Submitted:
27 June 2024
Posted:
28 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
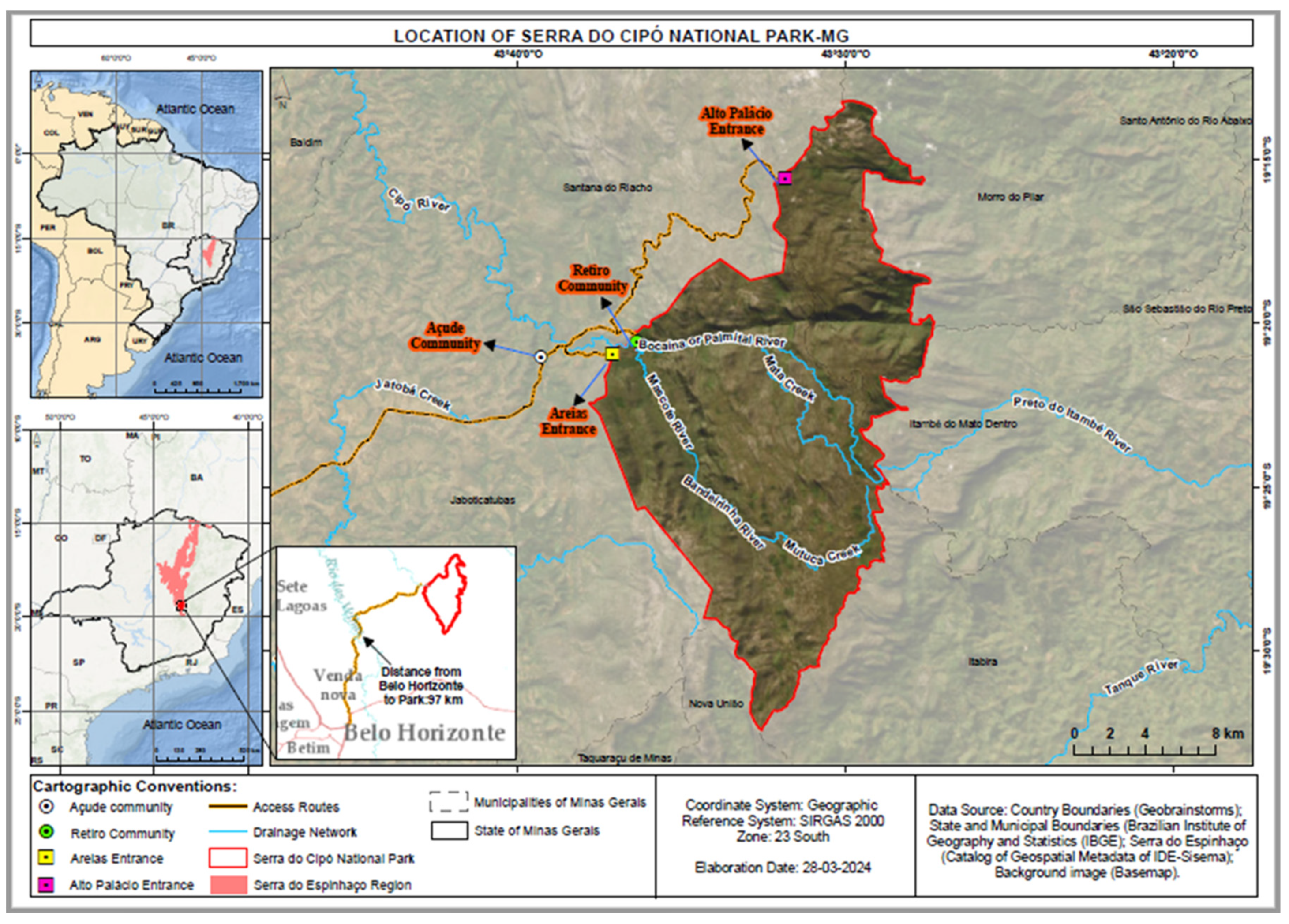
2.1. Fields of Study
2.2. Obtaining and Processing Samples
2.3. Statistical Analysis
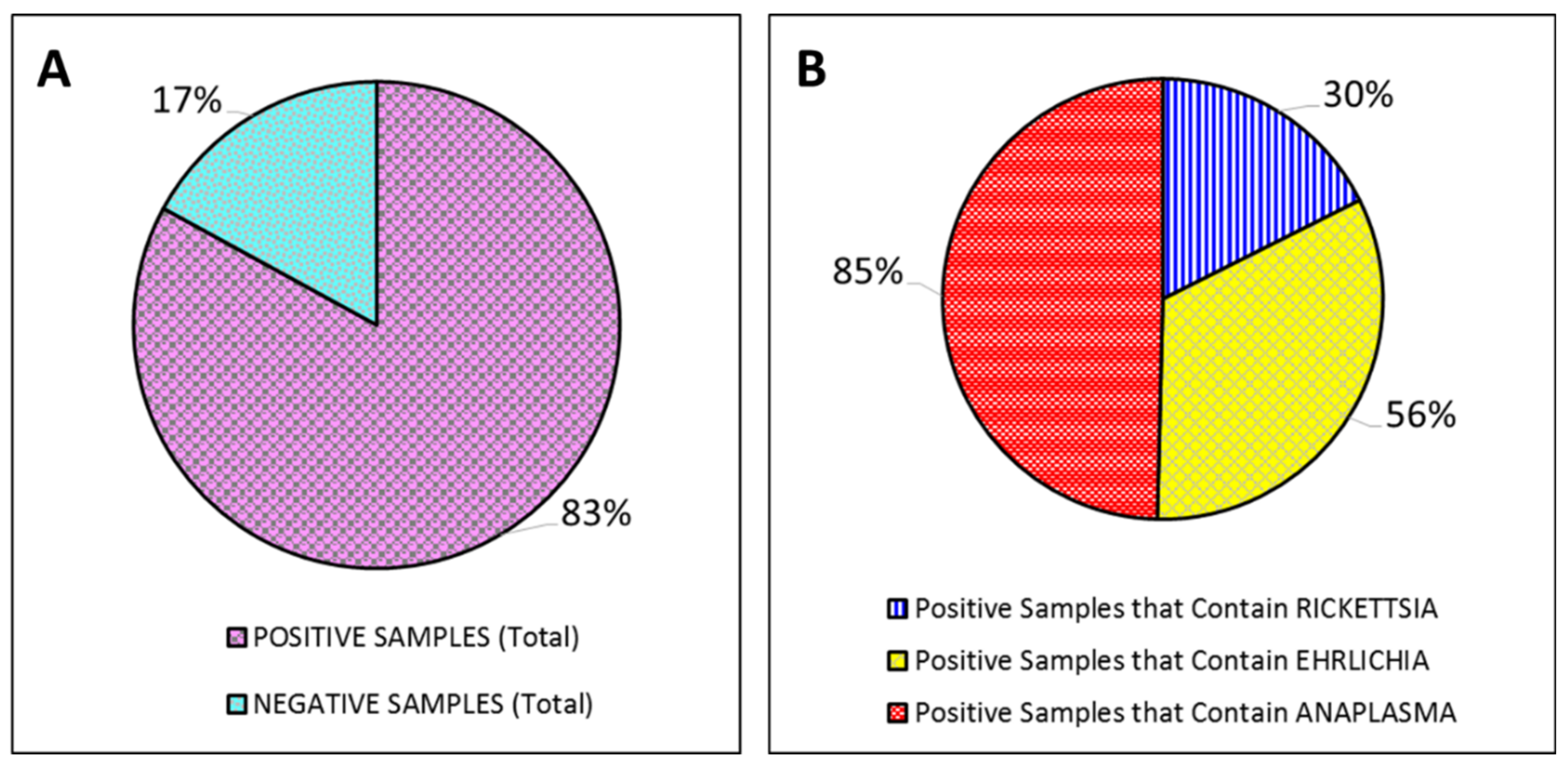
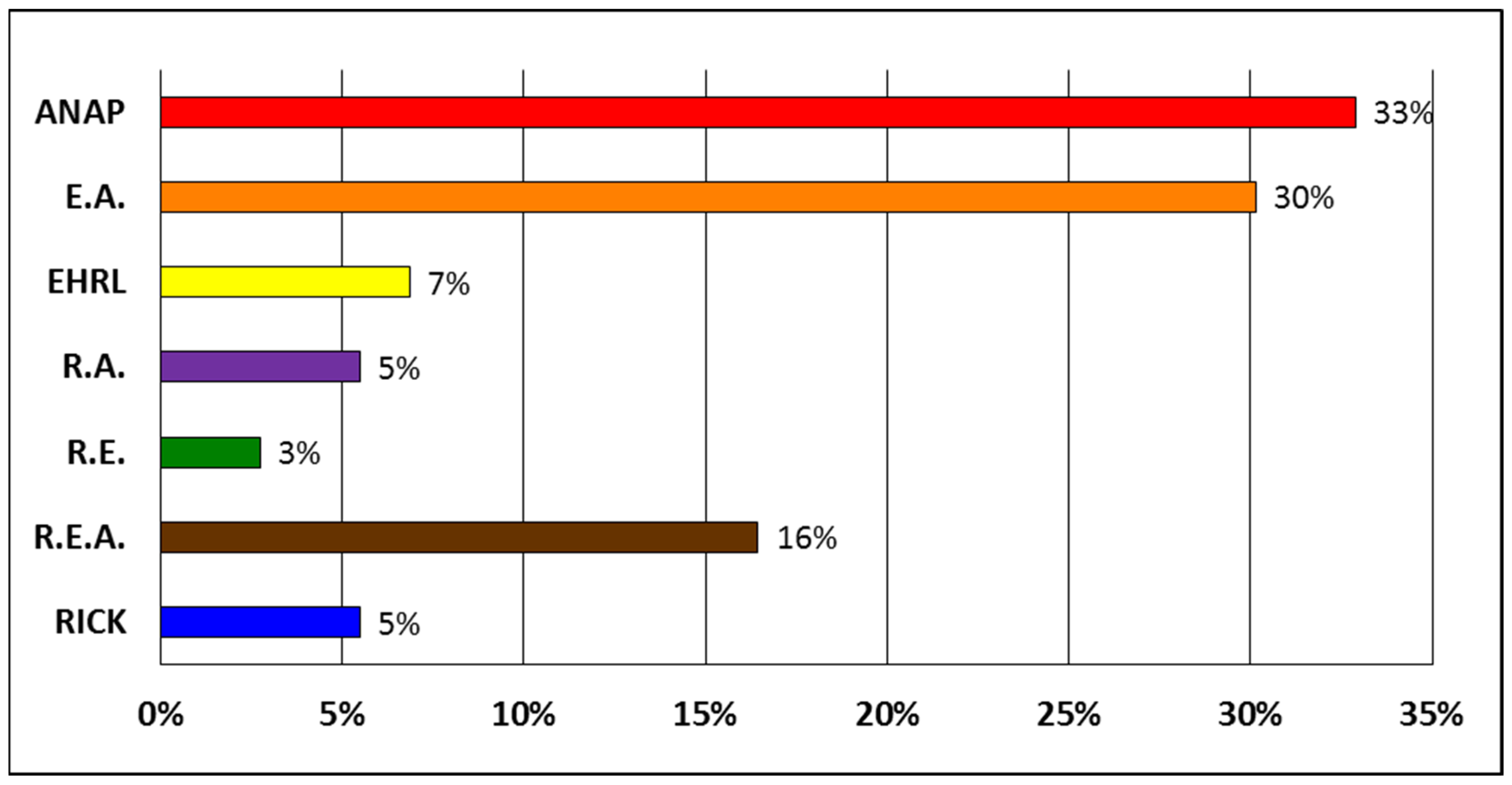
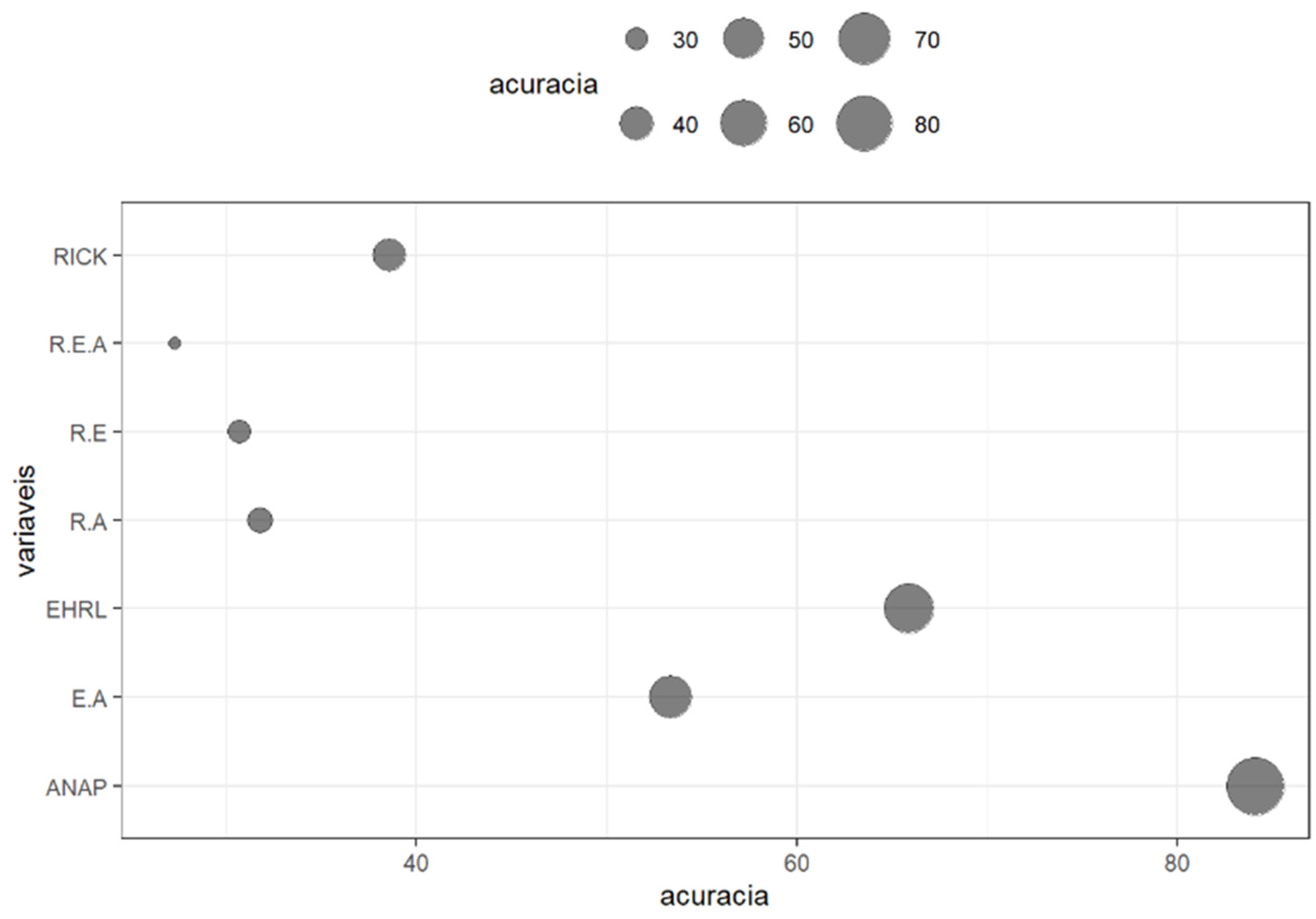
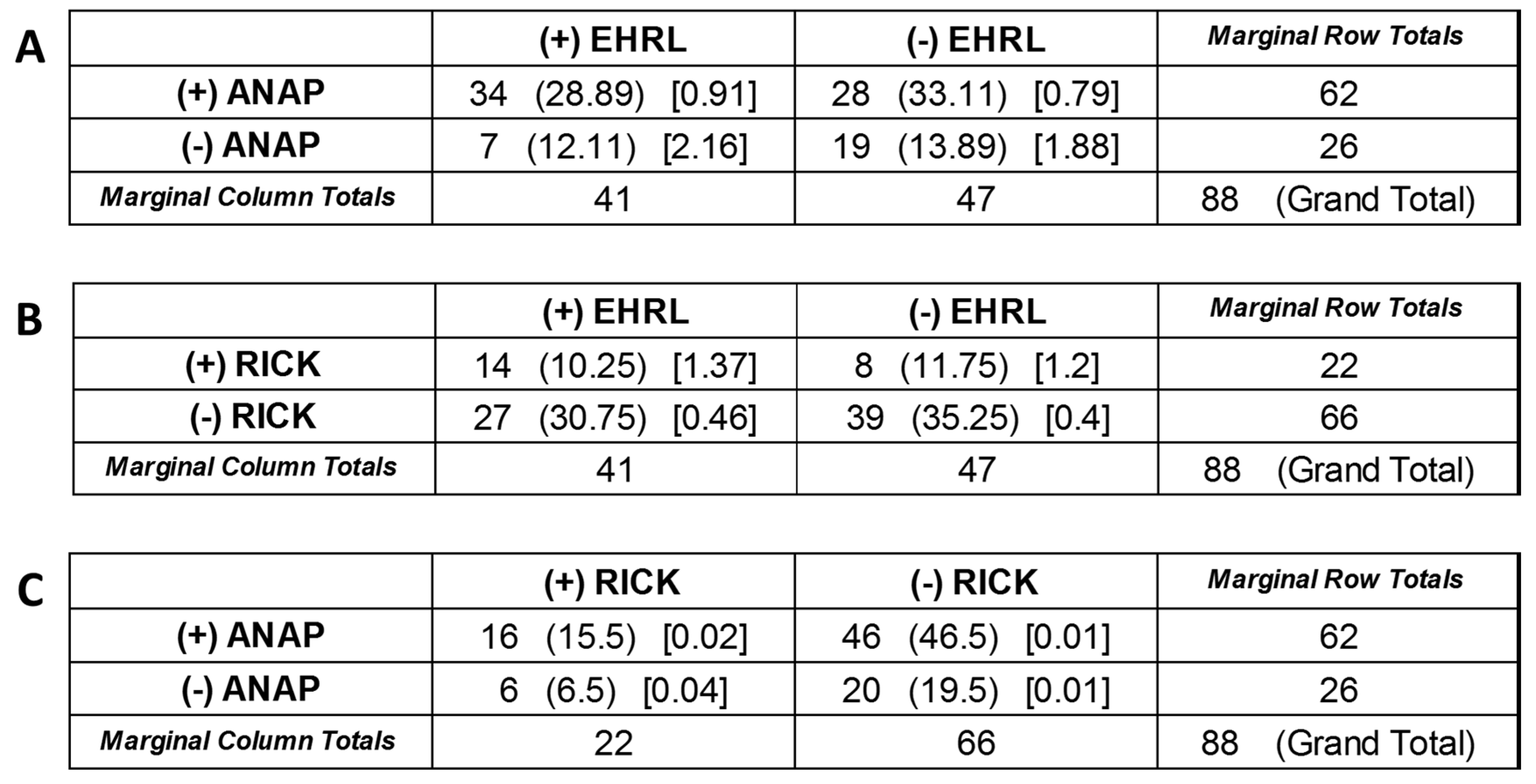
