1. Introduction
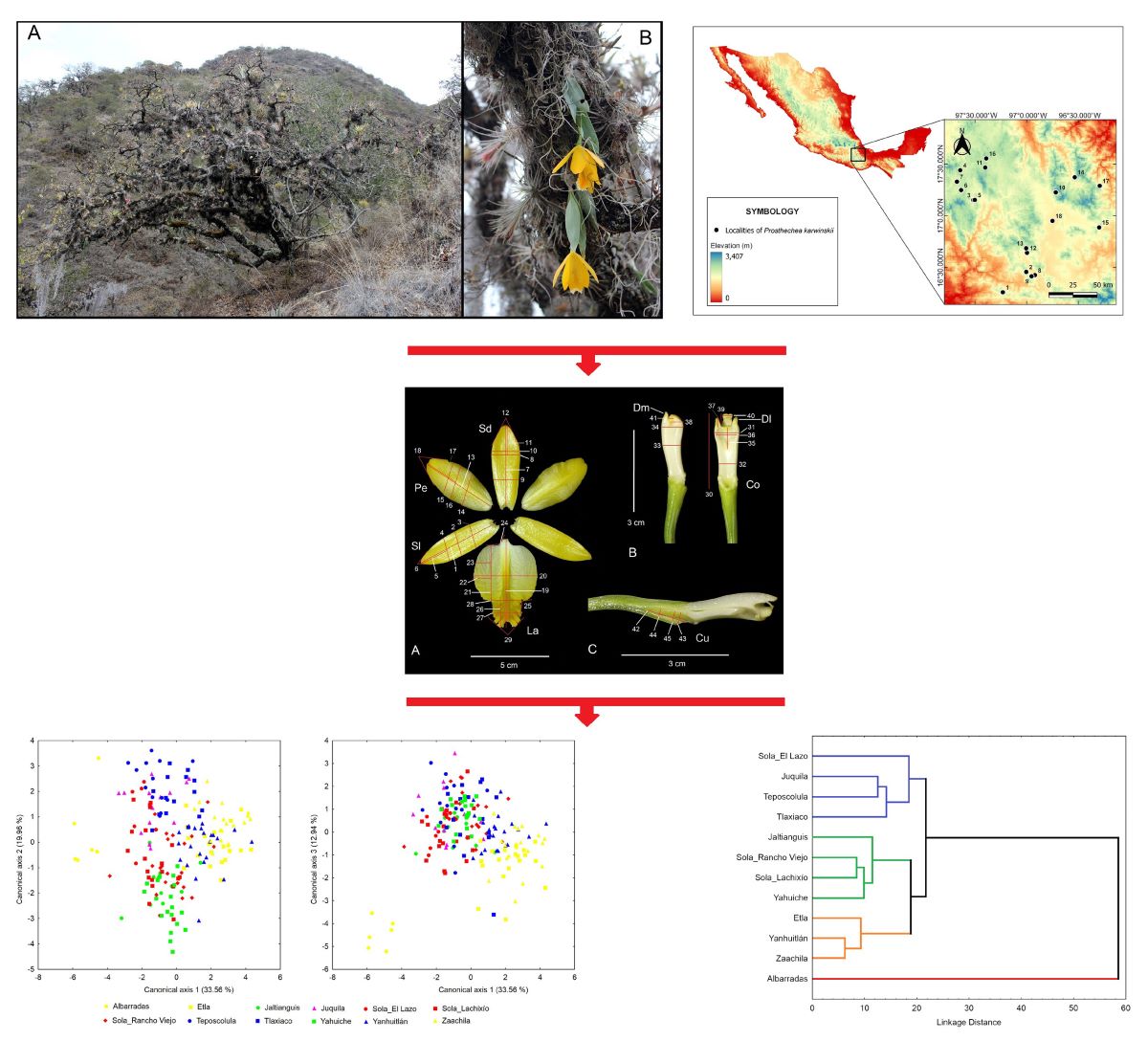
Prosthechea karwinskii (Mart.) J.M.H. Shaw is an orchid endemic to western and southern Mexico, inhabiting mountainous regions where it grows as a hanging epiphyte in oak or oak-pine forests subject to a well-marked seasonal drought (
Figure 1A). The inflorescence is a two-flowered (exceptionally three) raceme arising from the last developed pseudobulb (
Figure 1B). The taxon was described in 1830, but its taxonomic history has been linked to that of
Prosthechea citrina (Lex.) W.E. Higgins, or any of its nomenclatural synonyms, a very similar species, with which it becomes sympatric in some locations in Guerrero and Michoacán. In the past, the information available for
P. karwinskii was invariably attributed to
P. citrina; only in recent years have both species come to be considered different [
1,
2]. This species is one of the most distinctive orchids in the Mexican flora, valued for the ornamental beauty and pleasant aroma of its flowers, as well as having cultural significance since pre-Hispanic times. It was one of the orchids from which mucilage was obtained and used as an adhesive in feather art in pre-Hispanic times and during the colonial period [
3]. In traditional medicine, it has been used to soothe coughs, heal wounds and burns, treat diabetes, prevent miscarriage, and aid in childbirth [
4,
5,
6,
7]. The flowers are used as decorations in homes, commercial stands, and temples during Easter commemorations [
8,
9]. Additionally, due to the beauty, color, and aroma of its flowers, this plant is cultivated in a rustic manner in orchards in many communities in Oaxaca.
In most of the locations where
P. karwinskii grows, it is locally scarce and survives in forest fragments surrounded by a matrix of environments modified by anthropogenic causes (conversion into crop fields, human settlements, opening of new roads, goat farming). In Oaxaca, the most abundant populations of the species occur, but they face the risk of extraction for temporary adornment in local trade [
8,
9,
10], for religious purposes [
8], and to a lesser extent, for medicinal use [
4]. This practice occurs annually, mainly during the species’ flowering season, leading to its inclusion in the list of species of Mexican wild flora at risk [
11]. For other epiphytic orchids growing in the mountain forests of Mexico, it has been demonstrated that extraction for local trade has effects on species subjected to this practice, such as reductions in population size and rates of fertility and recruitment, as well as loss of genetic diversity [
12,
13,
14,
15].
In the localities of P. karwinskii in Oaxaca, variation has been observed in some floral traits, such as flower size and coloration, the shape of the labellum, and the shape of the apical teeth of the column. This leads to the assumption of the existence of intraspecific variation, at least among populations in the state, which has not been analyzed either through the use of morphological or molecular markers. The analysis of this variation will be important for identifying morphotypes with ornamental potential and desirable in a management program, recognizing phytogenetic diversity present in the species, identifying forms or subspecies within the orchid, as well as determining a possible geographical pattern associated with morphological variation.
Morphometric studies have been employed in some species of Orchidaceae, primarily analyzing variation associated with floral morphology [
16,
17,
18,
19,
20,
21,
22], although vegetative morphology has also been considered, including attributes of leaf anatomy [
17,
22]. Studies in this regard seek to find intraspecific differences [
18,
23] and interspecific differentiation to delimit similar taxa considered as cryptic species [
16,
22], recognize taxa of hybrid origin [
24], or identify morphotypes with phytogenetic value [
19,
20]. The quantitative analysis of characters identified in the labellum has been valuable for recognizing and characterizing intraspecific variation in orchids [
19,
20], although the use of traits present in other floral structures has also been useful [
18]. Morphometry has also been used to trace the geographical origin of samples of unknown origin, particularly for species or products of economic importance [
26,
27,
28,
29,
30]. Although various sources of information (
e.g., genetic and chemical) and analytical tools can address this issue, the use of multivariate methods with morphological offers the advantage of low cost [
27,
28,
29,
30] and relatively easy data collection for a large number of individuals [
26,
27]. However, this topic is analytically complex and requires caution in its implementation and interpretation due the need for a robust reference and the requirements of the analyzes [
26,
28].
The objective of this study was to analyze the variation among populations from different localities in Oaxaca, Mexico, and to identify variables with taxonomic potential, through a morphometric analysis. Additionally, an attempt was made to determine if this set of characters allows relating specimens extracted from their habitat and whose origin is unknown, which were recovered after having had a religious use. Predictions for this study are as follows: 1) the floral morphology of P. karwinskii, analyzed using morphometric methods, will allow us to recognize the interpopulation variation of the species; and 2) the floral traits of P. karwinskii could serve as a morphological marker to associate the geographical origin of specimens from unknown locations.
2. Results
2.1. ANOVA and Kruskal-Wallis Tests
Most of the 40 floral characters analyzed with ANOVA showed significant differences among the localities of
P. karwinski; however, the characters SlLa, SlAa, SdA1, SdLa, SdAa, and LaAbm did not show significant differences among localities (
Table 1). The specimens from Teposcolula exhibited the highest values for the length and width of floral segments, which determine flower size, such as SlLt (61.69 ± 1.85 mm), SlAm (16.94 ± 0.67 mm), SdLt (60.35 ± 1.58 mm), SdAm (18.42 ± 0.65 mm), PeLt (58.71 ± 1.71 mm), PeAm (26.25 ± 1.43 mm), LaLt (65.51 ± 1.68 mm), LaAm (44.90 ± 1.20 mm), and CoLt (29.69 ± 0.51 mm). In contrast, the specimens from Sola_Rancho Viejo appeared to have the smallest flowers, as characters like (SdLt 46.09 ± 1.10 mm), SdAm (15.26 ± 0.52 mm), PeLt (45.02 ± 0.99 mm), PeAm (19.81 ± 0.77 mm), LaLt (51.39 ± 1.15 mm), and LaAm (37.40 ± 1.19 mm) showed the lowest values (see
Table S1). Additionally, the locality of Albarradas showed the highest values in traits of the column and cuniculus, such as AnDlDm (2.11 ± 0.41 mm), CuAm (3.47 ± 0.06 mm), CuA1 (2.22 ± 0.13 mm).
The Kruskal-Wallis test revealed significant differences among localities for the characters CoGm (X
2 = 39.759, df=11, p-value < 0.05), CoGa (X
2 = 40.521, df=11, p-value < 0.05), and DmAn (X
2 = 27.5236, df=11, p-value < 0.05); however, there were no differences among localities for the characters LaAam (X
2 = 18.374, df=11, p-value > 0.05) and CoAe (X
2 = 17.823, df=11, p-value > 0.05). The specimens from Teposcolula and Sola_El Lazo recorded the highest values for CoGa (9.08 ± 0.22 mm and 9.08 ± 0.31 mm, respectively), while the individuals from Zaachila and Albarradas (7.44 ± 0.177 mm and 44 ± 0.26 mm, respectively) had the lowest values for these characters. For CoGm, the individuals from Teposcolula had the highest value (5.30 ± 0.32 mm). For the character DmAn, the individuals from Sola_El Lazo had the highest value (3.74 ± 0.22 mm), while those from Albarradas had the lowest value (2.88 ± 0.13 mm) (
Table S1).
