Introduction
Globally, Precision medicine (PM) promotes the healthcare system and improves the quality life of patients. According to the Dr. Wei, a Canadian researcher, PM stands for the preventive, precision, and people medicine (3-P theory) [
1]. Sometimes PM also referred as personalized medicines, stratified medicines, tailored medicines or individualized medicine. In the field of medicine disease prevention, diagnosis, and treatment are tailored to individual patients based on information derived from genetic and genomic data. The conventional one-size-fits-all method of providing healthcare could be replaced with PM. It acknowledges that people vary in their genetic make-up, exposure to the environment, and the way of life, all of which have an impact on how they react to medicine. The idea behind PM is that physicians could make better, more informed decisions regarding a patient’s care by knowing more about their genes and genome.
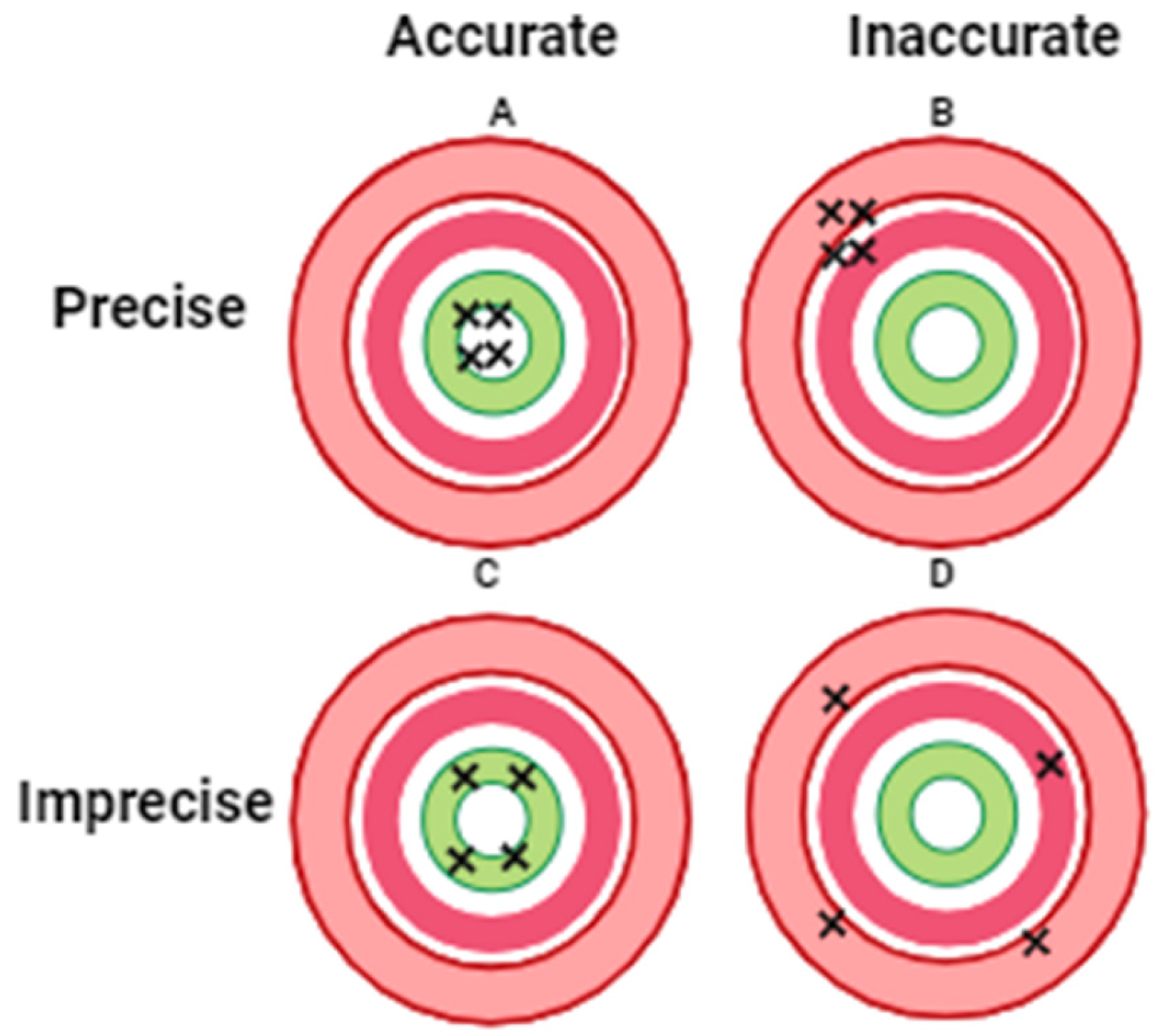
PM encompasses both accuracy and precision; however the term “accuracy” does not occur in the name. The concept of PM with target shooting is illustrated in
Figure 1 [
2].
This concept is basically a continuation of conventional medicine, which considers a single approach that is administered to all patients. However, taking into account each individual’s unique genetic and genomic make-up. The concept of tailored medicine was surfaced in the 1990s; however it wasn’t revolutionary at the time [
3]. This was due to increased automation and throughput in DNA sequencing technologies. These discoveries shed light on long-observed medical phenomena, such as the fact that some individuals respond more favourably to a given treatment than others and that some patients have extremely serious adverse drug reactions. Developments in pharmacogenetics (PGx) and pharmacogenomics have significantly aided in the knowledge of the molecular mechanisms. It emerges the influence of an individual’s genetic constitution on disease and therapeutics. The study of how various genetic variations influence; how people react to pharmacological treatments and the research of how genetic factors contribute to individual variability in how people react to pharmaceuticals [
4].
In the domains of clinical application, the PM came to innovative site due to tailored medicine, especially through translatory clinical research and health information technology, which includes computerized processing and storage of the patient data [
5,
6]. Developments in genetics and genomics, notably the use of electronic health records (EHRs) that store data on test results, demographics, medication histories, and patient histories have made it easier to integrate data from these domains into clinical settings [
7].
Importance of PMs in Tailoring Treatments
Personalized therapies have proven to be highly effective in a variety of contexts. According to the patient’s genetic composition, lifestyle choices, and physical attributes, PM could be a game-changer [
8].
The major aim of the PM is to develop a treatment strategy that is as distinct as the patient’s ailment. The technique is based on the genetic discovery, epigenomic, and clinical data that aids in understanding how an individual’s unique genomic profile makes them prone to various diseases. More patient categorization would enable better use of PM and proactive treatment programs, which will reduce cost and enhance the quality of life [
9].
Genomics and PMs
A comprehensive understanding of the range of rare diseases (e.g., filariasis, achondroplasia, etc.,) and cancers is made possible by genomic approaches. These include completely hereditary diseases, common diseases with a Mendelian subset, polygenic complicated disorders, and somatic mosaic abnormalities. The field of PM is rapidly expanding in biomedical applications due to its overall acceptability. However, a thorough review of the evidence foundation and impact of each intervention is required. Comparing each human genome to the reference sequence reveals about 3-5 million genetic variants. It is crucial to look for positive evidence of pathogenicity in any particular mutation while also considering the phenotypic, family structure, and inheritance pattern. Therefore, it is recommended to use a multidisciplinary strategy comprising physicians and clinical scientists [
10].
