1. Introduction
Endometriosis affects one in every ten women today, as reported by published data. [
1,
2]. Recognizing the growing interest in this complex pathology, which profoundly impacts women’s health, fertility, and healthcare systems worldwide, the search for the newest and most effective systems of professional assessment of this disease is of highest importance.
Endometriosis surgery has become one of the most complex and advanced domains of contemporary surgery, presenting challenges that affect the entire team of the gynecology ward and the operating theatre. Consequently, the need for precise diagnosis and predictive planning is crucial for effective and safe surgery.[
3]
The safety of the patient and surgeon, adequately planned time and equipment in the operating theatre and the advantage of discussing a comprehensive and adapted consent form with the patient before the operation are among the most valuable issues in a surgeon’s work today.
The diagnostic system for deep endometriosis, #ENZIAN, has been crucial since its inception. [
4] This intuitive and anatomical classification, created by Professor J.Keckstein, is tailored for endometriosis surgeons. It enhances the surgical approach and improves communication between radiologists and gynecologists, as well as interpersonal communication on an international level. Therefore, the aim of our study was to assess the concordance of the preoperative application of the #Enzian classification (#ENZIANi) with the postoperative result ( #ENZIANs) using surgical findings as reference standard in a group of complex endometriosis cases surgically treated in Centre of Oncology in Opole, Poland.
2. Materials and Methods
The project received approval from the bioethics committee Medical Faculty, University of Opole reference number UO/0020/KB/2022, and was conducted in line with its assumptions. This report conforms to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guideline for observational studies.[
6]
Using the #ENZIAN classification system at our specialized center, the Oncological Gynecology Department at the Oncology Centre in Opole, Poland, we have been analyzing the preoperative staging of endometriosis and postoperative reassessment. This approach aims to enhance our ability to accurately predict the type and duration of procedures required for each patient.
2.1. Population
The database of the Oncological Gynecology Clinical Department at the Oncology Centre in Opole, Poland, was retrospectively searched from 01.01.2020 to 31.12.2023.
Exclusion criteria included a history of co-existing cancer, post-menopausal status, incomplete preoperative exam data (MRI, ultrasound, detailed medical and surgical history). The ward does not admit juvenile, pregnant, or male patients. We included patients who underwent surgery for deep endometriosis and were prepared with an adapted preoperative protocol, with mandatory expert ultrasound and MRI diagnosis. Since the new #ENZIAN classification was published in 2021, retrospective reassessment was conducted for patients in 2020 and 2021, as we began using #ENZIAN in 2022.
Overall, 282 consecutive patients with deep endometriosis were involved in this study. These findings are intended to improve our surgical methods, enhance patient safety, and increase the effectiveness of endometriosis treatment, ultimately leading to better surgical planning and detailed explanation of the procedure to the patient.
2.2. Ultrasound Examination
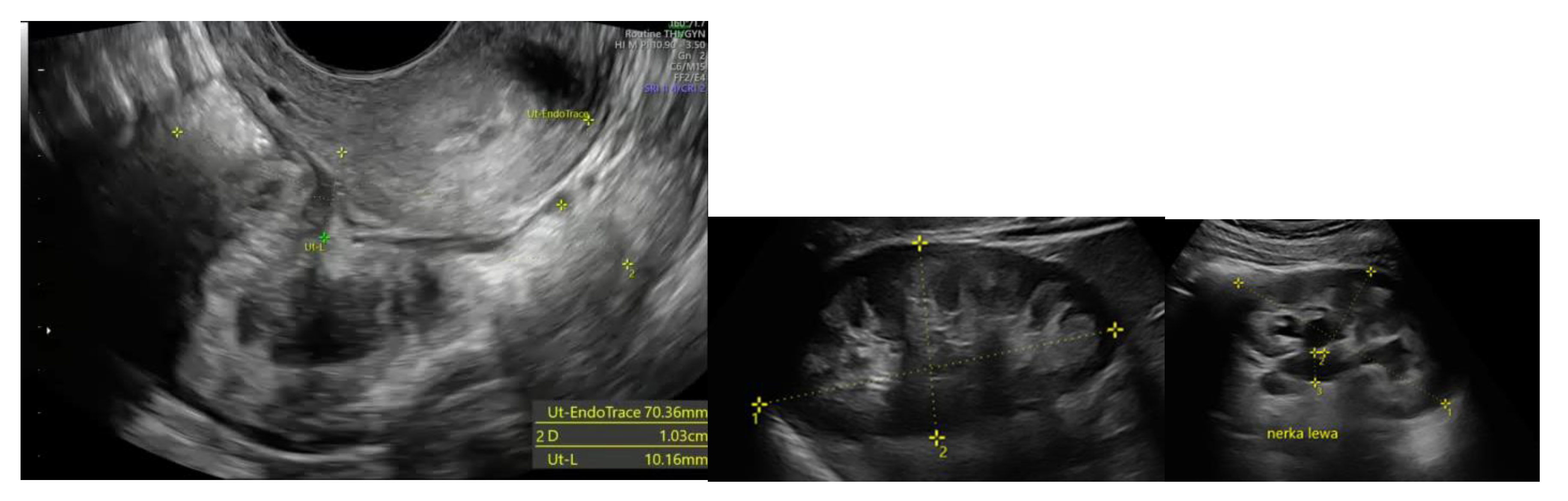
All patients underwent preoperative ultrasound examination by an expert surgeon-gynecologist – (E.M.-N.), with detailed pelvic compartments assessment via transvaginal sonography (TVS) and with kidney congestion assessment via transabdominal sonography (TAS), following an adapted protocol based on IDEA group guidelines. [
7] Examinations were performed on Voluson E8 Expert with the use of vaginal probe RIC5-9-D (depth max 16 cm) and abdominal probe RAB2-5-D. Detailed report was recorded for each patient. Examples of ultrasound examination images are shown in
Figure 1.