3. Results
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Jones, B.A.; Grace, D.; Kock, R.; Alonso, S.; Rushton, J.; Said, M.Y.; McKeever, D.; Mutua, F.; Young, J.; McDermott, J.; Pfeiffer, D.U. Zoonosis emergence linked to agricultural intensification and environmental change. Proc Natl Acad Sci U S A 2012, 110, 8399–8404. [Google Scholar] [CrossRef]
- Karesh, W.B.; Dobson, A.; Lloyd-Smith, J.O.; Lubroth, J.; Dixon, M.A.; Bennett, M.; Aldrich, S.; Harrington, T.; Formenty, P.; Loh, E.H.; Machalaba, C.C.; Thomas, M.J.; Heymann, D.L. Ecology of zoonoses: natural and unnatural histories. Lancet 2012, 380, 1936–1945. [Google Scholar] [CrossRef] [PubMed]
- Barcellos, C.D.C.; Monteiro, A.M.V.; Corvalán, C.; Gurgel, H.C.; Carvalho, M.S.; Artaxo, P.; Hacon, S.; Ragoni, V. Mudanças climáticas e ambientais e as doenças infecciosas: cenários e incertezas para o Brasil. Epidemiol. Serv. Saúde, 2009; 18, 285–304. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change. Climate change 2014: mitigation of climate change. Available online: https://archive.ipcc.ch/report/ar5/syr/ (accessed on 21 March 2024).