Genomics is a major contributor to the rise of PM, as it provides a very specific molecular window into individual differences. It allows for the prediction of an individual’s risk of disease, which can help a patient choose the best preventive strategy. In certain instances, it also allows physician to select the proper medication at the right dose for the right patient, rather than taking a “one size fits all” approach to drug management. PGx, is the study of how a person’s genes influence their pharmacological reaction. It serves as an essential for PM since it enables drug selection and dose to be tailored to a patient’s genetic profile [
11]. In PM, genomics plays an important role because it gives insights into the unique biological differences across individuals, enabling the estimation of illness risk. It is expected that pharmacogenomics will eventually affect the implementation of a personalized strategy in all areas of medicine [
12].
By replacing the conventional treatment strategies with a personalized preventive and predictive approach i.e., PM which could help to lower the healthcare cost. Even though, at present PM’s is the fasted growing concern in cancer therapy. In addition, PM and pharmacogenomics can aid in the treatment of rare or challenging illnesses, and also benefits for the incurable disorders [
13].
Pharmacogenomics
According to the European Agency for the evaluation of medicinal products (EMEA), pharmacogenomics is the “study of the variability of individual gene expression relevant to disease susceptibility as well as drug response at the cellular, tissue, individual, or population level” [
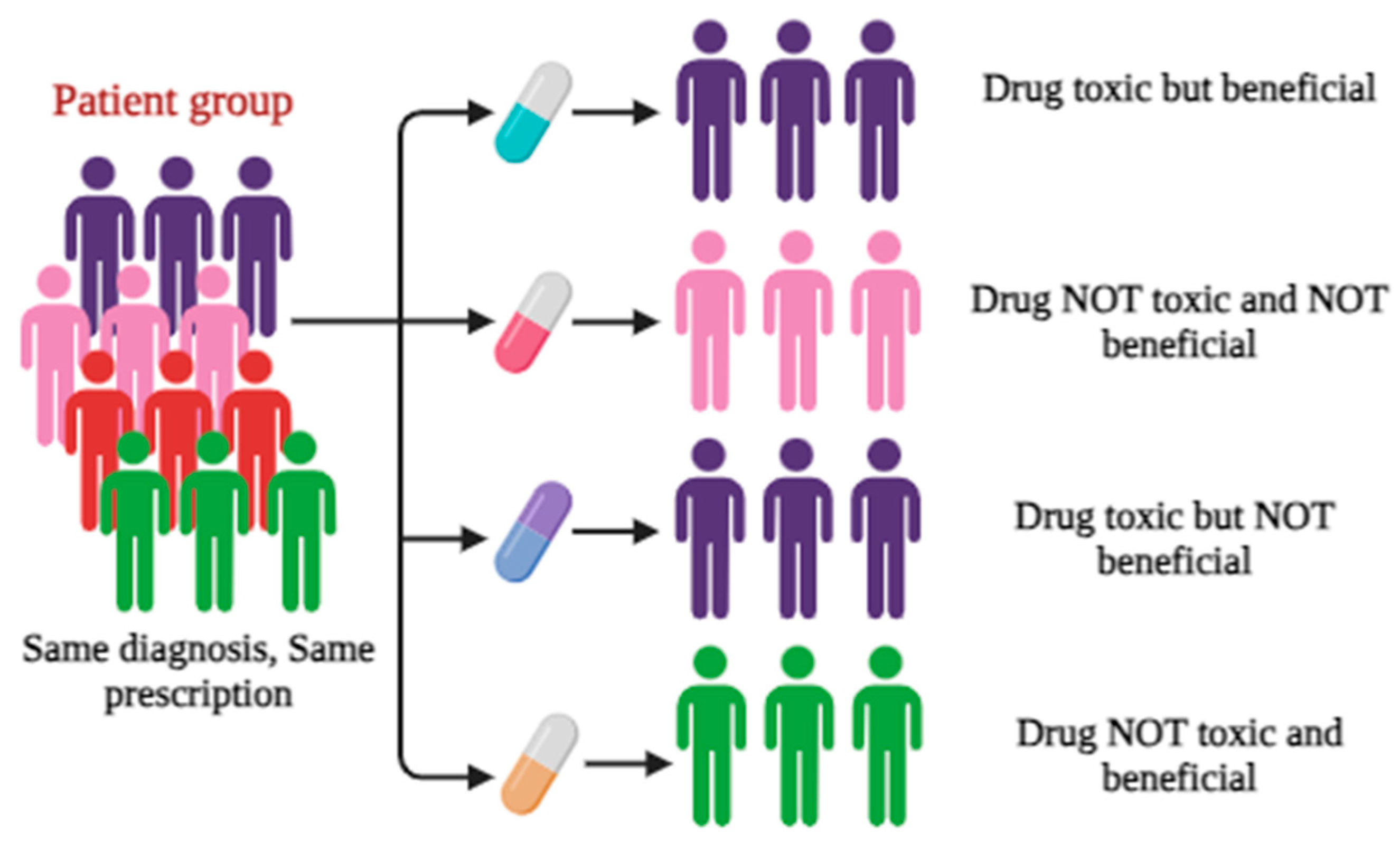
14]. PGx is “the study of interindividual variations in DNA sequence related to drug response.” Pharmacogenomics is an essential part of PM. Literature reveals that the field of PM is growing significantly with a strong genetic and molecular foundation. As it usually deals with the effect of single gene on drug response. The inter-individual variability with drug response is illustrated in
Figure 2 [
15].
PGx and pharmacogenomics are recognized as key stages towards personalized treatment. Genetic variations in medication response have the potential to transform pharmacological therapy by tailoring it to individual genotypes. The core idea is that a patient’s age, gender, and/or use of other medications. In addition, to environmental, genetic, epigenomic, and other factors, all have an impact on the interindividual variability in drug response. The ultimate goal of PM is to accurately tailor each therapy activity to the patient’s biological profile. Since two decades, modern sequencing technology has transformed the genetics in terms of human health. This can be achieved by combining various pharmacological information layers, including gene expression, pharmacokinetic (PK) profiles, novel statistical, and bioinformatic techniques to account for interpatient variations in medication effects.
Significance of Pharmacogenomics in PM for Cancer
In PM, ideally pharmacogenomics has serves two primary purposes. In the beginning, it oversees pharmaceutical firms’ efforts to find new chemical entities. In addition, it assists physicians in selecting the most appropriate drug for each patient based on their genetic composition, preventing negative drug responses, and optimizing pharmaceutical efficacy through dosage adjustments.[
17] Genetic variations among patient can affect nearly every aspect of a disease and its management with regards to the following criteria as shown in
Table 1. All of these genetic characteristics can have an impact on the risk-benefit ratio of medications. [
18,
19]
The drug also modifies the body’s physicochemical functions. The term “PD” describes the way a drug works in the body as well as its effects. It determines how well the medicine works on the target cells, such as heart tissue or neurons. The drug manufacturer achieves a delicate balance between PK and PD to guarantee that the treatment has the desired benefit while providing the fewest potential side effects to patients [
21,
22,
23].