2.3. The Pelvic MRI Scan
The project assumed that each included patient would undergo an MRI examination at a specialized MRI facility collaborating with the Gynecological Oncology Department. As an exception, 11 scans performed at other MRI facilities were accepted, considering the risk of decreasing the accuracy of the description, setting a condition that the examinations would be conducted according to similar protocols. The current preparation protocol for MRI examinations and the technical parameters were consistent with ESUR guidelines. [
8]
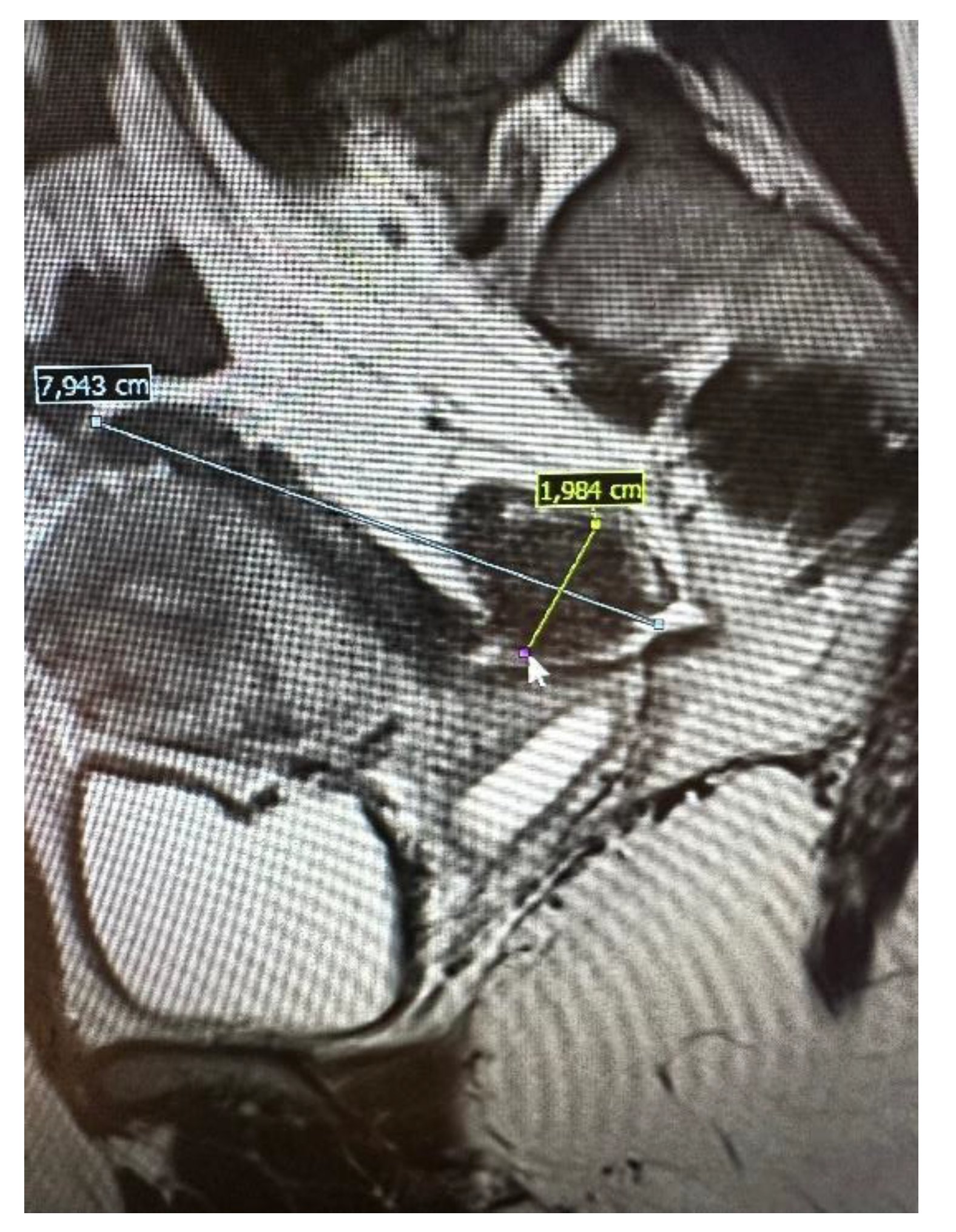
The pelvic MRI was conducted using a 3T scanner, following the established protocol for endometriosis. This included scans before and after the intravenous administration of a gadolinium-based contrast agent, as well as contrast administration into the vagina (using hyaluronic acid gel and a vaginal tampon) and rectum (using a water solution). Patients were positioned supine with a moderately filled bladder, and received an intravenous anti-peristaltic agent (butyl-scopolamine). The procedure was performed following bowel preparation (BPP) and fasting the previous day. The examination typically lasted approximately 60 minutes. The report included the abdominal wall structures. An example of an MRI scan is shown in
Figure 2.