- World Health Organization. World Malaria Report 2019. Available online: https://www.who.int/publications/i/item/9789241565721 (accessed on 21 March 2024).
- Domingos, A.; Antunes, S.; Borges, L.; Rosario, V.E.D. Approaches towards tick and tick-borne diseases control. Rev. Soc. Bras. Med. 2013, 46, 265–269. [Google Scholar] [CrossRef]
- Jongejan, F.; Uilenberg, G. The global importance of ticks. Parasitology 2004, 129. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, J.; Antunes, S.; Bonnet, S.; Cabezas-Cruz, A.; Domingos, A. G.; Estrada-Peña, A.; Johnson, N.; Kocan, K.M.; Mansfield, K.L.; Nijhof, A.M.; Papa, A.; Rudenko, N.; Villar, M.; Alberdi, P.; Torina, A.; Ayllón, N.; Vancova, M.; Golovchenko, M.; Grubhoffer, L.; Caracappa, S.; Fooks, A.R.; Gortazar, C.; Rego, R.O. Tick-pathogen interactions and vector competence: identification of molecular drivers for tick-borne diseases. Front. cell. infect. microbiol. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.F.; Magnarelli, L.A. Biology of ticks. Infect Dis Clin North Am 2008, 22, 195–215. [Google Scholar] [CrossRef]
- Parola, P.; Paddock, C.D.; Socolovschi, C.; Labruna, M.B.; Mediannikov, O.; Kernif, T.; Abdad, M.Y.; Stenos, J.; Bitam, I.; Fournier, P.-E.; Raoult, D. Update on tick-borne rickettsioses around the world: a geographic approach. Clin Microbiol Rev 2013, 26, 657–702. [Google Scholar] [CrossRef]
- Polo, G.; Labruna, M.B.; Ferreira, F. Satellite hyperspectral imagery to support tick-borne infectious diseases surveillance. PLoS One 2015, 10. [Google Scholar] [CrossRef]
- Oliveira, S.V.D.; Guimarães, J.N.; Reckziegel, G.C.; Neves, B.M.D.C.; Araújo-Vilges, K.M.D.; Fonseca, L.X.; Pinna, F.V.; Pereira, S.V.C.; Caldas, E.P.; Gazeta, G.S.; Gurgel-Gonçalves, R. An update on the epidemiological situation of spotted fever in Brazil. J. Venom. Anim. Toxins Incl. Trop. Dis 2016, 22. [Google Scholar] [CrossRef]
- Satjanadumrong, J.; Robinson, M.T.; Hughes, T.; Blacksell, S.D. Distribution and ecological drivers of spotted fever group Rickettsia in Asia. Ecohealth 2019, 16, 611–626. [Google Scholar] [CrossRef]
- Piotrowski, M.; Rymaszewska, A. Expansion of tick-borne rickettsioses in the world. Microorganisms 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Springer, A.; Glass, A.; Probst, J.; Strube, C. Tick-borne zoonoses and commonly used diagnostic methods in human and veterinary medicine. Parasitol. Res. 2021, 120, 4075–4090. [Google Scholar] [CrossRef]
- Ministerio da Saúde do Brasil. Guia de vigilância em saúde, 5th ed. 2022. Available online: https://bvsms.saude.gov.br/bvs/publicacoes/guia_vigilancia_saude_5ed_rev_atual.pdf (accessed on 21 March 2024).
