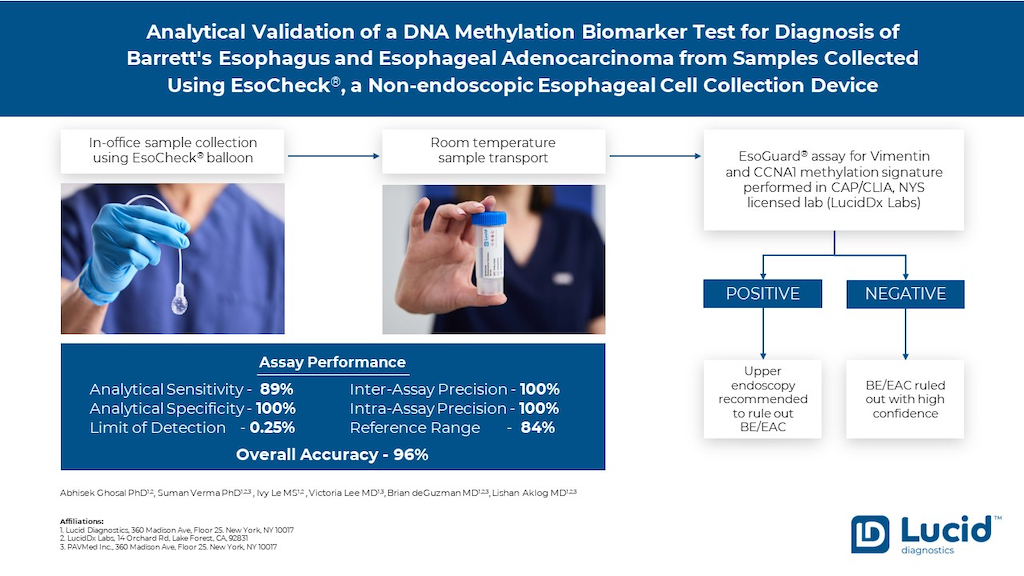
1. Introduction
Esophageal adenocarcinoma (EAC) is the second most lethal cancer in the United States, with over 500% increase in the last four decades and a dismal 5-year survival of only 20% [
1,
2,
3,
4]. Barrett’s esophagus (BE) is the only known, direct precursor to EAC [
5,
6]. Importantly, in contrast to EAC, BE can be successfully treated using endoscopic techniques with 80-90% success rates [
7,
8,
9]. Screening for BE is supported by multiple gastroenterology society guidelines and clinical practice recommendations [
10,
11,
12]. However, compliance with screening has traditionally been poor [
13]. To improve compliance and accessibility of BE screening, the American College of Gastroenterology (ACG), and American Gastroenterology Association (AGA) now recommend non-endoscopic esophageal cell collection in combination with biomarker testing as an acceptable alternative to screening endoscopy [
14,
15].
The specific methylation signature of 31 CpG sites in
VIM and
CCNA1 genes has been shown as a promising biomarker for detection of all stages of BE as well as EAC [
16]. Performance of this combined
VIM and
CCNA1 two-gene methylated biomarker panel, EsoGuard
® (EG), for detection of BE/EAC was first published in 2018 [
16]. The EG panel demonstrated a sensitivity and specificity of 94% and 91%, respectively for detection of BE in cytology brushings (n=313) and 88% and 92% respectively in samples collected with the non-endoscopic EsoCheck
® (EC) balloon device (n=86), where esophagogastroduodenoscopy (EGD) and biopsies were used as the comparator. EAC was detected with 96% sensitivity in brushings and 88% sensitivity in EC balloon samples.
More recently, two independent clinical validation studies (one case-control and one screening study) reported clinical performance of the commercial EG assay (LucidDx Labs, Lake Forest CA)[
17,
18]. In both studies, samples were collected using EC then preserved and transported at ambient temperature. The performance of EG was highly consistent to that shown in the pivotal 2018 study where the assay had been performed in an academic laboratory. [
16] with clinical sensitivity for detecting BE of 81% and 92%, specificity of 72% and 85%, and detection of EAC of 100% in both studies.
EsoGuard is a Next Generation Sequencing (NGS) DNA methylation biomarker assay that investigates methylation signatures in the
VIM and
CCNA1 genes. EG is performed in a CAP accredited, CLIA certified, NY state licensed laboratory (LucidDx Labs, Lake Forest, CA). The assay reports qualitative, binary results based on previously clinically validated cut-off [
18] of >1% methylation for
VIM or >0.5% methylation of
CCNA1 gene. A positive EG result suggests the presence of intestinal metaplasia or disease along the BE progressive spectrum (non-dysplastic BE, dysplastic BE and EAC), and therefore should be further investigated and staged by EGD. EG has a high negative predictive value (NPV, 98.6%), suggesting a negative result [
17] likely does not warrant further diagnostic evaluation. Clinically, the assay has been used as a triage to EGD when evaluating patients who meet guideline criteria for BE screening[
19,
20,
21].
EsoCheck is an FDA 510(k)-cleared, swallowable, non-endoscopic, balloon capsule device that circumferentially collects surface cells from a targeted region of the esophagus. It is performed without sedation or anesthesia and allows for rapid, in-office, point-of-service collection. Cell samples collected with EC can be utilized for cytology or other diagnostic testing, including tests like EG. Cell collection with EC had a success rate of 96.9%-99.6% in three clinical utility studies analyzing real-world data [
19,
20,
21]. The EG assay provided qualitative binary results in 94%-97% of the EC-collected samples, demonstrating that EC as an office-based esophageal cell collection device is highly effective in providing sufficient quality and quantity of DNA for EG analysis.