An individual’s intrinsic genetic polymorphism may cause a variation in the ratio of PK to PD, affecting how the medicine (or its metabolites) interacts with the body. Patients may receive precision medicines with the help of PGx based on their surroundings, nutrition, age, lifestyle, and present health status in addition to information on PG testing [
24].
In molecular technology, PM seeks to detect genomic indications in target individuals in order to provide more effective treatments and avoid negative effects. Developing a diagnostic test to assist patient treatment decisions involves: Identifying biomarkers to stratify patients into risk categories for treatment decisions, followed by a validation study with appropriately treated patients [
25].
PM classifies the patients into subgroups based on genomic biomarkers, allowing for tailored treatment. It uses biomarkers to predict a patient’s sickness condition or how effectively a treatment will perform for e.g., precision medicine for curing Alzheimer’s disease [
26].
PM currently utilises small biomarker panels to achieve some degree of stratification of patients into subgroups of diseases. However, a single molecular marker, such as the mutation status of a single gene, is still largely stratified in patients. BRAF (V600E) in melanoma, MYCN in neuroblastoma and the BRCA genes in breast and ovarian cancer are single prognostic gene biomarkers which are used clinically [
27,
28,
29].
Somatic Genetic Mutation for Selection of Molecularly Targeted Drugs
Driving somatic genetic mutations causing cancer development/progression are the scientific basis of the development of molecularly targeted drugs, which have been used clinically. Hence, cancer genome analysis could be a promising tool for selection of drugs such as gefitinib, erlotinib. Amongst crizotinib. gefitinib, erlotinib crizotinib are preferred drugs for non-small cell lung cancer (NSCLC) [
30,
31]. The impact of the implementation of personalized treatment has also been revealed by Schwaederle and co-workers who analysed 346 phase-I clinical trials. They found that patients who received personalized treatment (biomarker-based drug selection) had a significantly higher median response rate and a longer median progression-free survival period compared with those in the nonpersonalized treatment group. It shows the biomarker selection for molecularly targeted drugs could be of great importance. Furthermore, a small subset of cancer patients with actionable mutations could be expected to benefit from targeted therapy with either commercially available drugs or drugs in clinical trials. However, the most of the patients has developed resistance within a year [
32]. For example, 10% of NSCLCs in the USA harbor somatic EGFR mutations that are good targets for EGFR-targeted therapy (e.g., gefitinib and erotinib). Although they show a marked initial response, almost all patients subsequently experience progression of the disease due to drug resistance, which is mostly caused by secondary mutations in the EGFR gene. Thus, most cancer patients are treated with one or more cytotoxic anticancer drugs for which no good predictive markers are available to select those patients who can be expected to benefit or to exclude patients who have a higher risk of severe adverse events. Hence personalized immunotherapy based on genetic mutation could be the promising approach for cancer therapy [
33].
Personalized Immunotherapy
The probable promising therapy could be the immune checkpoint antibodies, which kill cancer cells through activation of antitumor cytotoxic T lymphocytes (CTLs). Meanwhile, anti-programmed cell death protein-1 (PD1)/PD-1 ligand-1 (PD-L1) antibodies can block the immunosuppressive co-signal mediated by the interaction of PD1 and PD-L1. Due to its activity of immuno-signal could become the most attractive anticancer drugs. However, high-cost of the drug could be the rate limiting factor and few patients experience the relapse and subsequent death. For example, 26 courses of pembrolizumab can cost almost USD1 million [
34]
In addition, cancer immunotherapies, including immune checkpoint inhibitors, would be one of the potential precise approaches to apply the results of genomic sequencing most effectively. Recently, highly cancer-specific antigens derived from somatic mutations i.e., neoantigen has become a topic of discussion in individual cancers. Precise prediction of neoantigens should accelerate the development of personalized immunotherapy including cancer vaccines and T-cell receptor-engineered T-cell therapy for a broader range of cancer patients [
35]
Patient Stratification: The Key to Delivering PM
An innovative healthcare method known as “patient stratification” aims to tailor medical therapy to each patient’s unique characteristics. Instead, then taking a one-size-fits-all strategy, patient stratification investigates the distinct characteristics of a patient’s genetic composition, lifestyle, and environmental factors. Analysing these factors allows healthcare practitioners to identify patient subgroups that are likely to react differently to a given treatment [
36]. The goal of patient stratification in cancer patients is to identify groups with different disease presentations, levels of severity, and expected life spans. Many stratification techniques that rely on high-throughput gene expression data have been effectively put into practice. This method paves the way for completely tailored medication by lowering adverse effects while enhancing therapeutic efficacy [
37].
Accurate patient stratification drives better understanding of the factors underpinning disease risk, rate of progression, therapy response, and presents us with a new palette of opportunities to impact patient care. Clinical decision support systems are beginning to apply patient stratification insights to inform treatment choices at the point of care. By increasing the chance that patients will get the right drug or combination of drugs first time, such precision medicine tools can reduce the cost of delivering care at the same time as maximizing patient benefit. Two approaches can be taken to delivering PM. Either stratified disease sub-groups can be studied to find new targets for drug discovery, or the same detailed patient stratification information can be used to identify the best treatment (or set of treatments) from the existing formulary to apply to an individual patient given their genetic makeup, phenotype, co-morbidities, and co-prescriptions [
38].
To date, the most effective applications of PM for complex, multi-factorial diseases such as cancer, dementia, diabetes, and monogenic disorders (e.g., sickle cell anaemia, Huntingdon’s disease, cystic fibrosis) [
39]. This is relatively simple, being very largely determined by a single pathogenic mutation, or in some cases different mutations in the same gene that have similar phenotypic effects. Molecularly distinct subgroups that may benefit from certain treatment methods have been found by genetic sequencing. However, additional approaches are required because this strategy is not suitable for common polygenic complex illnesses. For instance, type 2 diabetes has a complex underlying pathophysiology and a large range of available glucose-lowering therapeutic options with distinct mechanisms of action; it is an excellent candidate for a precision medicine strategy [
40].