- Durães, L.S.; Bitencourth, K.; Ramalho, F.R.; Nogueira, M.C.; Nunes, E.D.C.; Gazêta, G.S. Biodiversity of potential vectors of rickettsiae and epidemiological mosaic of spotted fever in the State of Paraná, Brazil. Front. Public Health 2021, 9. [Google Scholar] [CrossRef]
- Vieira, R.F.D.C.; Biondo, A.W.; Guimarães, A.M.S.; Santos, A.P.D.; Santos, R.P.D.; Dutra, L.H.; Diniz, P.P.V.P.; Morais, H.A.; Messick, J.B.; Labruna, M.B.; Vidotto, O. Ehrlichiosis in Brazil. Rev. Bras. Parasitol. Vet. 2011, 20, 01–12. [Google Scholar] [CrossRef] [PubMed]
- Vieira, R.F.D.C.; Vieira, T.S.W.J.; Nascimento, D.D.A.G.; Martins, T.F.; Krawczak, F.S.; Labruna, M.B.; Chandrashekar, R.; Marcondes, M.; Biondo, A.W.; Vidotto, O. Serological survey of Ehrlichia species in dogs, horses and humans: zoonotic scenery in a rural settlement from southern Brazil. Rev Inst Med Trop Sao Paulo 2013, 55, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Guedes, P.E.B.; Oliveira, T.N.D.A.; Carvalho, F.S.; Carlos, R.S.A.; Albuquerque, G.R.; Munhoz, A.D.; Wenceslau, A.A.; Silva, F.L. (2015). Canine ehrlichiosis: prevalence and epidemiology in northeast Brazil. Rev. Bras. Parasitol. Vet. 2015, 24, 115–121. [Google Scholar] [CrossRef]
- Aziz, M.U.; Hussain, S.; Song, B.; Ghauri, H.N.; Zeb, J.; Sparagano, O.A. Ehrlichiosis in Dogs: A Comprehensive Review about the Pathogen and Its Vectors with Emphasis on South and East Asian Countries. Vet. Sci. 2023, 10. [Google Scholar] [CrossRef]
- Perez, M.; Bodor, M.; Zhang, C.; Xiong, Q.; Rikihisa, Y. Human Infection with Ehrlichia canis accompanied by Clinical Signs in Venezuela. Ann. N. Y. Acad. Sci. 2006, 1078, 110–117. [Google Scholar] [CrossRef]
- Silva, A.B.; Canseco, S.P.; De la Torre, M.D.P.G.; Silva, A.M.; Mayoral, M.Á.; Mayoral, L.P.C.; Martínez, J.L.; Pérez-Campos, E. Asymptomatic human infection from contact with dogs: a case of human ehrlichiosis. Gaceta Médica de México 2014, 150, 171–174. [Google Scholar]
- Machado, R.Z.; Duarte, J.M.B.; Dagnone, A.S.; Szabó, M.P.J. Detection of Ehrlichia chaffeensis in Brazilian marsh deer (Blastocerus dichotomus). Vet. Parasitol. 2006, 139, 262–266. [Google Scholar] [CrossRef]
- Carrade, D.D.; Foley, J.E.; Borjesson, D.L.; Sykes, J.E. Canine granulocytic anaplasmosis: a review. J. Vet. Intern. Med. 2009, 23, 1129–1141. [Google Scholar] [CrossRef] [PubMed]
- Santos, H.A.; Thomé, S.M.; Baldani, C.D.; Silva, C.B.; Peixoto, M.P.; Pires, M.S.; Vitari, G.L.V.; Costa, R.L.; Santos, T.M.; Angelo, I.C.; Santos, L.A.; Faccini, J.L.H.; Massard, C.L. Molecular epidemiology of the emerging zoonosis agent Anaplasma phagocytophilum (Foggie, 1949) in dogs and ixodid ticks in Brazil. Parasit. Vectors. 2013, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, T.M.; Roier, E.C.R.; Pires, M.S.; Santos, H.A.; Vilela, J.A.R.; Peckle, M.; Paulino, P.G.; Baldani, C.D.; Massard, C.L. Molecular evidence of Anaplasma phagocytophilum and Theileria equi coinfection in horses from Rio de Janeiro, Brazil. VAS 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Dumler, J.S.; Choi, K.S.; Garcia-Garcia, J.C.; Barat, N.S.; Scorpio, D.G.; Garyu, J.W.; Grab, D.J.; Bakken, J.S. Human granulocytic anaplasmosis and Anaplasma phagocytophilum. Emerg. Infect. Dis. 2005, 11, 1828–1834. [Google Scholar] [CrossRef] [PubMed]
- Atif, F.A.; Mehnaz, S.; Qamar, M.F.; Roheen, T.; Sajid, M.S.; Ehtisham-ul-Haque, S.; Kashif, M.; Said, M.B. Epidemiology, Diagnosis, and Control of Canine Infectious Cyclic Thrombocytopenia and Granulocytic Anaplasmosis: Emerging Diseases of Veterinary and Public Health Significance. Vet. Sci. 2021, 8, 2–20. [Google Scholar] [CrossRef] [PubMed]
- Silveira, J.A.G.; Reis, I.A.; Estevam, L.G.T.M.; Pinto, M.C.C.; Zweygarth, E.; Passos, L.M.F.; Paz, G.F. 2017. Important frequency of Anaplasma phagocytophilum infection in a population of domiciled dogs in an urbanized area in south-eastern Brazil. Pesq. Vet. 2017, 37, 958–962. [Google Scholar] [CrossRef]
- Dantas-Torres, F.; Figueredo, L.A.; Sales, K.G.D.S.; Miranda, D.E.D.O.; Alexandre, J.L.D.A.; da Silva, Y.Y.; da Silva, L.G.; Valle, G.R.; Ribeiro, V.M.; Otranto, D.; Deuster, K.; Pollmeier, M.; Altreuther, G. Prevalence and incidence of vector-borne pathogens in unprotected dogs in two Brazilian regions. Parasit Vectors 2020, 13, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sousa, V.R.F.; Avaliação clínica, morfológica, hematológica, bioquímica e biomolecular de cães naturalmente infectados por Ehrlichia canis e Anaplasma platys. Universidade Federal Rural do Rio de Janeiro 2006. Available online: https://rima.ufrrj.br/jspui/handle/20.500.14407/9664 (accessed on 21 March 2024).
