Submitted:
28 June 2024
Posted:
28 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
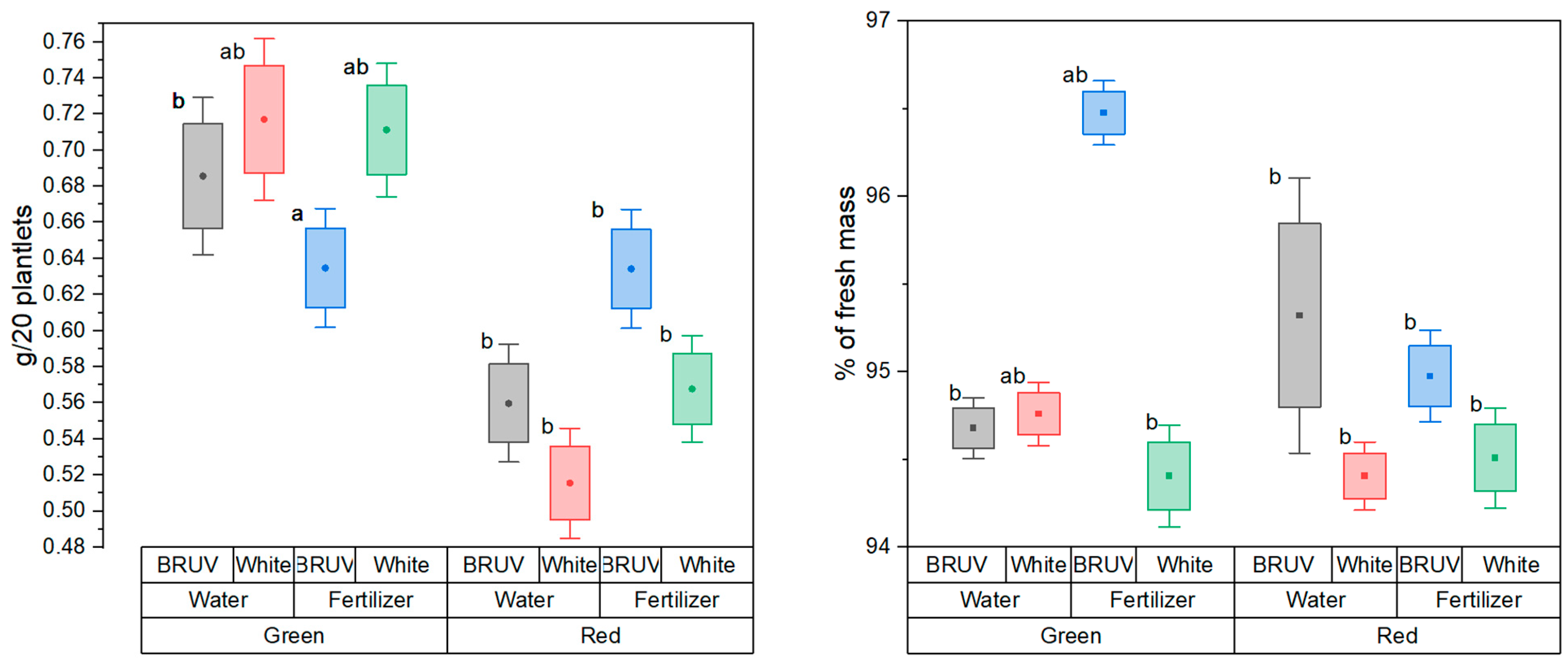
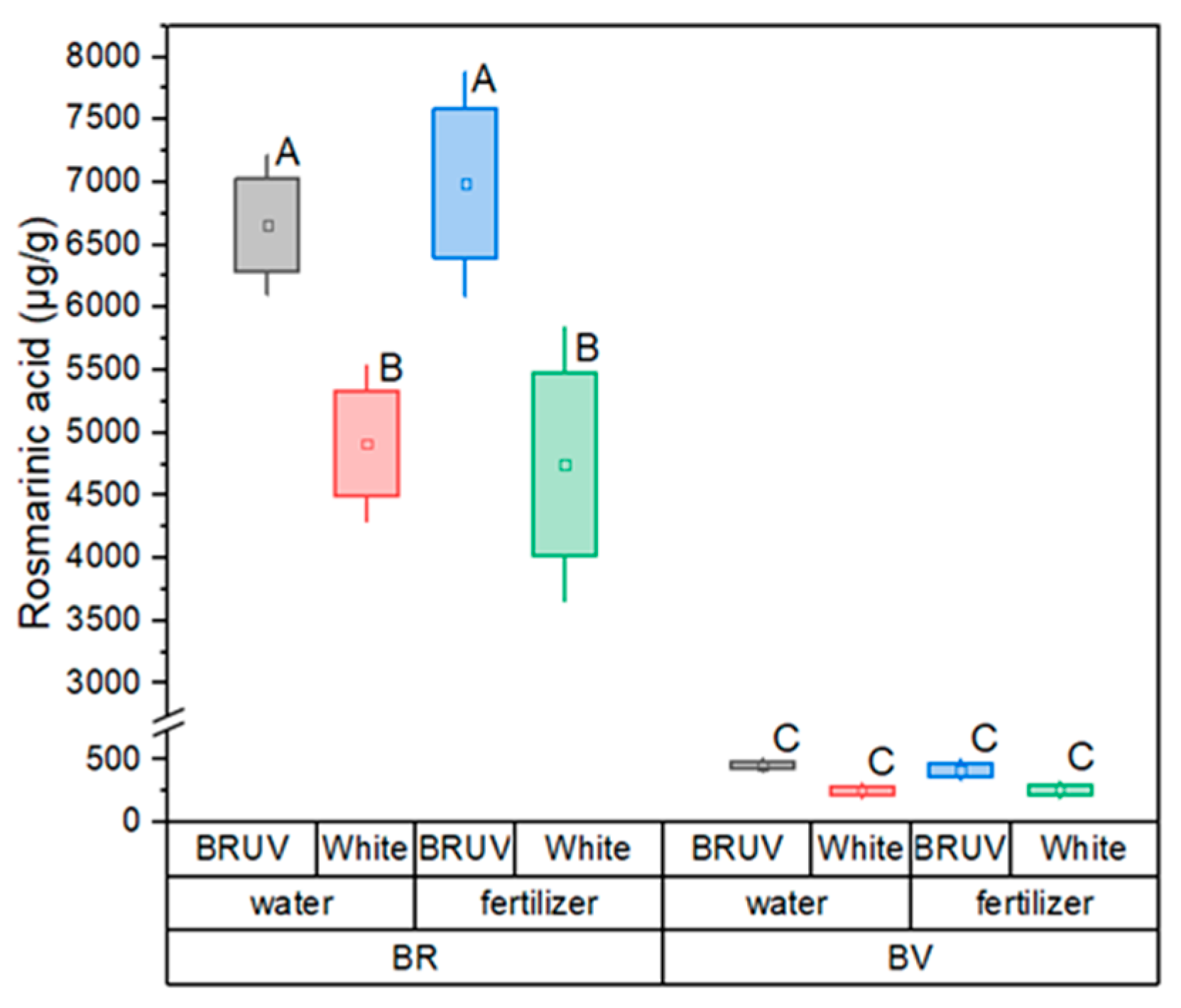
2. Results
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Analyses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rahman, Md. M.; Rahaman, Md. S.; Islam, Md. R.; Rahman, F.; Mithi, F. M.; Alqahtani, T.; Almikhlafi, M. A.; Alghamdi, S. Q.; Alruwaili, A. S.; Hossain, Md. S.; et al. Role of Phenolic Compounds in Human Disease: Current Knowledge and Future Prospects. Molecules 2021, 27, 233. [Google Scholar] [CrossRef] [PubMed]
- Ambriz-Perez, D. L.; Leyva-Lopez, N.; Gutierrez-Grijalva, E. P.; Heredia, J. B. Phenolic Compounds: Natural Alternative in Inflammation Treatment. A Review. Cogent Food Agric. 2016, 2. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative Stress, Aging, and Diseases. Clin. Interv. Aging 2018, Volume 13, 757–772. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Sathyapalan, T.; Atkin, S. L.; Sahebkar, A. Molecular Mechanisms Linking Oxidative Stress and Diabetes Mellitus. Oxid. Med. Cell. Longev. 2020, 2020, 1–13. [Google Scholar] [CrossRef]
- Blesa, J.; Trigo-Damas, I.; Quiroga-Varela, A.; Jackson-Lewis, V. R. Oxidative Stress and Parkinson’s Disease. Front. Neuroanat. 2015, 9. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-J.; Zhang, X.; Chen, W.-W. Role of Oxidative Stress in Alzheimer’s Disease. Biomed. Rep. 2016, 4, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Cianciosi, D.; Forbes-Hernández, T.; Afrin, S.; Gasparrini, M.; Reboredo-Rodriguez, P.; Manna, P.; Zhang, J.; Bravo Lamas, L.; Martínez Flórez, S.; Agudo Toyos, P.; et al. Phenolic Compounds in Honey and Their Associated Health Benefits: A Review. Molecules 2018, 23, 2322. [Google Scholar] [CrossRef] [PubMed]
- Saha, S. K.; Lee, S. B.; Won, J.; Choi, H. Y.; Kim, K.; Yang, G.-M.; Dayem, A. A.; Cho, S. Correlation between Oxidative Stress, Nutrition, and Cancer Initiation. Int. J. Mol. Sci. 2017, 18, 1544. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Zhong, Y.; Duan, Y.; Chen, Q.; Li, F. Antioxidant Mechanism of Tea Polyphenols and Its Impact on Health Benefits. Anim. Nutr. 2020, 6, 115–123. [Google Scholar] [CrossRef]