2.4. Other Imaging Techniques
In individual cases of diaphragmatic, pulmonary, or upper abdominal endometriosis, additional diagnostic tests specific to the affected organ/area were performed. The chest was examined using computed tomography (CT) [
9], as this is the first-line diagnostic modality for bronchopulmonary and pleuropulmonary endometriosis. The diaphragm and upper abdominal organs were examined with targeted magnetic resonance imaging (MRI) of the abdomen, including the diaphragm, as the modality of choice. [
10,
11,
12]
2.5. Surgery
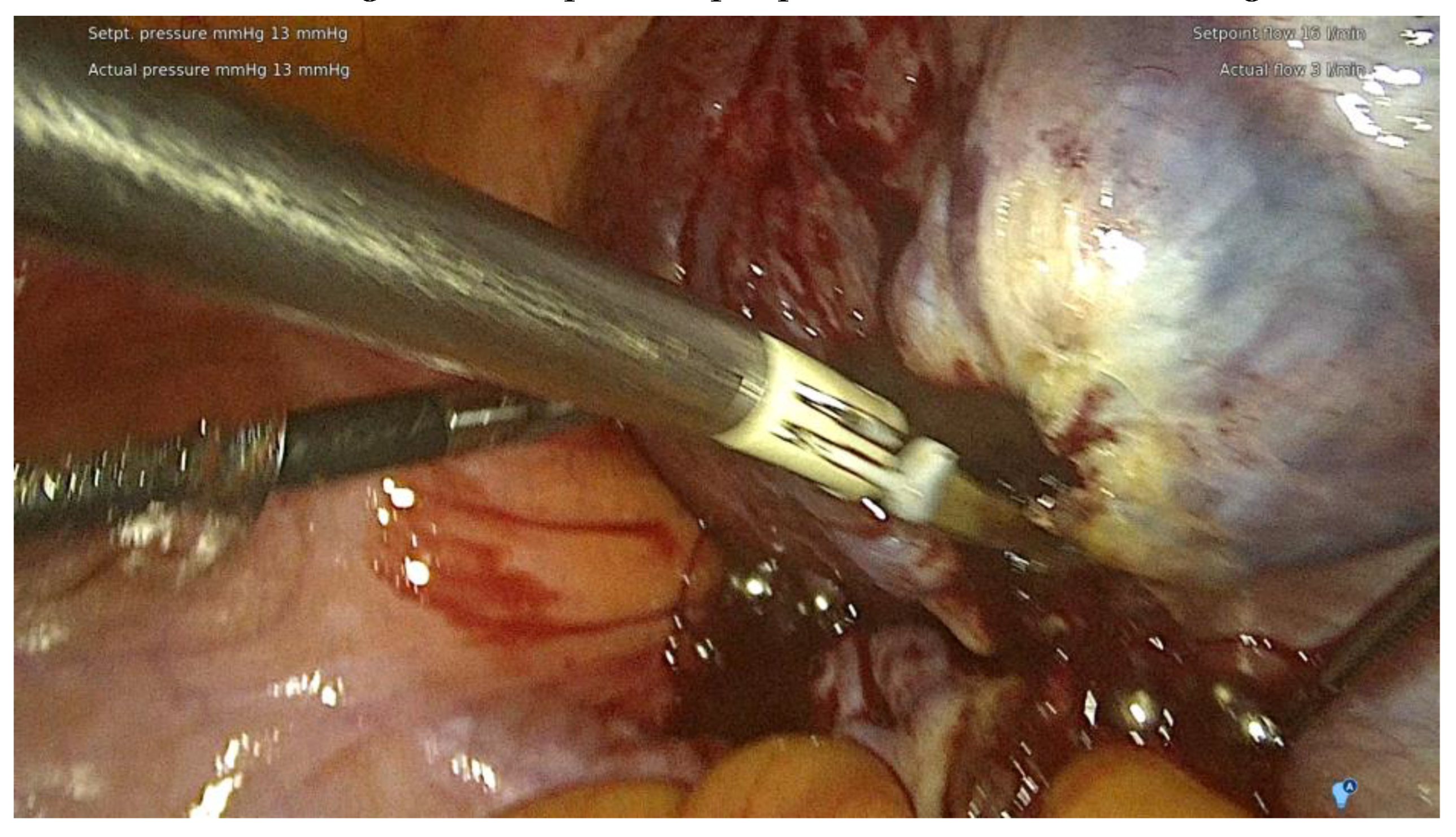
The surgical procedures were conducted at the ESGO-accredited Training & Surgical Center, one of the few hospitals in Poland performing the most complex surgeries and handling the highest number of deep endometriosis cases per year. The lead surgeon for all procedures was (E.M.-N.) assisted by her specialized team. Surgery was tailored to each patient, respecting their needs and following the scheme created with the compilation of modern and safe minimally invasive techniques with the use of ICG was the approach of choice. The support of multidisciplinary team was available if needed ( thoracic surgeon, urologist, oncology surgeon, neurosurgeon). The guidelines of the working group of the European Society for Gynecological Endoscopy (ESGE), European Society for Human Reproduction and Embryology (ESHRE) and the World Endometriosis Society (WES) recommendations on the practical aspects of surgery for treatment of deep endometriosis [
13] and surgical safety measures were adequately respected.
The range of the surgery was wide and consisted of modified radical hysterectomy, unilateral or bilateral infiltration of USLs and RVS resection, unilateral or bilateral ovarian cystectomy or drainage, large bowel resection procedures ( shaving, discoid resection, segmental resection of the rectum, sigmoid colon, caecum) , partial bladder resection, unilateral or bilateral ureterolysis sometimes with ureteral retransplantation, small intestine segmental resection, adnexectomy, nephrectomy, diaphragmatic lesions resection, bone, nerve and vessel lesion resection, and 2 cases of thoracic interventions. An image of the laparoscopic procedure is shown in
Figure 3.
2.6. Study Design
Given the personal experience and the availability of both imaging methods (TVS and MRI) for each patient qualified for surgery, the following sequence for assessing disease progression was decided upon.
Patients were evaluated on the #ENZIAN scale using two main criteria:
-
Preoperative assessment (#ENZIANi = imaging, TVS+MRI):
- ○
This assessment combined the findings from ultrasonography performed by the first surgeon qualifying and performing the surgery at each of these patients (E.M.-N.), using both vaginal and transabdominal probes, with the changes described in MRI reports by a radiology expert, using a 3T machine.
-
Postoperative assessment (#ENZIANs = surgery):
- ○
This assessment evaluated the changes observed during surgery.
The choice of combined imaging (TVS+MRI) facilitated the compilation of results and the preparation of a single, summed #ENZIANi scale before surgery. This approach combined the advantages of both imaging methods, allowing for quicker conclusions regarding disease progression and improved communication within the team.