Advancements of PM in Cancer
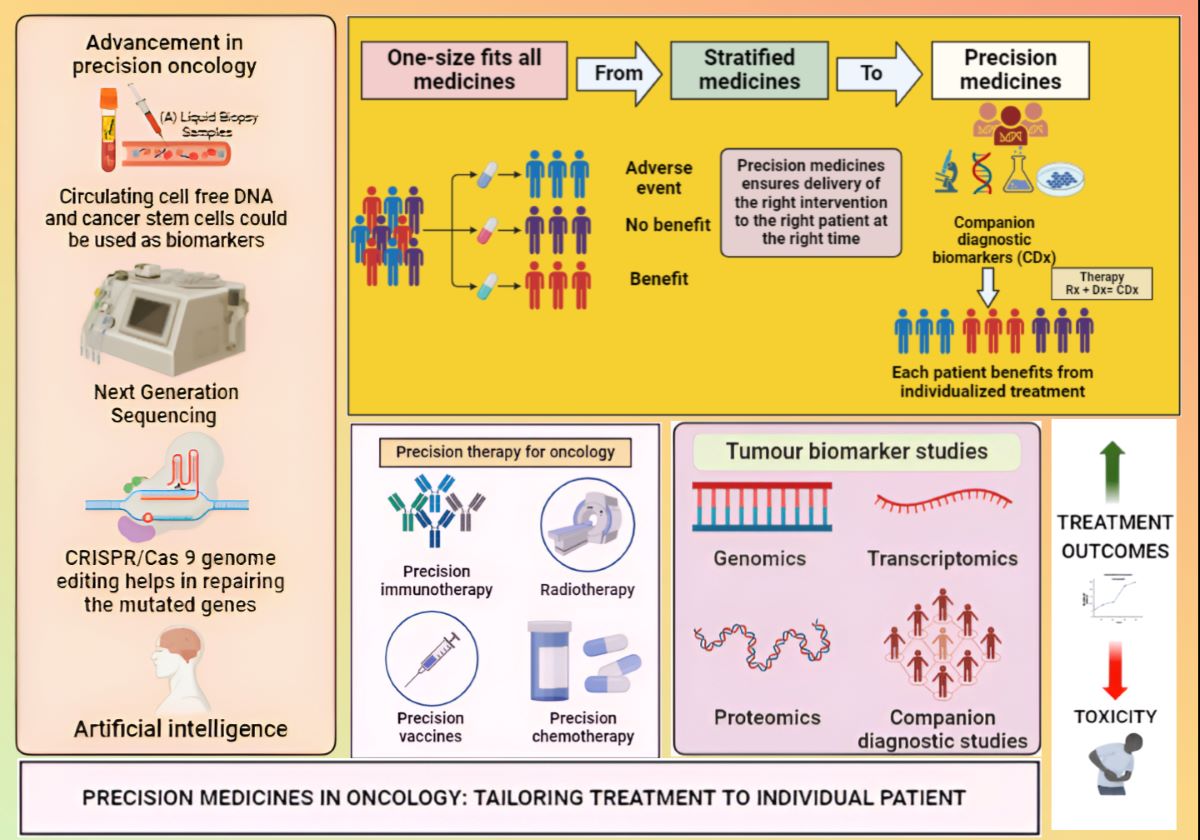
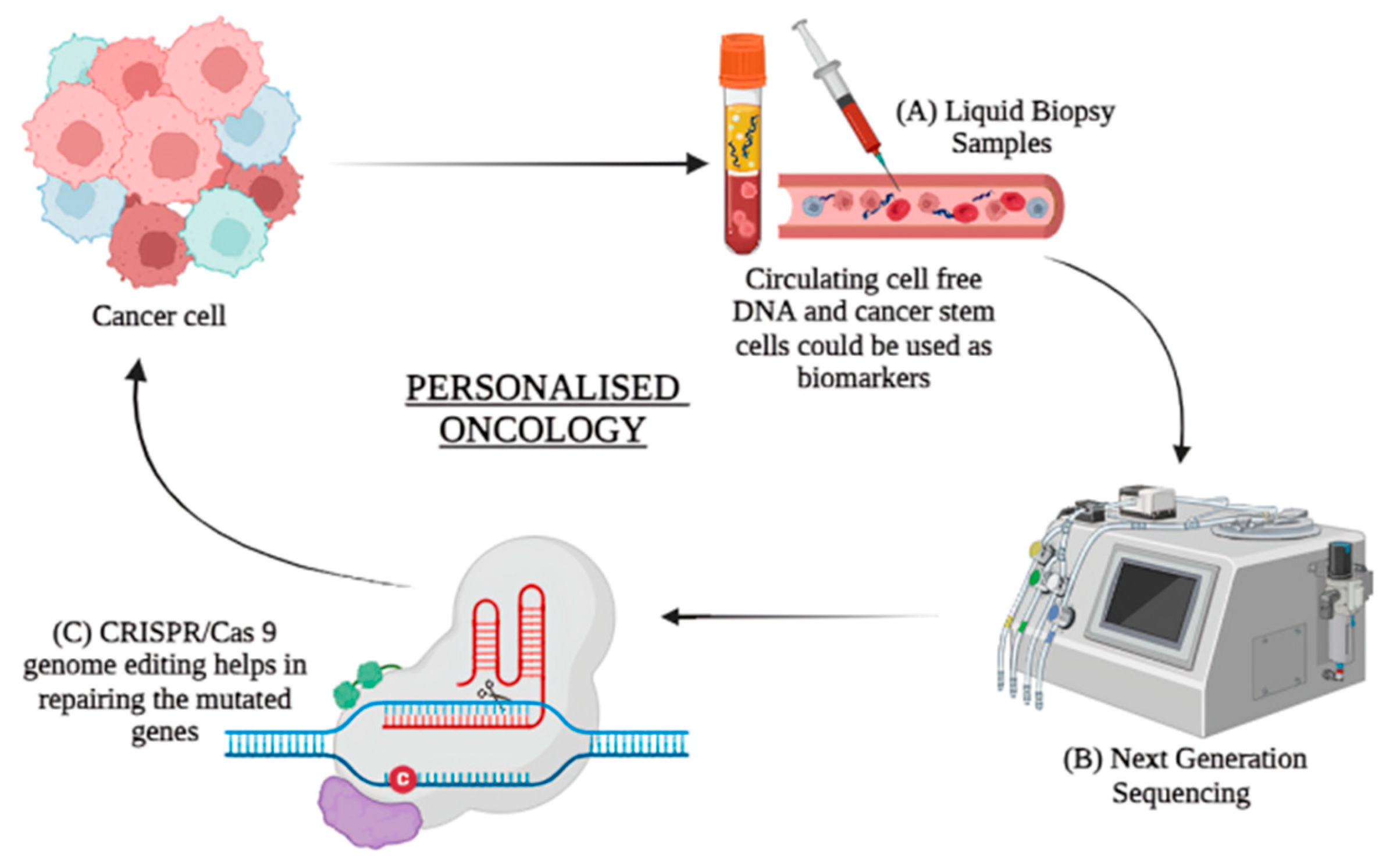
Next-generation sequencing (NGS): The speed, accuracy, and increasing affordability of NGS have helped spur the advent of PM, which involves designing treatment based on a person’s disease-driving molecular alterations [
41,
42,
43]. While NGS has been tested across multiple health care settings, its use is most advanced in oncology. The main objective of personalized molecular medicine is to target very specific disease-causing genes while minimizing the danger of target consequences. CRISPR as a genome-editing technique could aid in the development of more efficient gene-targeted modification technology. The plausible applications of NGS and CRISPR/Cas9 in PM are depicted in
Figure 4 [
44]. Cancer can be diagnosed without non-invasive biopsy samples with the help of circulation biomarkers (liquid biopsy) like cell free DNA and cancer stem cells. The advancement of NGS has helped in identification of various mutations in the cancer cells that cannot be identified by other methods like PCR. This helps in personalising the treatment for cancer. CRISPR/Cas9 genome editing tool locates the mutated gene and modifies it by creating double strand breaks. This is corrected by non-homologous end joining or homology directed repair which is now being studied for personalised oncology [
45].
