1. Introduction
Rasmussen’s encephalitis (RE) is a rare chronic inflammatory encephalopathy. The clinical profile encompasses severe refractory epilepsy, hemiplegia, impairments in motor skills and speech, dementia, and encephalitis condition marked by brain inflammation leading to progressive atrophy of one cerebral hemisphere [
1]. The annual incidence per 10 million people was described to be 2.4 in Germany and 1.7 in the UK. Currently, the 2005 European consensus reported by Bien is still the accepted guideline for pathogenesis, diagnosis, and treatment of RE [
2].
The primary cause of RE remains unknown, histopathological hallmarks include cortical inflammation, neuronal loss, and gliosis localized to one cerebral hemisphere, whereas the involvement of T lymphocytes was also described [
3,
4]. At this moment, surgery is the only potential cure. Early diagnosis of RE is imperative to initiate interventions aimed at arresting disease progression and ameliorating patient outcomes [
1,
5]. Therefore, a deeper understanding of the molecular mechanisms of the disease is needed to develop non-invasive treatments and novel biomarkers.
Here, we report a RE patient that underwent hemispherectomy and remains seizure-free after more than 6 months after seizure, with progressive motor improvement. Further, we performed molecular analysis of resected brain tissue and found a downregulation of cell cycle-related genes, possibly due to an increase in BDNF protein levels.
2. Case Report
A 13-year-old boy who initially presented a bilateral tonic-clonic seizure. A few weeks prior he had a self-limited viral illness, and his medical history and development had been otherwise uneventful. The onset of symptoms began approximately 2 years earlier with spasms in the limbs on the left, eventually associated with generalized tonic-clonic seizures. In addition, there was cephalic and ocular rotation to the right and hypertonia of the limbs on the left lasting an average of 5 minutes. He was started on valproate but a few months later developed difficult-to-control seizures with eye and head version to the left and left hypertonia. Later on, he presented focal myoclonic jerks in his left arm several times a day, at times progressing to his left leg and face.
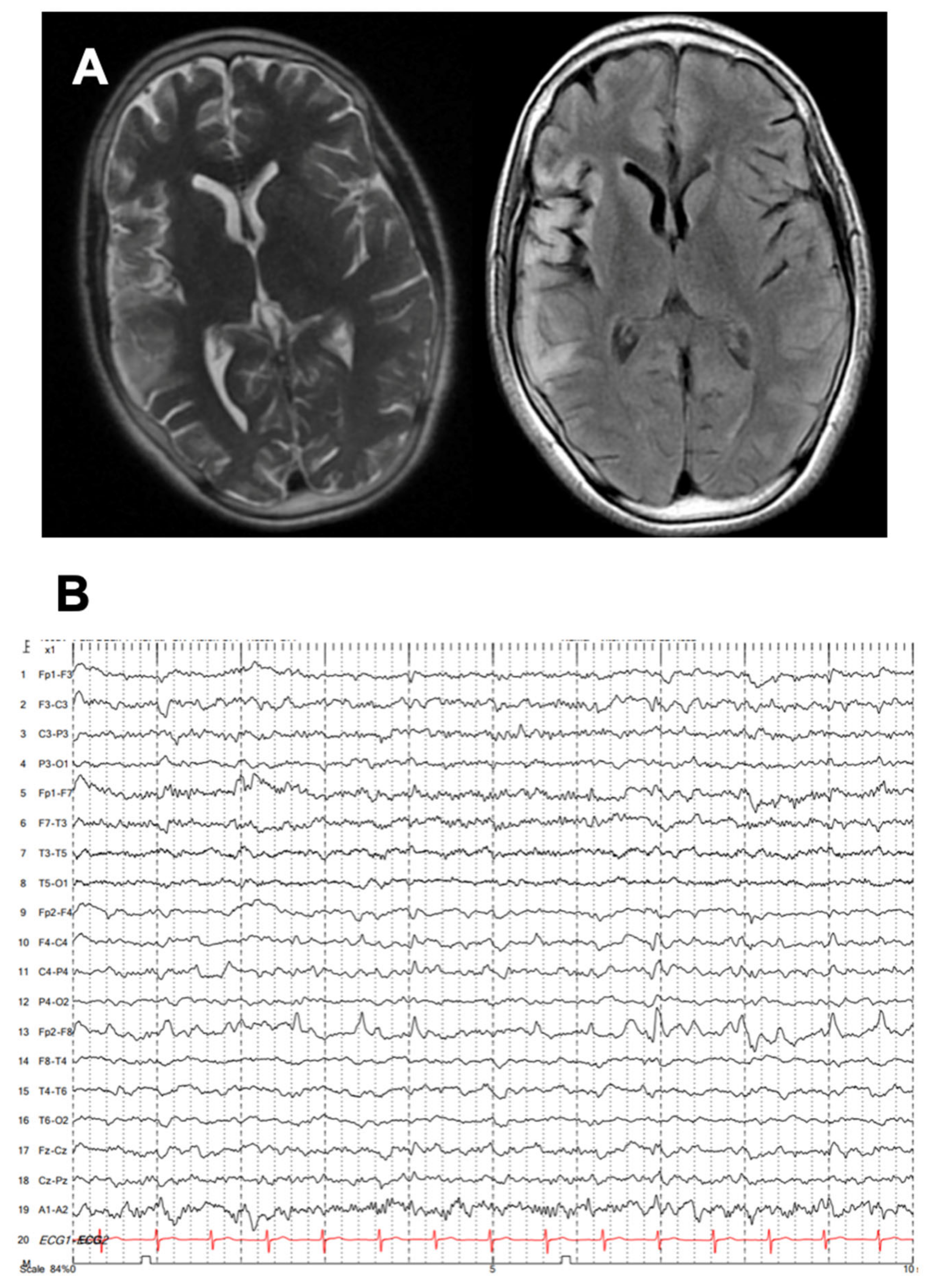