2.7. Statistical Analysis
Data were collected in an Excel spreadsheet (Microsoft Excel 2013; Microsoft Corp., Redmond, USA) and statistically analyzed using MedCalc v. 19.5.3 (MedCalc Soft-ware Ltd., Ostend, Belgium). Data were presented as means with standard deviation, medians, ranges, numbers, and percentages depending on the type of data distribution. The concordance between preoperative TVS, TAS, and MRI examinations and surgical assessments for the involvement of #ENZIAN compartments was evaluated using Cohen’s kappa. This coefficient refers to measures of the agreement between two raters or measurements. Cohen’;s kappa is used to evaluate how well two or more judges agree on their assessments or classifications, adjusting for agreement that could happen by chance. Interpretation of Cohen’s kappa values: a value was interpreted following Altman’s recommendations as poor (<;0.20), fair (0.21-0.40), moderate (0.41-0.60), good (0.61-0.80), and very good (0.81-1.00). [
14] To graphically present concordance, correlation heatmaps were used. In addition, a diagnostic test evaluation was conducted. The sensitivity and specificity of TVS, TAS, and MRI for detecting endometriotic lesions/adhesions in each #ENZIAN compartment were calculated along with the positive predictive value (PPV), negative predictive value (NPV), and accuracy of TVS and TAS in identifying endometriotic lesions/adhesions in the various #Enzian compartments. The results of diagnostic test evaluation were presented as percentages with 95% confidence intervals (CIs). [
15] For the diagnostic test evaluation, the following assumptions were made: true negative was defined when both were negative, true positive was defined when both results were above 0, the false negative was defined when imaging was negative and surgery indicated lesion of 1-3, and false positive was defined when imaging detected a lesion of 1-3 and surgery was negative. Cases with m,x, and missing data were excluded.
3. Results
3.1. Population
Out of the 307 women operated for deep endometriosis ( DE) in of the Gynecological Oncology Ward at the Oncology Centre in Opole, Poland, between 01.05.2020 and 31.12.2023, according to the criteria 24 were excluded from the final cohort. The group meeting the inclusion criteria comprised 282 women. The mean age of women was 39.22±5.78 years.
3.2. Concordance
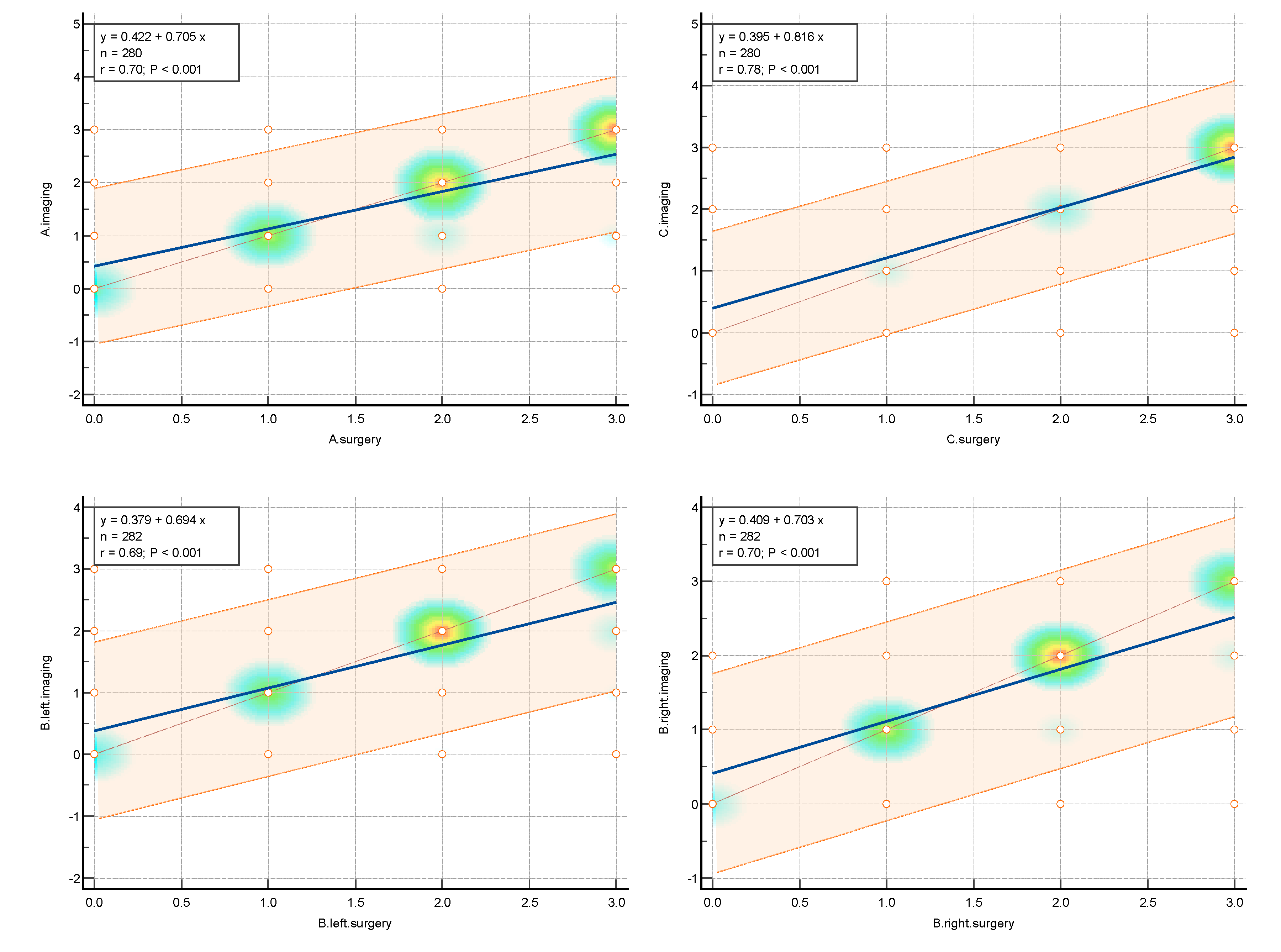