In this report we now present analytical validation studies for the EG assay, including analytical accuracy, analytical sensitivity, analytical specificity, linearity, lower limit of detection (LLOD), limit of blank (LOB), inter and intra-assay precision, reference interval and reportable range. Additionally, the study presents data for sample stability in preservative media during room temperature sample transportation as well as an interfering substance study.
2. Materials & Methods
2.1. Cell Culture
NCI-H1975 cell line (ATCC, CRL-5908) and SK-TG-4 cell line (Millipore Sigma,11012007) were utilized to create contrived specimens. The cells were cultured in RPMI-1640 medium (ATCC, 30-2001) supplemented with 10% fetal bovine serum (ATCC, 30-2020) in presence of 1x antibiotics mixture (Penicillin and Streptomycin). The cells were passaged either in 1:2 or 1:3 ratio following trypsinization and total cell count was determined by trypan blue staining with counting in Countess 3 FL-Automated Cell counter (Thermo Fisher Scientific Inc., CA). Each of the cell lines (SK-TG-4 (100% methylated) and NCI-H1975 (0% methylated) were mixed in 1:100 ratio for creating 1% spike-in.
2.2. Sample Collection
Esophageal cell samples were collected using EC as per its Instructions for Use (IFU). The sample was placed in a proprietary liquid preservative, cells suspended via shaking (or mixing), then transported and stored at room temperature until DNA extraction.
2.3. DNA Extraction from EsoCheck Balloon Samples
The cells were harvested by centrifugation from the preservative media at 4000 rpm for 5 min after removing the balloon by disposable forceps. Cells were lysed at 56 ± 2ºC in shaking heat block (1200 rpm for 30 min) in presence of proteinase K. The lysed cells were subjected to automated bead-based purification using NuCleoMag Tissue Kit (Macherey-Nagel, Germany) in KingFisher Apex instrument (Thermo Fisher Scientific Inc., CA). Eluted DNA was quantified by Qubit™ dsDNA HS Assay reagent (Thermo Fisher Scientific Inc., CA) following standard protocol.
2.4. EsoGuard Assay
The extracted DNA was bisulfite converted using the EZ DNA methylation lightning kit (Zymo Research, CA) and cleaned using the KingFisher Apex instrument (Thermo Fisher Scientific Inc., CA). Purified bisulfite converted DNA was either tested in singleplex or multiplex for the
VIM and
CCNA1 genes using primers and polymerase chain reaction (PCR) conditions as previously described elsewhere [
16]. In multiplex testing, each bisulfite converted DNA sample was divided into three technical replicates prior to PCR. The indexed amplicon library was pooled from multiple samples and subjected to AMPureXP (Beckman Coulter Life Sciences, CA) bead clean up followed by end-repair, A-tailing step using NEBNext
® Ultra™ II kit (New England Biolabs, MA) and dual index adapter ligation using NEXTflex
® Dual-Indexed DNA Barcodes (Perkin Elmer Inc., MA). The final library was quantified by Qubit™ dsDNA HS Assay (Thermo Fisher Scientific Inc., CA) and sequenced on MiSeq™ or NextSeq 1000 sequencing platform (Illumina Inc., CA).
Each EG run included one low positive control (1% contrived cells), one negative control (0% contrived cells), one PCR blank, and one bisulfite blank. Each run was evaluated for controls prior to analyzing sample results.
2.5. Bioinformatics Analysis
DNA sequencing reads were processed in the data analysis pipeline as described in a previous publication[
16]. The methylation status of each gene was determined based on a cut-off’s established from clinical validation studies [
16,
18]. The final EG result was considered positive if either or both the
VIM or
CCNA1 gene were positive, and negative if both genes were negative. In the case of triplicate testing, the methylation status of each sample was determined based on a majority call from all three technical replicates of the sample. Each sample was evaluated for mapping efficiency, bisulfite conversion efficiency, percent reads covering full length CpG, and depth of coverage of each gene (>10,000X coverage), as quality control (QC) parameters. If the sample failed any of these QC criteria, the sample was reported by the pipeline as non-diagnostic.
2.6. Sanger Sequencing
The extracted DNA from SK-TG-4 and NCI-H1975 were bisulfite converted as above and amplified with specific primers for VIM and CCNA1 separately. The amplified PCR products were cleaned by the NucleoSpin Gel and PCR cleanup kits (Macherey -Nagel, Germany) following manufacturer’s recommendations and the cleaned PCR product was sent to an outside sequencing laboratory (Genewiz from Azenta Life Sciences Inc., CA) for sequencing of both forward and reverse strands. The full-length DNA sequence was aligned by basic alignment tool (blastn, NCBI).
Statistical Methods:
The sample mean was calculated by averaging the replicates and plotted with standard deviation. % Coefficient of Variation (%CV) was calculated by dividing standard deviation with sample mean. Assay linearity was assessed using R2 value from scatterplot.