Bioinformatics: Bioinformatics tools may provide better diagnoses in genomic level with earlier detection of disease and better targeted therapy through efficient PM development. Multi-omics approaches such as genomics (e.g, whole genome sequencing, whole exome sequencing, clinical exome sequencing), transcriptomics (e.g., RNA sequencing, mRNA microarray), proteomics (e.g., proteome expression profiling, proteome mining, protein-protein interactions, post translational modifications), epigenomics (e.g., whole genome bisulfite sequencing, chromatin Immuno-precipitation sequencing), metabolomics (e.g., targeted analysis, metabolome profiling, metabolic fingerprinting), microbiomics (e.g., shotgun metagenomic sequencing, all omics techniques), and its analysis provides a great assistance in the development of PM [
46]. These devices may furnish better conclusions in genomic level with prior identification of infection and better focused on treatment through productive PM improvement [
47,
48]. Bioinformatics tools help in diagnosis ((e.g., web technology, cytoscape, gene expression profiling interactive analysis (GEPIA)), various stages of drug development (e.g., BLAST-basic local alignment search tool, STRING-search tool for the retrieval of interacting genes/proteins, Autodock, GROMACS-GROningen MAchine for chemical simulations, MOE-molecular operating environment, ADMET Predictor, pkCSM, RNA-Seq Tools, GATK-genome analysis toolkit, OpenClinica, CBioPortal) therapy, and personalized vaccination. Personalized vaccine means for an optimized prevention of disease with minimized reactogenicity and side effect. They are developed to take care of haplotypes and polymorphism can become risk of an adverse vaccine reaction [
49].
The new cutting-edge technology like vaccinomics a combination of immunogenomics, bioinformatics and immunogenetics could be helpful in the personalized vaccine development. The enhancement of bioinformatics tools and databases development for new diseases would be helpful for the advancement of PM [
50]. In future, looking for more coverage, affordability in genome data processing, accuracy in data interpretation, fast genetic data processing, and development of more bioinformatics tools the understanding of disease at the molecular level, and bioinformatics advancement for data interpretation. This would help the elevated success rate in this new young health care field. Discovery of bioinformatics tools would help to integrate the huge genomics data analysis and speed up the PM research.
CRISPR-Cas9: Previously, researchers have relied on naturally occurring mutations found in existing biological samples, often through the use of genome-wide association studies (GWASs), to study their effects on phenotypes [
51,
52]. However, this approach was restricted to mutations present in specific samples. Genome editing methods, such as the CRISPR‒Cas system, facilitate the study of genetic variants associated with diseases. These methods are applicable to both protein-coding and noncoding regions of the genome, offering a comprehensive approach to understanding the genetic influences on disease [
53]. One of the key advantages of CRISPR technology lies in the ease of designing and synthesizing multiple guide RNAs (gRNAs), facilitating its application in high-throughput assays as well as in PM. The CRISPR-Cas9 system has revolutionized the field of precision medicine by enabling precise and efficient gene manipulation and helps in repairing the mutated genes as shown in
Figure 4. Since its adaptation as a programmable gene editing tool in mammalian cells, CRISPR-Cas9 has transformed the landscape of genome engineering, offering a powerful and preferred gene editing tool with its cost-effectiveness, facile design, and high efficiency. CRISPR-Cas9 has been applied in various aspects of precision medicine, including: Target identification, disease modelling, diagnostics, epigenome editing, base and prime editing [
54].
Big data and artificial intelligence (AI): AI is transforming medical research by revealing data patterns that can be used to predict disease and treatment outcomes for individual patients. The centres for AI Research are characterizing patients by collecting genomic and clinical data as well as other ‘omics’ data, which provides information about gene expression and metabolism. Information about the microbes in the body, environmental exposures, and lifestyle factors that can affect human health is also gathered. These data are high dimensional and nonlinear, making them difficult to analyse with conventional statistical methods [
55,
56]. However, by developing and applying AI and mathematical approaches to these data, we are able to differentiate disease states and predict the efficacy of particular drugs and future outcomes. For example, when applied machine learning techniques based on the 32 blood markers in ovarian cancer patients before treatment, it was found a group of early-stage cancer patients with poor prognosis. This finding will help researchers develop new treatments for this group of patients. Predictive algorithms can help identify disease groups that haven’t been recognized by clinicians, as well as guide the selection of personalized treatment options for these patients [
57]. The integration of AI and machine learning technologies into precision medicine approaches holds immense promise for advancing neurological and cardiac health [
58]. By enabling more accurate diagnosis, personalized treatment strategies, and proactive disease management, AI-driven precision medicine has the potential to revolutionize healthcare delivery and improve the quality of life.
All cancers are caused by gene changes of some kind. Cancer cells are abnormal versions of normal cells, meaning something changed in the genes of a normal cell to make it turn into a cancer cell [
59]. For example, genes that normally help keep cells from growing out of control might get turned off, or genes that normally help cells grow and divide might get turned on all the time. Tumour genomes are shaped by mutational processes of exogenous (e.g., cigarette smoking and UV-light) or endogenous (such as defective DNA repair or 5-methylcytosine deamination occurring over time) nature that imprint alterations of different types and patterns in the tumour DNA. Mutational processes can act in parallel, sequentially, or during short periods of time in tumour evolution, and occur in either a clonal or a sub-clonal manner. PMs as classified by the PM Coalition. How PM is beneficial especially in cancer patients is shown in
Figure 5.
Table 2.
Few examples of FDA-approved PM used in oncology since 2023-24 [
61].
Table 2.
Few examples of FDA-approved PM used in oncology since 2023-24 [
61].
