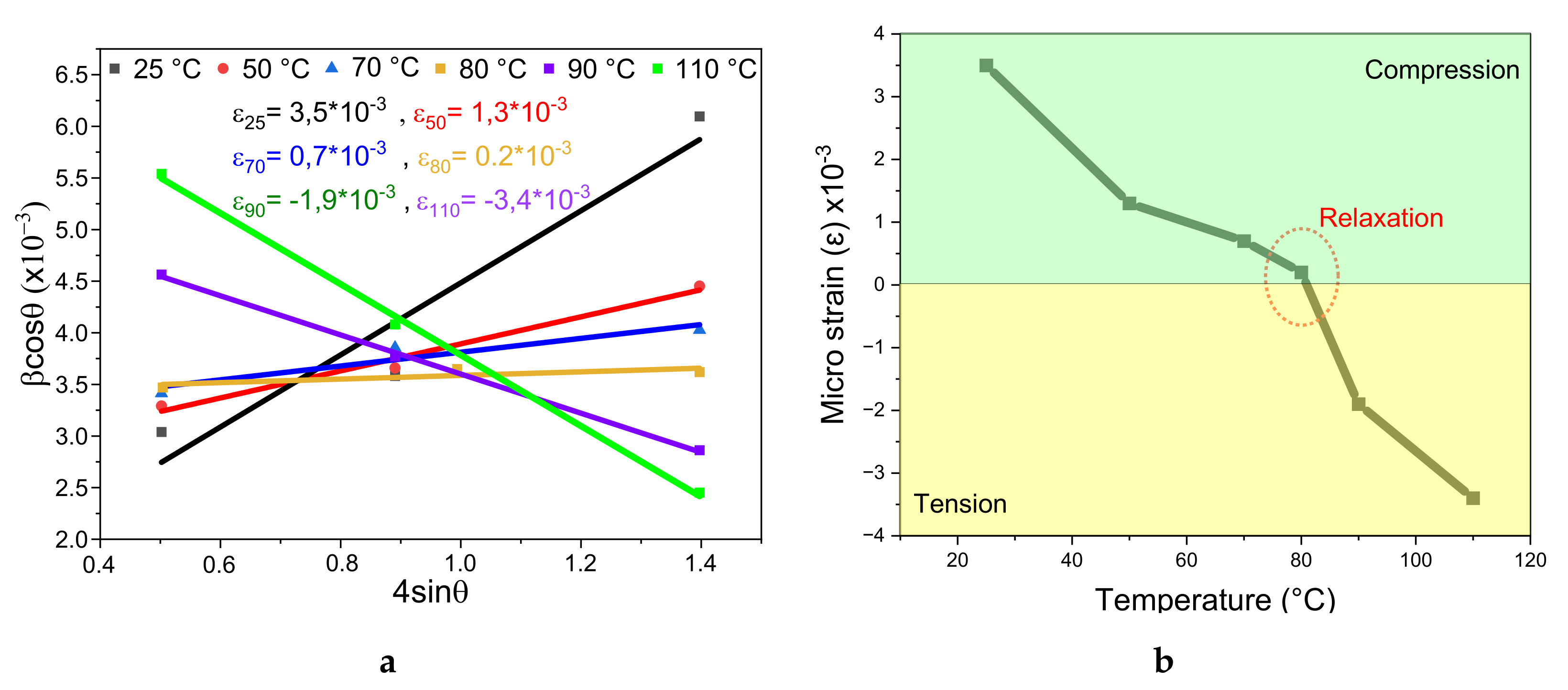
3.1. Williamson Hall Characterization
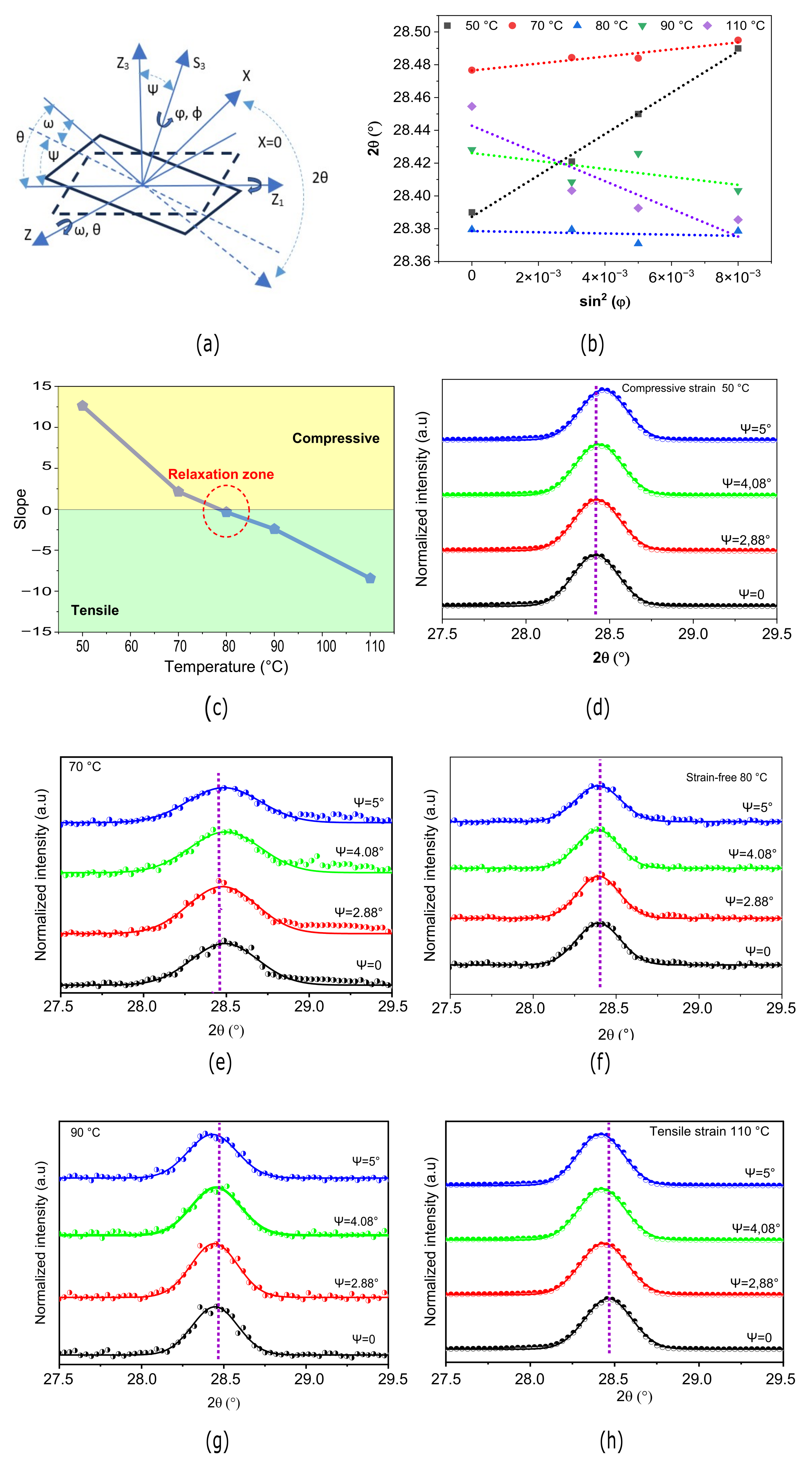
3.2. GIXRD Characterization
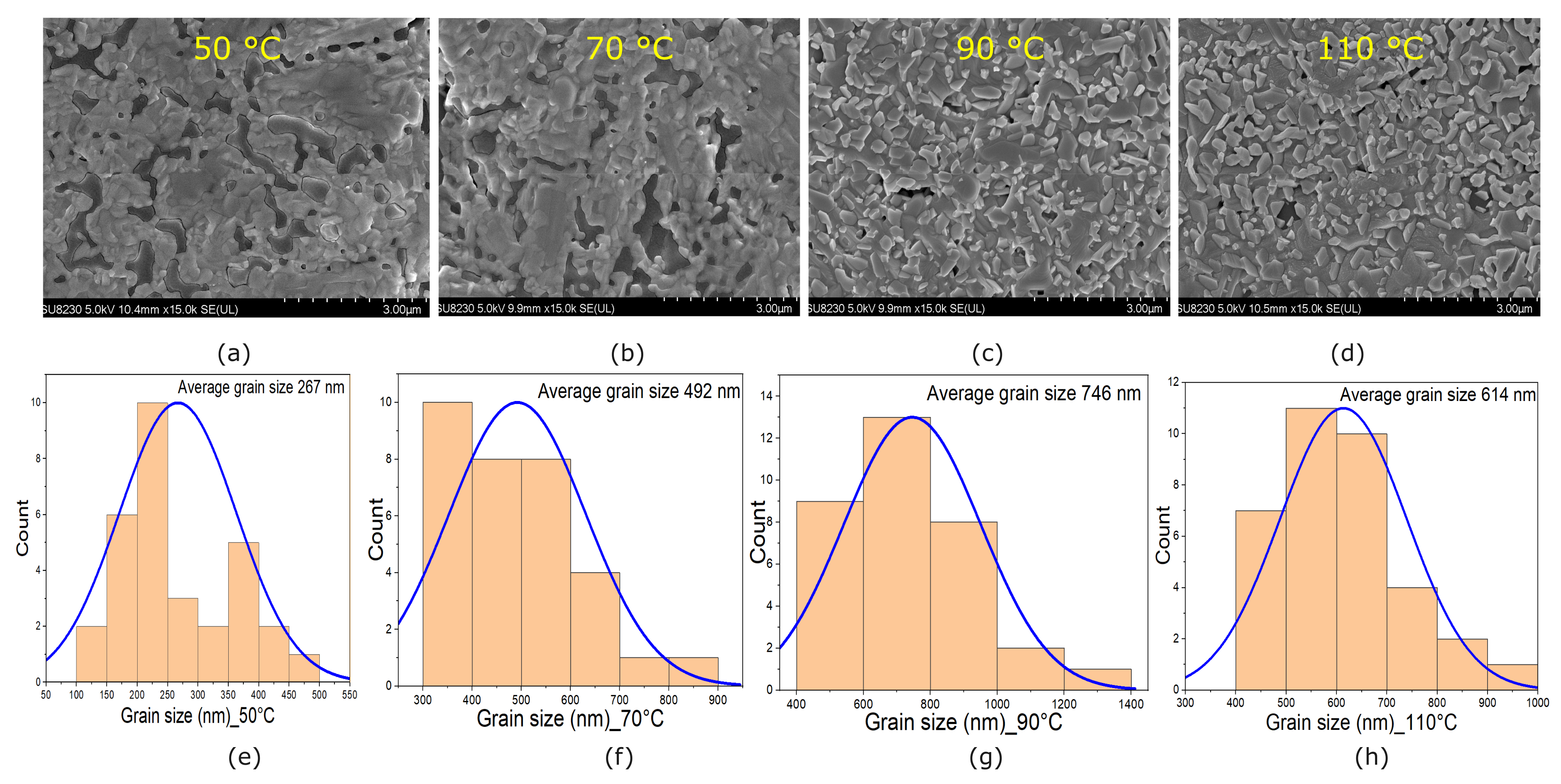
3.3. SEM Characterization
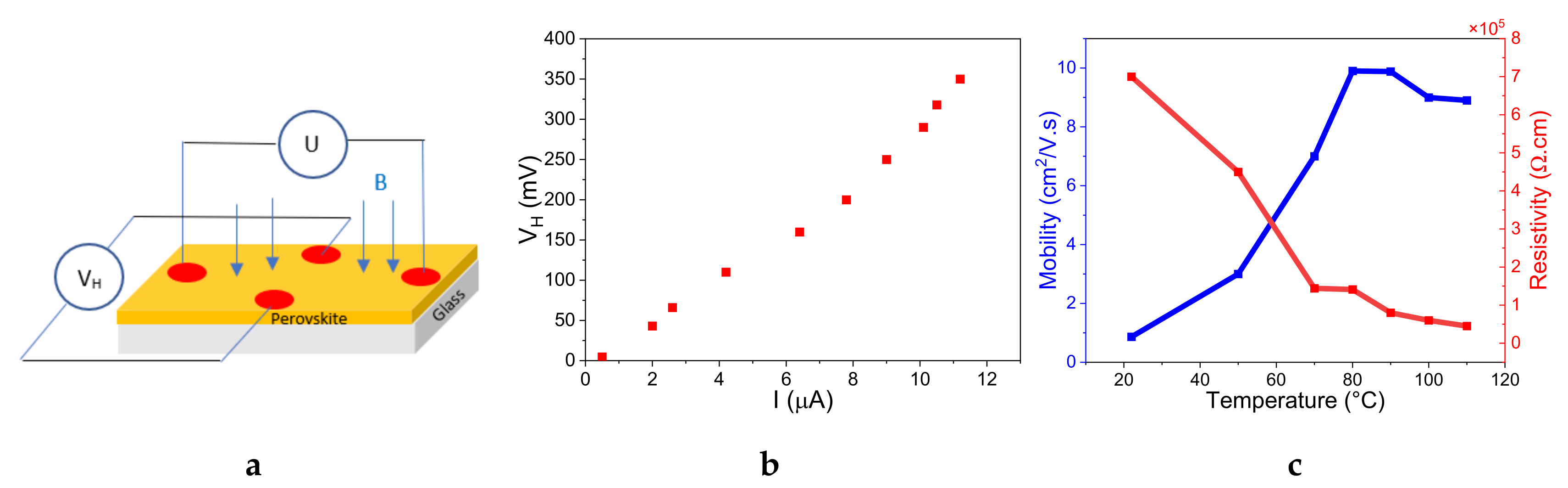
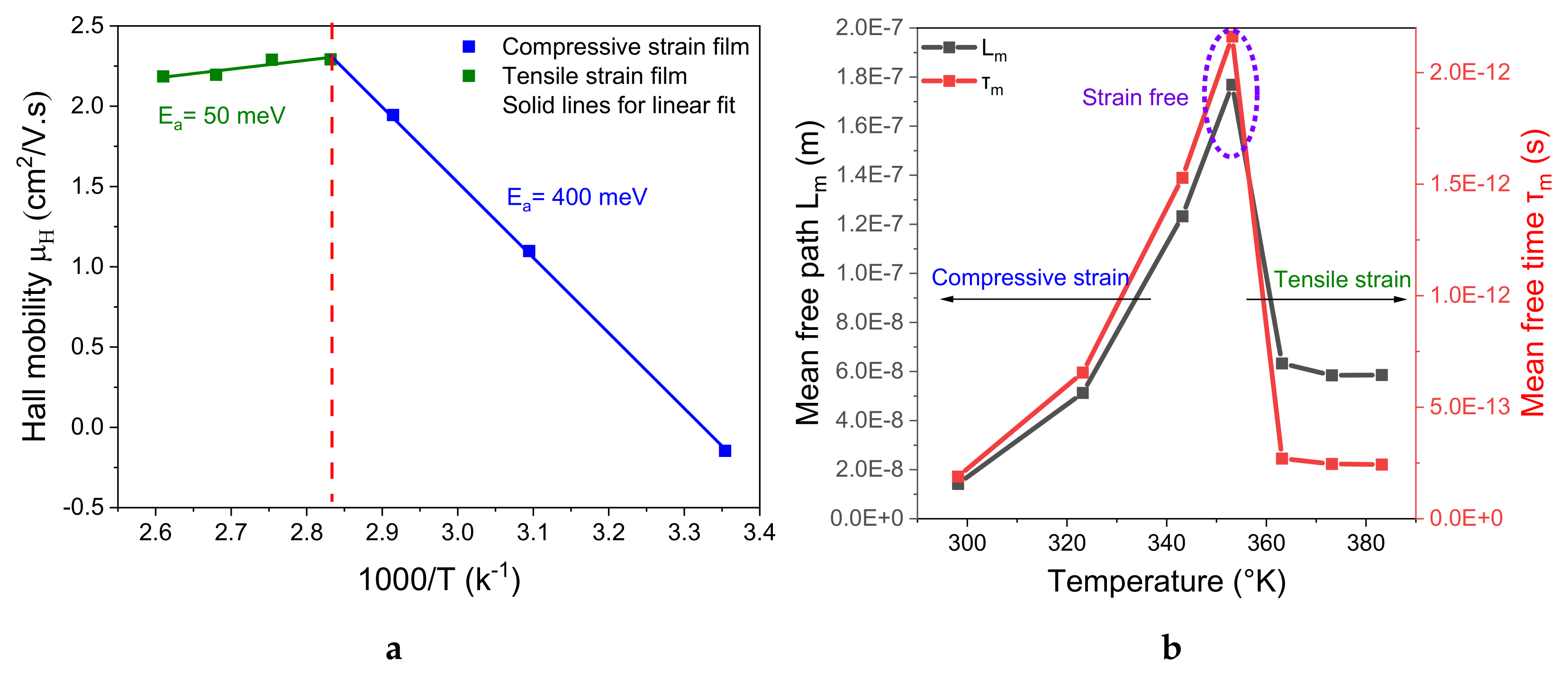
3.4. Hall Effect Characterization
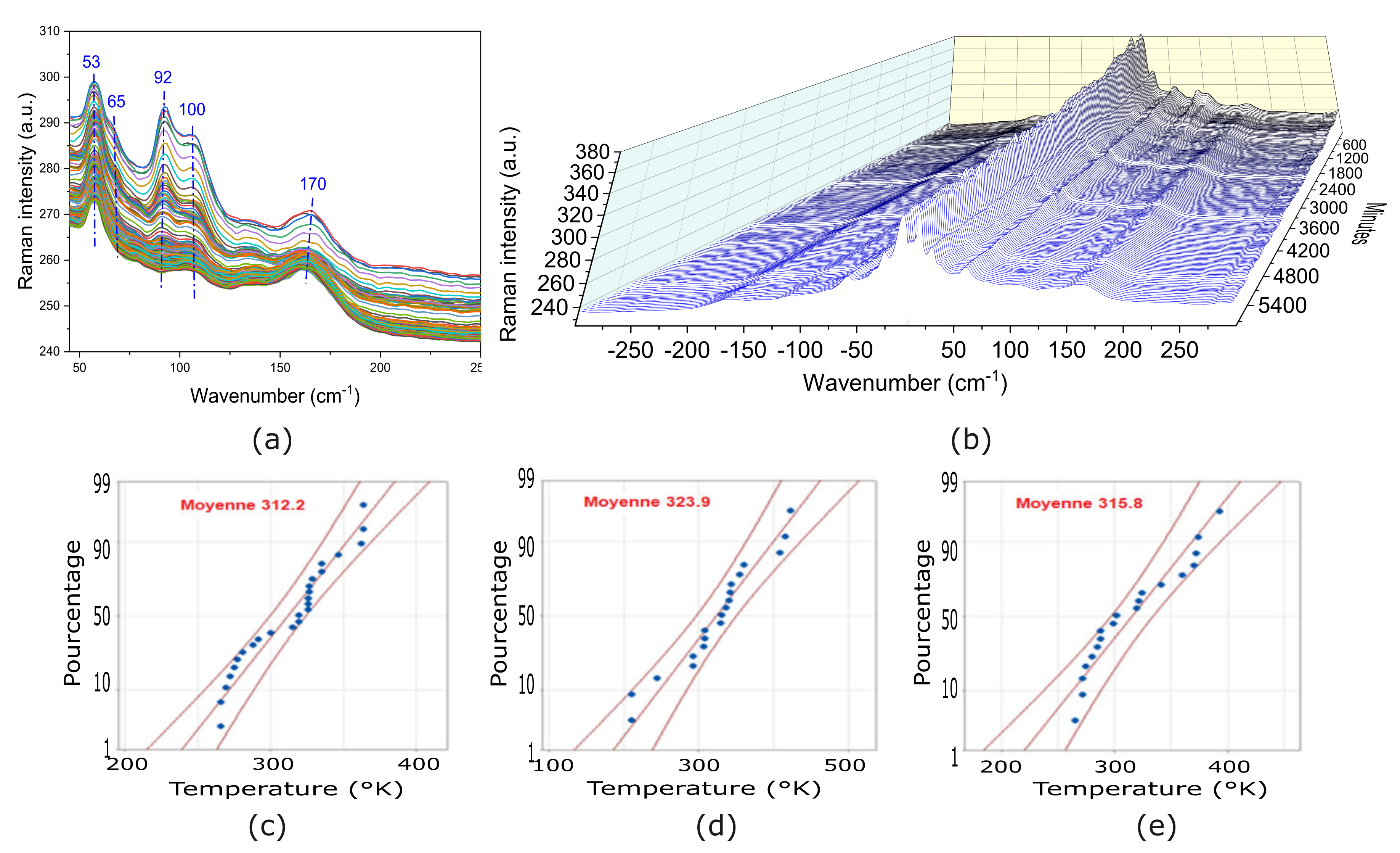
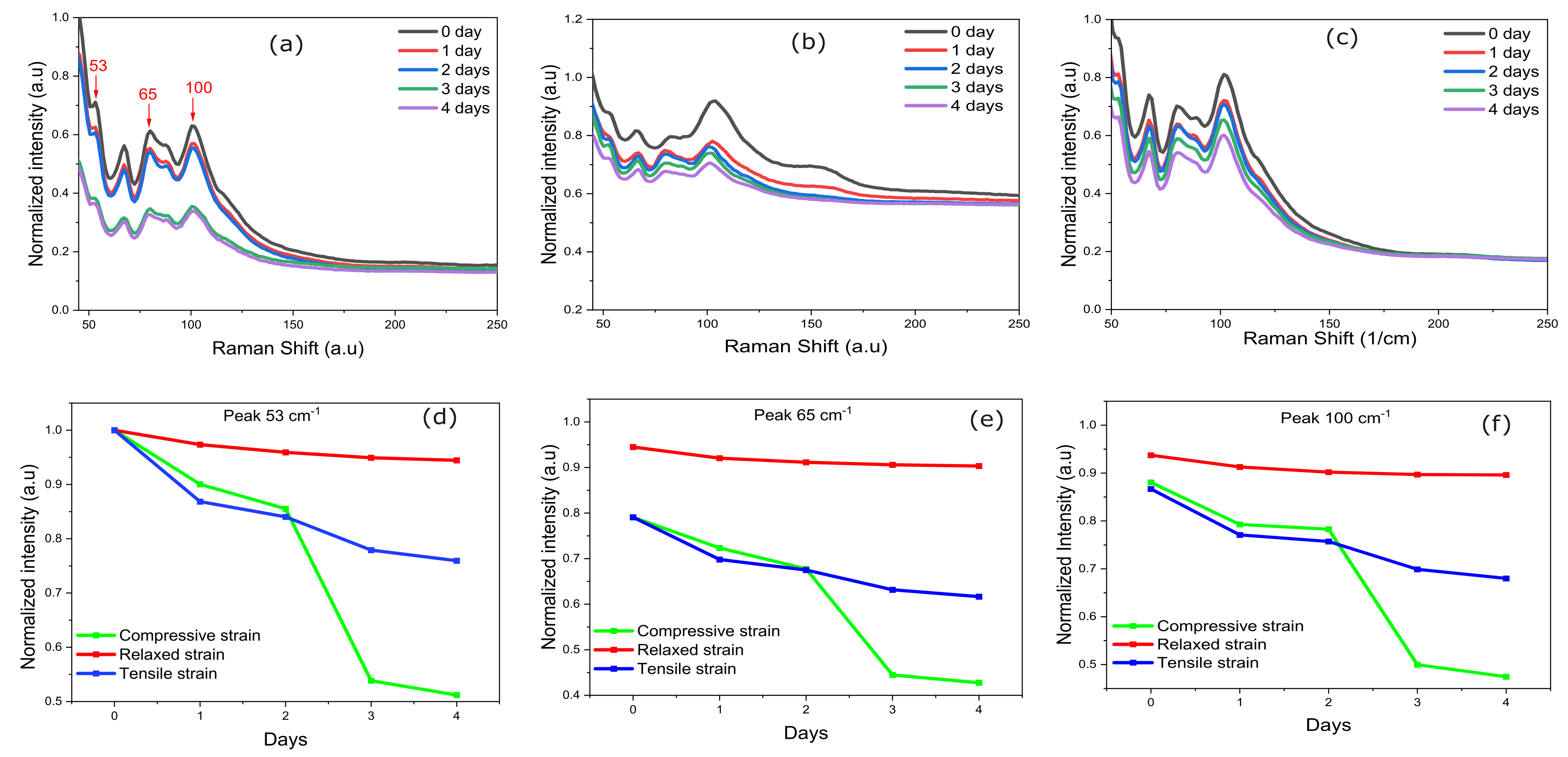