2.2. Multivariate Analyses (PCA, CVA, and Cluster Analyses)
Both the PCA and CVA produced similar results whether the samples obtained in Zaachila were excluded (data not shown) or included. Therefore, for both methods, only the results of the analyses including the floral samples obtained from this community in Oaxaca are presented. For the final analyses (PCA and CVA), 185 individuals and 33 morphological variables of P. karwinskii were included.
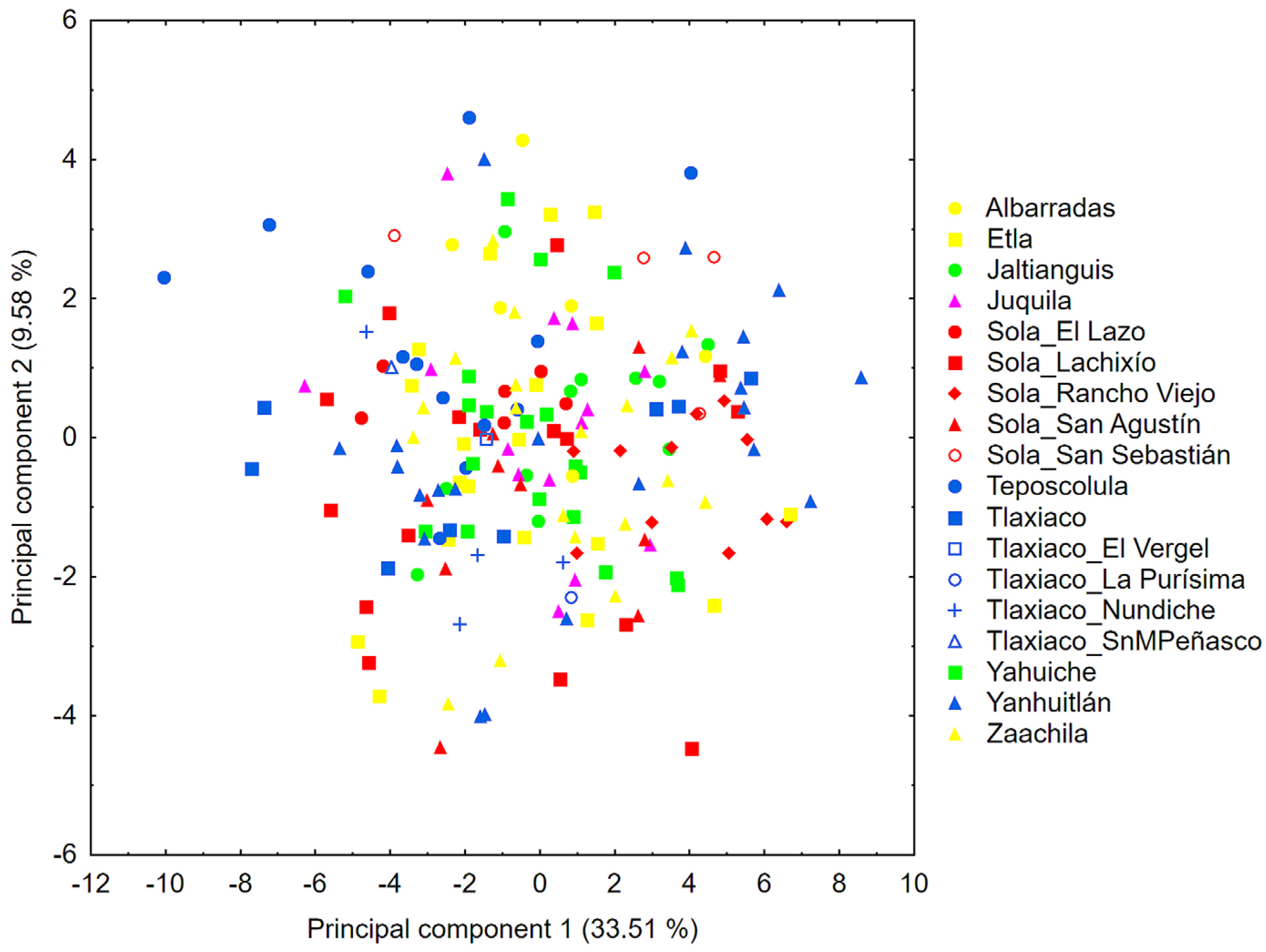
The PCA showed that eight principal components had eigenvalues > 1.0, which together accounted for 71.91% of the total variance (
Table 2). Among the eight components that retained the highest percentage of variance, the first one explained a third of it (33.51%) and was most correlated with the variables LaAm (-0.861), PeAm (-0.853), and SlAm (-0.852). This analysis showed a high overlap among individuals from different populations; however, along the axis that accounted for the highest percentage of variance, some individuals from Teposcolula and the populations of Yanhuitlan, Etla, and Sola_Rancho Viejo appeared at the extremes (
Figure 2).
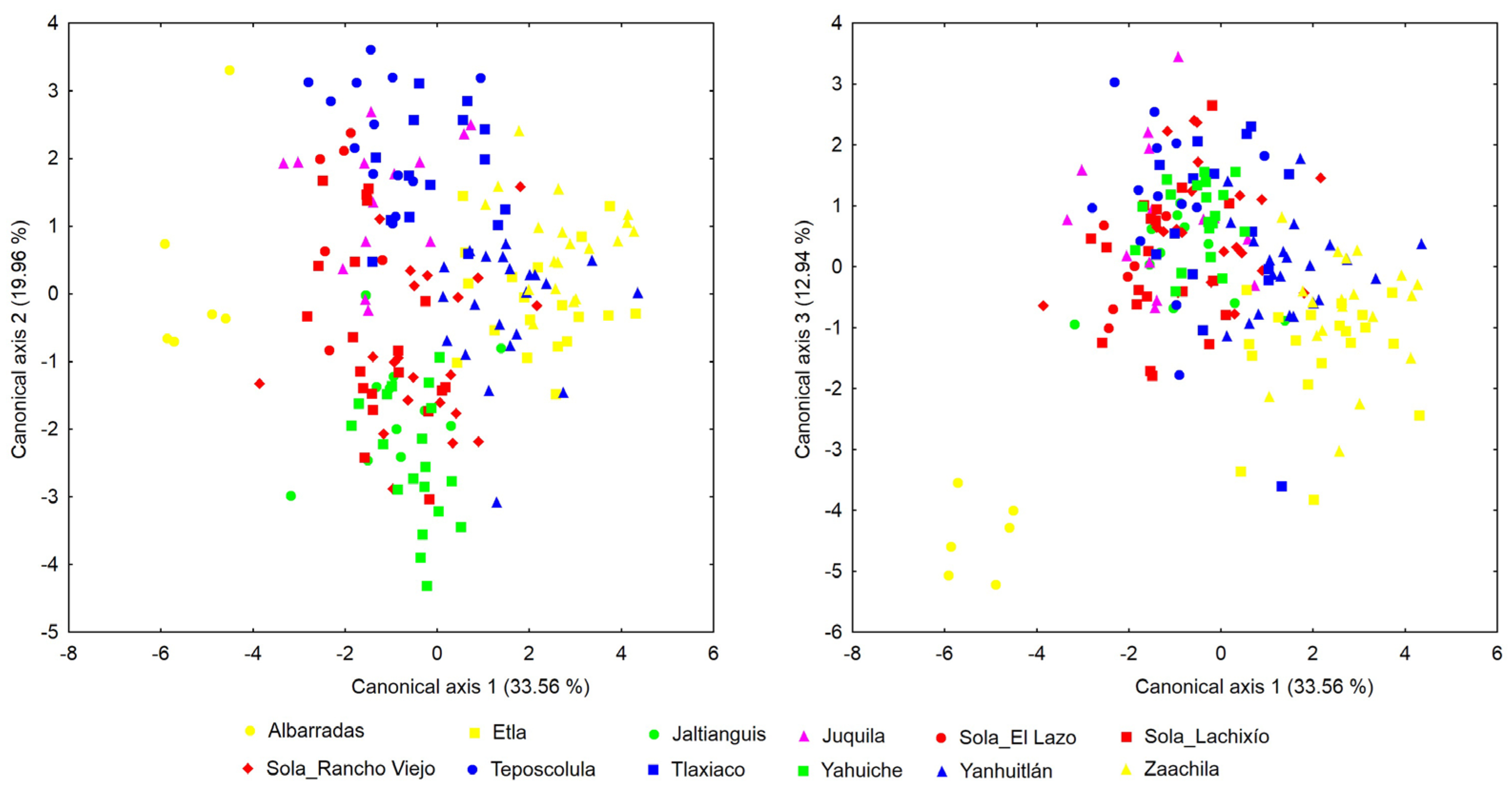
In the CVA, three canonical axes had eigenvalues > 1.0 and together explained 66.46% of the total variance (
Table 2). The first canonical axis explained 33.56% of the variance and reflected a greater contribution from the variables DmAl (0.950), SlAm (-0.551), and LaAml (-0.533). On this axis, there was a clear separation of individuals from the Albarradas population from the rest of the other ones (
Figure 3A). The samples from Jaltianguis and Yahuiche showed less dispersion along the first three canonical axes and overlapped with each other. Along axis 1, individuals from these two populations were completely separated from those from Etla and individuals from Zaachila (except for one individual from Jaltianguis). Axis 1 also showed a complete separation of the samples from Zaachila from those from Juquila and the two populations of Sola (El Lazo and Lachixío), with only a marginal overlap with the remaining Sola population (Rancho Viejo) and Tlaxiaco. Except for one individual, Teposcolula was also almost completely separated from those from Zaachila and Etla.
The second axis explained 19.96% of the variance and had a greater contribution from the variables LaLt (1.015), SlLt (-0.941), and DlAl (0.594). On axis 2, individuals from the Yahuiche and Jaltianguis populations were completely separated from those of Tlaxiaco, and Teposcolula (
Figure 3A), and present a marginal overlap with Juquila and Sola_El Lazo. On this axis, Yanhuitlan is completely separated of Teposcolula, and partially overlaps with Sola_Rancho Viejo. The third axis accounted for 12.94% of the variance and was more closely related to the variables LaAul (0.628), CoGa (0.484), and LaAm (0.480). On this axis, the separation of individuals from Albarradas from all other populations was again highlighted (
Figure 3B), as seen on axis 1. Along the first three axes, the samples from Zaachila showed greater overlap with those from the populations of Yanhuitlan and Etla (
Figure 3).