3. Results
3.1. Accuracy of Methylation Calling
The accuracy of the methylation calls made by EG at all 31 CpG sites (10 VIM CpG sites and 21 CCNA1 CpG sites) was confirmed against Sanger sequencing as a gold standard. A total of 62 unique data points were evaluated (2 cell lines at 31 CpG sites across two gene locus) in triplicates. All thirty-one CpG sites showed methylation level as expected based on Sanger sequencing (Supplementary
Figure 1 and
Table 1 A, B). The methylation percentages at each CpG site for both the VIM and CCNA1 genes were within the expected range of variance considering Sanger sequencing is only semi-quantitative with a limit of resolution of approximately 15-30% allele fraction. Moreover, when data was analyzed using EG analysis pipeline, which provides methylation% based on each sequencing read evaluating all 10 CpG sites for VIM locus and 21 CpG sites for CCNA1 locus the data obtained was highly concordant to Sanger sequencing (
Table 1C) on both regions.
3.2. Analytical Sensitivity, Specificity and Accuracy
Analytical accuracy of EG was first measured by sample exchange with Case Western Reserve University (CWRU), where the assay method was originally developed. The accuracy dataset consisted of blinded patient DNA specimens collected using EC (n=49) and contrived cell line mixtures (n=40). The balloon samples showed analytical sensitivity, specificity, and accuracy of 97%, 81% and 92%, respectively, while the contrived cell lines showed 100% sensitivity, specificity, and accuracy. Overall assay sensitivity, specificity and accuracy was 96%, 95% and 96% when compared to the CWRU reference lab (
Table 2 A, B and C).
Recently, the EG assay was upgraded to allow multiplex testing of the
VIM and
CCNA1 genes and sequencing on the MiSeq™ or NextSeq 1000 platforms based on sample volume. Additionally, samples are now tested in triplicates instead of singly. The analytical sensitivity, specificity, and accuracy of the multiplex EG assay was performed against the singleplex assay in a total of 77 EC-collected samples (27 positive and 50 negative by EG singleplex assay). The assay displayed 89% analytical sensitivity, 100% analytical specificity, and 96% analytical accuracy (
Table 3). Three false negative samples were discordant for the VIM gene only, and methylation percentages were close to the cutoff of >1.0% for VIM (1.1%, 1.1% and 1.2%) (Supplementary
Table 1).
3.3. Bioinformatic Pipeline Accuracy
The accuracy for the bioinformatic pipeline was evaluated against the reference laboratory (CWRU) bioinformatics pipeline by testing the same data set (n=32) between the sites. The accuracy was 100% for the pipeline with R
2 of 1 suggesting identical data obtained using both pipelines. Recent updates to the pipeline at LucidDx Labs included updates to the bioinformatics tools and automated initiation of data analysis were all validated against the previous version of the pipeline and showed accuracy of 100% (n= 207) (Supplementary
Figure 2).
3.4. Accuracy for Sequencing Platform
Accuracy of the NextSeq1000 sequencing platform against the MiSeq platform was performed. The same library was sequenced both in MiSeq and NextSeq platforms. The sample cohort consisted of 62 EC-collected samples (33 positive and 29 negatives on MiSeq platform) and 30 contrived cell line samples. The accuracy for the data set between the platforms was 100% with R
2 of 0.9976 for
VIM and 0.9982 for
CCNA1 (Supplementary
Figure 3).
4. Intra-Assay and Inter-Assay Precision
4.1. EsoGuard® Assay Precision
The intra-assay and inter-assay precision was performed by testing a sample cohort of 10 unique samples (four contrived and six EC-collected samples). The contrived sample set consisted of a mixture of positive and negative cell lines mimicking medium and low-positive samples and one negative sample (10%,1%, 0.5%, and 0%), while EC samples consisted of three positive and three negative clinical discard specimens. The intra-assay precision was performed by testing each sample, minimum three times on the same day, on the same run, and by a single operator while inter-assay precision was performed by testing all ten samples at least three times, on three different runs, on three different days, by different operators. The inter and intra-assay precision for the EG assay was 100% for all samples; the %CV (Coefficient of Variation) ranged from 1%-34% for
VIM and 2%-67% for
CCNA1 in positive samples and was 0% for all negative samples (
Table 4 and 5). As expected, the %CV was higher in the low-positive samples, however, the EG binary results were consistent across all replicates. This demonstrates a higher %CV does not impact EG results as the results are based on both the
VIM and
CCNA1 genes, and on triplicate data points.