3.5. Raman Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| GIXRD | grazing incident X-ray diffraction |
| CTE | coefficient of thermal expansion |
| PSC | perovskite solar cell |
| XRD | X-ray diffraction |
| FWHM | full-width half-maximum |
References
- Rong, Y.; Hu, Y.; Mei, A.; Tan, H.; Saidaminov, M.I.; Seok, S.I.; McGehee, M.D.; Sargent, E.H.; Han, H. Challenges for commercializing perovskite solar cells. Science 2018, 361, eaat8235. [Google Scholar] [CrossRef]
- Kim, H.; Seo, J.; Park, N. Material and device stability in perovskite solar cells. ChemSusChem 2016, 9, 2528–2540. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.C.; Yang, Y.; Li, Z.; Xue, D.J.; Hu, J.S. GeSe thin-film solar cells. Materials Chemistry Frontiers 2020, 4, 775–787. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, C.; Liu, L.; Ma, S.; Liu, P.; Wu, J.; Shi, C.; Du, Q.; Hao, Y.; Xiang, S. Interfacial residual stress relaxation in perovskite solar cells with improved stability. Advanced Materials 2019, 31, 1904408. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liu, S.C.; Li, Z.; Wang, S.; Xue, D.J.; Lin, Y.; Hu, J.S. Strain in perovskite solar cells: origins, impacts and regulation. National science review 2021, 8, nwab047. [Google Scholar] [CrossRef] [PubMed]
- Grote, C.; Berger, R.F. Strain tuning of tin–halide and lead–halide perovskites: a first-principles atomic and electronic structure study. The Journal of Physical Chemistry C 2015, 119, 22832–22837. [Google Scholar] [CrossRef]
- Hutchinson, J.W. Stresses and failure modes in thin films and multilayers. Notes for a Dcamm Course. Technical University of Denmark, Lyngby 1996, 1, 14. Type: Journal Article.
- Peng, J. Determination of residual stresses in coating by pseudo-grazing incidence X-Ray diffraction method. Thesis, 2006.