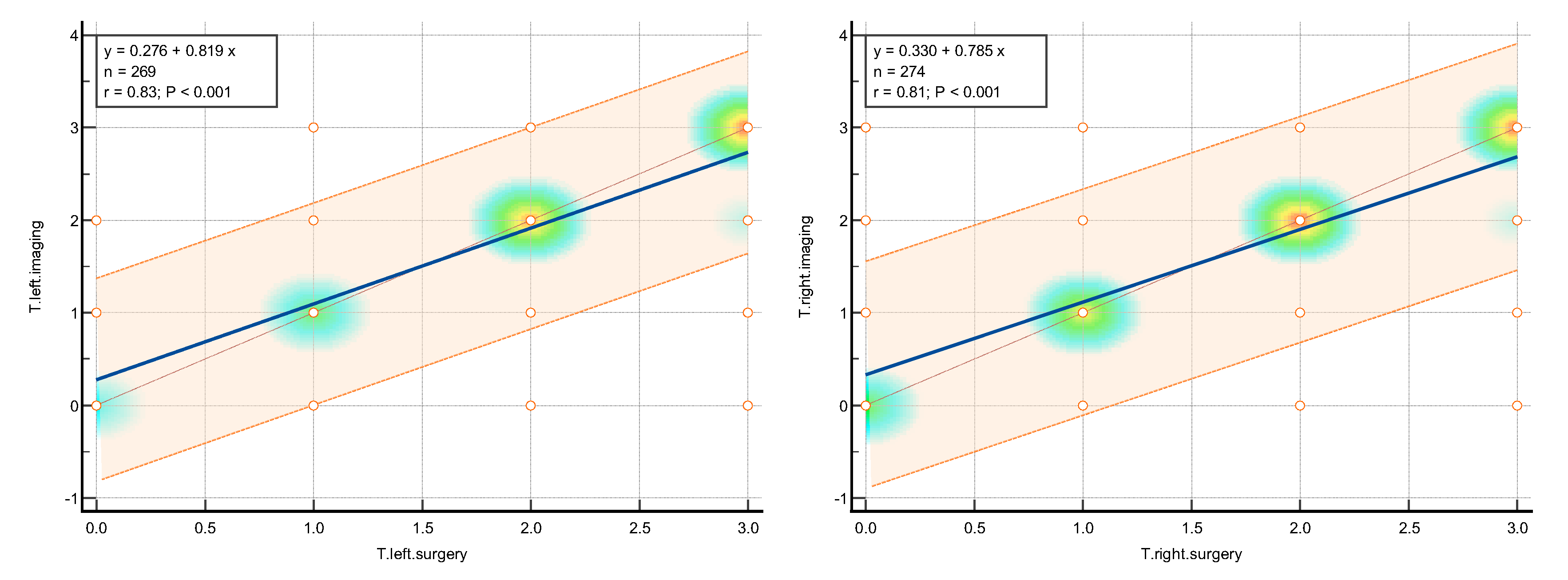
The statistical analysis was aimed to assess the level of concordance between the imaging techniques, consisted of the expert TVS/TAS and expert MRI, assessed together as #ENZIANi and intraoperative classification result, described as #ENZIANs.
The K value was interpreted following Altman's recommendations as poor (<0.20), fair (0.21-0.40), moderate (0.41-0.60), good (0.61-0.80), and very good (0.81-1.00).(Altman 1990)
Amongst the pelvic locations, the highest concordance with the highest k Cohen factor was observed in the tuboovarian area T left k Cohen 0.795 and T right k Cohen 0.791, in Fa (adenomyosis) k Cohen 0.776; C (rectum) k Cohen 0.766; and both ovaries O right k Cohen 0.75 and O left k Cohen 0.72.
The slightly lower results were achieved in the A and B and Fi intestines, respectively. Compartment A (vagina, retrovaginal space) had a k Cohen coefficient of0.In compartment B (sacrouterine ligaments, cardinal ligaments, pelvic sidewall): forB right, k Cohen was 0.653 and for B left, k Cohen was 0.For the intestines (16 cm above the Z line), the k Cohen coefficient equaled 0.The lowest concordance wasobserved for the peritoneum P with k Cohen of 0.519, alongside the F bladder k Cohen0.448; F ureter, P nerve, F muscle, F bone, F lung, as represented in the table below.
Values x, m, and cases where measurements were missing were excluded from the calculations.
The highest concordance was observed in the abdominal wall endometriosis – additionally assessed in this study- k Cohen 0,837 as those locations were diagnosed with special attention to the indicated location, often with additional measures.
The values of concordance are shown in
Table 1 Graphically, concordance was illustrated by correlation heatmaps depicted in
Figure 4.
3.3. Diagnostic Test Evaluation
The highest sensitivity was presented for the P and T compartments, and the following A, B, C, corresponding with deep endometriosis. Diagnostic test evaluation is shown in
Table 2.
4. Discussion
The matter of effective deep endometriosis imaging has been intensively analyzed in the gynecological publications lately. As the multiple specialized centers started introducing their own protocols, the number of comparable studies is consistently growing and the guidelines of working groups are being published, the future seems to be promising.