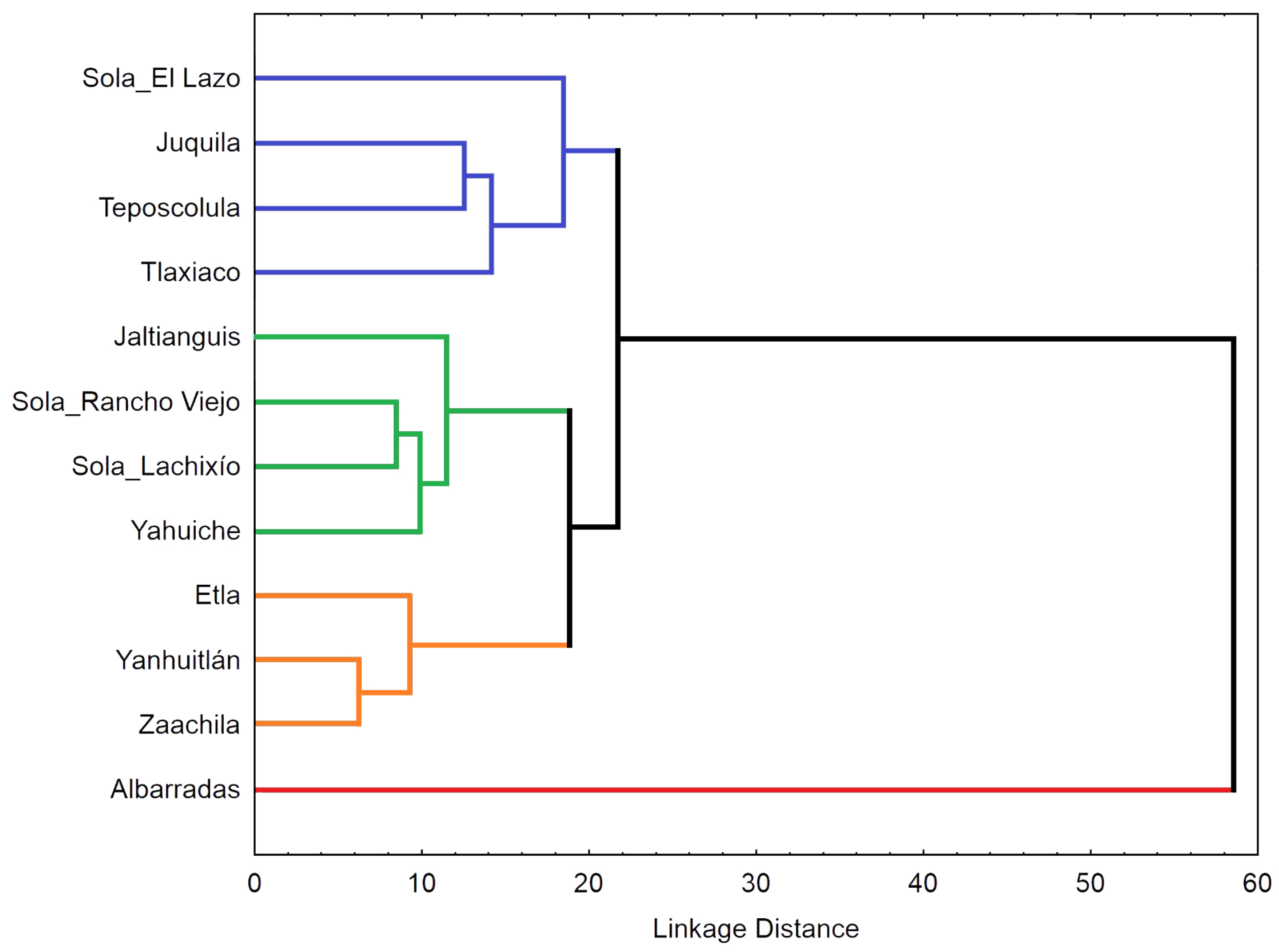
The cluster analysis also revealed the high morphological divergence of Albarradas population (
Figure 4). In the UPGMA dendrogram, this population was externally linked to the group containing the remaining populations. This latter group is divided into three subgroups: the first formed by intermixed populations from the southern regions of Oaxaca (Sola_El Lazo and Juquila) and two populations from the Mixteca (Teposcolula and Tlaxiaco); the second includes the remaining populations from Sola de Vega (Sola_Rancho Viejo and Sola_Lachixío) nested between those of the Sierra Norte (Jaltianguis and Yahuiche); and the third subgroup comprises Zaachila linked to Yanhuitlan, with Etla also joined to them.
2.3. Figures and Tables
Figure 1.
A) Habitat of Prosthechea karwinskii, an oak forest in San Pedro y San Pablo Teposcolula, Oaxaca. B) Prosthechea karwinskii growing in situ as a hanging epiphyte on Quercus sp. in Santo Domingo Yanhuitlan, Oaxaca. Photographs by R. Solano.
Figure 1.
A) Habitat of Prosthechea karwinskii, an oak forest in San Pedro y San Pablo Teposcolula, Oaxaca. B) Prosthechea karwinskii growing in situ as a hanging epiphyte on Quercus sp. in Santo Domingo Yanhuitlan, Oaxaca. Photographs by R. Solano.
Figure 2.
Representation of the axes 1 and 2 of the PCA resulting from the variation of 33 floral variables in 185 individuals of Prosthechea karwinskii from 18 localities (including Zaachila) in Oaxaca, Mexico.
Figure 2.
Representation of the axes 1 and 2 of the PCA resulting from the variation of 33 floral variables in 185 individuals of Prosthechea karwinskii from 18 localities (including Zaachila) in Oaxaca, Mexico.
Figure 3.
Representation of the axes 1-2 (A) and 1-3 (B) of the CVA resulting from the variation of 33 floral variables in 185 individuals of Prosthechea karwinskii from 12 expanded populations in Oaxaca, Mexico. See Material and Methods to see how the expanded populations were integrated.
Figure 3.
Representation of the axes 1-2 (A) and 1-3 (B) of the CVA resulting from the variation of 33 floral variables in 185 individuals of Prosthechea karwinskii from 12 expanded populations in Oaxaca, Mexico. See Material and Methods to see how the expanded populations were integrated.
Figure 4.
Phenogram showing the relationships between 12 expanded populations of Prosthechea karwinskii from Oaxaca, Mexico. The dendrogram was constructed using the UPGMA clustering algorithm and the squared Mahalanobis distances between the population centroids, calculated from 33 floral variables.
Figure 4.
Phenogram showing the relationships between 12 expanded populations of Prosthechea karwinskii from Oaxaca, Mexico. The dendrogram was constructed using the UPGMA clustering algorithm and the squared Mahalanobis distances between the population centroids, calculated from 33 floral variables.
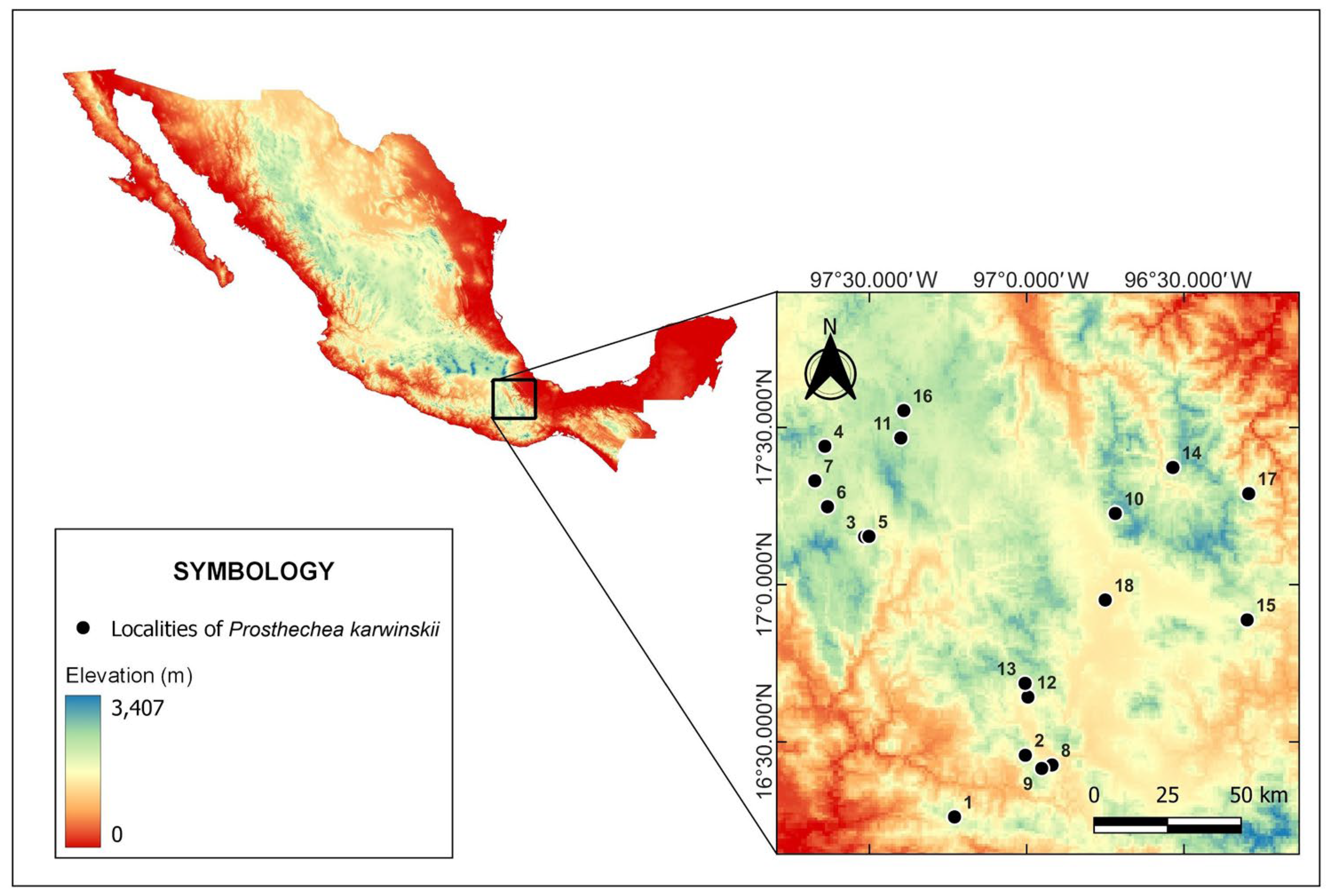
Figure 5.