- Tennyson, E.M.; Doherty, T.A.; Stranks, S.D. Heterogeneity at multiple length scales in halide perovskite semiconductors. Nature Reviews Materials 2019, 4, 573–587. [Google Scholar] [CrossRef]
- Abadias, G.; Chason, E.; Keckes, J.; Sebastiani, M.; Thompson, G.B.; Barthel, E.; Doll, G.L.; Murray, C.E.; Stoessel, C.H.; Martinu, L. Stress in thin films and coatings: Current status, challenges, and prospects. Journal of Vacuum Science & Technology A: Vacuum, Surfaces, and Films 2018, 36, 020801. [Google Scholar]
- Namvar, A.; Dehghany, M.; Sohrabpour, S.; Naghdabadi, R. Thermal residual stresses in silicon thin film solar cells under operational cyclic thermal loading: A finite element analysis. Solar Energy 2016, 135, 366–373. [Google Scholar] [CrossRef]
- Rolston, N.; Bush, K.A.; Printz, A.D.; Gold-Parker, A.; Ding, Y.; Toney, M.F.; McGehee, M.D.; Dauskardt, R.H. Engineering stress in perovskite solar cells to improve stability. Advanced Energy Materials 2018, 8, 1802139. [Google Scholar] [CrossRef]
- Lee, J.H.; Deng, Z.; Bristowe, N.C.; Bristowe, P.D.; Cheetham, A.K. The competition between mechanical stability and charge carrier mobility in MA-based hybrid perovskites: insight from DFT. Journal of Materials Chemistry C 2018, 6, 12252–12259. [Google Scholar] [CrossRef]
- Qiu, L.; He, S.; Ono, L.K.; Liu, S.; Qi, Y. Scalable fabrication of metal halide perovskite solar cells and modules. ACS Energy Letters 2019, 4, 2147–2167. [Google Scholar] [CrossRef]
- Mohammad, A.; Mahjabeen, F. Promises and Challenges of Perovskite Solar Cells: A Comprehensive Review. BULLET: Jurnal Multidisiplin Ilmu 2023, 2, 1147–1157. [Google Scholar]
- Hu, Q.; Chen, W.; Yang, W.; Li, Y.; Zhou, Y.; Larson, B.W.; Johnson, J.C.; Lu, Y.H.; Zhong, W.; Xu, J. Improving efficiency and stability of perovskite solar cells enabled by a near-infrared-absorbing moisture barrier. Joule 2020, 4, 1575–1593. [Google Scholar] [CrossRef]
- Rong, Y.; Hou, X.; Hu, Y.; Mei, A.; Liu, L.; Wang, P.; Han, H. Synergy of ammonium chloride and moisture on perovskite crystallization for efficient printable mesoscopic solar cells. Nature communications 2017, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Liu, W.; Li, C.; Da, S.; Zheng, Y.; Wu, Y.; Ran, F. Microstress for metal halide perovskite solar cells: from source to influence and management. Nanoscale.
- Zhu, C.; Niu, X.; Fu, Y.; Li, N.; Hu, C.; Chen, Y.; He, X.; Na, G.; Liu, P.; Zai, H.; et al. Strain engineering in perovskite solar cells and its impacts on carrier dynamics. Nature Communications 2019, 10, 815. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.W.; Osherov, A.; Alsari, M.; Sponseller, M.; Duck, B.C.; Jung, Y.K.; Settens, C.; Niroui, F.; Brenes, R.; Stan, C.V. Lattice strain causes non-radiative losses in halide perovskites. Energy & Environmental Science 2019, 12, 596–606. [Google Scholar]
- Qiao, L.; Fang, W.H.; Long, R.; Prezhdo, O.V. Elimination of Charge Recombination Centers in Metal Halide Perovskites by Strain. Journal of the American Chemical Society 2021, 143, 9982–9990. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Hu, J.; Wang, A.; Cui, Y.; Chen, B.; Niu, X.; Hao, F. Facile lattice tensile strain compensation in mixed-cation halide perovskite solar cells. Journal of Energy Chemistry 2022, 66, 422–428. [Google Scholar] [CrossRef]
- Zheng, X.; Chen, B.; Dai, J.; Fang, Y.; Bai, Y.; Lin, Y.; Wei, H.; Zeng, X.C.; Huang, J. Defect passivation in hybrid perovskite solar cells using quaternary ammonium halide anions and cations. Nature Energy 2017, 2, 1–9. [Google Scholar] [CrossRef]
- Ghosh, D.; Aziz, A.; Dawson, J.A.; Walker, A.B.; Islam, M.S. Putting the squeeze on lead iodide perovskites: pressure-induced effects to tune their structural and optoelectronic behavior. Chemistry of Materials 2019, 31, 4063–4071. [Google Scholar] [CrossRef] [PubMed]
- Steele, J.A.; Jin, H.; Dovgaliuk, I.; Berger, R.F.; Braeckevelt, T.; Yuan, H.; Martin, C.; Solano, E.; Lejaeghere, K.; Rogge, S.M. Thermal unequilibrium of strained black CsPbI3 thin films. Science 2019, 365, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Jin, B.; Cao, J.; Yuan, R.; Cai, B.; Wu, C.; Zheng, X. Strain relaxation for perovskite lattice reconfiguration. Advanced Energy and Sustainability Research 2023, 4, 2200143. [Google Scholar] [CrossRef]
- Zhao, J.; Deng, Y.; Wei, H.; Zheng, X.; Yu, Z.; Shao, Y.; Shield, J.E.; Huang, J. Strained hybrid perovskite thin films and their impact on the intrinsic stability of perovskite solar cells. Science advances 2017, 3, eaao5616. [Google Scholar] [CrossRef] [PubMed]
- Głowienka, D.; Miruszewski, T.; Szmytkowski, J. The domination of ionic conductivity in tetragonal phase of the organometal halide perovskite CH3NH3PbI3-xClx. Solid State Sciences 2018, 82, 19–23. [Google Scholar] [CrossRef]
- Zak, A.K.; Majid, W.A.; Abrishami, M.E.; Yousefi, R. X-ray analysis of ZnO nanoparticles by Williamson–Hall and size–strain plot methods. Solid State Sciences 2011, 13, 251–256. [Google Scholar]
- Hall, W. X-ray line broadening in metals. Proceedings of the Physical Society. Section A 1949, 62, 741. [Google Scholar] [CrossRef]
- Mote, V.; Purushotham, Y.; Dole, B. Williamson-Hall analysis in estimation of lattice strain in nanometer-sized ZnO particles. Journal of theoretical and applied physics 2012, 6, 1–8. [Google Scholar] [CrossRef]
- Nishimura, K.; Hirotani, D.; Kamarudin, M.A.; Shen, Q.; Toyoda, T.; Iikubo, S.; Minemoto, T.; Yoshino, K.; Hayase, S. Relationship between lattice strain and efficiency for Sn-perovskite solar cells. ACS applied materials & interfaces 2019, 11, 31105–31110. [Google Scholar]
- Pramanick, A.; Wang, X.; Hoffmann, C.; Diallo, S.; Jørgensen, M.; Wang, X.L. Microdomain dynamics in single-crystal BaTi O 3 during paraelectric-ferroelectric phase transition measured with time-of-flight neutron scattering. Physical Review B 2015, 92, 174103. [Google Scholar] [CrossRef]
- Robinson, I.; Harder, R. Coherent X-ray diffraction imaging of strain at the nanoscale. Nature materials 2009, 8, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Song, H. Analyse expérimentale et numérique de la distribution des contraintes résiduelles induites par choc-laser dans les alliages d’aluminium. Thesis, 2010.