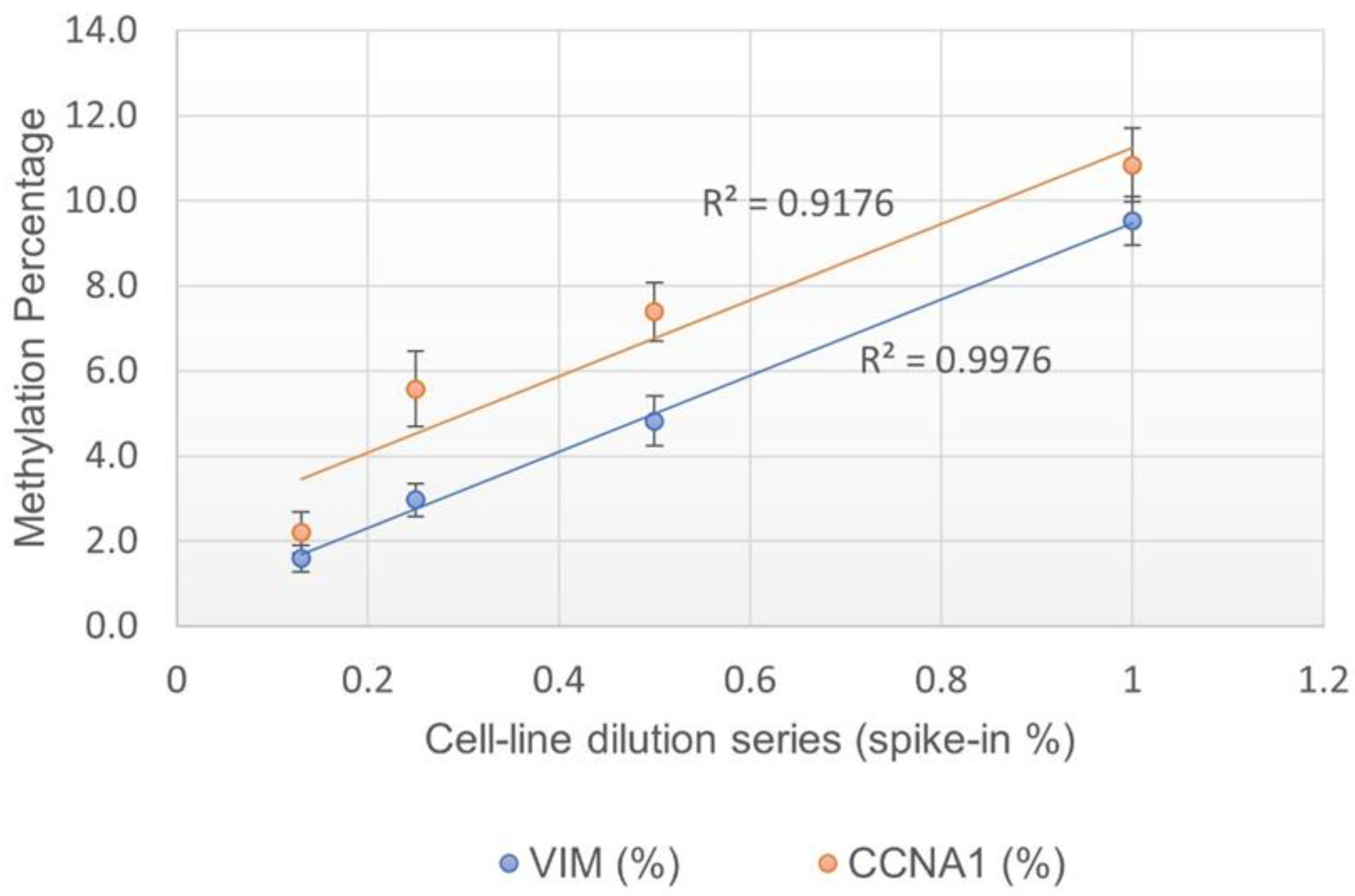
4.2. Assay Linearity and Limit of Detection (LOD)
The assay linearity was measured by testing serially diluted contrived cell line mixtures (1% ,0.5% ,0.25% and 0.13% spike-in). Each dilution was tested in at least 20 replicates. A positive correlation was observed for both
VIM and
CCNA1 genes with R
2 at 0.99 and 0.92 respectively, suggesting the assay is linear (
Figure 1). The LOD of the assay was determined to be 0.25% spike-in, or one methylated cell in the background of 400 unmethylated cells, as 0.25% was the lowest dilution at which >95% of data points were positive (
Table 6).
4.3. Assay Input Range
The DNA input range for the EG assay was tested using EC-collected samples (three positive and three negative) along with contrived specimens mimicking low-positive samples (0.5% and 1%, spike-in). All samples were tested at 50, 80,100, and 300 ng DNA input. EG concordance was 100% for the range (data not shown). However, for the EG minimum DNA requirement was kept at 100 ng to remain consistent with clinical validation studies which were performed at the 100 ng DNA input.
4.4. Reference Range
The assay reference range was determined by testing 82 samples that were confirmed negative for BE by endoscopy. In the sample cohort 69 samples were detected as true negative, whereas 13 samples were false positive suggesting a specificity at 84.1% in normal healthy patients (
Table 7).
4.5. Reportable Range
The EG assay report provides a qualitative, binary (“Positive” or “Negative”) result. Infrequently, cell samples may yield an insufficient amount of DNA for EG analysis, or the samples may have quality issues prohibiting analysis (3-6%) [
19,
20,
21]. These results are reported as ““Quantity Not Sufficient” (QNS) or “Unevaluable”, respectively. If this occurs, patients and providers may repeat the EC cell collection to provide an analyzable sample.
4.6. Limit of Blank (LOB)
Negative cell-line control with known methylation status (H-1975) was tested in multiple replicates across multiple runs (n=60) by different operators. Methylation percentages for all the data points were reported at 0% both for VIM and CCNA1 (data not shown).