Carbamazepine and lacosamide were added on with no improvement, and he developed progressive weakness in his left arm and leg. There was no family history of epilepsy. He was then referred to our tertiary epilepsy center, at which time neurological examination showed spastic left hemiparesis and moderate to severe dysarthria, with continuous left hemiclonic motor seizures (epilepsia partialis continua [EPC]). By arrival, his MRI showed progressive right hemisphere and caudate atrophy, as well as hyperintensities in the right temporal and frontal lobes, extending throughout the right insula (
Figure 1A). EEG also showed progressive slowing in the right hemisphere with periodic sharp waves over the right frontotemporal and parietal regions as well as frequent electrographic seizures (
Figure 1B).
Despite all measures and antiseizure drugs, he was refractory to treatment and had been admitted to the pediatric ICU for uncontrolled seizures and status epilepticus. This constellation of symptoms led us to diagnose probable Rasmussen’s encephalitis (RE) and he underwent a right functional hemispherectomy. On the first post-operative day, he had no more seizures and was awake and responsive, however developed a transitory right third nerve palsy. He remains seizure-free for the last six months post-surgery (Engel IA) and has shown progressive motor improvement of the left leg and arm. The set of findings allows us to consider a diagnosis of Rasmussen’s encephalitis. Cortical tissue sample obtained after functional hemispherectomy, and histopathology confirmed RE. Excised tissue was subjected to molecular analysis to evaluate the expression of cell cycle-related genes, and protein levels of inflammatory cytokines as well as neurotrophic factors. Methodology for molecular analyses are available online as supportive information (Appendix S1).
3. Results
3.1. Cortex Sample Characterization
The histological sections were bright grayish white with heterogeneous areas in the transition between white and gray matter of two turns. The brain parenchyma showed areas of reactive gliosis, zones of edema, neuronal loss in the subpial region and foci of lymphocytic infiltrate without atypia. The set of histopathological findings associated with clinical data is compatible with RE.