As for now, the #ENZIAN classification seems to be of the most frequent use and its value has been confirmed in many trials, attributing not only to the education of surgeons, radiologists and researchers, but also to introducing new, wider than before, way of operation planning. [
16]
According to the newest publication of the International Consensus published in May 2024 [
17], the golden standard in diagnosis of deep endometriosis is the expert TVS/TAS performed by the surgeon supplemented by an expert MRI, what is consistent with our approach of choice, presented in this work. [
18] This has also been widely analyzed throughout the years and has recently been tested in a fusion exam mode. [
19]
To the best of our knowledge, this study represents the largest retrospective investigation into the preoperative use of the #ENZIAN classification for endometriosis based in one center, performed by the same assessing and operating team: 1 surgeon performing TVS/TAS (E.M.-N.), the same expert as the leading surgeon (E.M.-N.), 1 expert radiologist (except for 11 cases, as mentioned above) and 1 gynecologist specializing in endometriosis (Z.B.) collecting the data and scoring #ENZIAN for each patient. [
10]
The MRI-based ENZIAN score correlates well with the intraoperative findings, enabling a better planning of the surgical procedure for patients and physicians. [
20]
Amongst the pelvic locations, the highest concordance with the highest k Cohen factor was observed in Fa (adenomyosis); C (rectum); T ( tuboovarian) and O ( ovaries) .
In Di Paola's retrospective study, which involved 82 women with deep endometriosis, MRI-based Enzian coding demonstrated good accuracy in detecting lesions across the various Enzian compartments: 81% for compartment A, 89% for compartment B, 82% for compartment C, 100% for compartment FA, and 37% for compartment FB. [
21], which results correspond very well with presented in this study.
We are also aware of the time factor – the period of time between the ultrasound assessment, the MRI exam and the operation. Even though the aim was to perform the above procedures shortly one after another, the service availability, patients’ personal reasons, the COVID-19 pandemic and other arbitrary situations inevitably may have influenced the final results in some patients. The lesions observed in imaging could obviously evolve due to time, pharmacological treatment and other diseases by the day of surgical intervention. We consider, though, that this is a universal condition that occur in each specialized center.
4.1. The B Compartment
The literature reports the poorest diagnostic performance for compartment B among all the individual parts of the Enzian classification. [
5] This finding of the study by Burla et al. are aligned. This was also a retrospective analysis, but including 63 patients.
Sensitivities and NPVs for compartments A, B, C, FA, FB and FI were 95.2% and 91.7%,78.4% and 56%, 91.4% and 89.7%, 57.1% and 94.1%, 85.7% and 98.3%, and 73.3% and 92.2%, respectively. The authors concluded that the Enzian classification can act as an anatomical reference and enhance dialog between specialists, including radiologists and gynecologists. [
20] Another valuable study was a retrospective MRI-based prediction analysis conducted by Thomassin-Naggara et al. which considered 150 women. [
3] This study demonstrated concordance with the surgical Enzian coding of 78.7% for compartment A, 34.7% for compartment B, and 82.7% for compartment C. Consistent with our findings, MRI showed the poorest diagnostic performance in compartment B. Accurate preoperative assessment should help surgeons anticipate challenging complications and the potential need for preoperative hormonal treatment, such as a gonadotropin-releasing hormone analog. Predicting the occurrence of de novo voiding dysfunction is of major importance. Although rated as a Clavien-Dindo grade II complication, it is one of the most dreaded complications; it requires self-catheterization and is a major determinant of postoperative alteration of quality of life with a risk of definitive sequelae in up to 3% of patients. [
3,
22,
23] In our study, the B compartment was of moderate concordance, which in our opinion, owes to our experienced radiology Center, assessing MRI exams. It was with huge enthusiasm that we greeted the #ENZIAN classification [
15,
24] after many years of inconvenience and communication problems when trying to describe the disease to the other physicians or to the patient, as grade IV was the only way to express the severity of the disease. For now, when we are fluent with the #ENZIAN and as most of the tertiary centers, we use it as our everyday work syllabus, we have a modest proposition of postulating some new development suggestions to the main scheme. Future development of the #ENZIAN, as it is clear that the classification is ameliorating with the improvement of surgery, is warmly awaited.
4.2. Rectum (C)
Considering that the concordance in the C parameter is relatively high, one may say that the result of this predictive factor is satisfying. Nevertheless, regarding the capacity of an expert surgeon or radiologist of precise rectum examination, we would have the need to express it properly in the pure C parameter.
As an illustration, the same C3 score is given for a 3 cm flat infiltration as well as for, for example, three large full-thickness lesions of 3 cm each, which narrow the intestine, significantly changing the scope of the procedure.
Therefore, in our opinion, the C parameter would need to be expanded with some additional information to define precisely the scope of the disease: 1. number of tumors, 2. narrowing of the bowel lumen, 3. the depth of the bowel wall infiltration according to the layer, and 4. the distance from the anal verge seems to be necessary. Hence, if possible, the app would be a field to be modified with this additional module, which could be facultative, added for tertiary care with intestinal surgery departments.
4.3. Location in Abdominal Wall (F w)
The #ENZIAN does not specify the parameter: abdominal wall endometriosis, where the lesions may also be extensive and may influence the scope of surgery and treatment planning. The parameter Fw can evidently be described in the F subgroup, but as it is much more prevalent than other locations, it’s separate description may be worth attention.