Localities where individuals of
Prosthechea karwinskii were sampled in Oaxaca, Mexico. See
Table 3 for the names of the localities.
Figure 5.
Localities where individuals of
Prosthechea karwinskii were sampled in Oaxaca, Mexico. See
Table 3 for the names of the localities.
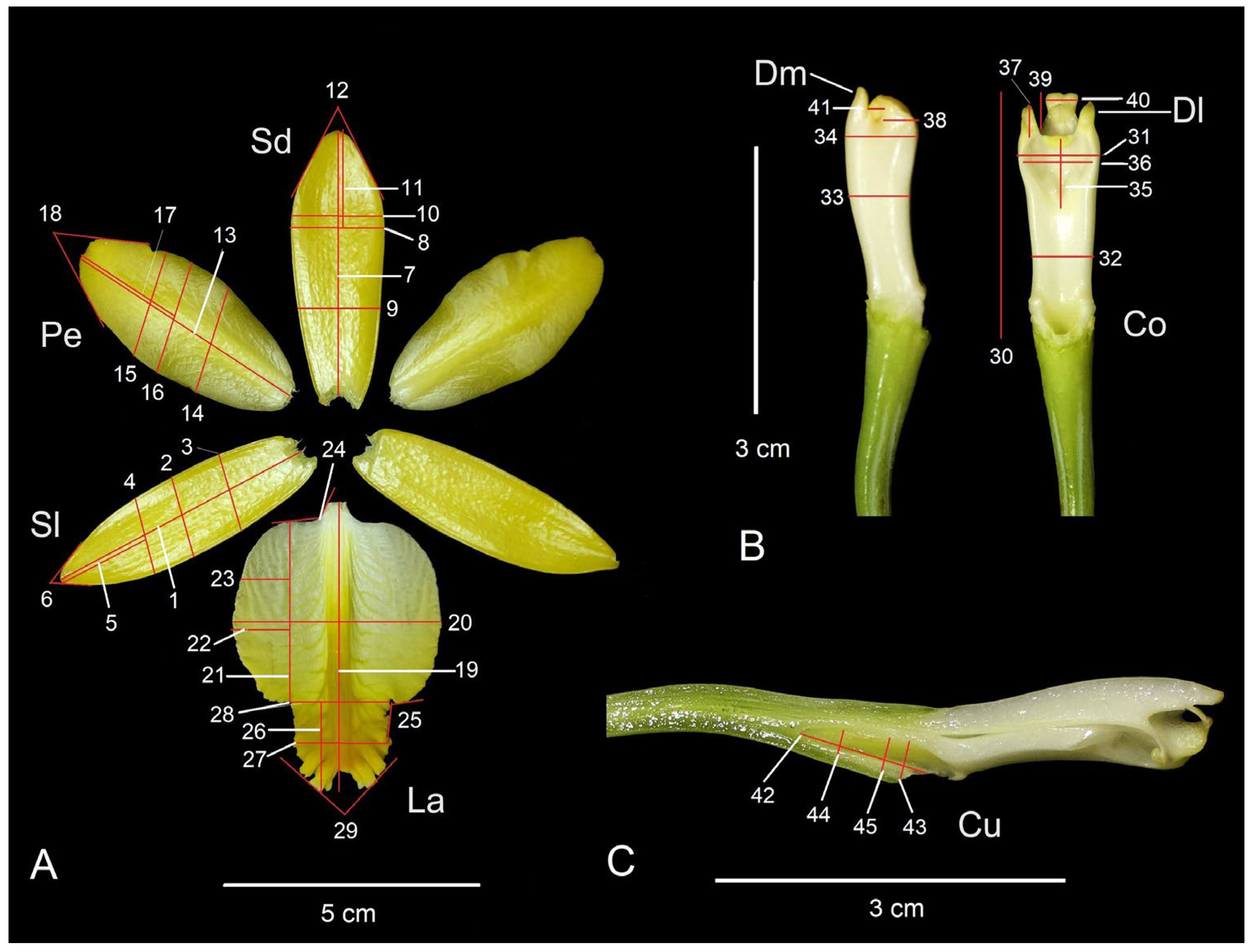
Figure 6.
Floral structures showing the variables evaluated in the morphometric analyzes of the 18 populations (including Zaachila) of
Prosthechea karwinskii from Oaxaca, Mexico. A) Flower dissection. B) Lateral and ventral views of the column C) Longitudinal section of the column and ovary, showing the cuniculus. Sl = lateral sepal, Sd = dorsal sepal, Pe = petal, La = labellum, Co = column, Dl = lateral tooth of the column, Dm = median tooth of the column, Cu = cuniculus. See
Table 4 for the names of the variables. Photographs by R. Solano.
Figure 6.
Floral structures showing the variables evaluated in the morphometric analyzes of the 18 populations (including Zaachila) of
Prosthechea karwinskii from Oaxaca, Mexico. A) Flower dissection. B) Lateral and ventral views of the column C) Longitudinal section of the column and ovary, showing the cuniculus. Sl = lateral sepal, Sd = dorsal sepal, Pe = petal, La = labellum, Co = column, Dl = lateral tooth of the column, Dm = median tooth of the column, Cu = cuniculus. See
Table 4 for the names of the variables. Photographs by R. Solano.
Table 1.
Results of the analysis of variance (ANOVA) for 40 of the 45 floral characters that met the assumption of normality, recorded in 185 individuals from 12 expanded populations of of Prosthechea karwinskii, including Zaachila. df = degrees of freedom, ss = sum of squares, ms = mean square. Differences are significant at p < 0.05.
Table 1.
Results of the analysis of variance (ANOVA) for 40 of the 45 floral characters that met the assumption of normality, recorded in 185 individuals from 12 expanded populations of of Prosthechea karwinskii, including Zaachila. df = degrees of freedom, ss = sum of squares, ms = mean square. Differences are significant at p < 0.05.
| Variable |
df |
ss |
ms |
F-Value |
p-value |
| SlLt |
11 |
1546 |
140.51 |
3.214 |
0.000528 |
| SlAm |
11 |
120.4 |
10.944 |
2.213 |
0.0157 |
| SlA1 |
11 |
127.1 |
11.552 |
2.096 |
0.0229 |
| SlA2 |
11 |
135.1 |
12.278 |
2.209 |
0.0229 |
| SlLa |
11 |
463 |
42.13 |
1.35 |
0.201 |
| SlAa |
11 |
1846 |
167.86 |
1.702 |
0.076 |
| SdLt |
11 |
2005 |
182.27 |
4.338 |
9.98e-06 |
| SdAm |
11 |
142 |
12.913 |
2.262 |
0.0134 |
| SdA1 |
11 |
121.9 |
11.084 |
1.72 |
0.0723 |
| SdA2 |
11 |
137.1 |
12.462 |
2.177 |
0.0177 |
| SdLa |
11 |
467 |
42.47 |
1.718 |
0.0728 |
| SdAa |
11 |
2178 |
198 |
1.513 |
0.13 |
| PeLt |
11 |
1777 |
161.52 |
4.154 |
1.92e-05 |
| PeAm |
11 |
516.8 |
46.98 |
3.065 |
0.000888 |
| PeA1 |
11 |
473.5 |
43.05 |
3.222 |
0.000514 |
| PeA2 |
11 |
351.4 |
31.95 |
2.592 |
0.0045 |
| PeLa |
11 |
515.5 |
46.86 |
2.643 |
0.00377 |
| PeAa |
11 |
8608 |
862.5 |
4.436 |
4.96e-06 |
| LaLt |
11 |
2123 |
193 |
4.351 |
9.53e-06 |
| LaAm |
11 |
677 |
61.55 |
2.497 |
0.00619 |
| LaLl |
11 |
678 |
61.63 |
2.99 |
0.00115 |
| LaAml |
11 |
3.817 |
0.347 |
4.113 |
2.21e-05 |
| LaA1l |
11 |
172.9 |
15.714 |
3.881 |
5.04e-05 |
| LaAul |
11 |
14463 |
1314.8 |
9.014 |
1.27e-12 |
| LaAlm |
11 |
3.817 |
0.347 |
4.113 |
2.21e-05 |
| LaLm |
11 |
5.668 |
0.5152 |
4.874 |
1.50e-06 |
| LaAbm |
11 |
172.6 |
15.695 |
1.839 |
0.0509 |
| LaAmm |
11 |
169.7 |
15.423 |
1.816 |
0.0545 |
| CoLt |
11 |
262.2 |
23.836 |
6.174 |
1.60e-08 |
| CoA1 |
11 |
46.92 |
4.266 |
2.917 |
0.00148 |
| RoAl |
11 |
48.02 |
4.366 |
3.471 |
0.000214 |
| RoAn |
11 |
42.36 |
3.85 |
2.119 |
0.0165 |
| DlAl |
11 |
95.07 |
8.642 |
14.04 |
<2e-16 |
| DlAn |
11 |
27.24 |
2.4768 |
3.08 |
0.00844 |
| DmAl |
11 |
109.69 |
9.972 |
18.58 |
<2e.16 |
| AnDlDm |
11 |
12.45 |
1.1315 |
6.602 |
3.68e-09 |
| CuLt |
11 |
112.9 |
10.264 |
2.263 |
0.0134 |
| CuAm |
11 |
12.1 |
1.1 |
3.215 |
0.000526 |
| CuA1 |
11 |
1.27 |
0.11542 |
3.472 |
0.000214 |
| CuA2 |
11 |
7.75 |
0.7046 |
2.245 |
0.0142 |
Table 2.
(on page 10)
. Contribution between variables and axes with eigenvalues > 1.00 of PCA (18 populations, including Zaachila) and CVA (12 expanded populations) of
Prosthechea karwinskii from Oaxaca, Mexico. Values in bold correspond to the three highest for each axis (A). See
Table 4 for variable names.
Table 2.