- Zeb, A. Concept, Mechanism, and Applications of Phenolic Antioxidants in Foods. J. Food Biochem. 2020, 44. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M. C. B.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell. Longev. 2016, 2016, 1–9. [Google Scholar] [CrossRef]
- Lobiuc, A.; Pavăl, N.-E.; Mangalagiu, I. I.; Gheorghiță, R.; Teliban, G.-C.; Amăriucăi-Mantu, D.; Stoleru, V. Future Antimicrobials: Natural and Functionalized Phenolics. Molecules 2023, 28, 1114. [Google Scholar] [CrossRef] [PubMed]
- Lee, B. H.; Nam, T. G.; Park, N. Y.; Chun, O. K.; Koo, S. I.; Kim, D.-O. Estimated Daily Intake of Phenolics and Antioxidants from Green Tea Consumption in the Korean Diet. Int. J. Food Sci. Nutr. 2016, 67, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Stepaniak, U.; Topor-Mądry, R.; Szafraniec, K.; Pająk, A. Estimated Dietary Intake and Major Food Sources of Polyphenols in the Polish Arm of the HAPIEE Study. Nutrition 2014, 30, (11–12). [Google Scholar] [CrossRef] [PubMed]
- Kapolou, A.; Karantonis, H. C.; Rigopoulos, N.; Koutelidakis, A. E. Association of Mean Daily Polyphenols Intake with Mediterranean Diet Adherence and Anthropometric Indices in Healthy Greek Adults: A Retrospective Study. Appl. Sci. 2021, 11, 4664. [Google Scholar] [CrossRef]
- Wisnuwardani, R. W.; De Henauw, S.; Ferrari, M.; Forsner, M.; Gottrand, F.; Huybrechts, I.; Kafatos, A. G.; Kersting, M.; Knaze, V.; Manios, Y.; et al. Total Polyphenol Intake Is Inversely Associated with a Pro/Anti-Inflammatory Biomarker Ratio in European Adolescents of the HELENA Study. J. Nutr. 2020, 150, 1610–1618. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ros, R.; Knaze, V.; Rothwell, J.A.; Hémon, B.; Moskal, A.; Overvad, K.; Tjønneland, A.; Kyrø, C.; Fagherazzi, G.; Boutron-Ruault, M.-C.; et al. Dietary Polyphenol Intake in Europe: The European Prospective Investigation into Cancer and Nutrition (EPIC) Study. Eur. J. Nutr. 2016, 55, 1359–1375. [Google Scholar] [CrossRef] [PubMed]
- Wisnuwardani, R. W.; De Henauw, S.; Androutsos, O.; Forsner, M.; Gottrand, F.; Huybrechts, I.; Knaze, V.; Kersting, M.; Le Donne, C.; Marcos, A.; et al. Estimated Dietary Intake of Polyphenols in European Adolescents: The HELENA Study. Eur. J. Nutr. 2019, 58, 2345–2363. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Williamson, G. Dietary Intake and Bioavailability of Polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef]