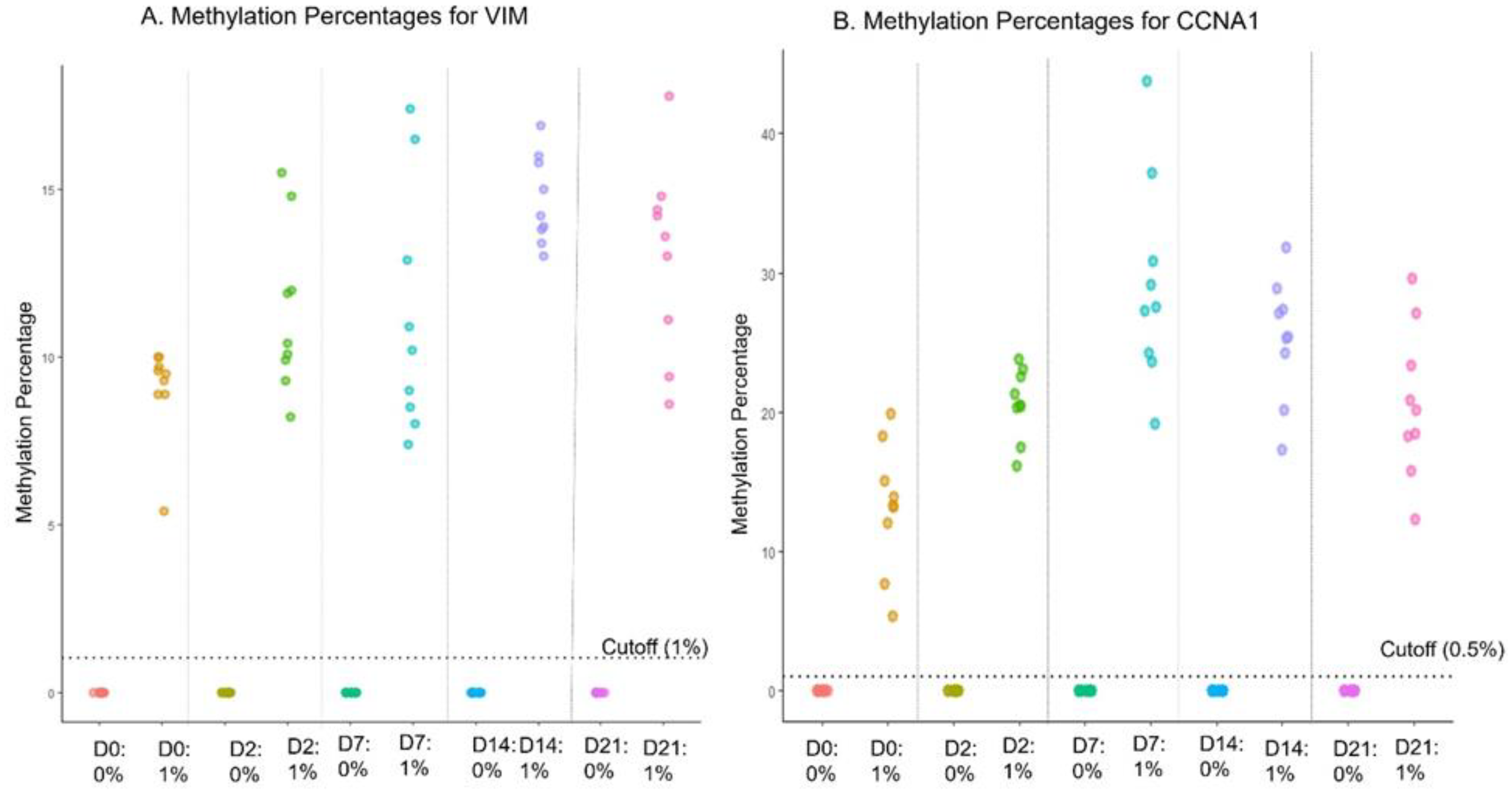
4.7. Sample Stability in Preservative Media
Sample stability in the proprietary preservative was tested for up to 21 days of storage. Low-positive cell line spike-in (1% contrived specimens) were used along with negative cell lines (0%). Each cell line sample was incubated for 0, 2, 7, 14, and 21 days (n=6 for each) in preservative media at room temperature (25 ± 3°C). The EG assay data showed 100% concordance for both positive and negative samples for all time points up to 21 days (Supplementary
Table 2). The dot plot shows the distribution of methylation reads at different days for both genes (
Figure 2 A and B). Further, the potential effect of any extreme temperatures during shipping was evaluated by incubating the low-positive and negative cell line controls at -20ºC, 4ºC, Room Temperature (25 ± 3 ºC), 37ºC and 50 ºC for 2 days, followed by incubation at room temperature for an additional 12 days. The assay displayed 100% concordance at all temperatures (Supplementary
Table 3).