- Šlapeta, J.; Halliday, B.; Chandra, S.; Alanazi, A. D.; Abdel-Shafy, S. Rhipicephalus linnaei (Audouin, 1826) recognised as the “tropical lineage” of the brown dog tick Rhipicephalus sanguineus sensu lato: Neotype designation, redescription, and establishment of morphological and molecular reference. Ticks Tick Borne Dis 2022, 13. [Google Scholar] [CrossRef]
- Peixoto, C.S.; Alterações oculares e hematológicas em cães acometidos por Ehrlichia canis e co-infecções. Universidade de Brasília 2019. Available online: http://www.realp.unb.br/jspui/handle/10482/37471 (accessed on 21 March 2024).
- Schmidt, P.L. Companion animals as sentinels for public health. Vet Clin North Am Small Anim Pract 2009, 39, 241–250. [Google Scholar] [CrossRef]
- Silva, M.E.; Ribeiro, R.R.; Costa, J.O.; Moraes-Filho, J.; Pacheco, R.C.; Labruna, M.B. Prevalência de anticorpos anti-Rickettsia spp. em cães da cidade de Belo Horizonte, MG. Arq. Bras. Med. Vet. Zootec. 2010, 62, 1007–1010. [Google Scholar] [CrossRef]
- Clow, K.M.; Leighton, P.A.; Pearl, D.L.; Jardine, C.M. A framework for adaptive surveillance of emerging tick-borne zoonoses. One Health 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Durães, L.S.; Bitencourth, K.; de Oliveira, S.V.; Gazêta, G.S. Fiebre maculosa en Brasil: contexto histórico y actual. In Enfermedades rickettsiales en Latinoamérica, 1st ed.; Arias, S.P., Jaramillo, A.C., Buriticá, S.M., Eds.; Fondo Editorial Biogénesis: Medellín, Colombia, 2020; pp. 240–266. ISBN 978-958-5596-67-2. [Google Scholar]
- Campos, S.D. , Cunha, N.C.D., Machado, C.S., de Souza, T.V., Fonseca, A.B.M., Pinter, A.; Fonseca, A.H.; Almosny, N.R.P. Circulação de Rickettsias do Grupo da Febre Maculosa em cães no entorno de Unidades de Conservação Federais do estado do Rio de Janeiro: evidência sorológica e fatores associados. Pesq. Vet. 2017, 37, 1307–1312. [Google Scholar] [CrossRef]
- Springer, A.; Glass, A.; Topp, A.K.; Strube, C. Zoonotic tick-borne pathogens in temperate and cold regions of Europe—A review on the prevalence in domestic animals. Front. vet. sci. 2020, 7. [Google Scholar] [CrossRef] [PubMed]
- Davitt, C.; Traub, R.; Batsukh, B.; Battur, B.; Pfeffer, M.; Wiethoelter, A.K. Knowledge of Mongolian veterinarians towards canine vector-borne diseases. One Health 2022, 15. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, F.S.; Gazeta, G.S.; Souza, E.R.; Ribeiro, A.; Marrelli, M.T.; Schumaker, T.T.S. Rickettsia rickettsii, Rickettsia felis and, Rickettsia sp TwKM03 infecting Rhipicephalus sanguineus and Ctenocephalides felis collected from dogs in a Brazilian Spotted Fever focus in the state of Rio de Janeiro/Brazil. Clin Microbiol Infect 2009, 15, 267–268. [Google Scholar] [CrossRef] [PubMed]
- Moraes-Filho, J.; Pinter, A.; Pacheco, R.C.; Gutmann, T.B.; Barbosa, S.O.; Gonzáles, M.A.R. M.; Muraro, M.A.; Cecílio, S.R.M.; Labruna, M.B. New Epidemiological Data on Brazilian Spotted Fever in an Endemic Area of the State of São Paulo, Brazil. Vector Borne Zoonotic Dis 2009, 9, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Rozental, T.; Ferreira, M.S.; Gomes, R.; Costa, C.M.; Barbosa, P.R.A.; Bezerra, I.O.; Garcia, M.H.O.; Oliveira E Cruz, D.M.; Galliez, R.; Oliveira, S.; Brasil, P.; Rezende, T.; De Lemos, E.R.S. A cluster of Rickettsia rickettsii infection at an animal shelter in an urban area of Brazil. Epidemiol Infect 2015, 143, 2446–2450. [Google Scholar] [CrossRef] [PubMed]
- Lemos, E.R.S.; Machado, R.D.; Coura, J.R.; Guimarães, M.A.A.; Freire, N.M.S. Infestation by ticks and detection of antibodies to spotted fever group Rickettsiae in wild animals captured in the State of São Paulo, Brazil. A preliminary report. Mem Inst Oswaldo Cruz 1996, 91, 701–702. [Google Scholar] [CrossRef] [PubMed]
- Souza, C.E.; Camargo, L.B.; Pinter, A.; Donalisio, M.R. High seroprevalence for Rickettsia rickettsii in equines suggests risk of human infection in silent areas for the Brazilian spotted fever. PLoS ONE 2016, 11, 1–9. [Google Scholar] [CrossRef]
- Mahachi, K.; Kontowicz, E.; Anderson, B.; Toepp, A. J.; Lima, A. L.; Larson, M.; Wilson, G.; Grinnage-Pulley, T.; Bennett, C.; Ozanne, M.; Anderson, M.; Fowler, H.; Parrish, M.; Saucier, J.; Tyrrell, P.H.; Palmer, Z.; Buch, J.; Chandrashekar, R.; Scorza, B.; Brown, G.; Oleson, J.J.; Petersen, C.A. Predominant risk factors for tick-borne co-infections in hunting dogs from the USA. Parasit Vectors 2020, 13, 1–12. [Google Scholar] [CrossRef]
- Instituto Chico Mendes de Conservação da Biodiversidade. Parque Nacional da Serra do Cipó. Available online: https://www.icmbio.gov.br/parnaserradocipo/guia-do-visitante.html. (accessed on 20 May 2024).