(on page 10)
. Contribution between variables and axes with eigenvalues > 1.00 of PCA (18 populations, including Zaachila) and CVA (12 expanded populations) of
Prosthechea karwinskii from Oaxaca, Mexico. Values in bold correspond to the three highest for each axis (A). See
Table 4 for variable names.
| Variable |
PCA (N =185) |
CVA (N =185) |
| |
A1 |
A2 |
A3 |
A4 |
A5 |
A6 |
A7 |
A8 |
A1 |
A2 |
A3 |
| SlLt |
-0.835 |
0.367 |
0.015 |
0.063 |
0.114 |
0.132 |
0.030 |
0.031 |
-0.128 |
-0.941 |
-0.066 |
| SlAm |
-0.852 |
-0.089 |
-0.055 |
0.070 |
-0.247 |
0.085 |
0.067 |
-0.019 |
-0.551 |
0.230 |
-0.146 |
| SlLa |
-0.671 |
0.369 |
-0.209 |
0.087 |
-0.050 |
0.187 |
-0.059 |
-0.117 |
0.435 |
-0.159 |
-0.004 |
| SlAa |
-0.281 |
-0.701 |
0.027 |
0.372 |
-0.134 |
0.054 |
-0.001 |
-0.050 |
0.411 |
0.241 |
0.222 |
| SdLa |
-0.657 |
0.403 |
-0.166 |
0.028 |
-0.041 |
0.161 |
-0.051 |
-0.242 |
-0.230 |
0.006 |
0.112 |
| SdAa |
-0.174 |
-0.696 |
-0.001 |
0.407 |
-0.121 |
-0.033 |
0.056 |
0.161 |
-0.242 |
-0.121 |
-0.024 |
| PeAm |
-0.853 |
0.104 |
-0.045 |
0.114 |
-0.221 |
0.025 |
0.059 |
0.092 |
0.062 |
0.352 |
-0.007 |
| PeLa |
-0.706 |
0.407 |
-0.128 |
0.124 |
0.002 |
0.043 |
0.032 |
-0.164 |
0.082 |
-0.188 |
0.205 |
| PeAa |
-0.176 |
-0.632 |
0.114 |
0.457 |
0.059 |
0.189 |
0.016 |
0.129 |
0.341 |
-0.104 |
0.191 |
| LaLt |
-0.827 |
0.323 |
0.106 |
0.103 |
0.121 |
0.063 |
-0.067 |
0.153 |
0.158 |
1.015 |
0.088 |
| LaAm |
-0.861 |
0.069 |
0.069 |
0.236 |
-0.244 |
-0.081 |
-0.007 |
0.081 |
0.291 |
-0.420 |
0.480 |
| LaLl |
-0.804 |
0.201 |
0.147 |
0.205 |
-0.006 |
-0.021 |
-0.040 |
0.150 |
0.143 |
0.241 |
-0.436 |
| LaAml |
-0.630 |
0.199 |
0.145 |
0.204 |
-0.376 |
-0.276 |
-0.052 |
0.184 |
-0.533 |
0.040 |
-0.050 |
| LaAul |
0.251 |
-0.043 |
-0.518 |
-0.069 |
0.206 |
0.137 |
0.018 |
-0.242 |
0.082 |
-0.230 |
0.628 |
| LaAlm |
-0.074 |
0.020 |
-0.022 |
-0.010 |
0.474 |
0.441 |
0.375 |
0.404 |
0.034 |
-0.040 |
0.157 |
| LaLm |
-0.604 |
0.329 |
0.096 |
0.049 |
0.147 |
0.192 |
-0.094 |
-0.031 |
0.227 |
-0.273 |
-0.064 |
| LaAmm |
-0.661 |
-0.047 |
-0.018 |
0.131 |
-0.121 |
0.270 |
0.028 |
-0.166 |
-0.432 |
-0.050 |
-0.139 |
| LaAam |
0.016 |
-0.220 |
0.070 |
0.026 |
-0.102 |
0.458 |
-0.717 |
-0.011 |
0.049 |
-0.104 |
-0.053 |
| CoLt |
-0.622 |
0.168 |
-0.107 |
0.016 |
0.249 |
-0.313 |
-0.037 |
0.161 |
-0.152 |
0.557 |
-0.050 |
| CoAe |
-0.729 |
-0.390 |
-0.297 |
-0.277 |
-0.039 |
-0.125 |
0.024 |
0.078 |
-0.497 |
-0.334 |
0.034 |
| CoA1 |
-0.711 |
-0.311 |
-0.327 |
-0.208 |
-0.065 |
0.094 |
0.204 |
-0.065 |
0.031 |
-0.074 |
-0.076 |
| CoGa |
-0.750 |
-0.274 |
-0.345 |
-0.195 |
-0.002 |
-0.090 |
0.233 |
-0.026 |
0.081 |
0.142 |
0.484 |
| RoAl |
-0.394 |
-0.147 |
-0.248 |
-0.405 |
0.094 |
-0.156 |
-0.488 |
0.207 |
-0.327 |
-0.198 |
0.354 |
| RoAn |
-0.588 |
-0.372 |
-0.350 |
-0.404 |
0.009 |
-0.062 |
-0.121 |
0.145 |
0.285 |
0.219 |
-0.269 |
| DlAl |
-0.339 |
0.008 |
0.067 |
0.189 |
0.580 |
-0.345 |
-0.163 |
0.260 |
0.162 |
0.594 |
0.339 |
| DlAn |
-0.543 |
-0.199 |
-0.204 |
0.021 |
0.137 |
-0.316 |
0.028 |
-0.297 |
-0.240 |
-0.393 |
0.309 |
| DmAl |
-0.252 |
-0.253 |
0.140 |
0.261 |
0.505 |
0.237 |
-0.057 |
-0.193 |
0.950 |
-0.243 |
-0.160 |
| DmAn |
-0.494 |
-0.329 |
-0.096 |
-0.040 |
0.262 |
0.044 |
-0.071 |
-0.283 |
0.108 |
-0.213 |
-0.342 |
| AnDlDm |
-0.089 |
-0.043 |
0.019 |
-0.489 |
-0.278 |
0.463 |
0.081 |
0.314 |
-0.311 |
-0.402 |
-0.360 |
| CuLt |
-0.466 |
-0.051 |
0.303 |
-0.273 |
0.306 |
0.073 |
-0.035 |
-0.019 |
-0.098 |
0.211 |
-0.088 |
| CuAm |
-0.424 |
-0.186 |
0.651 |
-0.201 |
-0.088 |
-0.131 |
-0.102 |
-0.133 |
0.144 |
0.206 |
-0.107 |
| CuA1 |
-0.486 |
-0.126 |
0.647 |
-0.315 |
0.108 |
0.013 |
0.117 |
-0.088 |
0.217 |
0.221 |
-0.202 |
| CuA2 |
-0.484 |
-0.218 |
0.622 |
-0.356 |
-0.026 |
-0.042 |
0.146 |
-0.169 |
-0.096 |
-0.088 |
0.016 |
| Eigenvalue |
11.06 |
3.16 |
2.35 |
1.93 |
1.65 |
1.42 |
1.13 |
1.03 |
3.29 |
1.96 |
1.27 |
| Accumulated variance (%) |
33.51 |
43.10 |
50.23 |
56.09 |
61.08 |
65.37 |
68.80 |
71.91 |
33.56 |
53.52 |
66.46 |
Table 3.
Geographic information for the localities of
Prosthechea karwinskii in Oaxaca, Mexico. See Materials and Methods to know how the expanded populations were integrated. QF =
Quercus forest, QPF =
Quercus-
Pinus forest. N = number of individuals represented in the sample size per locality/population. The locality/population numbers correspond to those shown in the map of
Figure 5. The species is an orchid protected by Mexican environmental legislation; therefore, the coordinates of the localities were omitted. NA = the data does not apply.
Table 3.
Geographic information for the localities of
Prosthechea karwinskii in Oaxaca, Mexico. See Materials and Methods to know how the expanded populations were integrated. QF =
Quercus forest, QPF =
Quercus-
Pinus forest. N = number of individuals represented in the sample size per locality/population. The locality/population numbers correspond to those shown in the map of
Figure 5. The species is an orchid protected by Mexican environmental legislation; therefore, the coordinates of the localities were omitted. NA = the data does not apply.
| Number |
Locality/Population |
N |
Expanded population (N) |
Elevation |
Vegetation |
| 1 |
Amialtepec, Santa Catarina Juquila |
14 |
Juquila (14) |
2105 m |
QF |
| 2 |
El Lazo, Sola de Vega |
6 |
Sola_El Lazo (6) |
1840 m |
QPF |
| 3 |
El Vergel, Tlaxiaco |
1 |
Tlaxiaco (15) |
1900 m |
QPF |
| 4 |
La Purisima, Tlaxiaco |
1 |
2160 m |
QPF |
| 5 |
San Mateo Peñasco, Tlaxiaco |
1 |
2150 m |
QF |
| 6 |
Tlaxiaco |
8 |
2160 m |
QPF |
| 7 |
Santiago Nundiche, Tlaxiaco |
4 |
2200 m |
QPF |
| 8 |
Rancho Viejo, Sola de Vega |
11 |
Sola_Rancho Viejo (21) |
1838 m |
QF |
| 9 |
San Agustin, Sola de Vega |
10 |
2091 m |
QPF |
| 10 |
San Agustin, Etla |
19 |
Etla (19) |
1950 m |
QPF |
| 11 |
San Pedro y San Pablo Teposcolula |
13 |
Teposcolula (13) |
2410 m |
QPF |
| 12 |
El Vado-San Sebastian de las Grutas, Sola de Vega |
4 |
Sola_Lachixío (20) |
2100 m |
QF |
| 13 |
San Vicente Lachixío, Sola de Vega |
16 |
2240 m |
QPF |
| 14 |
Santa Maria Jaltianguis |
11 |
Jaltianguis (11) |
2164 m |
QPF |
| 15 |
San Lorenzo Albarradas |
6 |
Albarradas (6) |
2230 m |
QPF |
| 16 |
Santo Domingo Yanhuitlan |
22 |
Yanhuitlan (22) |
2400 m |
QPF |
| 17 |
Yahuiche, Ixtlan de Juarez |
19 |
Yahuiche (19) |
2017 m |
QF |
| 18 |
Zaachila |
19 |
Zaachila (19) |
NA |
NA |
Table 4.
Floral morphologic variables of
Prosthechea karwinskii and their coding used in the present study. The variable numbers correspond to those shown in
Figure 6.
Table 4.