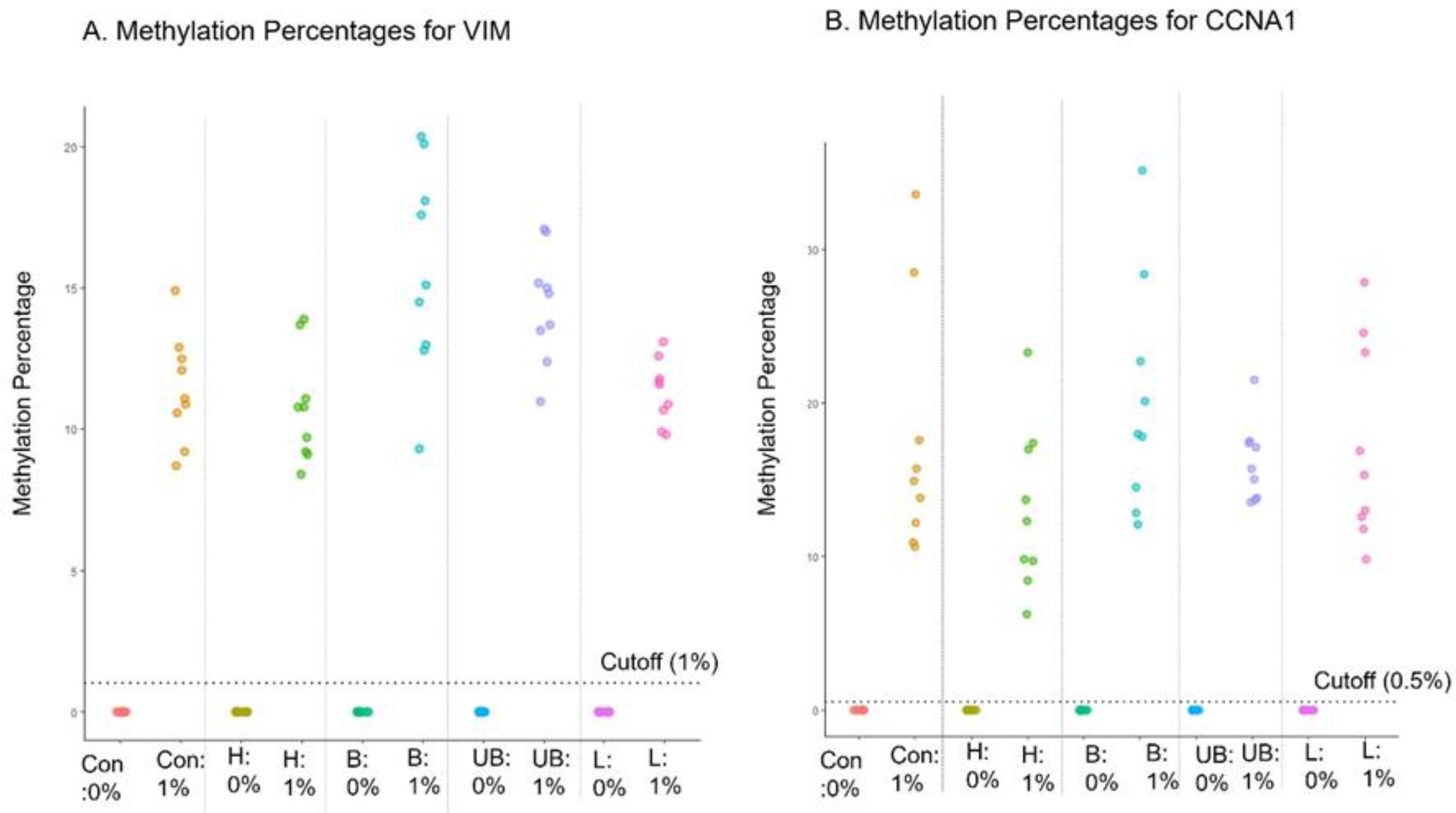
4.8. Interference Testing
Interference of the EG assay by hemolysate (heme), bile (conjugated or unconjugated bilirubin) and triglyceride-rich lipoprotein was evaluated, as these substances are generally present in blood and the proximal GI tract, and if large amounts of blood or these compounds are present in a sample collected with EC, it would be important to discern the potential impact on EG test results. Known negative (0%) (n=3) and low-positive contrived cell line samples (1%) (n=3) were incubated in the preservative media for 2 days with the interfering substances and tested with the EG assay after DNA extraction. The methylation data from the EG assay confirmed 100% concordance for both negative and positive samples, indicating no interference was observed due to any of the tested substances (
Figure 3 A, B).
4.9. Percentage of Samples Near Cutoff
As with any qualitative, binary assay, EG’s precision may be lower near the cutoff (as seen with higher %CV closer to cutoff). We quantified the percentage of samples near the cutoff, i.e., the “grey zone”, in a real-world cohort to assess whether a binary assay was justified, or an “Indeterminate” result should be reported for such samples. First, the grey zone for each gene was calculated using EC specimens with known EGD results (8 EGD negative and 82 EGD positive). Positive samples were supplemented with contrived low positive specimens (n=21) near the cutoff. A total of 333 data points were evaluated (87 positive and 246 negative). The grey zone for VIM was determined to be 0.58%-1.95% and for CCNA1 was 0.24%-1.57% (95% CI). Two thousand six hundred and seventeen (495 EG positive, and 2122 EG negative) real-world samples that were previously tested in the clinical lab were retrospectively evaluated to determine whether the methylation percentage fell within the grey zone for either VIM, CCNA1 or both. It was determined that 12.2% of samples were in the grey zone for VIM and1.3% for CCNA1. However, only 1.7% of samples were in the grey zone for both VIM and CCNA1 (data not shown).
