Submitted:
27 June 2024
Posted:
01 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Dur/Tre Treatment, Evaluation of Adverse Events, and Changes in Liver Function
2.3. Determination of Antitumor Response
2.4. Evaluation of Changes in AFP, DCP, and AFP-L3
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics at Baseline
3.2. Antitumor Response at 8 Weeks after Dur/Tre Initiation According to RECIST and mRECIST
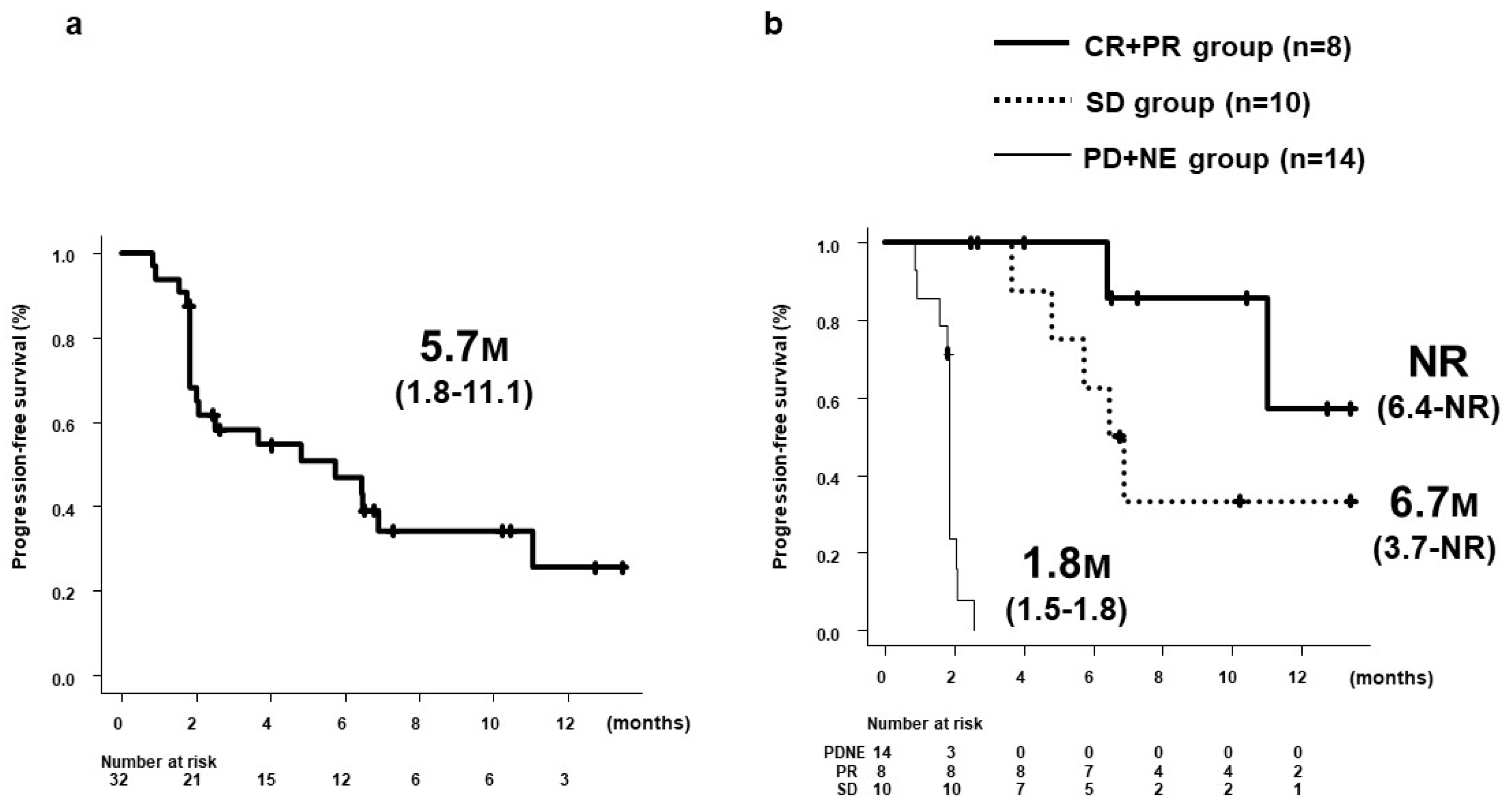
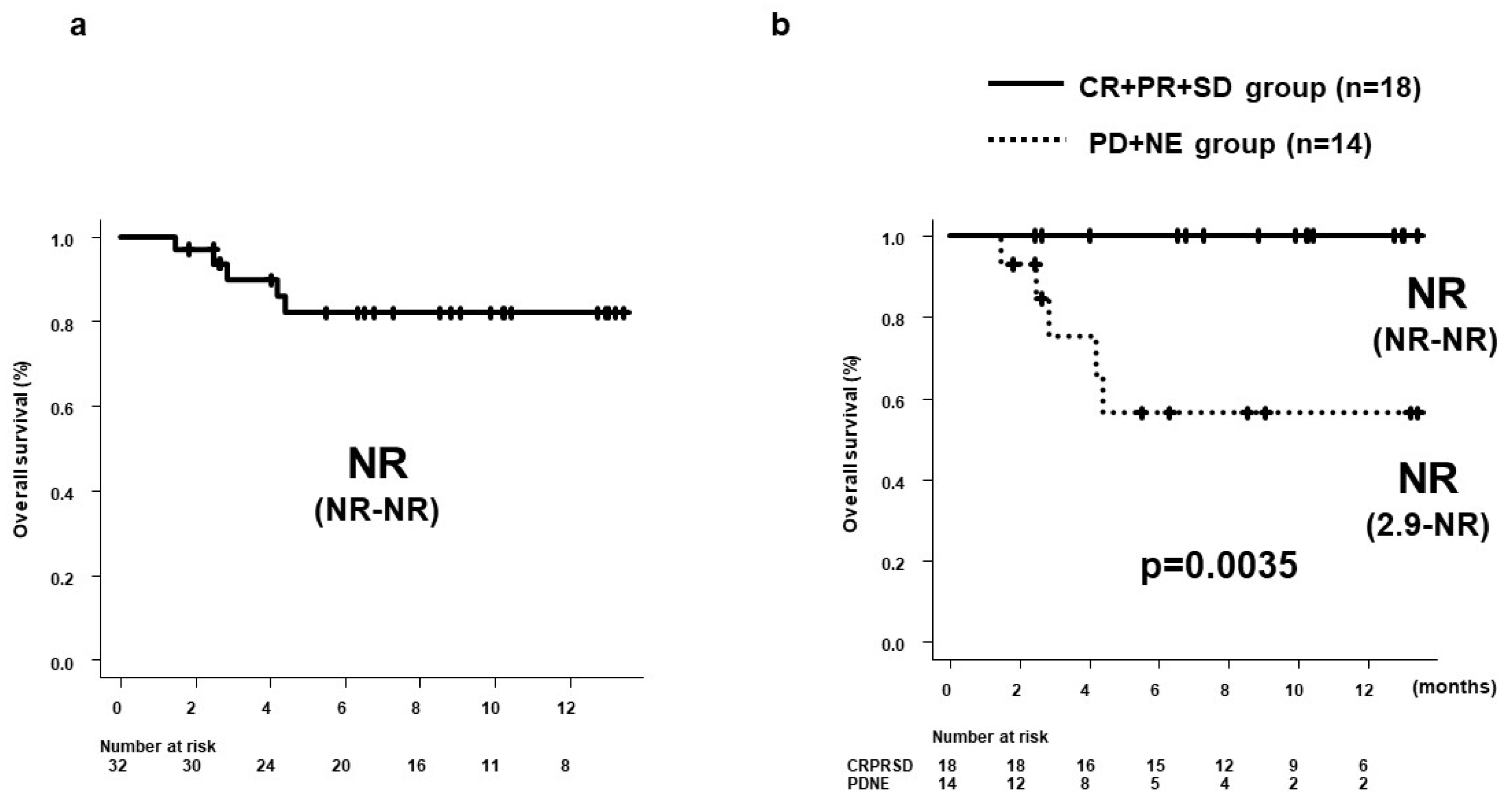
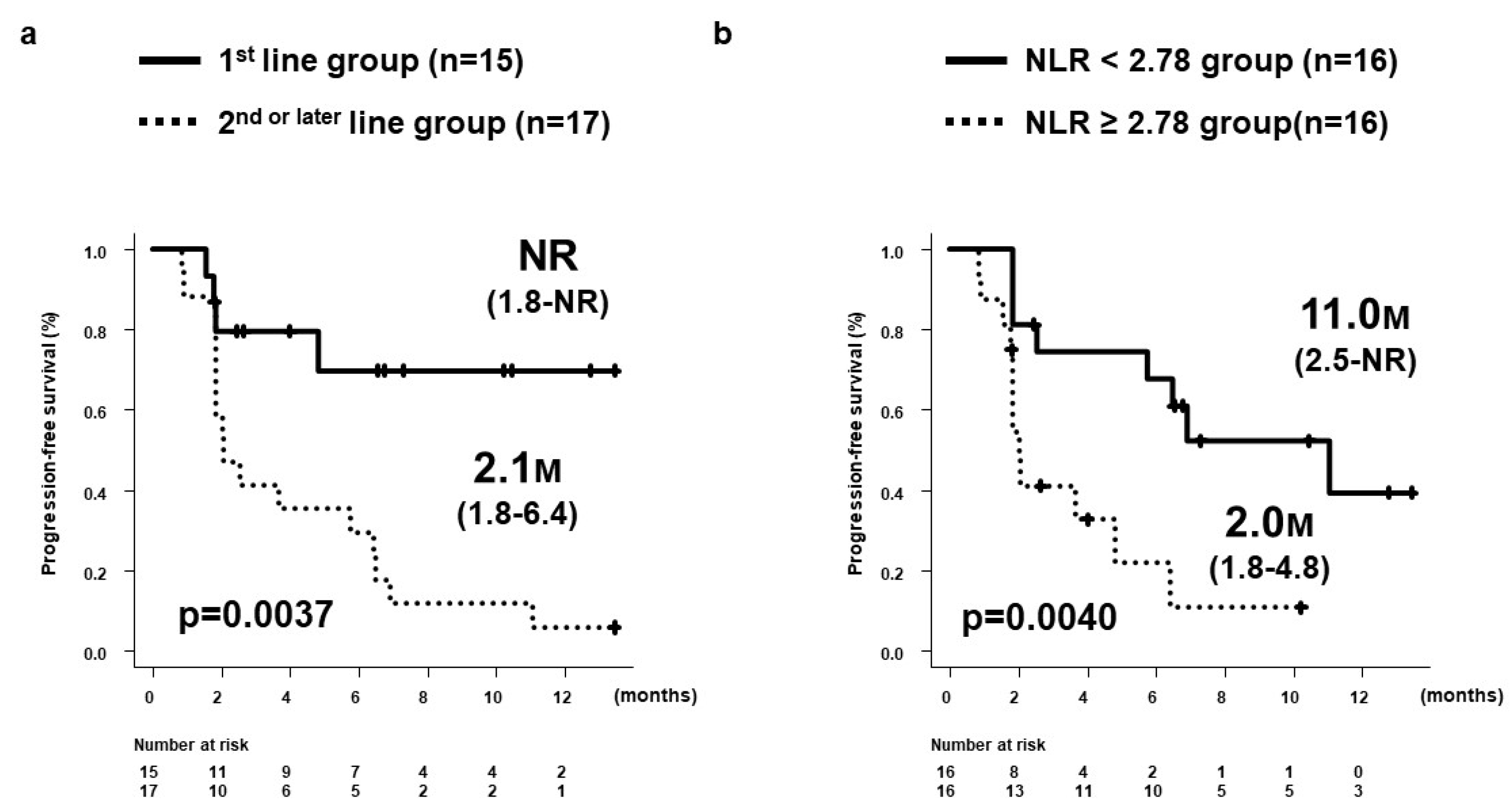
3.3. PFS and OS by 8W-RESIST
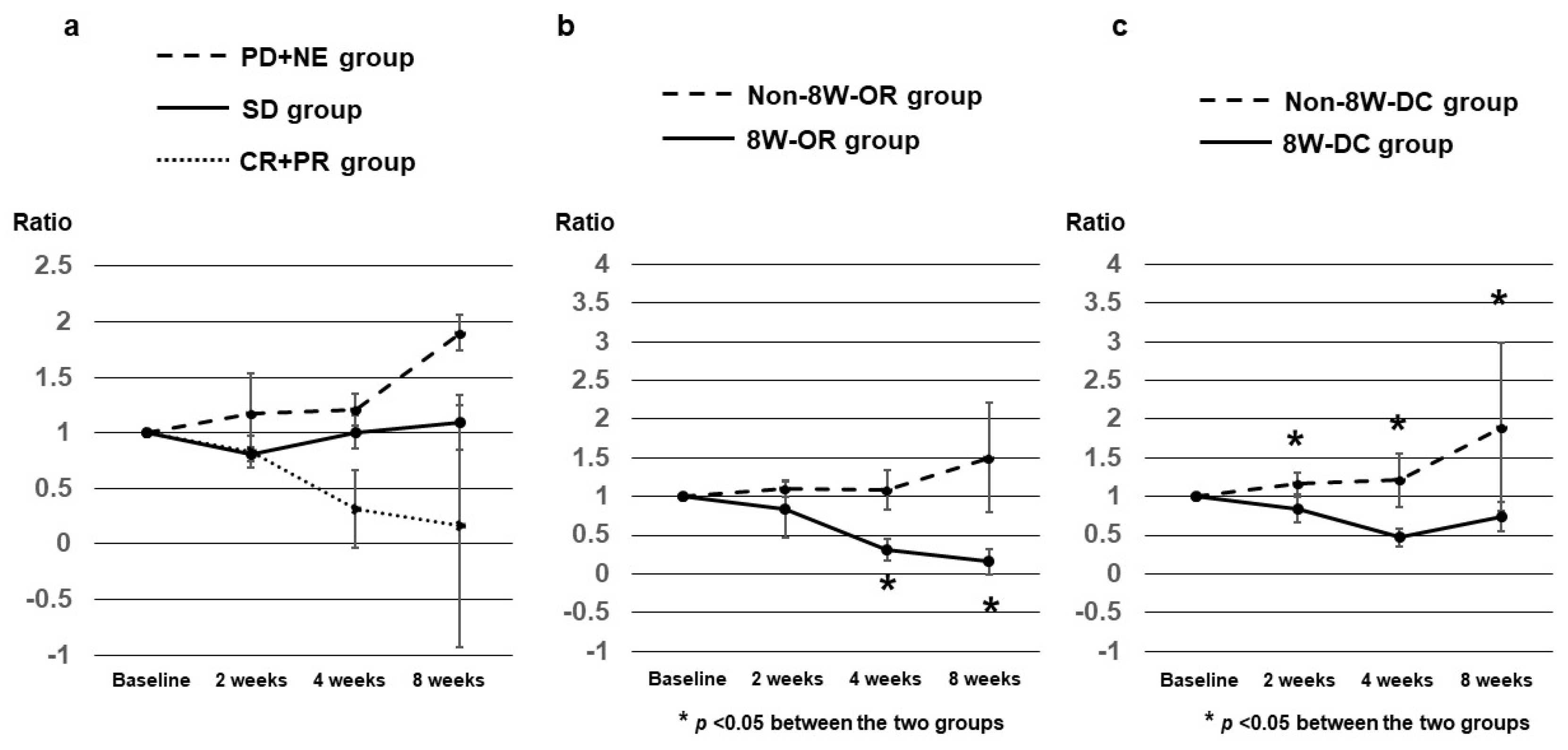
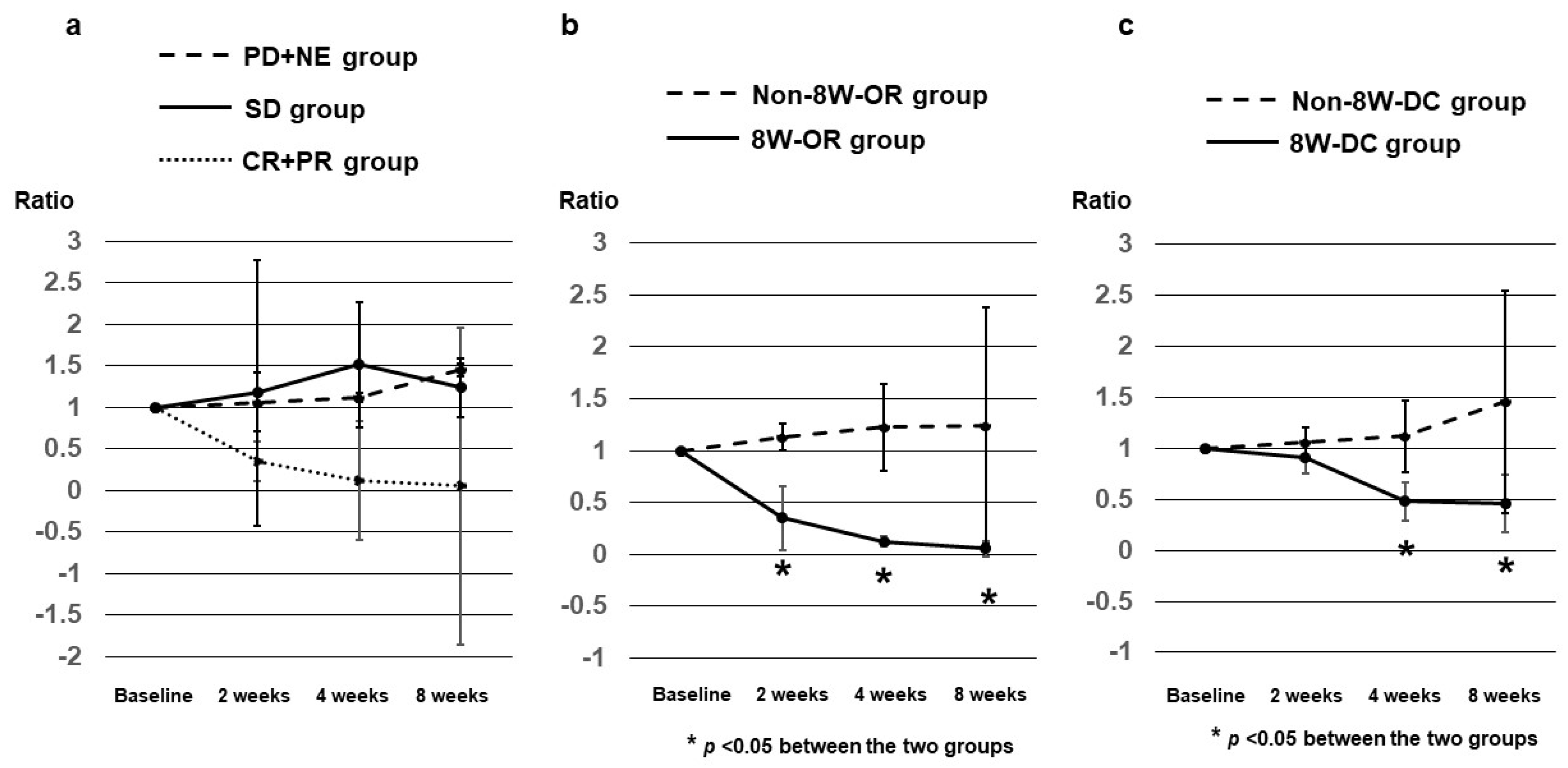
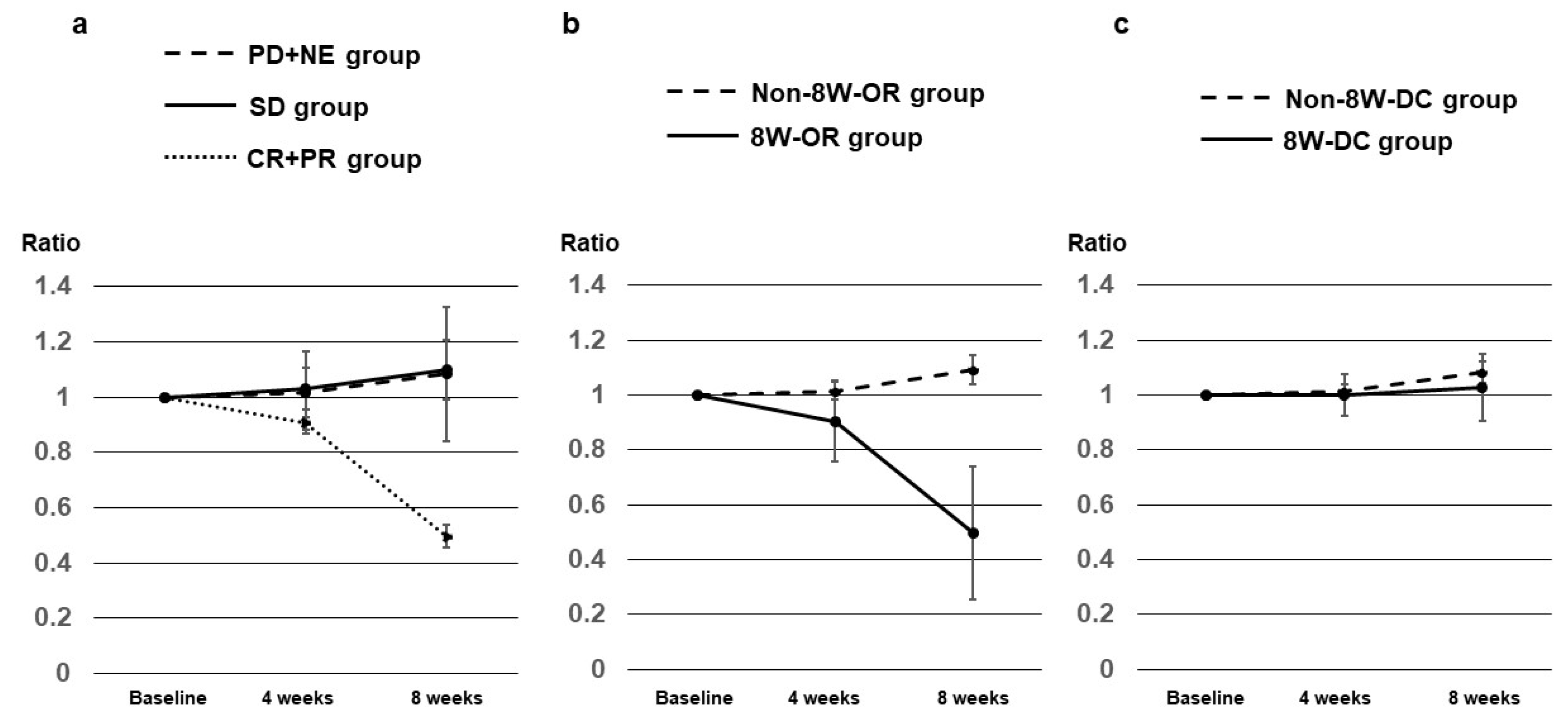
3.4. AFP Ratios at 2, 4, and 8 Weeks after Dur/Tre Initiation, Stratified by 8W-RECIST
3.5. DCP Ratios at 2, 4, and 8 Weeks after Dur/Tre Initiation, Stratified by 8W-RECIST
3.6. AFP-L3 Ratios at 4 and 8 Weeks after Dur/Tre Initiation, Stratified by 8W-RECIST
3.7. Prognostic Factors at Start of Dur/Tre Associated with Good PFS