3.2. PCR Array for Cell-Cycle Related Genes
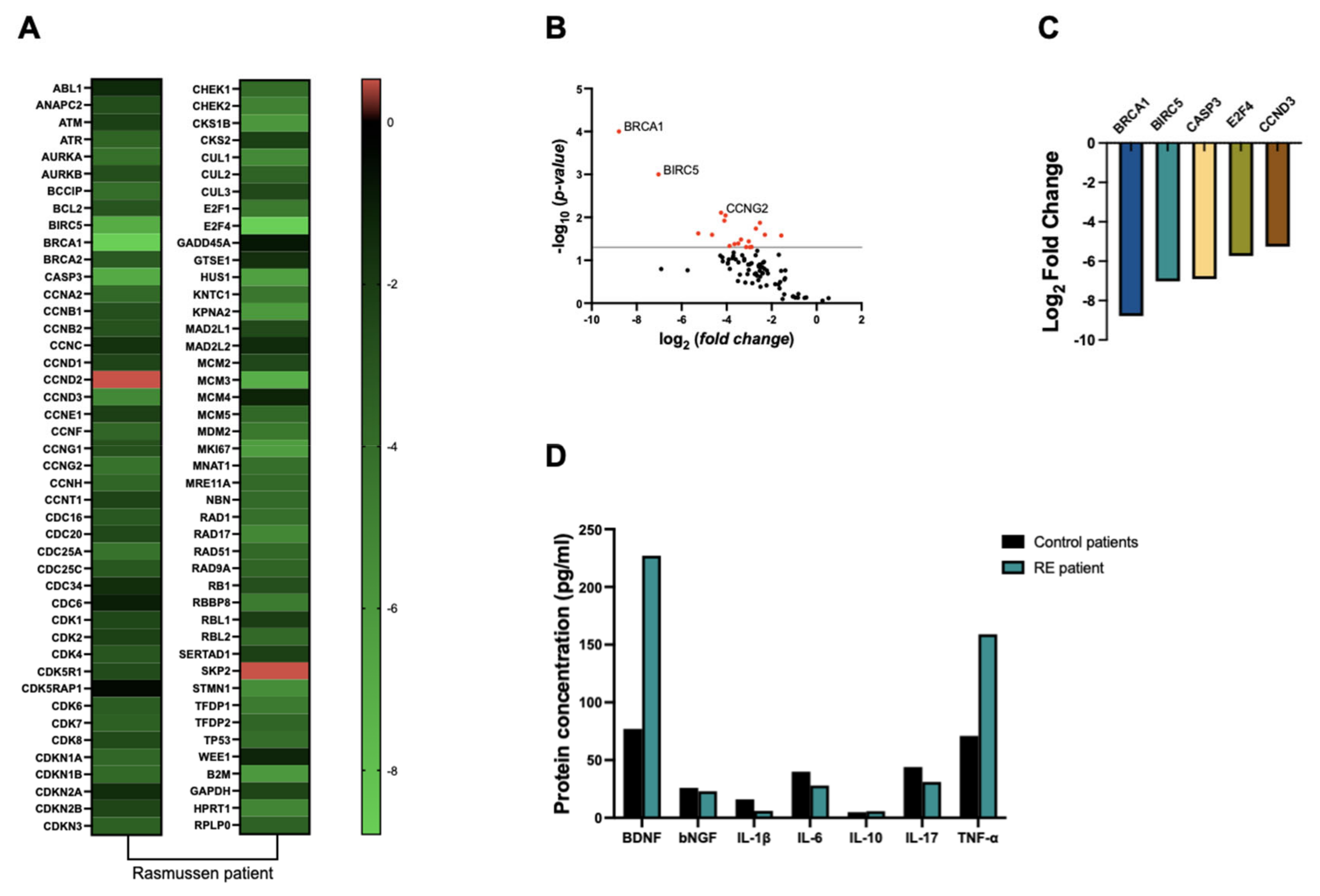
In order to analyze the expression of genes associated with cell cycle regulation, we performed a RT-qPCR Array using the RT 2 Profiler™ PCR Array Human Cell Cycle to evaluate expression of up to 88 genes. Our findings revealed an overall downregulation of cell cycle-related genes, particularly BRCA1 (8-fold), BIRC5 (7-fold), CASP3 (6.9-fold), E2F4 (5.7-fold), and CCDN (5.2-fold), as depicted in
Figure 2A-C, when compared to control samples.
3.3. Cytokines and Neurotrophic Factors Protein Levels
As inflammation is considered a hallmark of RE, we decided to analyze the concentration of inflammatory cytokines in the resected brain tissue. Our analysis revealed a twofold increase in TNF-α levels in RE tissue compared to control samples. However, no significant differences were observed in the concentrations of other cytokines, including IL-1β, IL-6, IL-10, and IL-17, between RE and control groups (
Figure 2D). Our findings revealed a substantial 2.9-fold increase in BDNF protein levels in RE tissue compared to control samples. Conversely, NGF levels remained relatively consistent between RE and control groups (
Figure 2D).
4. Discussion
RE is a rare epileptic disorder, typically emerging during childhood, marked by a gradual unilateral hemispheric degeneration of the brain [
2]. While the exact etiology of RE remains elusive, it is increasingly recognized as an autoimmune-mediated disorder, leading to investigations into treatments targeting the immune response [
6]. In contrast to many cell types, neurons are thought to lose their ability to divide once they have matured, remaining predominantly quiescent within the adult nervous system. Yet, reactivation of the cell cycle in adult neurons is an initial indicator of neurodegeneration and CNS injury [
7]. Therefore, we decided to evaluate expression of cell cycle-related genes in the brain tissue of a RE patient.