4.4. Ureters’ Assessment (Fu)
In our Center’s experience, MRI poorly detects enlarged ureters - changes are most often described when significant ureteral enlargement is visible, often associated with kidney sclerosis. This is probably due to ureter’s anatomy, as it becomes distended over the pelvic brim, which is located over the area covered by the pelvic MRI. “The diagnosis of ureteral endometriosis entails a high index of suspicion for the disorder. Imaging techniques are of limited value in providing an accurate depiction of extension of ureteral lesions. Preliminary results suggest that magnetic resonance urography is accurate in differentiating between intrinsic and extrinsic forms of ureteral involvement, but further studies are required to define its role in directing better treatment. [
25] Taking into account the scientific publications over the last few years, the approach seems to be similar. The first argument for a routine renal assessment during an endometriosis scan is: “as many as 50% of patients with ureteral endometriosis are asymptomatic. It is, therefore, difficult to obtain a prompt diagnosis and ureteral endometriosis can lead to subtle loss of renal function. [
26] “Delayed diagnosis of UE can lead to significantly decreased kidney function or even loss of obstruction of ureteral and hydroureteronephrosis. [
27,
28] “Additionally, ureteral hydronephrosis should first be evaluated with renal ultrasound.” [
26] “Renal ultrasound is the ideal first-line examination for ureteral endometriosis. In fact, it is most useful in detecting hydronephrosis and it is noninvasive, re-producible and cost effective. Once the diagnosis is confirmed or suspected by means of renal ultrasound, several tools are available to confirm this diagnosis”. [
27] Ureteral endometriosis is inevitably accompanied by endometriosis in the pelvic wall or the parametrium. Therefore, all patients who suffer from deep endometriosis should be offered a renal ultrasound every six months to rule out urinary obstruction, which is an absolute indication for treatment. In the case of hydronephrosis, a urological diagnostic confirmation of renal function should be carried out preoperatively (retention parameters, renal scintigraphy, cysto-/ureteroscopy, MRI urography, excretory urography). [
11] “However, imaging techniques are of limited value in providing an accurate depiction of the extent of the disease and the infiltration of the ureteral wall. Abdominal ultrasound is routinely used as a screening tool to rule out urinary tract obstruction in patients with pelvic endometriosis, because of the high rate of silent presentations. The exam is simple and non-invasive, the evaluation requires no intravenous contrast and the findings are highly sensitive for hydronephrosis. Therefore, in clinical practice, periodic kidney ultrasound (every 6 months at first) from the time of diagnosis is generally suggested.“ [
29]. Magnetic resonance imaging (MRI) may reveal direct signs such as a nodule or a mass invading the ureter along its course or at the ureterovesical junction . Nevertheless, the ureter is a hollow organ only 4- 5 mm in diameter and sometimes it is difficult to analyze it with MRI as its spatial resolution reaches its limit. [
29] “The most reliable assessment of ureteral involvement is achieved through examining kidney congestion via transabdominal ultrasound (TAS).” [
15]
5. Limitations
Regarding the fact, that the group of patients was not random and selected in majority to only complex cases due to the number of them which needed advanced surgery and were admitted to the specialized oncological gynecology ward, which may correspond with a tertiary endometriosis centre, due to its admission criteria, the main limitations of this study might be the following: it is not representative for all endometriosis cases so do not show the full application of the #ENZIAN. The system of three level endometriosis care is under construction in Poland and our Center is nowadays one of the few, that can undertake such level of surgery.
The learning curve.
Both TVS and MRI must be considered as improving in efficacy with time, as both expert surgeon performing the TVSs and the expert radiologist specialized in DE protocol assessment were gaining experience with time, what is obviously an advantage, but might have biased the results.
6. Conclusions
Preoperative assessment using TVS/TAS+MRI with the ENZIANi score
correlates well with the ENZIANs postoperative score and demonstrates good concordance in the detection and localization of deep endometriosis, thereby minimizing false negative results and ensuring accurate preoperative staging.
The ultrasound examination performed by the surgeon:
I. enhances the utility of the scale through the direct application of visual
representation of the pelvis and concise, practical, repetitive coding.
II. highlights key aspects for the patient, better preparing them for surgery,
discussions with the patient, and obtaining full informed consent,
III. improves diagnostic efficiency as the surgeon performing the examination learns to analyze ultrasound images more effectively, which can be compared with images of endometrial changes during surgery. This correlation contributes to better assessment in
subsequent examinations.
The ENZIAN classification is perfectly tailored to surgeons’needs, and one of its most valuable advantages is its continuous development. Suggestions for futureimprovements, such as adding the expanded C module, may be considered during the upcoming new edition launch.
Author Contributions
Conceptualization, Zofia Borowiec and Ewa Milnerowicz-Nabzdyk; Formal analysis, Zofia Borowiec, Maja Mrugala and Ewa Milnerowicz-Nabzdyk; Investigation, Zofia Borowiec, Maja Mrugala, Wiktor Bek and Ewa Milnerowicz-Nabzdyk; Methodology, Zofia Borowiec and Ewa Milnerowicz-Nabzdyk; Resources, Zofia Borowiec; Software, Ewa Milnerowicz-Nabzdyk; Supervision, Ewa Milnerowicz-Nabzdyk; Validation, Zofia Borowiec and Ewa Milnerowicz-Nabzdyk; Visualization, Zofia Borowiec; Writing – original draft, Zofia Borowiec; Writing – review & editing, Zofia Borowiec, Krzysztof Nowak, Wiktor Bek and Ewa Milnerowicz-Nabzdyk. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki: and approved by the Institutional Review Board (or Ethics Committee) of Medical Faculty: University of Opole, Poland (UO/0020/KB/2022 and 20.12.2022).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Shafrir, A.L.; Farland, L.V.; Shah, D.K.; et al. Risk for and consequences of endometriosis: A critical epidemiologic review. Best Pract Res Clin Obstet Gynaecol 2018, 51, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Swift, B.; Taneri, B.; Becker, C.M.; et al. Prevalence, diagnostic delay and economic burden of endometriosis and its impact on quality of life: results from an Eastern Mediterranean population. Eur J Public Health 2024, 34, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Thomassin-Naggara, I.; Monroc, M.; Chauveau, B.; et al. Multicenter External Validation of the Deep Pelvic Endometriosis Index Magnetic Resonance Imaging Score. JAMA Netw Open 2023, 6, e2311686. [Google Scholar] [CrossRef]