3.8. PFS by Treatment Line and NLR
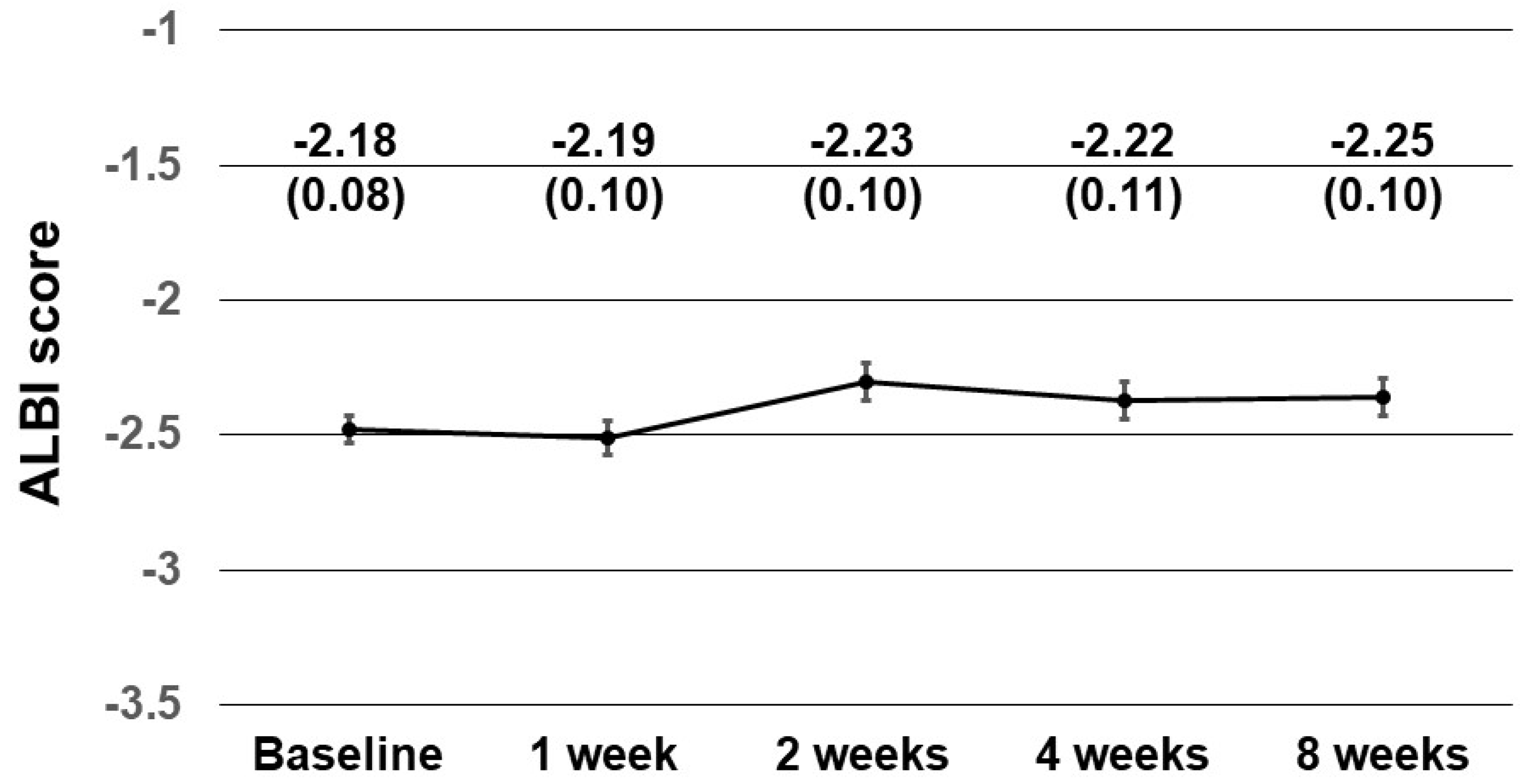
3.9. Safety and Changes in Liver Function
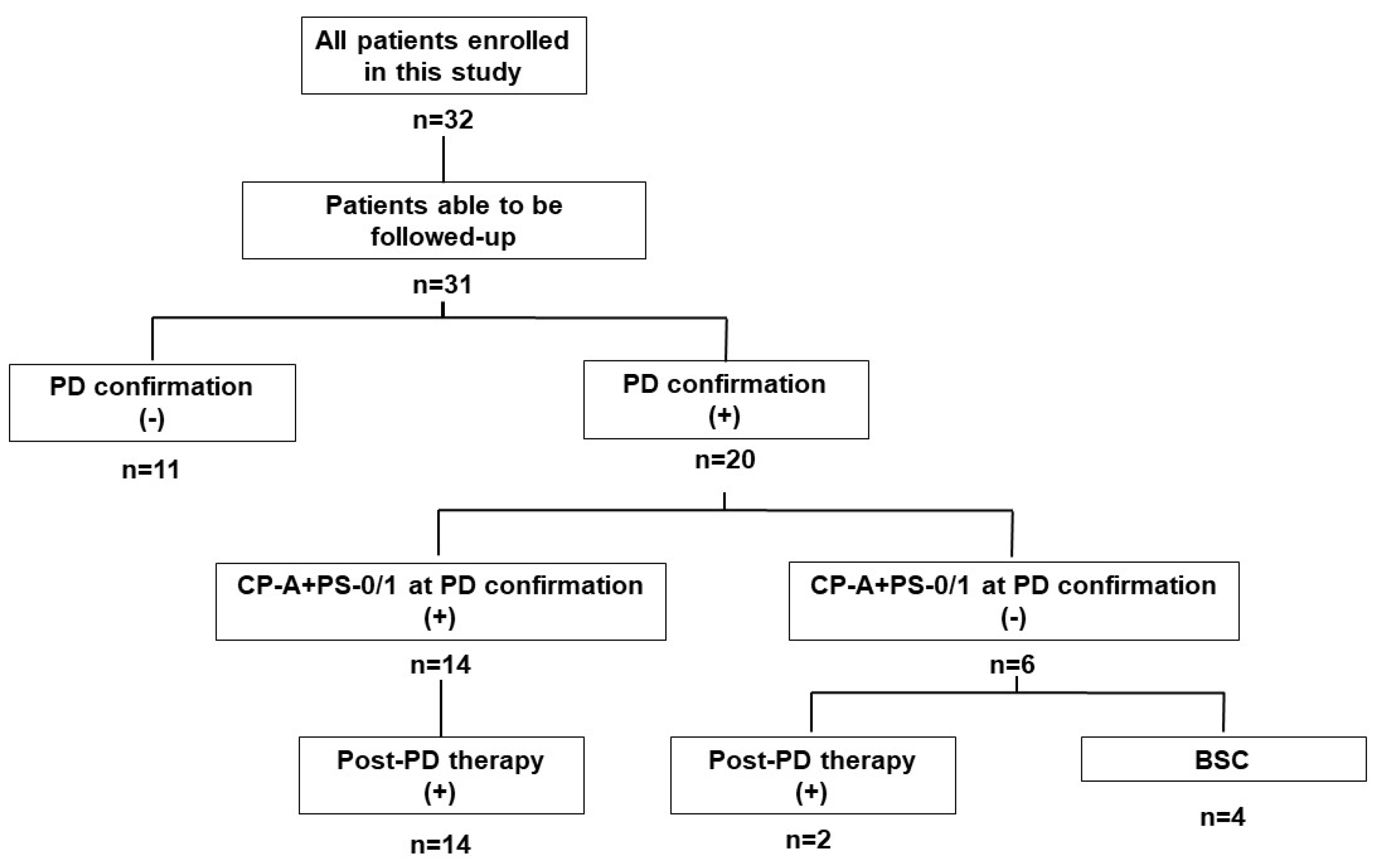
3.10. Post-Progression Therapy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abou-Alfa, G.K.; Lau, G.; Kudo, M.; Chan, S.L.; Kelley, R.K.; Furuse, J.; Sukeepaisarnjaroen, W.; Kang, Y.K.; Dao, T.V.; De Toni, E.N.; et al. Tremelimumab plus durvalumab in unresected hepatocellular carcinoma. N Eng J Med Evid 2022, 1, EVIDoa2100070. [Google Scholar]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol 2022, 76, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Gordan, J.D.; Kennedy, E.B.; Abou-Alfa, G.K.; Beal, E.; Finn, R.S.; Gade, T.P.; Goff, L.; Gupta, S.; Guy, J.; Hoang, H.T.; et al. Systemic therapy for advanced hepatocellular carcinoma: ASCO guideline update. J Clin Oncol 2024, 42, 1830–1850. [Google Scholar] [CrossRef] [PubMed]