- Knaze, V.; Rothwell, J.A.; Zamora-Ros, R.; Moskal, A.; Kyrø, C.; Jakszyn, P.; Skeie, G.; Weiderpass, E.; Santucci de Magistris, M.; Agnoli, C.; et al. A New Food-Composition Database for 437 Polyphenols in 19,899 Raw and Prepared Foods Used to Estimate Polyphenol Intakes in Adults from 10 European Countries. Am. J. Clin. Nutr. 2018, 108, 517–524. [Google Scholar] [CrossRef]
- Bhaswant, M.; Shanmugam, D. K.; Miyazawa, T.; Abe, C.; Miyazawa, T. Microgreens—A Comprehensive Review of Bioactive Molecules and Health Benefits. Molecules 2023, 28, 867. [Google Scholar] [CrossRef] [PubMed]
- Michell, K.A.; Isweiri, H.; Newman, S.E.; Bunning, M.; Bellows, L.L.; Dinges, M.M.; Grabos, L.E.; Rao, S.; Foster, M.T.; Heuberger, A.L.; et al. Microgreens: Consumer Sensory Perception and Acceptance of an Emerging Functional Food Crop. J. Food Sci. 2020, 85, 926–935. [Google Scholar] [CrossRef]
- Lobiuc, A.; Vasilache, V.; Oroian, M.; Stoleru, T.; Burducea, M.; Pintilie, O.; Zamfirache, M.-M. Blue and Red LED Illumination Improves Growth and Bioactive Compounds Contents in Acyanic and Cyanic Ocimum Basilicum L. Microgreens. Molecules 2017, 22, 2111. [Google Scholar] [CrossRef]
- Hosseini, A.; Zare Mehrjerdi, M.; Aliniaeifard, S. Alteration of Bioactive Compounds in Two Varieties of Basil ( Ocimum Basilicum ) Grown Under Different Light Spectra. J. Essent. Oil Bear. Plants 2018, 21, 913–923. [Google Scholar] [CrossRef]
- Viršilė, A.; Laužikė, K.; Sutulienė, R.; Brazaitytė, A.; Kudirka, G.; Samuolienė, G. Distinct Impacts of UV-A Light Wavelengths on Nutraceutical and Mineral Contents in Green and Purple Basil Cultivated in a Controlled Environment. Horticulturae 2023, 9, 1168. [Google Scholar] [CrossRef]
- Shiga, T.; Shoji, K.; Shimada, H.; Hashida, S.; Goto, F.; Yoshihara, T. Effect of Light Quality on Rosmarinic Acid Content and Antioxidant Activity of Sweet Basil, Ocimum Basilicum L. Plant Biotechnol. 2009, 26, 255–259. [Google Scholar] [CrossRef]