Floral morphologic variables of
Prosthechea karwinskii and their coding used in the present study. The variable numbers correspond to those shown in
Figure 6.
| Floral structure |
Variable |
Number |
Code |
| Lateral sepal |
Total length |
1 |
SlLt |
| Maximum width |
2 |
SlAm |
| Width at 1/3 |
3 |
SlA1 |
| Width at 2/3 |
4 |
SlA2 |
| Length between maximum width and apex |
5 |
SlLa |
| Angle at the apex |
6 |
SlAa |
| Dorsal sepal |
Total length |
7 |
SdLt |
| Maximum width |
8 |
SdAm |
| Width at 1/3 |
9 |
SdA1 |
| Width at 2/3 |
10 |
SdA2 |
| Length between maximum width and apex |
11 |
SdLa |
| Angle at the apex |
12 |
SdAa |
| Petal |
Total length |
13 |
PeLt |
| Maximum width |
14 |
PeAm |
| Width at 1/3 |
11 |
PeA1 |
| Width at 2/3 |
16 |
PeA2 |
| Length between maximum width and apex |
17 |
PeLa |
| Angle at the apex |
18 |
PeAa |
| Labellum |
Total length |
19 |
LaLt |
| Maximum width |
20 |
LaAm |
| Length of the lateral lobe |
21 |
LaLl |
| maximum width of the lateral lobe |
22 |
LaAml |
| Width at 1/3 of lateral lobe |
23 |
LaA1l |
| Angle between lateral lobe and claw |
24 |
LaAul |
| Angle between lateral and middle lobes |
25 |
LaAlm |
| Length of middle lobe |
26 |
LaLm |
| Maximum width of middle lobe |
27 |
LaAmm |
| Width at the base of the middle lobe |
28 |
LaAbm |
| Angle at the apex of the middle lobe |
29 |
LaAam |
| Column |
Total length |
30 |
CoLt |
| Width at the stigma level |
31 |
CoAe |
| Width at 1/3 |
32 |
CoA1 |
| Thickness in the middle part |
33 |
CoGm |
| Thickness at the level of the anther |
34 |
CoGa |
| Rostellum |
Height of the stigmatic cavity |
35 |
RoAl |
| Width of the stigmatic cavity |
36 |
RoAn |
| Teeth of the column |
Height of the lateral tooth |
37 |
DlAl |
| Width of the lateral tooth |
38 |
DlAn |
| Height of the middle tooth |
39 |
DmAl |
| Width of the middle tooth |
40 |
DmAn |
| Width of the gap between the middle and the lateral teeth |
41 |
AnDlDm |
| Cuniculus |
Total length |
42 |
CuLt |
| Maximum width |
43 |
CuAm |
| Width at 1/3 |
44 |
CuA1 |
| Width at 2/3 |
45 |
CuA2 |
3. Discussion
3.1. Morphological Variation in Natural Populations of Prosthechea karwinskii
Plants have the ability to modify their phenotype in response to environmental conditions. However, the variation within a species due to the environment is expected to be smaller and more limited in floral characters compared to vegetative ones, since the former are related to reproductive success and must maintain their function [
31,
32]. The variation in floral morphology can be interpreted as an adaptation to selection by different pollinators [
33,
34]. Interactions plant-pollinators and climatic influence can explain the variation in floral traits, suggesting that the variation expressed on them is a product of an adaptive response [
35]. However, other processes can produce divergence among geographically separated populations of flowering plants, such as random genetic drift, isolation, indirect selection, and genetic factors [
36,
37]. Because floral traits are considered phenotypically more stable than vegetative ones, their variation tends to be less within populations, making them valuable for recognizing infraspecific variation [
19,
23]. Studies evaluating infraspecific morphological variation in orchids have so far been conducted using floral traits and have generally been useful in identifying such variation [
16,
17,
18,
19,
20,
21,
22,
23,
24,
25].
Of the floral characters evaluated in the ANOVA most (38 out of 40) showed significant variation among the populations of P. karwinskii analyzed here, as demonstrated by the ANOVA and Kruskal-Wallis tests. Of the other five floral characters that did not show normality and were evaluated with the Kruskal-Wallis test, three of them showed significant differences among populations. These tests identified Teposcolula (Mixteca region) as the population in Oaxaca that includes individuals with the largest flowers (showing the highest values for sepal, petal, and labellum length and width), while those from Sola_Rancho Viejo (Sierra Sur region) include individuals with the smallest flowers. Among orchids of ornamental value, specimens with larger flowers are preferred for cultivation and are selected as mother plants or as parent for artificial hybrids. Since P. karwinskii is an orchid appreciated for its ornamental value, the Teposcolula population holds greater importance in horticulture compared to other localities in Oaxaca.
The PCA conducted with the set of floral characters for
P. karwinskii showed a high overlap between individuals from the 17 sampled locations and those obtained from Zaachila. Thus, it seems that the traits of floral morphology do not allow for detecting differences between populations, or their number is not sufficient to discriminate the infraspecific variation of
P. karwinskii. Nevertheless, along the axis that accumulated the highest percentage of variance, several individuals from Teposcolula and Yanhuitlan appear at the extremes. The characters associated with this pattern were related to the width of the perianth segments (labellum, petal and lateral sepal). Among all the populations analyzed, Teposcolula has the widest segments, while Yanhuitlan, Etla, and Sola_Rancho Viejo have the narrowest segments, as also revealed by the univariate analyses. Ibáñez [
27] suggested that complex patterns revealed through multivariate analyzes of morphological data may be associated with the life history of the organisms. The localities of Teposcolula and Yanhuitlan host two of the largest populations of the species in Oaxaca, where the highest percentage of individuals are reproductive. Possibly, these two populations present high levels of genetic variation, which could be related to how their individuals are dispersed in the PCA graph. Other studies that have evaluated interspecific [
17,
22] or intraspecific variation [
23] in orchids have also not found differentiation between populations that are geographically separated when analyzing floral characters with PCA. Such results are common given the method’s assumptions, particularly the lack of
a priori categorization into groups that would typically minimize intragroup variance and maximize intergroup variance [
38]. However, this multivariate method has been useful for recognizing morphotypes in orchid species represented by wild specimens [
20] or cultivated ones [
19,
25] in Mexico.
The analysis of
P. karwinskii’s floral morphology with the CVA and cluster analysis were informative for discriminating infraspecific variation. These analyses showed that individuals from Alabarradas are well separated from the rest of the localities. Differences in the teeth of the column discriminate these individuals from those from other localities. Additionally, individuals from Jaltianguis and Yahuiche showed low dispersion and tend to overlap with each other. This morphological pattern is congruent with geography, since these two locations are very close to each other in the region known as Sierra Norte of Oaxaca. Interestingly, individuals from Etla, the locality closest to the previous two, tend to show low dispersion among themselves and separate from those of Jaltianguis-Yahuiche. The morphological differentiation that is present with respect to Teposcolula being more expected. The CVA showed that the variables with the greatest contribution are traits of the column (height of the middle and the lateral teeth, and thickness at the level of the anther), lateral sepal (maximum width, total length), labellum (maximum width of the lateral lobe, total length, angle between lateral lobe and claw, and maximum width). Due to the nature of the characters revealed by the analyses as the most important for the morphological patterns of the species, these traits could be related to the attraction of pollinators, as has been hypothesized in other studies analyzing morphological variation in orchids [
22,
39]. We recommend paying attention to the most important variables presented in this work, as they can be useful as taxonomic markers at the intraspecific level and possibly at the level of the
Prosthechea citrina complex, the species group to which
P. karwinskii belongs.
The population of Albarradas turned out to be the most differentiated among those of
P. karwinskii from Oaxaca. The individuals from this locality exhibit the lowest height of the middle and lateral teeth of the column, the smaller angle between lateral lobe and claw of the labellum, and the thinnest thickness of the column at the level of the anther. Additionally, they have the greatest maximum width of the lateral lobe of the labellum. This population could be recognized as a variety or geographic form of the species, geographically isolated in the central part of the state of Oaxaca. Moreover, the locality of Albarradas is a priority for the conservation of the species, as it represents a unique morphological variant. Unfortunately, the forest where this form grows in Albarradas hosts one of the least numerous populations of this orchid in Oaxaca. It will be interesting to verify if the morphological differentiation of the Albarradas population is related to genetic differentiation, as has been corroborated for other orchid species using vegetative characters [
17].
3.2. Morphological Patterns of Zaachila Flowers of Unknown Origin
Among the natural populations of P. karwinskii used as reference in this study to compare flowers obtained from Easter celebrations in Zaachila (2017-2019), morphometric analyses revealed greater overlap and morphological similarity between the Zaachila material and populations from Yanhuitlan and Etla. Flowers from Zaachila are distinct from those in Albarradas, Jaltianguis and Yahuiche (Sierra Norte), Teposcolula (Mixteca), Juquila and Sola_El Lazo (Southern mountains of Oaxaca). Conversely, Zaachila specimens exhibit varying degrees of overlap with individuals from the Sierra Sur (Juquila, Sola_El Lazo, Sola_Lachixío, Sola_Rancho Viejo) and Mixteca (Tlaxiaco) regions. While the species is relatively common across various forests in Oaxaca, our inference suggests that individuals rescued from Zaachila could come from the Mixteca or vicinity of Etla. However, the available material spans more than one year of festivities, indicating participants likely gathered flowers from multiple locations over three years. The findings presented are specific to the study period, and we caution that they may vary annually depending on where extraction occurs.
Morphometric methods have been successfully employed to determine the unknown origins of organisms in various animal groups, utilizing live specimens, preserved in museums or commercialized [
26,
27,
28,
29,
30]. However, they are relatively unexplored in plant species for this purpose. This study represents an initial attempt to infer the origins of Zaachila individuals based on morphological data. We point out the challenges (even with other types of data, e.g., genetics), including potential variance due to collection from multiple localities during the sampling period, gaps in samples from other distribution sites [
26,
28], and varying population sizes [
40], which are intrinsic to orchids and other epiphytes [
41].
Several populations studied here are situated in areas where specimens are harvested for local markets and religious use [
3,
8,
9], impacting genetic variability and effective population sizes, as well increasing the chances of experiencing inbreeding depression and bottleneck events [
13,
14,
15]. Genetic analysis could help elucidate the causes of floral morphological variation in
P. karwinskii and its biogeographical patterns, though correlations between genetic and morphological variability aren’t always straightforward.