- Yeo, Y.H.; Lee, Y.T.; Tseng, H.R.; Zhu, Y.; You, S.; Agopian, V.G.; Yang, J.D. Alpha-fetoprotein: past, present, and future. Hepatol Commun 2024, 8, e0422. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, H.; Kumada, T.; Tada, T.; Sone, Y.; Kaneoka, Y.; Maeda, A. Tumor markers for hepatocellular carcinoma: simple and significant predictors of outcome in patients with HCC. Liver Cancer 2015, 4, 126–136. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kuzuya, T.; Asahina, Y.; Tsuchiya, K.; Tanaka, K.; Suzuki, Y.; Hoshioka, T.; Tamaki, S.; Kato, T.; Yasui, Y.; Hosokawa, T.; et al. Early decrease in α-fetoprotein, but not des-γ-carboxy prothrombin, predicts sorafenib efficacy in patients with advanced hepatocellular carcinoma. Oncology 2011, 81, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Kuzuya, T.; Ishigami, M.; Ishizu, Y.; Honda, T.; Hayashi, K.; Katano, Y.; Hirooka, Y.; Ishikawa, T.; Nakano, I.; Goto, H. Early clinical response after 2 weeks of sorafenib therapy predicts outcomes and anti-tumor response in patients with advanced hepatocellular carcinoma. PLoS One 2015, 10, e0138776. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sánchez, A.I.P.; Roces, L.V.; García, I.Z.; López, E.L.; Hernandez, M.A.C.; Parejo, M.I.B.; Peña-Díaz, J. Value of α-fetoprotein as an early biomarker for treatment response to sorafenib therapy in advanced hepatocellular carcinoma. Oncol Lett 2018, 15, 8863–8870. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kuzuya, T.; Ishigami, M.; Ito, T.; Ishizu, Y.; Honda, T.; Ishikawa, T.; Fujishiro, M. Favorable radiological antitumor response at 2 weeks after starting lenvatinib for patients with advanced hepatocellular carcinoma. Hepatol Res 2020, 50, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Kodama, K.; Kawaoka, T.; Namba, M.; Uchikawa, S.; Ohya, K.; Morio, K.; Nakahara, T.; Murakami, E.; Yamauchi, M.; Hiramatsu, A.; et al. Correlation between early tumor marker response and imaging response in patients with advanced hepatocellular carcinoma treated with lenvatinib. Oncology 2019, 97, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Saeki, I.; Yamasaki, T.; Yamashita, S.; Hanazono, T.; Urata, Y.; Furutani, T.; Yokoyama, Y.; Oishi, T.; Maeda, M.; Kimura, T.; et al. Early predictors of objective response in patients with hepatocellular carcinoma undergoing lenvatinib treatment. Cancers 2020, 12, 779. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kuzuya, T.; Kawabe, N.; Hashimoto, S.; Miyahara, R.; Sawaki, A.; Nakano, T.; Nakaoka, K.; Tanaka, H.; Miyachi, Y.; Mii, A.; et al. Early changes in alpha-fetoprotein are a useful predictor of efficacy of atezolizumab plus bevacizumab treatment in patients with advanced hepatocellular carcinoma. Oncology 2022, 100, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Tsuji, K.; Hiraoka, A.; Tada, T.; Hirooka, M.; Kariyama, K.; Tani, J.; Atsukawa, M.; Takaguchi, K.; Itobayashi, E.; et al. Usefulness of tumor marker score for predicting the prognosis of hepatocellular carcinoma patients treated with atezolizumab plus bevacizumab: a multicenter retrospective study. Cancers 2023, 15, 4348. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kinami, T.; Amioka, K.; Kawaoka, T.; Uchikawa, S.; Yamasaki, S.; Kosaka, M.; Johira, Y.; Yano, S.; Naruto, K.; Ando, Y.; et al. Evaluation of response to atezolizumab plus bevacizumab in patients with advanced hepatocellular carcinoma using the combination of response evaluation criteria in solid tumors and alpha-fetoprotein. Cancers 2023, 15, 2304. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Campani, C.; Bamba-Funck, J.; Campion, B.; Sidali, S.; Blaise, L.; Ganne-Carrié, N.; Demory, A.; Sutter, O.; Larrey, E.; Evain, M.; et al. Baseline ALBI score and early variation of serum AFP predicts outcomes in patients with HCC treated by atezolizumab-bevacizumab. Liver Int 2023, 43, 708–717. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health, National Cancer Institute, U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. Published 27 November 2017. Available online: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf (accessed on 20 June 2020).