- Murali, B.; Yengel, E.; Peng, W.; Chen, Z.; Alias, M.S.; Alarousu, E.; Ooi, B.S.; Burlakov, V.; Goriely, A.; Eddaoudi, M. Temperature-induced lattice relaxation of perovskite crystal enhances optoelectronic properties and solar cell performance. The journal of physical chemistry letters 2017, 8, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Francisco-López, A.; Charles, B.; Alonso, M.I.; Garriga, M.; Campoy-Quiles, M.; Weller, M.T.; Goñi, A.R. Phase diagram of methylammonium/formamidinium lead iodide perovskite solid solutions from temperature-dependent photoluminescence and Raman spectroscopies. The Journal of Physical Chemistry C 2020, 124, 3448–3458. [Google Scholar] [CrossRef]
- Ren, X.; Yang, Z.; Yang, D.; Zhang, X.; Cui, D.; Liu, Y.; Wei, Q.; Fan, H.; Liu, S.F. Modulating crystal grain size and optoelectronic properties of perovskite films for solar cells by reaction temperature. Nanoscale 2016, 8, 3816–3822. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Hou, Z.; Zou, S.; Lu, X.; Yang, J.; Hao, T.; Zhou, Z.; Xu, J.; Zeng, Y.; Xiao, W. Direct observation on p-to n-type transformation of perovskite surface region during defect passivation driving high photovoltaic efficiency. Joule 2021, 5, 467–480. [Google Scholar] [CrossRef]
- Xie, Z.; Feng, K.; Xiong, Y.; Chen, X.; Liang, Y.; Abid, K.; Xu, L. A High Seebeck Voltage Thermoelectric Module with P-type and N-type MAPbI3 Perovskite Single Crystals. Advanced Electronic Materials 2021, 7, 2001003. [Google Scholar] [CrossRef]
- Yin, W.J.; Shi, T.; Yan, Y. Unusual defect physics in CH3NH3PbI3 perovskite solar cell absorber. Applied Physics Letters 2014, 104, 063903. [Google Scholar] [CrossRef]
- Karim, A.; Khan, M.; Hossain, M. Temperature dependency of excitonic effective mass and charge carrier conduction mechanism in CH3NH3PbI3- xClx thin films. Scientific reports 2021, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Shao, Y.; Xie, H.; Lyu, L.; Liu, X.; Gao, Y.; Huang, J. Qualifying composition dependent p and n self-doping in CH3NH3PbI3. Applied Physics Letters 2014, 105, 163508. [Google Scholar] [CrossRef]
- Frolova, L.A.; Dremova, N.N.; Troshin, P.A. The chemical origin of the p-type and n-type doping effects in the hybrid methylammonium–lead iodide (MAPbI 3) perovskite solar cells. Chemical Communications 2015, 51, 14917–14920. [Google Scholar] [CrossRef]
- Wehrenfennig, C.; Eperon, G.E.; Johnston, M.B.; Snaith, H.J.; Herz, L.M. High charge carrier mobilities and lifetimes in organolead trihalide perovskites. Advanced materials 2014, 26, 1584–1589. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Yu, L.; Wang, H.; Zou, T.; Luo, L.; Liu, Z.; Lu, Z. Cubic structure of the mixed halide perovskite CH 3 NH 3 PbI 3- x Cl x via thermal annealing. RSC Advances 2015, 5, 85480–85485. [Google Scholar] [CrossRef]
- Saffari, M.; Mohebpour, M.A.; Soleimani, H.R.; Tagani, M.B. DFT analysis and FDTD simulation of CH3NH3PbI3- xClx mixed halide perovskite solar cells: role of halide mixing and light trapping technique. Journal of Physics D: Applied Physics 2017, 50, 415501. [Google Scholar] [CrossRef]
- Leguy, A.M.; Azarhoosh, P.; Alonso, M.I.; Campoy-Quiles, M.; Weber, O.J.; Yao, J.; Bryant, D.; Weller, M.T.; Nelson, J.; Walsh, A. Experimental and theoretical optical properties of methylammonium lead halide perovskites. Nanoscale 2016, 8, 6317–6327. [Google Scholar] [CrossRef]
- Berdiyorov, G.R.; Kachmar, A.; El-Mellouhi, F.; Carignano, M.A.; El-Amine Madjet, M. Role of cations on the electronic transport and optical properties of lead-iodide perovskites. The Journal of Physical Chemistry C 2016, 120, 16259–16270. [Google Scholar] [CrossRef]
- Berdiyorov, G.; Madjet, M.; El-Mellouhi, F.; Peeters, F. Effect of crystal structure on the electronic transport properties of the organometallic perovskite CH3NH3PbI3. Solar Energy Materials and Solar Cells 2016, 148, 60–66. [Google Scholar] [CrossRef]
- Shan, D.; Tong, G.; Cao, Y.; Tang, M.; Xu, J.; Yu, L.; Chen, K. The effect of decomposed PbI2 on microscopic mechanisms of scattering in CH3NH3PbI3 films. Nanoscale Research Letters 2019, 14, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Brenner, T.M.; Egger, D.A.; Kronik, L.; Hodes, G.; Cahen, D. Hybrid organic—inorganic perovskites: low-cost semiconductors with intriguing charge-transport properties. Nature Reviews Materials 2016, 1, 1–16. [Google Scholar] [CrossRef]