- Fuller Laboratories. Anaplasma phagocytophilum (EED-120) Instructions for Use, Original 7/95, Current Version D (1/04). Fullerton, California, United States of America. Available online: https://fullerlaboratories.com/wp-content/uploads/2023/05/EED-120-English.pdf. (accessed on 15 May 2024).
- Pagano, M.; Gauvreau, K. Princípios de Bioestatística, 2nd ed.; Pioneira Thomson Learning: São Paulo, SP, Brazil, 2004. [Google Scholar]
- Ministério da Saúde do Brasil. Casos confirmados de Febre Maculosa. Brasil, Grandes Regiões e Unidades Federadas (Infecção) - 2007 a 2024. Available online: https://www.gov.br/saude/pt-br/assuntos/saude-de-a-a-z/f/febre-maculosa/situacao-epidemiologica/casos-confirmados-de-febre-maculosa-brasil-grandes-regioes-e-unidades-federadas-infeccao-2007-a-2024/view (accessed on 15 May 2024).
- Ministério da Saúde do Brasil. Óbitos confirmados de Febre Maculosa. Brasil, Regiões e Unidades Federadas (Infecção) - 2007 a 2024. Available online: https://www.gov.br/saude/pt-br/assuntos/saude-de-a-a-z/f/febre-maculosa/situacao-epidemiologica/obitos-de-febre-maculosa-brasil-grandes-regioes-e-unidades-federadas-infeccao-2007-a-2024/view (accessed on 21 March 2024).
- De Paiva Diniz, P.P.V.; Schwartz, D.S.; De Morais, H.S.A.; Breitschwerdt, E.B. Surveillance for zoonotic vector-borne infections using sick dogs from southeastern Brazil. Vector Borne Zoonotic Dis 2007, 7, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Breitschwerdt, E.B.; Papich, M.G.; Hegarty, B.C.; Gilger, B.; Hancock, S.I.; Davidson, M.G. Efficacy of Doxycicline, azithromicin, or trovafloxacin for treatment of experimental rocky mountain spotted fever in dogs. Antimicrob Agents Chemother 1999, 43, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Sangioni, L.A.; Horta, M.C.; Vianna, M.C.B.; Gennari, S.M.; Soares, R.M.; Galvão, M.A.M.; Schumaker, T.T.S.; Ferreira, F.; Vidotto, O.; Labruna, M.B. Rickettsial infection in animals and Brazilian spotted fever endemicity. Emerg. Infect. Dis. 2005, 11, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Inokuma, H.; Nane, G.; Uechi, T.; Yonahara, Y.; Brouqui, P. , Okuda; M.; Onishi, T. Survey of tick infestation and tick-borne ehrlichial infection of dogs in Ishigaki Island, Japan. J Vet Med Sci 2001, 63, 1225–1227. [Google Scholar] [CrossRef] [PubMed]
- Dreher, U.M.; De La Fuente, J.; Hofmann-Lehmann, R.; Meli, M. L.; Pusterla, N.; Kocan, K. M.; Woldehiwet, Z.; Braun, U.; Regula, G.; Staerk, K.D.C.; Lutz, H. Serologic cross-reactivity between Anaplasma marginale and Anaplasma phagocytophilum. Clin Vaccine Immunol 2005, 12, 1177–1183. [Google Scholar] [CrossRef] [PubMed]
- Beall, M.J.; Chandrashekar, R.; Eberts, M.D.; Cyr, K.E.; Diniz, P.P.V.P.; Mainville, C. , Hegarty, B.C.; Crawford, J.M.; Breitschwerdt, E.B. Serological and molecular prevalence of Borrelia burgdorferi, Anaplasma phagocytophilum, and Ehrlichia species in dogs from Minnesota. Vector Borne Zoonotic Dis 2008, 8, 455–464. [Google Scholar] [CrossRef]
- Bowman, D.; Little, S.E.; Lorentzen, L.; Shields, J.; Sullivan, M.P.; Carlin, E.P. Prevalence and geographic distribution of Dirofilaria immitis, Borrelia burgdorferi, Ehrlichia canis, and Anaplasma phagocytophilum in dogs in the United States: results of a national clinic-based serologic survey. Vet. Parasitol. 2009, 160, 138–148. [Google Scholar] [CrossRef]
- Cetinkaya, H.; Matur, E.; Akyazi, I.; Ekiz, E.E.; Aydin, L.; Toparlak, M. Serological and molecular investigation of Ehrlichia spp. and Anaplasma spp. in ticks and blood of dogs, in the Thrace Region of Turkey. Ticks Tick Borne Dis 2016, 7, 706–714. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