- Vodnik, D.; Vogrin, Ž.; Šircelj, H.; Grohar, M. C.; Medič, A.; Carović-Stanko, K.; Safner, T.; Lazarević, B. Phenotyping of Basil (Ocimum Basilicum L.) Illuminated with UV-A Light of Different Wavelengths and Intensities. Sci. Hortic. 2023, 309, 111638. [Google Scholar] [CrossRef]
- Chutimanukul, P.; Wanichananan, P.; Janta, S.; Toojinda, T.; Darwell, C. T.; Mosaleeyanon, K. The Influence of Different Light Spectra on Physiological Responses, Antioxidant Capacity and Chemical Compositions in Two Holy Basil Cultivars. Sci. Rep. 2022, 12, 588. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, L.; Zou, H.; Qiu, L.; Zheng, Y.; Yang, D.; Wang, Y. Effects of Light on Secondary Metabolite Biosynthesis in Medicinal Plants. Front. Plant Sci. 2021, 12, 781236. [Google Scholar] [CrossRef]
- Gholamnia, A.; Mosleh Arani, A.; Sodaeizadeh, H.; Tarkesh Esfahani, S.; Ghasemi, S. Expression Profiling of Rosmarinic Acid Biosynthetic Genes and Some Physiological Responses from Mentha Piperita L. under Salinity and Heat Stress. Physiol. Mol. Biol. Plants Int. J. Funct. Plant Biol. 2022, 28, 545–557. [Google Scholar] [CrossRef]
- Rizi, M. R.; Azizi, A.; Sayyari, M.; Mirzaie-Asl, A.; Conti, L. Increased Phenylpropanoids Production in UV-B Irradiated Salvia Verticillata as a Consequence of Altered Genes Expression in Young Leaves. Plant Physiol. Biochem. 2021, 167, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R. A.; Paiva, N. L. Stress-Induced Phenylpropanoid Metabolism. Plant Cell 1995, 1085–1097. [Google Scholar] [CrossRef]
- Koshiba, T.; Saito, E.; Ono, N.; Yamamoto, N.; Sato, M. Purification and Properties of Flavin- and Molybdenum-Containing Aldehyde Oxidase from Coleoptiles of Maize. Plant Physiol. 1996, 110, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Boudet, A. M.; Kajita, S.; Grima-Pettenati, J.; Goffner, D. Lignins and Lignocellulosics: A Better Control of Synthesis for New and Improved Uses. Trends Plant Sci. 2003, 8, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.; Husler, E.; Karwatzki, B.; Meinhard, J. Proposed Biosynthetic Pathway for Rosmarinic Acid in Cell Cultures of Coleus Blumei Benth. Planta 1993, 189. [Google Scholar] [CrossRef]
- Petersen, M.; Abdullah, Y.; Benner, J.; Eberle, D.; Gehlen, K.; Hücherig, S.; Janiak, V.; Kim, K. H.; Sander, M.; Weitzel, C.; et al. Evolution of Rosmarinic Acid Biosynthesis. Phytochemistry 2009, 70, 1663–1679. [Google Scholar] [CrossRef] [PubMed]