- Eames, C.; Frost, J.M.; Barnes, P.R.; O’regan, B.C.; Walsh, A.; Islam, M.S. Ionic transport in hybrid lead iodide perovskite solar cells. Nature communications 2015, 6, 1–8. [Google Scholar] [CrossRef]
- Bhargava, R.; Sharma, P.K.; Kumar, S.; Pandey, A.C.; Kumar, N. Consequence of doping mediated strain and the activation energy on the structural and optical properties of ZnO: Cr nanoparticles. Journal of Solid State Chemistry 2010, 183, 1400–1408. [Google Scholar] [CrossRef]
- Miyata, A.; Mitioglu, A.; Plochocka, P.; Portugall, O.; Wang, J.T.W.; Stranks, S.D.; Snaith, H.J.; Nicholas, R.J. Direct measurement of the exciton binding energy and effective masses for charge carriers in organic–inorganic tri-halide perovskites. Nature Physics 2015, 11, 582–587. [Google Scholar] [CrossRef]
- Yang, J.; Meissner, M.; Yamaguchi, T.; Zhang, X.; Ueba, T.; Cheng, L.; Ideta, S.; Tanaka, K.; Zeng, X.; Ueno, N. Band dispersion and hole effective mass of methylammonium lead iodide perovskite. Solar RRL 2018, 2, 1800132. [Google Scholar] [CrossRef]
- Davies, C.L.; Filip, M.R.; Patel, J.B.; Crothers, T.W.; Verdi, C.; Wright, A.D.; Milot, R.L.; Giustino, F.; Johnston, M.B.; Herz, L.M. Bimolecular recombination in methylammonium lead triiodide perovskite is an inverse absorption process. Nature communications 2018, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kauffmann, T.H.; Kokanyan, N.; Fontana, M.D. Use of Stokes and anti-Stokes Raman scattering for new applications. Journal of Raman Spectroscopy 2019, 50, 418–424. [Google Scholar] [CrossRef]
- Barbillat, J.; Bougeard, D.; Buntinx, G.; Delhaye, M.; Dhamelincourt, P.; Fillaux, F. Spectrométrie raman. Techniques de l’ingénieur. Analyse et caractérisation 1999, 4, P2865.1–P2865.31. [Google Scholar] [CrossRef]
- Chang, A.Y.; Cho, Y.; Chen, K.; Chen, C.; Kinaci, A.; Diroll, B.T.; Wagner, M.J.; Chan, M.K.; Lin, H.; Schaller, R.D. Slow organic-to-inorganic sub-lattice thermalization in methylammonium lead halide perovskites observed by ultrafast photoluminescence. Advanced Energy Materials 2016, 6, 1600422. [Google Scholar] [CrossRef]
- Singh, R.K.; Jain, N.; Singh, J.; Kumar, R. Stability behavior of chemically synthesized organic electrolyte salts and methylammonium lead halide perovskite light harvester. Adv Mater Lett 2017, 8, 707–711. [Google Scholar] [CrossRef]
- Ardimas.; Pakornchote, T.; Sukmas, W.; Chatraphorn, S.; Clark, S.J.; Bovornratanaraks, T. Phase transformations and vibrational properties of hybrid organic–inorganic perovskite MAPbI3 bulk at high pressure. Scientific Reports 2023, 13, 16854. [CrossRef] [PubMed]
- Liang, Z.; Zhang, S.; Xu, X.; Wang, N.; Wang, J.; Wang, X.; Bi, Z.; Xu, G.; Yuan, N.; Ding, J. A large grain size perovskite thin film with a dense structure for planar heterojunction solar cells via spray deposition under ambient conditions. RSC advances 2015, 5, 60562–60569. [Google Scholar] [CrossRef]
- Leguy, A.M.; Hu, Y.; Campoy-Quiles, M.; Alonso, M.I.; Weber, O.J.; Azarhoosh, P.; Van Schilfgaarde, M.; Weller, M.T.; Bein, T.; Nelson, J. Reversible hydration of CH3NH3PbI3 in films, single crystals, and solar cells. Chemistry of Materials 2015, 27, 3397–3407. [Google Scholar] [CrossRef]
- Segovia, R.; Qu, G.; Peng, M.; Sun, X.; Shi, H.; Gao, B. Evolution of Photoluminescence, Raman, and Structure of CH 3 NH 3 PbI 3 Perovskite Microwires Under Humidity Exposure. Nanoscale research letters 2018, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Byranvand, M.M.; Saliba, M. Defect passivation of perovskite films for highly efficient and stable solar cells. Solar Rrl 2021, 5, 2100295. [Google Scholar] [CrossRef]
- Chen, B.; Rudd, P.N.; Yang, S.; Yuan, Y.; Huang, J. Imperfections and their passivation in halide perovskite solar cells. Chemical Society Reviews 2019, 48, 3842–3867. [Google Scholar] [CrossRef] [PubMed]
- Aydin, E.; De Bastiani, M.; De Wolf, S. Defect and contact passivation for perovskite solar cells. Advanced Materials 2019, 31, 1900428. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