- Johnson, P.J.; Berhane, S.; Kagebayashi, C.; Satomura, S.; Teng, M.; Reeves, H.L.; O’Beirne, J.; Fox, R.; Skowronska, A.; Palmer, D.; et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach—the ALBI grade. J Clin Oncol 2015, 33, 550–558. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Lencioni, R.; Llovet, J.M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 2010, 30, 52–60 . [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant 2013, 48, 452–458 . [Google Scholar] [CrossRef] [PubMed]
- Sangro, B.; Chan, S.L.; Kelley, R.K.; Lau, G.; Kudo, M.; Sukeepaisarnjaroen, W.; Yarchoan, M.; De Toni, E.N.; Furuse, J.; Kang, Y.K.; et al. Four-year overall survival update from the phase III HIMALAYA study of tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. Ann Oncol 2024, 35, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Ueshima, K.; Kudo, M.; Takita, M.; Nagai, T.; Tatsumi, C.; Ueda, T.; Kitai, S.; Ishikawa, E.; Yada, N.; Inoue, T.; et al. Des-γ-carboxyprothrombin may be a promising biomarker to determine the therapeutic efficacy of sorafenib for hepatocellular carcinoma. Dig Dis 2011, 29, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Murata, K.; Suzuki, H.; Okano, H.; Oyamada, T.; Yasuda, Y.; Sakamoto, A. Cytoskeletal changes during epithelial-to-fibroblastoid conversion as a crucial mechanism of des-gamma-carboxy prothrombin production in hepatocellular carcinoma. Int J Oncol 2009, 35, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Eso, Y.; Takeda, H.; Taura, K.; Takai, A.; Takahashi, K.; Seno, H. Pretreatment neutrophil-to-lymphocyte ratio as a predictive marker of response to atezolizumab plus bevacizumab for hepatocellular carcinoma. Curr Oncol 2021, 28, 4157–4166. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, J.H.; Chen, Y.Y.; Kee, K.M.; Wang, C.C.; Tsai, M.C.; Kuo, Y.H.; Hung, C.H.; Li, W.F.; Lai, H.L.; Chen, Y.H. The prognostic value of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in patients with hepatocellular carcinoma receiving atezolizumab plus bevacizumab. Cancers 2022, 14, 343. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tada, T.; Kumada, T.; Hiraoka, A.; Hirooka, M.; Kariyama, K.; Tani, J.; Atsukawa, M.; Takaguchi, K.; Itobayashi, E.; Fukunishi, S.; et al. Neutrophil-lymphocyte ratio predicts early outcomes in patients with unresectable hepatocellular carcinoma treated with atezolizumab plus bevacizumab: a multicenter analysis. Eur J Gastroenterol Hepatol 2022, 34, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Ochi, H.; Kurosaki, M.; Joko, K.; Mashiba, T.; Tamaki, N.; Tsuchiya, K.; Marusawa, H.; Tada, T.; Nakamura, S.; Narita, R.; et al. Usefulness of neutrophil-to-lymphocyte ratio in predicting progression and survival outcomes after atezolizumab-bevacizumab treatment for hepatocellular carcinoma. Hepatol Res 2023, 53, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, Y.; Oya, R.; Takemoto, N.; Inohara, H. Neutrophil-to-lymphocyte ratio as a prognostic marker for head and neck squamous cell carcinoma treated with immune checkpoint inhibitors: Meta-analysis. Head Neck 2022, 44, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, R.; Liu, D.; Li, W. Association of pretreatment neutrophil-to-lymphocyte ratio with clinical outcomes in cancer immunotherapy: an evidence synthesis from 30 meta-analyses. Int Immunopharmacol 2024, 132, 111936. [Google Scholar] [CrossRef] [PubMed]
- Kuzuya, T.; Ishigami, M.; Ito, T.; Ishizu, Y.; Honda, T.; Ishikawa, T.; Hirooka, Y.; Fujishiro, M. Clinical characteristics and outcomes of candidates for second-line therapy, including regorafenib and ramucirumab, for advanced hepatocellular carcinoma after sorafenib treatment. Hepatol Res 2019, 49, 1054–1065. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, A.; Kumada, T.; Fukunishi, S.; Atsukawa, M.; Hirooka, M.; Tsuji, K.; Ishikawa, T.; Takaguchi, K.; Kariyama, K.; Itobayashi, E.; et al. Post-progression treatment eligibility of unresectable hepatocellular carcinoma patients treated with lenvatinib. Liver Cancer 2020, 9, 73–83. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Persano, M.; Rimini, M.; Tada, T.; Suda, G.; Shimose, S.; Kudo, M.; Cheon, J.; Finkelmeier, F.; Lim, HY.; Presa, J.; et al. Sequential therapies after atezolizumab plus bevacizumab or lenvatinib first-line treatments in hepatocellular carcinoma patients. Eur J Cancer 2023, 189, 112933. [Google Scholar] [CrossRef] [PubMed]
| Patient characteristics | n = 32 |