- Kivimäenpä, M.; Mofikoya, A.; Abd El-Raheem, A. M.; Riikonen, J.; Julkunen-Tiitto, R.; Holopainen, J. K. Alteration in Light Spectra Causes Opposite Responses in Volatile Phenylpropanoids and Terpenoids Compared with Phenolic Acids in Sweet Basil ( Ocimum Basilicum ) Leaves. J. Agric. Food Chem. 2022, 70, 12287–12296. [Google Scholar] [CrossRef] [PubMed]
- Taulavuori, K.; Hyöky, V.; Oksanen, J.; Taulavuori, E.; Julkunen-Tiitto, R. Species-Specific Differences in Synthesis of Flavonoids and Phenolic Acids under Increasing Periods of Enhanced Blue Light. Environ. Exp. Bot. 2016, 121, 145–150. [Google Scholar] [CrossRef]
- Pech, R.; Volná, A.; Hunt, L.; Bartas, M.; Červeň, J.; Pečinka, P.; Špunda, V.; Nezval, J. Regulation of Phenolic Compound Production by Light Varying in Spectral Quality and Total Irradiance. Int. J. Mol. Sci. 2022, 23, 6533. [Google Scholar] [CrossRef]
- Ptushenko, O. S.; Ptushenko, V. V.; Solovchenko, A. E. Spectrum of Light as a Determinant of Plant Functioning: A Historical Perspective. Life 2020, 10, 25. [Google Scholar] [CrossRef]
- Pashkovskiy, P.; Ivanov, Y.; Ivanova, A.; Kreslavski, V. D.; Vereshchagin, M.; Tatarkina, P.; Kuznetsov, V. V.; Allakhverdiev, S. I. Influence of Light of Different Spectral Compositions on Growth Parameters, Photosynthetic Pigment Contents and Gene Expression in Scots Pine Plantlets. Int. J. Mol. Sci. 2023, 24, 2063. [Google Scholar] [CrossRef]
- Tarakanov, I. G.; Tovstyko, D. A.; Lomakin, M. P.; Shmakov, A. S.; Sleptsov, N. N.; Shmarev, A. N.; Litvinskiy, V. A.; Ivlev, A. A. Effects of Light Spectral Quality on Photosynthetic Activity, Biomass Production, and Carbon Isotope Fractionation in Lettuce, Lactuca Sativa L., Plants. Plants 2022, 11, 441. [Google Scholar] [CrossRef]
- Paradiso, R.; Proietti, S. Light-Quality Manipulation to Control Plant Growth and Photomorphogenesis in Greenhouse Horticulture: The State of the Art and the Opportunities of Modern LED Systems. J. Plant Growth Regul. 2022, 41, 742–780. [Google Scholar] [CrossRef]
- Lazzarin, M.; Meisenburg, M.; Meijer, D.; Van Ieperen, W.; Marcelis, L. F. M.; Kappers, I. F.; Van Der Krol, A. R.; Van Loon, J. J. A.; Dicke, M. LEDs Make It Resilient: Effects on Plant Growth and Defense. Trends Plant Sci. 2021, 26, 496–508. [Google Scholar] [CrossRef]