| Sr. no. |
Cancer type |
Target |
Treatment |
Approval |
Year |
| 1 |
Unresectable or metastatic esophageal squamous cell carcinoma (ESCC) after prior systemic chemotherapy |
PD-1 |
Tislelizumab (Tevimbra) |
FDA |
2024 |
| 2 |
Newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL) |
BCR-ABL |
Ponatinib (Iclusig) |
FDA |
2024 |
| 3 |
Unresectable or metastatic urothelial carcinoma |
Tumor PD-L1 expression |
Nivolumab (Opdivo) + Cisplatin and Gemcitabine |
FDA |
2024 |
| 4 |
Relapsed or refractory multiple myeloma |
B-cell maturation antigen (BCMA) |
Abecma (idecabtagene vicleucel) |
FDA |
2024 |
| 5 |
Metastatic NSCLC |
MET exon 14 skipping alterations |
Tepotinib (Tepmetko) |
FDA |
2024 |
| 6 |
Unresectable or metastatic melanoma |
Tumor-infiltrating lymphocytes (TIL) |
Lifileucel (Amtagvi) |
FDA |
2024 |
| 7 |
Metastatic NSCLC |
MET exon 14 skipping alterations |
Tepotinib (Tepmetko) |
FDA |
2024 |
| 8 |
Unresectable or metastatic melanoma |
None specified |
Lifileucel (Amtagvi) |
FDA |
2024 |
| 9 |
HER2-positive gastric or gastroesophageal junction adenocarcinoma, biliary tract cancer, NSCLC |
PD-L1 (CPS ≥ 1) |
Pembrolizumab (Keytruda) |
FDA |
2023 |
| 10 |
Nasopharyngeal carcinoma (NPC) |
PD-1 |
Toripalimab (Loqtorzi) |
FDA |
2023 |
| 11 |
Myelodysplastic syndromes (MDS) |
IDH1 mutation |
Ivosidenib (Tibsovo) |
FDA |
2023 |
| 12 |
Solid tumors in pediatric patients |
NTRK gene fusion |
Entrectinib (Rozlytrek) |
FDA |
2023 |
| 13 |
Stage IIB/C melanoma |
None specified |
Nivolumab (Opdivo) |
FDA |
2023 |
| 14 |
Metastatic NSCLC |
BRAF V600E mutation |
Encorafenib (Braftovi) with Binimetinib (Mektovi) |
FDA |
2023 |
| 15 |
Metastatic advanced nasopharyngeal carcinoma |
NTRK1-3 gene fusions. |
Augtyro (repotrectinib) |
FDA |
2023 |
| 16 |
Hematologic malignancies |
Prominin-1 |
Omisirge (omidubicel-onlv) |
FDA |
2023 |
| 17 |
Metastatic breast cancer |
Estrogen receptor (ER). |
Orserdu (elacestrant) |
FDA |
2023 |
| 18 |
Metastatic advanced nasopharyngeal carcinoma |
None specified |
Loqtorzi (toripalimab-tpzi) |
FDA |
2023 |
| 19 |
Metastatic breast cancer |
|
Truqap (capivasertib) |
FDA |
2023 |
| 20 |
Acute myeloid leukemia following consolidation chemotherapy |
|
Vanflyta (quizartinib) |
FDA |
2023 |
| 21 |
Metastatic Merkel cell carcinoma |
|
Zynyz (retifanlimab-dlwr) |
FDA |
2023 |
| 22 |
NSCLC |
KRAS G12C-mutated |
Krazati (adagrasib) |
FDA |
2022 |
| 23 |
HER2-low breast cancer. |
HER2 |
Enhertu (fam-trastuzumab deruxtecan-nxki) |
FDA |
2022 |
| 24 |
Uveal melanoma |
HLA-A |
Kimmtrak (tebentafusp-tebn) |
FDA |
2022 |
| 25 |
Metastatic castration-resistant prostate cancer |
PSMA |
Pluvicto (lutetium Lu 177 vipivotide tetraxetan) |
FDA |
2022 |
| 26 |
Multiple myeloma |
BCMA |
Carvykti (ciltacabtagene autoleucel) |
FDA |
2022 |
| 27 |
Melanoma |
PD-1 and LAG-3 |
Opdualag (nivolumab and relatlimab-rmbw) |
FDA |
2022 |
| 28 |
Solid tumors |
BRAF V600E |
Tafinlar (dabrafenib) + Mekinist (trametinib) |
FDA |
2022 |
| 29 |
Intrahepatic cholangiocarcinoma |
FGFR2 |
Lytgobi (futibatinib) |
FDA |
2022 |
| 30 |
Ovarian cancer |
FRα |
Elahere (mirvetuximab soravtansine-gynx) |
FDA |
2022 |
| 31 |
Thyroid cancer |
RET |
Retevmo (selpercatinib) |
FDA |
2022 |
| 32 |
Follicular lymphoma |
CD20 |
Lunsumio (mosunetuzumab-axgb) |
FDA |
2022 |
| 33 |
Colorectal cancer |
HER2 |
Tukysa (tucatinib) |
FDA |
2022 |
| 34 |
NSCLC -first line |
ALK |
Lorlatinib |
FDA |
2021 |
| 35 |
NSCLC |
MET |
Tepotinib |
FDA |
2021 |
| 36 |
NSCLC and thyroid cancer |
RET |
Selpercatinib |
FDA |
2021 |
| 37 |
Hepatobiliary cancer |
FGFR2 |
Pemigatinib |
FDA |
2021 |
Conclusions and Future Prospective
In conclusion, PM is a revolutionary approach in healthcare that tailors’ disease prevention, diagnosis, and treatment to individual patients based on their genetic and genomic profiles. This method recognizes the uniqueness of each person’s genetic makeup, environmental exposures, and lifestyle, which can affect how they respond to medications. PM aims to replace the conventional one-size-fits-all healthcare model with personalized treatments that are more effective and efficient. Personalized treatment for patients diagnosed with solid tumors has resulted in several advances in recent years. Precision cancer medicine has outgrown its beginning and demonstrated that the development of therapies based on a deeper understanding of specific disease mechanisms can improve patient outcomes and quality of life. The possibility to offer a molecular-based personalized approach for cancer patients represents an attractive possibility in oncology. Advancements in NGS, bioinformatics, and genome editing techniques like CRISPR-Cas9 have further propelled PM. These technologies allow for the identification of disease-causing genes and the modification of mutated genes, contributing to the development of personalized treatments. The integration of various cutting-edge technologies and bioinformatics tools is expected to further advance PM, making it a promising approach for overcoming multidrug resistance in cancer therapy and other complex diseases.
The future of precision oncology looks promising, with rapid advancements in NGS technologies and a growing understanding of cancer genomics. Despite the progress, significant challenges remain, such as the uncertainty around the impact of pharmacogenomics methods on patient outcomes and the need to further understand cancer pathogenesis at the molecular level. In addition, significant challenges remain, such as the uncertainty around the impact of pharmacogenomics on patient outcomes and the need to further understand cancer pathogenesis at the molecular level. Addressing these challenges will require continued research and innovation.
Author Contributions
S.K.: Formal analysis, Investigation, Writing-original draft; U.V.: Data curation and Review; A.P.: Investigation and Review; R.G.I.: Conceptualization, Visualization, Methodology, Review, and Editing.
Acknowledgments
The authors are thankful to the Datta Meghe College of Pharmacy, Datta Meghe Institute of Higher Education and Research, Wardha, India, for their support.
Conflicts of Interest
The authors declare there is no conflict of interest.
Abbreviations
ADME, absorbed, distributed, metabolized, and excreted; AI, artificial intelligence; CTLs, cytotoxic T lymphocytes; EHRs, electronic health records; EMEA, European agency for the evaluation of medicinal products; GEPIA, gene expression profiling interactive analysis; NGS, next-generation sequencing; NCE, new molecular entities; NSCLC, non-small cell lung cancer; PD, pharmacodynamics; PGx, pharmacogenetics; PK, pharmacokinetic; PM, precision medicine.