- Keckstein, J.; Saridogan, E.; Ulrich, U.A.; et al. The #Enzian classification: A comprehensive non-invasive and surgical description system for endometriosis. Acta Obstet Gynecol Scand 2021, 100, 1165–1175. [Google Scholar] [CrossRef]
- Enzelsberger, S.H.; Oppelt, P.; Nirgianakis, K.; et al. Preoperative application of the Enzian classification for endometriosis (The cEnzian Study): A prospective international multicenter study. Bjog 2022, 129, 2052–2061. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Bmj 2007, 335, 806–808. [Google Scholar] [CrossRef]
- Guerriero, S.; Condous, G.; van den Bosch, T.; et al. Systematic approach to sonographic evaluation of the pelvis in women with suspected endometriosis, including terms, definitions and measurements: a consensus opinion from the International Deep Endometriosis Analysis (IDEA) group. Ultrasound Obstet Gynecol 2016, 48, 318–332. [Google Scholar] [CrossRef] [PubMed]
- Bazot, M.; Bharwani, N.; Huchon, C.; et al. European society of urogenital radiology (ESUR) guidelines: MR imaging of pelvic endometriosis. Eur Radiol 2017, 27, 2765–2775. [Google Scholar] [CrossRef]
- Rousset, P.; Rousset-Jablonski, C.; Alifano, M.; et al. Thoracic endometriosis syndrome: CT and MRI features. Clin Radiol 2014, 69, 323–330. [Google Scholar] [CrossRef]
- Di Giovanni, A.; Montanari, E.; Hudelist, G.; et al. Comparison Between Sonography-Based and Surgical Evaluation of Endometriotic Lesions Using the #Enzian Classification - A Retrospective Data Analysis. Ultraschall Med 2023, 44, 290–298. [Google Scholar] [CrossRef]
- Frumkin, N.; Schmädecker, R.; Isermann, R.; et al. Surgical Treatment of Deep Endometriosis. Geburtshilfe Frauenheilkd 2023, 83, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Gui, B.; Valentini, A.L.; Ninivaggi, V.; et al. Shining light in a dark landscape: MRI evaluation of unusual localization of endometriosis, Diagn Interv Radiol 2017, 23, 272–281. [CrossRef]
- Keckstein, J.; Becker, C.M.; Canis, M.; et al. Recommendations for the surgical treatment of endometriosis. Part 2: deep endometriosis. Hum Reprod Open 2020, 2020, hoaa002. [Google Scholar] [CrossRef]
- Altman, D. , Practical Statistics for Medical Research. New York: Chapman and Hall/CRC, 1990, Vol.
- Montanari, E.; Bokor, A.; Szabó, G.; et al. Accuracy of sonography for non-invasive detection of ovarian and deep endometriosis using #Enzian classification: prospective multicenter diagnostic accuracy study. Ultrasound Obstet Gynecol 2022, 59, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Bendifallah, S.; Roman, H.; Rubod, C.; et al. Impact of hospital and surgeon case volume on morbidity in colorectal endometriosis management: a plea to define criteria for expert centers. Surg Endosc 2018, 32, 2003–2011. [Google Scholar] [CrossRef] [PubMed]
- Condous, G.; Gerges, B.; Thomassin-Naggara, I.; et al. Non-invasive imaging techniques for diagnosis of pelvic deep endometriosis and endometriosis classification systems: an International Consensus Statement(). Hum Reprod Open 2024, 2024, hoae029. [Google Scholar] [CrossRef]
- Keckstein, J.; Hoopmann, M.; Merz, E.; et al. Expert opinion on the use of transvaginal sonography for presurgical staging and classification of endometriosis. Arch Gynecol Obstet 2023, 307, 5–19. [Google Scholar] [CrossRef]
- Guerriero, S.; Ajossa, S.; Pagliuca, M.; et al. Advances in Imaging for Assessing Pelvic Endometriosis. Diagnostics (Basel) 2022, 12. [Google Scholar] [CrossRef]
- Burla, L.; Scheiner, D.; Samartzis, E.P.; et al. The ENZIAN score as a preoperative MRI-based classification instrument for deep infiltrating endometriosis. Arch Gynecol Obstet 2019, 300, 109–116. [Google Scholar] [CrossRef]
- Di Paola, V.; Manfredi, R.; Castelli, F.; et al. Detection and localization of deep endometriosis by means of MRI and correlation with the ENZIAN score. Eur J Radiol 2015, 84, 568–574. [Google Scholar] [CrossRef]
- de Resende, J.A.J.; Cavalini, L.T.; Crispi, C.P.; et al. Risk of urinary retention after nerve-sparing surgery for deep infiltrating endometriosis: A systematic review and meta-analysis. Neurourol Urodyn 2017, 36, 57–61. [Google Scholar] [CrossRef]
- Roman, H.; Desnyder, E.; Pontré, J.; et al. Combined vaginal-laparoscopic approach vs. laparoscopy alone for prevention of bladder voiding dysfunction after removal of large rectovaginal endometriosis. J Visc Surg 2021, 158, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Keckstein, J.; Hudelist, G. Classification of deep endometriosis (DE) including bowel endometriosis: From r-ASRM to #Enzian-classification. Best Pract Res Clin Obstet Gynaecol 2021, 71, 27–37. [Google Scholar] [CrossRef]
- Ghezzi, F.; Cromi, A.; Bergamini, V.; et al. Management of ureteral endometriosis: areas of controversy. Curr Opin Obstet Gynecol 2007, 19, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Li, P.; Liu, Q.; et al. Ureteral endometriosis in patients with deep infiltrating endometriosis: characteristics and management from a single-center retrospective study. Arch Gynecol Obstet 2019, 300, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Berlanda, N.; Vercellini, P.; Carmignani, L.; et al. Ureteral and vesical endometriosis. Two different clinical entities sharing the same pathogenesis. Obstet Gynecol Surv 2009, 64, 830–842. [Google Scholar] [CrossRef]
- Barra, F.; Scala, C.; Biscaldi, E.; et al. Ureteral endometriosis: a systematic review of epidemiology, pathogenesis, diagnosis,treatment, risk of malignant transformation and fertility. Hum Reprod Update 2018, 24, 710–730. [Google Scholar] [CrossRef]
- Maccagnano, C.; Pellucchi, F.; Rocchini, L.; et al. Ureteral endometriosis: proposal for a diagnostic and therapeutic algorithm with a review of the literature. Urol Int 2013, 91, 1–9. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).