- Pál, M.; Hamow, K. Á.; Rahman, A.; Majláth, I.; Tajti, J.; Gondor, O. K.; Ahres, M.; Gholizadeh, F.; Szalai, G.; Janda, T. Light Spectral Composition Modifies Polyamine Metabolism in Young Wheat Plants. Int. J. Mol. Sci. 2022, 23, 8394. [Google Scholar] [CrossRef]
- Marchant, M. J.; Molina, P.; Montecinos, M.; Guzmán, L.; Balada, C.; Castro, M. Effects of LED Light Spectra on the Development, Phytochemical Profile, and Antioxidant Activity of Curcuma Longa from Easter Island. Plants 2022, 11, 2701. [Google Scholar] [CrossRef]
- Makowski, W.; Tokarz, B.; Banasiuk, R.; Królicka, A.; Dziurka, M.; Wojciechowska, R.; Tokarz, K. M. Is a Blue–Red Light a Good Elicitor of Phenolic Compounds in the Family Droseraceae? A Comparative Study. J. Photochem. Photobiol. B 2019, 201, 111679. [Google Scholar] [CrossRef]
- Zhen, S.; Haidekker, M.; Van Iersel, M. W. Far--red Light Enhances Photochemical Efficiency in a Wavelength--dependent Manner. Physiol. Plant. 2019, 167, 21–33. [Google Scholar] [CrossRef]
- Tan, T.; Li, S.; Fan, Y.; Wang, Z.; Ali Raza, M.; Shafiq, I.; Wang, B.; Wu, X.; Yong, T.; Wang, X.; et al. Far-Red Light: A Regulator of Plant Morphology and Photosynthetic Capacity. Crop J. 2022, 10, 300–309. [Google Scholar] [CrossRef]
- Wang, M.; Leng, C.; Zhu, Y.; Wang, P.; Gu, Z.; Yang, R. UV-B Treatment Enhances Phenolic Acids Accumulation and Antioxidant Capacity of Barley Seedlings. LWT 2022, 153, 112445. [Google Scholar] [CrossRef]
- Rabelo, M. C.; Bang, W. Y.; Nair, V.; Alves, R. E.; Jacobo-Velázquez, D. A.; Sreedharan, S.; De Miranda, M. R. A.; Cisneros-Zevallos, L. UVC Light Modulates Vitamin C and Phenolic Biosynthesis in Acerola Fruit: Role of Increased Mitochondria Activity and ROS Production. Sci. Rep. 2020, 10, 21972. [Google Scholar] [CrossRef]
- Chai, W. Y.; Goh, J. K.; Kalavally, V.; Rahman, S.; Lim, Y. Y.; Choo, W. S. Enhancing Rosmarinic Acid Production and Regulating Enzyme Activity in Melissa Officinalis L. Using Spectrally Tunable Light-Emitting Diodes. Ind. Crops Prod. 2023, 204, 117332. [Google Scholar] [CrossRef]