References
- Wei, L.Y. Scientific advance in acupuncture. The American journal of Chinese Medicine 1979, 7, 53–75. [Google Scholar] [CrossRef]
- Wang, R.C.; Wang, Z. Precision Medicine: Disease Subtyping and Tailored Treatment. Cancers 2023, 15, 3837. [Google Scholar] [CrossRef] [PubMed]
- Miller, N.E.; Stoll, R.; Mahaffee, W.F.; Neill, T.M.; Pardyjak, E.R. An experimental study of momentum and heavy particle transport in a trellised agricultural canopy. Agric. For. Meteorol. 2015, 211–212, 100–114. [Google Scholar] [CrossRef]
- Naithani, N.; Sinha, S.; Misra, P.; Vasudevan, B.; Sahu, R. Precision medicine: Concept and tools. Med J. Armed Forces India 2021, 77, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Look, E.F. Personalised medicine for the European citizen. Towards more precise medicine for the diagnosis, treatment and prevention of disease (iPM); European Science Foundation: Strasbourg, 2012. [Google Scholar]
- Aguilera-Cobos, L.; García-Sanz, P.; Rosario-Lozano, M.P.; Claros, M.G.; Blasco-Amaro, J.A. An innovative framework to determine the implementation level of personalized medicine: A systematic review. Front. Public Heal. 2023, 11, 1039688. [Google Scholar] [CrossRef]
- Abul-Husn, N.S.; Kenny, E.E. Personalized Medicine and the Power of Electronic Health Records. Cell 2019, 177, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Goetz, L.H.; Schork, N.J. Personalized medicine: motivation, challenges, and progress. Fertil. Steril. 2018, 109, 952–963. [Google Scholar] [CrossRef] [PubMed]
- Malod-Dognin, N.; Petschnigg, J.; Pržulj, N. Precision medicine―A promising, yet challenging road lies ahead. Current Opinion in Systems Biology 2018, 7, 1–7. [Google Scholar] [CrossRef]
- Brittain, H.K.; Scott, R.; Thomas, E. The rise of the genome and personalised medicine. Clin. Med. 2017, 17, 545–551. [Google Scholar] [CrossRef]
- Ginsburg, G.S.; Willard, H.F. Genomic and personalized medicine: foundations and applications. Transl. Res. 2009, 154, 277–287. [Google Scholar] [CrossRef]
- Sadee, W. Genomics and personalized medicine. International journal of pharmaceutics 2011, 415, 2–4. [Google Scholar] [CrossRef] [PubMed]
- Goetz, L.H.; Schork, N.J. Personalized medicine: motivation, challenges, and progress. Fertil. Steril. 2018, 109, 952–963. [Google Scholar] [CrossRef] [PubMed]
- Mini, E.; Nobili, S. Pharmacogenetics: implementing personalized medicine. Clinical cases in mineral and bone metabolism 2009, 6, 17–24. [Google Scholar] [PubMed]
- Hassan, R.; Allali, I.; E Agamah, F.; Elsheikh, S.S.M.; E Thomford, N.; Dandara, C.; Chimusa, E.R. Drug response in association with pharmacogenomics and pharmacomicrobiomics: towards a better personalized medicine. Briefings Bioinform. 2020, 22. [Google Scholar] [CrossRef] [PubMed]
- Mirsadeghi, S.; Larijani, B. Personalized Medicine: Pharmacogenomics and Drug Development. Acta Medica Iranica 2017, 55, 150–165. [Google Scholar]
- Cascorbi, I. Significance of Pharmacogenomics in Precision Medicine. Clin. Pharmacol. Ther. 2018, 103, 732–735. [Google Scholar] [CrossRef] [PubMed]
- HHS. FDA. CDER. BER. CDRH. Guidance for industry—Clinical pharmacogenomics: premarket evaluation in early-stage clinical studies and recommendations for labeling. January 2013. https://www.fda.gov/downloads/Drug.
- Classen, D.C.; Pestotnik, S.L.; Evans, R.S.; Lloyd, J.F.; Burke, J.P. Adverse drug events in hospitalized patients: excess length of stay, extra costs, and attributable mortality. Jama 1997, 277, 301–6. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R. The role of pharmacogenomics in precision medicine. Medical Laboratory Observer (MLO) 2017, 49. [Google Scholar]
- Shalimova, A.; Babasieva, V.; Chubarev, V.N.; Tarasov, V.V.; Schiöth, H.B.; Mwinyi, J. Therapy response prediction in major depressive disorder: current and novel genomic markers influencing pharmacokinetics and pharmacodynamics. Pharmacogenomics 2021, 22, 485–503. [Google Scholar] [CrossRef]
- Zięba, A.; Matosiuk, D.; Kaczor, A.A. The Role of Genetics in the Development and Pharmacotherapy of Depression and Its Impact on Drug Discovery. Int. J. Mol. Sci. 2023, 24, 2946. [Google Scholar] [CrossRef]
- Adams, J. Pharmacogenomics and personalized medicine. Nature Education 2008, 1, 194. [Google Scholar]
- Grech, G.; Grossman, I. (Eds.) Preventive and predictive genetics: Towards personalised medicine. Springer; 2015 Jun 24.
- Vargas, A.J.; Harris, C.C. Biomarker development in the precision medicine era: lung cancer as a case study. Nat. Rev. Cancer 2016, 16, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Arafah, A.; Khatoon, S.; Rasool, I.; Khan, A.; Rather, M.A.; Abujabal, K.A.; Faqih, Y.A.H.; Rashid, H.; Rashid, S.M.; Ahmad, S.B.; et al. The Future of Precision Medicine in the Cure of Alzheimer’s Disease. Biomedicines 2023, 11, 335. [Google Scholar] [CrossRef]
- Yeh, I.; von Deimling, A.; Bastian, B.C. Clonal BRAF Mutations in Melanocytic Nevi and Initiating Role of BRAF in Melanocytic Neoplasia. JNCI J. Natl. Cancer Inst. 2013, 105, 917–919. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Tan, W.; Song, Q.; Pei, H.; Li, J. BRCA1 and Breast Cancer: Molecular Mechanisms and Therapeutic Strategies. Front. Cell Dev. Biol. 2022, 10, 813457. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, J.I.; Dyberg, C.; Fransson, S.; Wickström, M. Molecular mechanisms and therapeutic targets in neuroblastoma. Pharmacol. Res. 2018, 131, 164–176. [Google Scholar] [CrossRef]
- Miller, V.A.; Hirsh, V.; Cadranel, J.; Chen, Y.-M.; Park, K.; Kim, S.-W.; Zhou, C.; Su, W.-C.; Wang, M.; Sun, Y.; et al. Afatinib versus placebo for patients with advanced, metastatic non-small-cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX-Lung 1): A phase 2b/3 randomised trial. Lancet Oncol. 2012, 13, 528–538. [Google Scholar] [CrossRef]
- Shaw, A.T.; Kim, D.-W.; Nakagawa, K.; Seto, T.; Crinó, L.; Ahn, M.-J.; De Pas, T.; Besse, B.; Solomon, B.J.; Blackhall, F.; et al. Crizotinib versus Chemotherapy in AdvancedALK-Positive Lung Cancer. New Engl. J. Med. 2013, 368, 2385–2394. [Google Scholar] [CrossRef]
- Schwaederle, M.; Zhao, M.; Lee, J.J.; Lazar, V.; Leyland-Jones, B.; Schilsky, R.L.; Mendelsohn, J.; Kurzrock, R. Association of biomarker-based treatment strategies with response rates and progression-free survival in refractory malignant neoplasms: a meta-analysis. JAMA oncology 2016, 2, 1452–9. [Google Scholar] [CrossRef]
- Paez, J.G.; Jänne, P.A.; Lee, J.C.; Tracy, S.; Greulich, H.; Gabriel, S.; Herman, P.; Kaye, F.J.; Lindeman, N.; Boggon, T.J.; et al. EGFR Mutations in Lung Cancer: Correlation with Clinical Response to Gefitinib Therapy. Science 2004, 304, 1497–1500. [Google Scholar] [CrossRef]
- Andrews, L.P.; Yano, H.; Vignali, D.A.A. Inhibitory receptors and ligands beyond PD-1, PD-L1 and CTLA-4: breakthroughs or backups. Nat. Immunol. 2019, 20, 1425–1434. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, N.; Nakamura, Y. Importance of immunopharmacogenomics in cancer treatment: Patient selection and monitoring for immune checkpoint antibodies. Cancer Sci. 2016, 107, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y. Large-scale data analysis and integration to advance precision prognosis, therapy stratification and understanding of human disease. (Doctoral dissertation, University of Southampton).