Mexico’s use of plants to satisfy aspects of the cultural and social life of local communities is vast, given the high cultural and biological biodiversity [
42,
43,
44]. Among them, ceremonial uses constitute a cultural element of the peoples, making their transmission important. However, the extraction of wild plants used in such ceremonies often has a negative impact on their populations [
45,
46]. The conservation and use of wildlife are controversial [
47], but these topics must be addressed through collaborative strategies [Ticktin, Robinson 45, 48]. There are some preliminary initiatives for
P. karwinskii [10, Julia 49]. Our findings underscore the need for collaborative conservation efforts to preserve the morphological and genetic diversity of species vulnerable to flower extraction for traditional uses, ensuring their long-term survival. These efforts motivate us to develop collaborative conservation strategies that ensure the maintenance of the morphological and genetic variability of the species in regions most susceptible to flower extraction for traditional uses, as well as in the communities where these extracted specimens are destined. This will help guarantee the long-term preservation of both the species and its cultural significance.
4. Materials and Methods
4.1. Biological Material
During the flowering season of
P. karwinskii (March-April), localities (populations) representing its distribution in Oaxaca were visited between 2015 and 2021. The geographical information of the sampled localities is presented in
Table 3, while
Figure 5 shows their geographical distribution on an Oaxaca map. In each visited locality, one flower per individual was collected, ensuring they were on different host trees to avoid collecting ramets from the same individual; the proximal flower was chosen when the inflorescence had more than one. The sample size of each locality depended on the population abundance and is indicated in
Table 1. A voucher specimen (herbarium or spirit) was prepared from each locality, which was deposited in the Herbarium OAX (acronym according to [
50]. Additionally, flowers rescued from specimens used as decorations in Catholic temples during the Easter celebration in the Villa de Zaachila, Oaxaca (2017-2019) were obtained. These samples, whose original locality is unknown, were obtained with permission from the organizing committee of this commemoration in the community in 2019, once they were removed from the temples. Both the flowers collected in the field and the rescued ones were preserved in a fixing solution of water (78%), ethanol 96% (21%), lactic acid 85% (6%), benzoic acid (0.5% w/v), and glycerin (5%), and then deposited in the Laboratory of Extraction and Analysis of Natural Products (CIIDIR Oaxaca, Instituto Politecnico Nacional).
4.2. Selection of Floral Characters
Each flower of
P. karwinskii was dissected into sepals, petals, labellum, and column to select the characters used as variables in the morphometric analyses, following the procedure of Borba et al. [
18] and da Cruz et al. [
23] with some modifications. Photographs (Canon Rebel camera) were taken of the sepals, petals, and labellum, which were spread out as shown in
Figure 6A. The column was separated from the rest of the perianth for recording the characters as shows in
Figure 6B. The ovary and column were longitudinally sectioned to show the nectary or cuniculus and to record the characters shown in
Figure 6C. Since the flower of
P. karwinskii has bilateral symmetry, only the right-side sepal, petal, lateral lobe of the labellum, and lateral tooth of the column were considered. A total of 45 characters were selected from these structures, of which 39 were linear measurements and six were angles (
Table 4,
Figure 6). Each linear measurement was taken with a digital caliper, and the angles were measured with a protractor. Missing data in some individuals for certain traits, due herbivory, were replaced with the population average for that variable. The set of these continuous characters was recorded in a total of 185 individuals of
P. karwinskii. Specimens rescued from Zaachila were considered as a separate population. Specimens morphologically similar from populations very close to each other (< 5 km apart) were integrated as a single population (expanded population); when a locality was represented by fewer than five specimens, they were also integrated with those from the nearest locality, thus avoiding the effect of a non-representative population. Due to this, the CVA and cluster analysis included 12 expanded populations, as shown in
Table 3.
4.3. Statistial Analyses
An analysis of variance (ANOVA) and a Tukey test as a
post hoc test were conducted to find significant differences among each of the 45 floral characters and the origin locality of the individuals. Out of the 45 floral traits, five did not meet all the assumptions for applying an ANOVA, including normality. To assess if there are differences between populations for these five characters (LaAam, CoGa, CoGm, CoAe, and DmAn), a Kruskal-Wallis non-parametric test was applied, followed by Dunn’s test with Bonferroni correction as a
post-hoc analysis. These analyses were performed using R 2023.12.0 [
51] in the stats package.
Multivariate analyzes were implemented in Statistica 10 [
52]. First, a correlation test was conducted between all possible pairs of variables. Out of the 45 selected variables, 12 showed a correlation greater than 0.9 with each other and were therefore discarded for further analyses. By floral structure, the discarded variables were from the lateral sepal (width at 1/3 of its length, width at 2/3 of its length), dorsal sepal (total length, maximum width, width at 1/3 of its length, width at 2/3 of its length), petal (total length, width at 1/3 of its length, width at 2/3 of its length), labellum (width at 1/3 of its length, width of the base of the middle lobe), and column (thickness in the middle part). The remaining 33 characters were subjected to ordination analyses to evaluate morphological variation among populations, which allowed identifying the most significant variables for morphological patterns. To explore the data structure and detect possible outliers, a principal component analysis (PCA) based on the correlation matrix (correlated variables excluded) was performed. Since this exploratory analysis did not show the presence of outliers, no individuals were removed from the analyses. Subsequently, a canonical variate analysis (CVA) was performed using the expanded populations as categorical variables. We use the standardized coefficients for canonical variables to identify the most important in the observed patterns [
18]. Both the correlation test, PCA, and CVA were conducted including and excluding samples from Zaachila (of unknown origin). We calculated a matrix of squared Mahalanobis distances between the expanded population centroids (including Zaachila), using the expanded populations as the group variable and the 33 floral variables. This matrix was used to perform a cluster analysis using UPGMA (unweighted pair-group method) with arithmetic averages as the clustering algorithm.
5. Conclusions
The majority of floral characters analyzed here varied significantly among populations of P. karwinskii. The CVA was informative for discriminating the infraspecific variation in this orchid, but the PCA was not. Albarradas harbors the most differentiated population of P. karwinskii in Oaxaca, and it might be recognized as a variety or geographical form of the species and considered a priority for conservation. The Teposcolula population has value for horticultural management due to its individuals having the largest flowers in this species. The individuals rescued in Zaachila group with those from Etla and Yanhuitlan, suggesting that the origin of the former might be assigned to these two localities or others very close to them. Additional studies are recommended to compare morphological variation with genetic and geographical variation of the populations studied here.
6. Patents
No patent will be obtained from the results of the study.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Table S1: Results of the ANOVA and Tukey tests for the floral characters recorded in 12 localities of Prosthechea karwinskii from Oaxaca, including individuals rescued from Zaachila.
Author Contributions
Conceptualization, RS and GCL; methodology, MHSE, GCL, & RS; software, MHSE, GGL, & MACM; validation, MHSE, GCL, MACM, LLR, & RS; formal analysis, MHSE, GCL, & MACM; investigation, MHSE, GCL, & RS; resources, RS & LLR; data curation, MHSE, GCL, & RS; writing—original draft preparation, MHSE, GCL, MACM, LLR, & RSG; writing—review and editing, MHSE, GCL, MACM, LLR, & RS; visualization, MHSE, GCL & RS; supervision, GCL & RS; project administration, LLR & RS; funding acquisition, LLR & RS. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by Instituto Politécnico Nacional, through the grant number SIP-20220893.
Data Availability Statement
The authors assure that, by mutual agreement, we have shared data and information included in this study for its potential publication. We note that access to the supplementary material accompanying the manuscript should be restricted until publication is finalized.
Acknowledgments
We appreciate the permission granted by the municipal and community authorities of the visited localities where the floral samples were collected. Special thanks to Clarita Ibarra-Contreras, Gabriela Cruz-García, and Beatriz Pérez-López who provided floral material or assisted in their collection. Brenda Solis García for her assistance in maintaining the collection of flowers used in the study.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Solano, R.; Salazar-Chávez, G.A.; Jiménez-Machorro, R. New combinations in Orchidaceae of Mexico. Act. Bot. Mex. 2011, 97, 49–56. [Google Scholar] [CrossRef]
- Solano, R.; Salazar-Chávez, G.A.; Jiménez-Machorro, R.; Hágsater, E.; Cruz-García, G. Actualización del catálogo de autoridades taxonómicas de Orchidaceae de México. Instituto Politécnico Nacional. Centro Interdisciplinario de Investigación para el Desarrollo Integral Regional Unidad Oaxaca. Informe Final, SNIB-CONABIO, Proyecto No. KT005. Mexico City, 2020.
- Hágsater, E.; Soto-Arenas, M.A.; Salazar-Chávez, G.A.; Jiménez-Machorro, R.; López-Rosas, M.A.; Dressler, R.L. Las orquídeas de México, Instituto Chinoin: Mexico City, Mexico, 2007; 302 pp.
- Cruz-García, G.; Solano, R.; Lagunez-Rivera, L. Documentation of the medicinal knowledge of Prosthechea karwinskii (Orchidaceae) in a Mixtec community in Mexico. Rev. Bras. Farmacogn. 2014, 24, 153–158. [Google Scholar] [CrossRef]
- Barragán-Zarate, G.S.; Lagunez-Rivera, L.; Solano, R.; Pineda-Peña, E.A.; Landa-Juárez, AY.; Chávez-Piña, A.E.; Carranza-Álvarez, C.; Hernández-Benavides, D.M. Prosthechea karwinskii, an orchid used as traditional medicine, exerts anti-inflammatory activity and inhibits ROS. J. Ethnopharmacol. 2020, 253, 112632. [Google Scholar] [CrossRef] [PubMed]
- Barragán-Zarate, G.S.; Lagunez-Rivera, L.; Solano, R.; Carranza-Álvarez, C.; Hernández-Benavides, D.M.; Belmonte-Jiménez, S.I.; Vilarem, G. UPLC-ESI-qTOF-MS/MS characterization of bioactive constituents and ROS inhibition in Prosthechea karwinskii leaves, pseudobulbs, and flowers. Heliyon 2022, 8, e09867. [Google Scholar] [CrossRef] [PubMed]
- Lagunez-Rivera, L.; Barragan-Zarate, G.S.; Solano, R.; Alexander-Aguilera, A.; Chávez-Piña, A.E. Mexican orchid (Prosthechea karwinskii) and use in cardiovascular protection cellular and physiological aspects. In Ancient and Traditional Foods, Plants, Herbs and Spices; Rajendram, R., Patel, V.R., Patel, V.B., Eds.; Taylor & Francis: Boca Raton, U.S.A, 2023; pp. 259–279. [Google Scholar] [CrossRef]
- Solano, R.; Cruz-Lustre, G.; Martínez-Feria, A.; Lagunez-Rivera, A. Plantas utilizadas en la celebración de la Semana Santa en Zaachila, Oaxaca. Polibotánica 2010, 29, 263–279. [Google Scholar]
- Cruz-García, G.; Lagunez-Rivera, L.; Chávez-Angeles, M.G.; Solano, R. The wild orchid trade in a Mexican local market: diversity and economics. Econ. Bot. 2015, 69, 291–305. [Google Scholar] [CrossRef]
- Dutra, D. Demography, wild harvest patterns and trade of culturally important species: priorities for management and conservation. Ph.D. dissertation, The University of Hawaii at Manoa, U.S.A., 2014; 76 pp.