- Park, W. T.; Yeo, S. K.; Sathasivam, R.; Park, J. S.; Kim, J. K.; Park, S. U. Influence of Light-Emitting Diodes on Phenylpropanoid Biosynthetic Gene Expression and Phenylpropanoid Accumulation in Agastache Rugosa. Appl. Biol. Chem. 2020, 63, 25. [Google Scholar] [CrossRef]
- Marchica, A.; Cotrozzi, L.; Detti, R.; Lorenzini, G.; Pellegrini, E.; Petersen, M.; Nali, C. The Biosynthesis of Phenolic Compounds Is an Integrated Defence Mechanism to Prevent Ozone Injury in Salvia Officinalis. Antioxidants 2020, 9, 1274. [Google Scholar] [CrossRef] [PubMed]
- Khater, E.-S.; Bahnasawy, A.; Abass, W.; Morsy, O.; El-Ghobashy, H.; Shaban, Y.; Egela, M. Production of Basil (Ocimum Basilicum L.) under Different Soilless Cultures. Sci. Rep. 2021, 11, 12754. [Google Scholar] [CrossRef]
- Herald, T. J.; Gadgil, P.; Tilley, M. High--throughput Micro Plate Assays for Screening Flavonoid Content and DPPH--scavenging Activity in Sorghum Bran and Flour. J. Sci. Food Agric. 2012, 92, 2326–2331. [Google Scholar] [CrossRef]


| Cultivar | Fertilizer | Treatment | Fs | Fm’ | ΦPSII | Chlorophyll (AU) |
|---|---|---|---|---|---|---|
| Green | Water | BRUV | 474.63a±17.32 | 2106.89ab±72.83 | 0.78ab±0.01 | 6.33a±0.28 |
| White | 539.99a±21.1 | 2245.66a±80.99 | 0.77b±0.01 | 5.16a±1.91 | ||
| Fertilizer | BRUV | 561.67a±89.45 | 2288.25ab±343.99 | 0.76ab±0.02 | - | |
| White | 568.34a±25.77 | 2378.59a±99.4 | 0.77ab±0.01 | - | ||
| Red | Water | BRUV | 287.67b±16.19 | 1288.27c±64.63 | 0.79a±0.01 | 5.97a±0.37 |
| White | 321.5b±16.79 | 1388.1c±54.73 | 0.78ab±0.01 | 3.22b±0.37 | ||
| Fertilizer | BRUV | 415.59ab±20.99 | 1652.67bc±90.88 | 0.75b±0.01 | - | |
| White | 505.34a±27.53 | 2083a±133.11 | 0.76ab±0.01 | - |
| Cultivar | Fertilizer | Treatment | Total phenolic content (µg GAE/g dry plant mass) | Antioxidant activity (% inhibition) |
|---|---|---|---|---|
| Green | Water | BRUV | 3739.1b±202.11 | 88.08±1.71a |
| White | 3846.48b±200.94 | 88.19±4.83a | ||
| Fertilizer | BRUV | 5739.56b±313.12 | 85.28±1.54a | |
| White | 4858.96b±264.71 | 81.4±3.85a | ||
| Red | Water | BRUV | 8194.1ab±476.49 | 86.96±3.97a |
| White | 7219.1ab±425.58 | 92.73±1.8a | ||
| Fertilizer | BRUV | 14063.79a±755.69 | 87.84±1.26a | |
| White | 13145.41a±768.87 | 92.33±1.46a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