- Classen, S.; Staratschek-Jox, A.; Schultze, J.L. Use of Genome-Wide High-Throughput Technologies in Biomarker Development. Biomarkers Med. 2008, 2, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Gardner S, Das S, Taylor K. AI enabled precision medicine: patient stratification, drug repurposing and combination therapies. InArtificial intelligence in oncology drug discovery and development 2020 Sep 9. IntechOpen.
- Chial, H. Rare genetic disorders: learning about genetic disease through gene mapping, SNPs, and microarray data. Nature education 2008, 1, 192. [Google Scholar]
- Heo, C.U.; Choi, C.-I. Current Progress in Pharmacogenetics of Second-Line Antidiabetic Medications: Towards Precision Medicine for Type 2 Diabetes. J. Clin. Med. 2019, 8, 393. [Google Scholar] [CrossRef] [PubMed]
- Bode, A.M.; Dong, Z. Precision oncology- the future of personalized cancer medicine? npj Precis. Oncol. 2017, 1, 2. [Google Scholar] [CrossRef] [PubMed]
- Morash, M.; Mitchell, H.; Beltran, H.; Elemento, O.; Pathak, J. The Role of Next-Generation Sequencing in Precision Medicine: A Review of Outcomes in Oncology. J. Pers. Med. 2018, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Schwartzberg, L.; Kim, E.S.; Liu, D.; Schrag, D. Precision oncology: who, how, what, when, and when not? American Society of Clinical Oncology Educational Book 2017, 37, 160–9. [Google Scholar] [CrossRef] [PubMed]
- Morash, M.; Mitchell, H.; Beltran, H.; Elemento, O.; Pathak, J. The Role of Next-Generation Sequencing in Precision Medicine: A Review of Outcomes in Oncology. J. Pers. Med. 2018, 8, 30. [Google Scholar] [CrossRef]
- Soda, N.; Clack, K.; Shiddiky, M.J.A. Recent advances in liquid biopsy technologies for cancer biomarker detection. Sensors Diagn. 2022, 1, 343–375. [Google Scholar] [CrossRef]
- Naithani, N.; Sinha, S.; Misra, P.; Vasudevan, B.; Sahu, R. Precision medicine: Concept and tools. Med J. Armed Forces India 2021, 77, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Atsaves, V.; Leventaki, V.; Rassidakis, G.Z.; Claret, F.X. AP-1 Transcription Factors as Regulators of Immune Responses in Cancer. Cancers 2019, 11, 1037. [Google Scholar] [CrossRef]
- Poland, G.A.; Kennedy, R.B.; Ovsyannikova, I.G. Vaccinomics and personalized vaccinology: is science leading us toward a new path of directed vaccine development and discovery? PLoS pathogens 2011, 7, e1002344. [Google Scholar] [CrossRef] [PubMed]
- Sunil Krishnan, G.; Joshi, A.; Kaushik, V. Bioinformatics in personalized medicine. Advances in Bioinformatics 2021, 303–315. [Google Scholar]
- A Poland, G.; Ovsyannikova, I.G.; Jacobson, R.M. Personalized vaccines: the emerging field of vaccinomics. Expert Opin. Biol. Ther. 2008, 8, 1659–1667. [Google Scholar] [CrossRef] [PubMed]
- Visscher, P.M.; Wray, N.R.; Zhang, Q.; Sklar, P.; McCarthy, M.I.; Brown, M.A.; Yang, J. 10 years of GWAS discovery: biology, function, and translation. The American Journal of Human Genetics 2017, 101, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Lee, S.H.; Goddard, M.E.; Visscher, P.M. GCTA: A Tool for Genome-wide Complex Trait Analysis. Am. J. Hum. Genet. 2011, 88, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Doudna, J.A. CRISPR technology: A decade of genome editing is only the beginning. Science 2023, 379, 251. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kweon, J.; Kim, Y. Recent advances in CRISPR-based functional genomics for the study of disease-associated genetic variants. Exp. Mol. Med. 2024, 56, 861–869. [Google Scholar] [CrossRef]
- Filipp, F.V. Opportunities for Artificial Intelligence in Advancing Precision Medicine. Curr. Genet. Med. Rep. 2019, 7, 208–213. [Google Scholar] [CrossRef]
- Miyano, S. Revolutionizing cancer genomic medicine by AI and supercomputer with big data. In2018 IEEE Symposium on VLSI Technology 2018 Jun 18 (pp. 7-11). IEEE.
- Kawakami, E.; Tabata, J.; Yanaihara, N.; Ishikawa, T.; Koseki, K.; Iida, Y.; Saito, M.; Komazaki, H.; Shapiro, J.S.; Goto, C.; Akiyama, Y. Application of artificial intelligence for preoperative diagnostic and prognostic prediction in epithelial ovarian cancer based on blood biomarkers. Clinical cancer research 2019, 25, 3006–15. [Google Scholar] [CrossRef] [PubMed]
- Hussain, I.; Nazir, M.B. Precision Medicine: AI and Machine Learning Advancements in Neurological and Cardiac Health. Revista Espanola de Documentacion Cientifica 2024, 18, 150–79. [Google Scholar]
- Bertram, J.S. The molecular biology of cancer. Molecular aspects of medicine 2000, 21, 167–223. [Google Scholar] [CrossRef] [PubMed]
- Musyuni, P.; Bai, J.; Sheikh, A.; Vasanthan, K.S.; Jain, G.K.; Abourehab, M.A.; Lather, V.; Aggarwal, G.; Kesharwani, P.; Pandita, D. Precision medicine: Ray of hope in overcoming cancer multidrug resistance. Drug Resist. Updat. 2022, 65, 100889. [Google Scholar] [CrossRef]
- https://www.fda.gov/drugs/development-approval-process-drugs/drug-approvals-and-databases.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).