5. Discussion
EsoGuard
® is a novel DNA methylation biomarker assay which has previously shown excellent clinical sensitivity (81%—92%) and specificity (72% -92%) for detecting BE in esophageal cell samples collected non-endoscopically using the EsoCheck
® balloon cell collection device [
16,
17,
18]. EG investigates methylation at 31 CpG sites in the regulatory regions of the VIM and
CCNA1 genes, which has been correlated with metaplastic to neoplastic changes in esophageal cells. It utilizes a proprietary algorithm that calculates methylation per read for both genes and provides percentage of sequencing reads that are methylated. If either or both of the
VIM or
CCNA1 gene has methylated reads above pre-established cutoff based on prior clinical validation then the EG assay is reported positive. [
18]. A positive test result suggests the presence of BE or EAC and a confirmatory EGD is recommended for definitive diagnosis and histopathologic definition of the underlying disease. A negative result suggests BE or/EAC are not present (NPV=98.6%), and that further invasive diagnostic testing may not be needed [
19,
21].
We presented our EG analytical validation studies performed as per CLSI guidelines [
23] and have shown that EG has high analytical sensitivity (89%), specificity (100%), and accuracy (96%). Additionally, the assay is linear (VIM, R
2=0.998 and CCNA R
2=0.918) and has a limit of detection as low as one methylated cell in the background of 400 unmethylated cells, as measured using contrived cell line mixtures. The assay displayed 100% inter- and intra-assay precision in all tested samples (contrived and clinical samples). The samples collected with EC and preserved in room temperature preservation media displayed the expected results for up to 21 days of storage. The assay has shown concordant results in DNA input as low as 50 ng. However, as supporting clinical validation studies were performed using a minimum of 100 ng DNA input, the minimal DNA input requirement for the commercial assay remains at 100 ng. Additional clinical data may allow for reduction of the minimum DNA requirement of the assay in the future. The EG assay met all requirements as per the standards of CAP, CLIA and NY state to be approved as a Laboratory Developed Test (LDT).
We performed quantification of samples in a well-defined grey zone to assess the appropriateness of EG as a binary assay. Only 1.7% of samples in a real-world cohort were in the grey zone for both VIM and CCNA1 genes, confirming that providing a binary EG result is suitable for nearly all samples. Moreover, assigning an ‘Indeterminate” result for the very small percentage of patients in the grey zone would not provide the physician with an actionable result, therefore it would diminish the value of EG as a triage test in these patients. As with any triage screening test, EG’s cutoffs are optimized to minimize the false negative rate (i.e., maximize NPV). Negative predictive value of 98.6% provides high confidence that patients with negative EG results are unlikely to be false negatives [
17]. As any false positive patients reported by EG are not exposed to any additional risk since they are merely referred to the gold standard screening test, EGD, we have determined that EG binary results are appropriate as a triage test.
In summary, the EsoGuard® assay is an accurate, robust, and highly sensitive DNA methylation biomarker test for detection of epigenetic changes related to BE and EAC in distal esophageal cells collected using EsoCheck® device.
Author Contributions
Conceptualization, S.V, A.G. and I.L; Methodology, A.G and I.L; Software, A.G. and I.L; Validation, A.G. and I.L; Formal Analysis, S.V., A.G. and I.L; Data Curation, S.V, A.G and I.L.; Writing—Original Draft Preparation, S.V, A.G and I.L; Writing—Review & Editing, V.L, and L.A.; Supervision, S.V.; Project Administration, S.V, A.G; Funding Acquisition, L.A.
Funding
The work is supported by Lucid Diagnostics’s internal funding for Research and Development.
Acknowledgments
The authors acknowledge Case Western Reserve University and Dr Sanford D. Martowitz and his team for providing samples for EsoGuard® accuracy testing and the LucidDx Labs clinical team for collaboration and for providing clinical discard specimens for performing validation studies.
Conflicts of Interest
The authors are the employees of Lucid Diagnostics and/or PAVMed Inc.
References
- Devesa, S.S.; Blot, W.J.; Fraumeni, J.F. Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. 1998, 83, 2049–2053. [Google Scholar] [CrossRef]
- Hur, C.; Miller, M.; Kong, C.Y.; Dowling, E.C.; Nattinger, K.J.; Dunn, M.; Feuer, E.J. Trends in esophageal adenocarcinoma incidence and mortality. Cancer 2013, 119, 1149–1158. [Google Scholar] [CrossRef]
- National Cancer Institute: Surveillance, E.; , and End Results Program. Esophageal Cancer — Cancer Stat Facts. 2024. Available online: https://seer.cancer.gov/statfacts/html/esoph.html (accessed on 22 May 2024).