In this work, we found a general decreased expression of cell cycle-related genes in brain tissue from the RE patient, where the genes BRCA1, BIRC5, CASP3, E2F4 and CCDN were more than 5-fold downregulated.
The BRCA1 gene plays a vital role in DNA repair and cellular responses to DNA damage, with associations to senescence and various neurological disorders [
8,
9]. Negative regulation of BRCA1 expression in the brain, as seen in the RE patient, may impact neural tissue homeostasis, altering responses to medication and tissue excitability thresholds. Knockdown mouse models of BRCA1 show reduced cell size and dendritic branching, alongside impaired long-term potentiation, indicating BRCA role in synaptic plasticity crucial for learning and memory [
10].
Several studies suggest that neuronal death in conditions such as ischemia, seizures, and brain diseases involve programmed cell death, including apoptosis [
11]. Cyclin Ds, crucial for mitotic control, serve as markers to assess neuronal progression through the cell cycle under pathology [
12]. Brain tissue from the RE patient showed significantly reduced expression of CCND3 and E2F4 genes compared to controls. The negative expression of CCND3 in cortical tissue of the RE which may be an important factor in the instability of the damaged tissue and the progression of seizures and refractoriness because the expression of cell cycle regulators in healthy differentiated neurons is not related to neuronal proliferation but rather to a role possibly linked to neuronal plasticity and stability.
E2F4, a transcriptional repressor, plays a vital role in cell cycle arrest and is crucial for the proliferation and survival of mouse embryonic stem cells, decreasing histone acetylation at cell cycle gene promoters [
13]. In RE, brain tissue may undergo aberrant reorganization after seizures due to molecular deficiencies in DNA repair and cell cycle control, potentially leading to improper cell fate determination.
Another gene downregulated in RE tissue is BIRC5, which belongs to the inhibitor of apoptosis (IAP) gene family, which encodes proteins that prevent cell death by apoptosis. Evidence suggests that the IAP family is associated with regulating the progression of the intrinsic pathway during seizure-induced neuronal death [
14]. Therefore, the reduction in BIRC5 expression may be a consequence of the patient's successive seizures due to refractoriness. Marinowic et al. (2020) demonstrated through an induced pluripotent stem cell (iPSC) model from patients with Focal Cortical Dysplasia (FCD) Type 2b that the expression of CIAP1 was 20-fold decreased when compared to control brain tissue [
15].
Caspase-3 is a protein involved in apoptosis, found to be significantly elevated in the temporal cortex of epilepsy patients compared to controls. While a caspase-3 inhibitor didn't reverse neurodegeneration, it adversely affected axonal and dendritic integrity [
16]. Cortical tissue from RE patient presented a negative fold change in caspase-3 expression, where a possible decrease in apoptosis mediated by this negative regulation may not have an anti-epileptogenic effect but instead may impair the structure and integrity of neurons following a seizure.
BDNF is a protein that belongs to the neurotrophin family, which plays a crucial role in the survival, growth, and maintenance of neurons in the brain [
17]. Here we report that BDNF protein levels are increased in RE brain tissue. In the context of RE, an initial insult could lead to an increase in BDNF as a neuroprotective response. When excessive, BDNF inhibits cell cycle regulators, including apoptosis-related genes, thus promoting survival of damaged neurons [
18]. However, these damaged neurons then stimulate the production of more BDNF, establishing a feedback loop. This loop amplifies neurodegeneration, ultimately worsening the condition.
Surgery remains the sole solution for addressing the seizures induced by RE, with postoperative seizure-free rates ranging from 70% to 80%. After a failure to control the patient's symptoms using medication, we decided to proceed to a functional hemispherectomy. Since surgery was performed, the patient has been free from seizures for the past six months according to Engel IA classification. Additionally, he has displayed gradual motor enhancement in the left leg and arm.
Author Contributions
Conceptualization, J.G.; diagnostics and surgery, V.C., W.M., A.P. and E.N.; tissue processing, F.A.C.X.; experiment design and molecular analyses J.G.; investigation, J.G., D.M. and F.A.C.X.; resources, J.d.C.; writing—original draft preparation, J.G.; writing—review and editing, D.M. and J.G.; supervision, D.M., and A.P.; funding acquisition, general review and final revision of the manuscript, J.d.C. All authors have read and agreed to the published version of the manuscript.
Funding
No funding was received to conduct this research.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the research ethics committee of the Pontifical Catholic University of Rio Grande do Sul (CAAE: 19776619.9.0000.5336 under approval number: 3.577.035).