|---|---|
| Age, years; median (range) | 75 (40–89) |
| Sex, male/female | 27/5 |
| Etiology, HBV/HCV/non-viral | 5/6/21 |
| Treatment line, 1st/2nd/3rd/4th/5th | 15/6/7/2/2 |
| ECOG-PS, 0/1 | 28/4 |
| Child–Pugh score, 5/6/7/8 | 16/11/4/1 |
| mALBI grade, 1/2a/2b/3 | 7/8/16/1 |
| BCLC stage, A/B/C | 1/17/14 |
| Intrahepatic tumor number, <4/≥4 | 6/26 |
| Maximum intrahepatic tumor size, <50 mm/≥50 mm | 20/12 |
| Portal vein tumor thrombosis, 0/1/2/3/4 | 23/0/4/4/1 |
| Extrahepatic metastasis, -/+ | 22/10 |
| AFP level, ng/mL; median (range) | 138 (1.7–17,239) |
| DCP level, mAU/ml; median (range) | 2068 (10–162,000) |
| AFP-L3 level, %; median (range) | 19.1 (<0.5–88.1) |
| NLR; median (range) | 2.73 (1.36–10.95) |
| Observation period, months; median (range) | 8.0 (1.5–13.6) |
| CR n (%) |
PR n (%) |
SD n (%) |
PD n (%) |
NE n (%) |
CRR | ORR | DCR | |
|---|---|---|---|---|---|---|---|---|
| 8W-RECIST | 0 (0) |
8 (25.0) |
10 (31.3) |
13 (40.6) |
1 (3.1) |
0% | 25.0% | 56.3% |
| 8W-mRECIST | 3 (9.4) |
7 (21.9) |
8 (25.0) |
13 (40.6) |
1 (3.1) |
9.4% | 31.3% | 56.3% |
| 8W-RECIST | AFP ratios, median (SE) | p value | |||||
|---|---|---|---|---|---|---|---|
| CR + PR (8W-OR) n = 6 |
SD n = 6 |
PD + NE (Non-8W-DC) n = 11 |
CR + PR + SD (8W-DC) n = 12 |
SD + PD + NE (Non-8W-OR) n = 17 |
8W-OR vs Non-8W-OR |
8W-DC vs Non-8W-DC |
|
| At 2W | 0.830 (0.364) |
0.810 (0.062) |
1.170 (0.144) |
0.830 (0.159) |
1.105 (0.111) |
0.2475 | 0.0112 |
| At 4W | 0.310 (0.142) |
1.005 (0.149) |
1.210 (0.349) |
0.470 (0.120) |
1.080 (0.254) |
0.0020 | 0.0006 |
| At 8W | 0.160 (0.163) |
1.095 (0.243) |
1.900 (1.091) |
0.735 (0.191) |
1.500 (0.706) |
0.0020 | 0.0037 |
| 8W-RECIST | DCP ratios, median (SE) | p value | |||||
|---|---|---|---|---|---|---|---|
| CR + PR (8W-OR) n = 7 |
SD n = 9 |
PD + NE (Non-8W-DC) n = 11 |
CR + PR + SD (8W-DC) n = 16 |
SD + PD + NE (Non-8W-OR) n = 20 |
8W-OR vs Non-8W-OR |
8W-DC vs Non-8W-DC |
|
| At 2W | 0.350 (0.309) |
1.180 (0.095) |
1.065 (0.144) |
0.910 (0.157) |
1.130 (0.129) |
0.0357 | 0.3550 |
| At 4W | 0.125 (0.0531) |
1.520 (0.231) |
1.120 (0.349) |
0.480 (0.189) |
1.225 (0.418) |
0.0001 | 0.0255 |
| At 8W | 0.055 (0.073) |
1.240 (0.440) |
1.455 (1.0919 |
0.460 (0.284) |
1.240 (1.145) |
0.0001 | 0.0147 |
| 8W-RECIST | AFP-L3 ratios, median (SE) | p value | |||||
|---|---|---|---|---|---|---|---|
| CR + PR (8W-OR) n = 7 |
SD n = 9 |
PD + NE (Non-8W-DC) n = 11 |
CR + PR + SD (8W-DC) n = 16 |
SD + PD + NE (Non-8W-OR) n = 20 |
8W-OR vs Non-8W-OR |
8W-DC vs Non-8W-DC |
|
| At 4W | 0.905 (0.149) |
1.031 (0.074) |
1.016 (0.025) |
1.000 (0.078) |
1.016 (0.033) |
0.0779 | 0.5858 |
| At 8W | 0.496 (0.242) |
1.099 (0.106) |
1.084 (0.040) |
1.028 (0.122) |
1.092 (0.052) |
0.1213 | 0.3001 |
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Factors | HR (95%CI) | p value | HR (95%CI) | p value |
| Age (<75 years) | 0.913 (0.374-2.226) | 0.8412 | ||
| Sex (female) | 1.531 (0.506-4.636) | 0.4508 | ||
| Etiology (HBV or HCV) | 1.846 (0.749-4.552) | 0.1830 | ||
| Treatment line (1st) | 0.233 (0.078-0.702) | 0.0096 | 0.209 (0.069-0.637) | 0.0059 |
| ECOG-PS (0) | 0.597 (0.173-2.059) | 0.4141 | ||
| Child–Pugh score (5) | 0.655 (0.270-1.590) | 0.3498 | ||
| BCLC stage (A or B) | 0.600 (0.247-1.459) | 0.2603 | ||
| Number of intrahepatic tumors (≥4) | 1.111 (0.322-3.832) | 0.8677 | ||
| Maximum size of intrahepatic tumors (≥50 mm) | 0.941 (0.374-3.372) | 0.8978 | ||
| Portal vein tumor thrombosis (+) | 1.463 (0.577-3.707) | 0.4224 | ||
| Extrahepatic metastasis (+) | 0.519 (0.211-1.277) | 0.1534 | ||
| AFP level (≥163ng/mL) | 2.065 (0.822-5.191) | 0.1230 | ||
| DCP level (≥2294 mAU/ml) | 2.010 (0.818-4.938) | 0.1277 | ||
| AFP-L3 level (≥43.4%) | 2.206 (0.894-5.445) | 0.0860 | ||
| NLR (<2.78) | 0.2680 (0.100-0.717) | 0.0087 | 0.234 (0.085-0.647) | 0.0051 |
| Adverse event | Any grade n (%) |
Grade 1/2 n (%) |
Grade 3/4 n (%) |
|---|---|---|---|
| Anorexia | 7 (21.9) | 6 (18.8) | 1 (3.1) |
| General fatigue | 7 (21.9) | 7 (21.9) | 0 |
| Pruritus | 7 (21.9) | 7 (21.9) | 0 |
| Fever | 7 (21.9) | 7 (21.9) | 0 |
| Diarrhea | 6 (18.8) | 2 (6.3) | 4 (12.5) |
| Skin rash | 3 (9.4) | 3 (9.4) | 0 |
| Deterioration of liver function | 2 (6.3) | 0 | 2 (6.3) |
| Reduced adrenal function | 1 (3.1) | 1 (3.1) | 0 |
| Elevated aspartate aminotransferase | 2 (6.3) | 1 (3.1) | 1 (3.1) |
| Hypothyroidism | 1 (3.1) | 1 (3.1) | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