- Pera, M.; Cameron, A.J.; Trastek, V.F.; Carpenter, H.A.; Zinsmeister, A.R. Increasing incidence of adenocarcinoma of the esophagus and esophagogastric junction. Gastroenterology 1993, 104, 510–513. [Google Scholar] [CrossRef] [PubMed]
- Schoofs, N., R. Bisschops, and H. Prenen, Progression of Barrett’s esophagus toward esophageal adenocarcinoma: an overview. Ann. Gastroenterol. 2017, 30, 1–6. [Google Scholar] [PubMed]
- Tan, M.C. , et al., Systematic review with meta-analysis: prevalence of prior and concurrent Barrett’s oesophagus in oesophageal adenocarcinoma patients. Aliment. Pharmacol. Ther. 2020, 52, 20–36. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, N.J. , et al., Safety and efficacy of endoscopic spray cryotherapy for Barrett’s esophagus with high-grade dysplasia. Gastrointest Endosc. 2010, 71, 680–5. [Google Scholar] [CrossRef]
- Shaheen, N.J. , et al., Radiofrequency ablation in Barrett’s esophagus with dysplasia. N Engl J Med. 2009, 360, 2277–88. [Google Scholar] [CrossRef] [PubMed]
- Weusten, B.; Bisschops, R.; Coron, E.; Dinis-Ribeiro, M.; Dumonceau, J.-M.; Esteban, J.-M.; Hassan, C.; Pech, O.; Repici, A.; Bergman, J.; et al. Endoscopic management of Barrett’s esophagus: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy 2017, 49, 191–198. [Google Scholar] [CrossRef]
- Qumseya, B.; Sultan, S.; Bain, P.; Jamil, L.; Jacobson, B.; Anandasabapathy, S.; Agrawal, D.; Buxbaum, J.L.; Fishman, D.S.; Gurudu, S.R.; et al. ASGE guideline on screening and surveillance of Barrett’s esophagus. Gastrointest. Endosc. 2019, 90, 335–359. [Google Scholar] [CrossRef] [PubMed]
- Spechler, S.J. , Clinical practice. Barrett’s Esophagus. N Engl J Med. 2002, 346, 836–42. [Google Scholar] [CrossRef] [PubMed]
- Yadlapati, R.; Gyawali, C.P.; Pandolfino, J.E.; Chang, K.; Kahrilas, P.J.; Katz, P.O.; Katzka, D.; Komanduri, S.; Lipham, J.; Menard-Katcher, P.; et al. AGA Clinical Practice Update on the Personalized Approach to the Evaluation and Management of GERD: Expert Review. Clin. Gastroenterol. Hepatol. 2022, 20, 984–994. [Google Scholar] [CrossRef] [PubMed]
- Kolb, J.M. , et al. , Understanding Compliance, Practice Patterns, and Barriers Among Gastroenterologists and Primary Care Providers Is Crucial for Developing Strategies to Improve Screening for Barrett’s Esophagus. Gastroenterology, 2022, 162, 1568–1573. [Google Scholar] [PubMed]
- Muthusamy, V.R.; Wani, S.; Gyawali, C.P.; Komanduri, S.; Bergman, J.; Canto, M.I.; Chak, A.; Corley, D.; Falk, G.W.; Fitzgerald, R.; et al. AGA Clinical Practice Update on New Technology and Innovation for Surveillance and Screening in Barrett’s Esophagus: Expert Review. Clin. Gastroenterol. Hepatol. 2022, 20, 2696–2706. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, N.J. , et al. , Diagnosis and Management of Barrett’s Esophagus: An Updated ACG Guideline. Am J Gastroenterol, 2022, 117, 559–587. [Google Scholar] [PubMed]
- Moinova, H.R.; LaFramboise, T.; Lutterbaugh, J.D.; Chandar, A.K.; Dumot, J.; Faulx, A.; Brock, W.; Cabrera, O.D.l.C.; Guda, K.; Barnholtz-Sloan, J.S.; et al. Identifying DNA methylation biomarkers for non-endoscopic detection of Barrett’s esophagus. Sci. Transl. Med. 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Greer, K. , et al. , Acceptability of non-endoscopic screening for barrett’s esophagus (be) among veterans eligible for be screening. Gastrointestinal Endoscopy, 2023, 97, AB1057. [Google Scholar]
- Moinova, H.R.; Verma, S.; Dumot, J.; Faulx, A.; Iyer, P.G.; Canto, M.I.; Wang, J.S.; Shaheen, N.J.; Thota, P.N.; Aklog, L.; et al. Multicenter, Prospective Trial of Nonendoscopic Biomarker-Driven Detection of Barrett’s Esophagus and Esophageal Adenocarcinoma. Am. J. Gastroenterol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Lister, D.; Fine, A.; Maheshwari, S.; Bradley, P.S.; Lee, V.T.; Deguzman, B.J.; Verma, S.; Aklog, L. Clinical Utility of EsoGuard® on Samples Collected with EsoCheck® as a Triage to Endoscopy for Identification of Barrett’s Esophagus—Interim Data from the CLUE Study. Arch. Clin. Biomed. Res. 2023, 7, 626–634. [Google Scholar] [CrossRef]
- Rachelle Hamblin MD, M.V.T.L., MD; Brian, J. deGuzman, MD; Suman Verma MD, PhD; Lishan Aklog, MD, Clinical Utility of EsoGuard® as a Barrett’s Esophagus Triage Test for On-duty Firefighters. Journal of Gastrointestinal & Digestive System, 2023, 13.
- Richard Englehardt, J.B.S. , Nikolai A Bildzukewicz, Rachelle Hamblin, Victoria T Lee, Suman Verma, Brian J deGuzman and Lishan Aklog, Real World Experience and Clinical Utility of Esoguard®—Interim Data from the Lucid Registry. J. Gastroenterol. Dig. Syst. 2023, 7, 43–53. [Google Scholar]
- Greer, K.B. , et al., Non-endoscopic screening for Barrett’s esophagus and Esophageal Adenocarcinoma in at risk Veterans. medRxiv, 2024: p. 2024.03.15.24304354. [CrossRef]
- Evaluation of Qualitative, Binary Output Examination Performance. Clinical and Laboratory Standards Institute, 2023. 3rd ed.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).