Informed Consent Statement
All participants signed a free and informed consent form in accordance with Resolution No. 466/12 of the National Health Council of Brazil.
Data Availability Statement
All data from this article are available from the corresponding author under reasonable request.
Acknowledgments
This research was funded by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superi-or-Brazil (finance code 001) and Conselho Nacional de Desenvolvimento Científico e Tecnológico.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Varadkar, Sophia et al. “Rasmussen's encephalitis: clinical features, pathobiology, and treatment advances.” The Lancet. Neurology vol. 13,2 (2014): 195-205. [CrossRef]
- Bien, C G et al. “Pathogenesis, diagnosis and treatment of Rasmussen encephalitis: a European consensus statement.” Brain : a journal of neurology vol. 128,Pt 3 (2005): 454-71. [CrossRef]
- Pardo, Carlos A et al. “The pathology of Rasmussen syndrome: stages of cortical involvement and neuropathological studies in 45 hemispherectomies.” Epilepsia vol. 45,5 (2004): 516-26. [CrossRef]
- Schwab, Nicholas et al. “CD8+ T-cell clones dominate brain infiltrates in Rasmussen encephalitis and persist in the periphery.” Brain : a journal of neurology vol. 132,Pt 5 (2009): 1236-46. [CrossRef]
- Bien, Christian G, and Johannes Schramm. “Treatment of Rasmussen encephalitis half a century after its initial description: promising prospects and a dilemma.” Epilepsy research vol. 86,2-3 (2009): 101-12. [CrossRef]
- Freeman, John M. “Rasmussen's syndrome: progressive autoimmune multi-focal encephalopathy.” Pediatric neurology vol. 32,5 (2005): 295-9. [CrossRef]
- Frade, José M, and María C Ovejero-Benito. “Neuronal cell cycle: the neuron itself and its circumstances.” Cell cycle (Georgetown, Tex.) vol. 14,5 (2015): 712-20. [CrossRef]
- Leung, Emily, and Lili-Naz Hazrati. “Breast cancer type 1 and neurodegeneration: consequences of deficient DNA repair.” Brain communications vol. 3,2 fcab117. 27 May. 2021. [CrossRef]
- Pao, Gerald M et al. “Role of BRCA1 in brain development.” Proceedings of the National Academy of Sciences of the United States of America vol. 111,13 (2014): E1240-8. [CrossRef]
- Suberbielle, Elsa et al. “DNA repair factor BRCA1 depletion occurs in Alzheimer brains and impairs cognitive function in mice.” Nature communications vol. 6 8897. 30 Nov. 2015. [CrossRef]
- Chi, Hao et al. “Neuronal Cell Death Mechanisms in Major Neurodegenerative Diseases.” International journal of molecular sciences vol. 19,10 3082. 9 Oct. 2018. [CrossRef]
- Timsit, S et al. “Increased cyclin D1 in vulnerable neurons in the hippocampus after ischaemia and epilepsy: a modulator of in vivo programmed cell death?.” The European journal of neuroscience vol. 11,1 (1999): 263-78. [CrossRef]
- Hsu, Jenny et al. “E2F4 regulates transcriptional activation in mouse embryonic stem cells independently of the RB family.” Nature communications vol. 10,1 2939. 3 Jul. 2019. [CrossRef]
- Korhonen, L et al. “Regulation of X-chromosome-linked inhibitor of apoptosis protein in kainic acid-induced neuronal death in the rat hippocampus.” Molecular and cellular neurosciences vol. 17,2 (2001): 364-72. [CrossRef]
- Marinowic, Daniel Rodrigo et al. “Analysis of genes involved in cell proliferation, adhesion, and control of apoptosis during embryonic neurogenesis in Induced Pluripotent Stem Cells (iPSCs) from patients with Focal Cortical Dysplasia.” Brain research bulletin vol. 155 (2020): 112-118. [CrossRef]
- Tzeng, Tsai-Teng et al. “Caspase 3 involves in neuroplasticity, microglial activation and neurogenesis in the mice hippocampus after intracerebral injection of kainic acid.” Journal of biomedical science vol. 20,1 90. 6 Dec. 2013. [CrossRef]
- Wang, Camille S et al. “BDNF signaling in context: From synaptic regulation to psychiatric disorders.” Cell vol. 185,1 (2022): 62-76. [CrossRef]
- Boutahar, Nadia et al. “Brain-derived neurotrophic factor inhibits cell cycle reentry but not endoplasmic reticulum stress in cultured neurons following oxidative or excitotoxic stress.” Journal of neuroscience research vol. 88,10 (2010): 2263-71. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).