- SEMARNAT. Norma Oficial Mexicana NOM-059-SEMARNAT-2010, Protección ambiental – Especies nativas de México de flora y fauna silvestres – Categorías de riesgo y especificaciones para su inclusión, exclusión o cambio – Lista de especies en riesgo. Modificación del Anexo Normativo III. Diario Oficial de la Federación. November 14, 2019. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=5578808&fecha=14/11/2019 (accessed on 16 March 2024).
- Coutiño-Cortés, A.G.; Bertolini, V.; Archila-Morales, F.; Valle-Mora, J.; Iracheta-Donjuan, J.; García-Bautista, M.; Ruiz-Montoya, L. El uso ornamental de Guarianthe skinneri (Orchidaceae), en Chiapas y Guatemala, determina parcialmente su diversidad y estructura genética. Act. Bot. Mex. 2018, 124, 35–48. [Google Scholar] [CrossRef]
- Emeterio-Lara, A.; García-Franco, J.; Hernández-Apolinar, M.; Mora-Herrera, M.; Toledo-Hernández, V.; Valencia-Díaz, S.; Flores-Palacios, A. Endogamy cost and reproductive biology of Laelia autumnalis, an endemic orchid of Mexico. Pl. Ecol. 2018, 219, 1423–1434. [Google Scholar] [CrossRef]
- Emeterio-Lara, A.; García-Franco, J.; Hernández-Apolinar, M.; Mora-Herrera, M.; Toledo-Hernández, V.; Valencia-Díaz, S.; Flores-Palacios, A. Is pseudobulb harvest a sustainable management strategy in wild orchid populations? An experiment with Laelia autumnalis. Forest Ecol. Manag. 2021, 491, 119205. [Google Scholar] [CrossRef]
- Rojas-Méndez, K.J.; Peñaloza-Ramíerez, J.M.; Rocha-Ramírez, V.; Cortés-Palomec, A.; McCauley, R.A.; Oyama, K. Massive extraction of the orchid Laelia speciosa (HBK) Schltr. for trading in local markets affects its population genetic structure in a fragmented landscape in central Mexico. Trop. Conserv. Sci. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Catling, P.M. Malaxis salazarii, a new species from Mexico and Northern Mesoamerica. Orquidea (Mexico City) n.s. 1990, 12, 93–104. [Google Scholar]
- Borba, E.L.; Shepher, G.J.; van den Berg, C.; Semir, J. Floral and vegetative morphometrics of five Pleurothallis (Orchidaceae) species: correlation with taxonomy, phylogeny, genetic variability and pollination systems. Ann. Bot. 2002, 90, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Borba, E.L.; Funch, R.R.; Ribeiro, P.L.; Smidt, E.C.; Silva, P.V. Demography, and genetic and morphological variability of the endangered Sophronitis sincorana (Orchidaceae) in the Chapada Diamantina, Brazil. Pl. Syst. Evol. 2007, 267, 129–146. [Google Scholar] [CrossRef]
- Salazar-Rojas, V.M.; Herrera-Cabrera, B.E.; Soto-Arenas, M.A.; Castillo-González, F. Morphological variation in Laelia anceps subsp. dawsonii f. chilapensis Soto-Arenas Orchidaceae in traditional home gardens of Chilapa, Guerrero, México. Gen. Resour. Crop Evol. 2010, 57, 543–552. [Google Scholar] [CrossRef]
- Hernández-Ruíz, J.; Herrera-Cabrera, B. E.; Delgado-Alvarado, A. Variación morfológica del labelo de Vanilla pompona (Orchidaceae) en Oaxaca, México. Rev. Mex. Biodivers. 2019, 90, 2–90. [Google Scholar] [CrossRef]
- Melo, C.M.; Borba, E.L. Morphological variability in rupicolous species of the Acianthera prolifera complex (Orchidaceae) occurring in southeastern Brazil. Pl. Syst. Evol. 2011, 293, 135–145. [Google Scholar] [CrossRef]
- Cruz-Lustre, G.; Batista, J.A.N.; Radins, A.J.; González, A.; Borba, E.L. Morphometric analysis of the Habenaria parviflora complex (Orchidaceae). Pl. Syst. Evol. 2020, 306, 37. [Google Scholar] [CrossRef]
- da Cruz, D.T.; Selbach-Schnadelbach, A.; Mota-Lambert, S.; Ribeiro, P.L.; Borba, E.L. Genetic and morphological variability in Cattleya elongata Barb. Rodr. (Orchidaceae), endemic to the campo rupestre vegetation in northeastern Brazil. Pl. Syst. Evol. 2011, 294, 87–98. [Google Scholar] [CrossRef]
- Bateman, R.M.; Smith, R.J.; Fay, M.F. Morphometric and population genetic analyses elucidate the origin, evolutionary significance and conservation implications of Orchis angusticruris (O. purpurea x O. simia), a hybrid orchid new to Britain. Bot. J. Linn. Soc. 2008, 157, 687–711. [Google Scholar] [CrossRef]
- Lima-Morales, M.; Herrera-Cabrera, B.E.; Delgado-Alvarado, A. Intraspecific variation of Vanilla planifolia (Orchidaceae) in the Huasteca region, San Luis Potosi, Mexico: morphometry of floral labellum. Pl. Syst. Evol. 2021, 307, 40. [Google Scholar] [CrossRef]
- Chiari, Y.; Claude, J. Morphometric identification of individuals when there are more shape variables than reference specimens: a case study in Galápagos tortoises. C. R. Biol. 2008, 335(1), 62–68. [Google Scholar] [CrossRef]
- Ibánez, A.L. Fish traceability: guessing the origin of fish from a seafood market using fish scale shape. Fish. Res. 2015, 170, 82–88. [Google Scholar] [CrossRef]
- Trivellini, M.M.; Van der Molen, S.; Filun, L.; Márquez, F. Can shell shape be used to find the origin of South American mussels? Mar. Biol. Res. 2021, 17(2), 215–222. [Google Scholar] [CrossRef]
- Zheng, C.; Jiang, T.; Luo, R.; Chen, X.; Liu, H.; Yang, J. Geometric morphometric analysis of the Chinese mitten crab Eriocheir sinensis: a potential approach for geographical origin authentication. N. Am. J. Fish. Manage. 2021, 41(4), 891–903. [Google Scholar] [CrossRef]
- Oleksa, A.; Căuia, E.; Siceanu, A.; Puškadija, Z.; Kovačić, M.; Pinto, M.A.; Tofilski, A. Honeybee (Apis mellifera) wing images: a tool for identification and conservation. GigaScience 2023, 12, giad019. [Google Scholar] [CrossRef] [PubMed]
- Pélabon, C.; Armbruster, W.S.; Hansen, T.F. Experimental evidence for the Berg hypothesis: vegetative traits are more sensitive than pollination traits to environmental variation. Funct. Ecol. 2011, 25, 247–257. [Google Scholar] [CrossRef]
- Romero-Bravo, A.; Castellanos, M.C. Nectar and floral morphology differ in evolutionary potential in novel pollination environments. New Phytol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Clegg, M.T.; Durbin, M.L. Flower color variation: a model for the experimental study of evolution. Proc Natl Acad Sci USA 2000, 97, 7016–7023. [Google Scholar] [CrossRef] [PubMed]
- Mascó, M.; Noy-Meir, I.; Sersic, A.N. Geographic variation in flower color patterns within Calceolaria uniflora Lam. in Southern Patagonia. Plant. Syst. Evol. 2004, 244, 77–91. [Google Scholar] [CrossRef]
- Weber, U.K.; Nuismer, S.L.; Espíndola, A. Patterns of floral morphology in relation to climate and floral visitors. Ann. Bot. 2019, 125, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Podolsky, R.H.; Holtsford, T.P. Population structure of morphological traits in Clarkia, Dudleyana I, comparison of Fst between allozymes and morphological traits. Genetic 1995, 140, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Schemske, D.W.; Bierzychudek, P. Perspective: evolution of flower color in the desert annual Linanthus parryae: wright revisited. Evolution 2001, 55, 1269–1282. [Google Scholar] [PubMed]
- Palacio, F.X.; Apodaca, M.J.; Crisci, J.V. Análisis Multivariado para Datos Biológicos: Teoría y su Aplicación Utilizando el Lenguaje R; Fundación de Hstoria Natural Félix de Azara: Buenos Aires, Argentina, 2020. [Google Scholar]
- Benítez-Vieyra, S.; Pérez-Alquicira, J.; Sazatornil, F.D.; Domínguez, C.A.; Boege, K.; Pérez-Ishiwara, R.; Fornoni, J. Evolutionary transition between bee pollination and hummingbird pollination in Salvia: Comparing means, variances and covariances of corolla traits. J. Evol, Biol, 2019, 32, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Congiu, L.; Striebel-Greiter, B.; Gessner, J.; Boscari, E.; Boner, M.; Jahrl, J.; Della-Porte, S.; Ludwig, A. Identification and tracking of sturgeons and paddlefish products in trade: Implications for trade control and biodiversity management. Aquaculture 2023, 574, 739708. [Google Scholar] [CrossRef]
- Mondragón, D.M.; Valverde-Valdés, M.T.; Hernández-Apolinar, M. Population ecology of epiphytic angiosperms: a review. Trop. Ecol. 2015, 56, 01–39. [Google Scholar] [CrossRef]
- Beltrán-Rodríguez, L.A. , Martínez-Rivera, B.; Maya, A.P. Etnoecología de la flor de catarina-Laelia autumnalis (La Llave & Lex.) Lindl.)-(Orchidaceae) en una comunidad campesina al sur del estado de Morelos, México: conservando un recurso y preservando saberes populares. Etnobiología, 2012, 10, 1–17. [Google Scholar]
- Casas, A.; Blancas, J.; Lira, R. (2016). Mexican ethnobotany: interactions of people and plants in Mesoamerica. Ethnobotany of Mexico: Interactions of People and Plants in Mesoamerica; Springer: New York, USA.
- Briseño-Tellez, J.M.; Pulido-Silva, M.T.; Bautista, K.; García-Mera, A.; Larios-Lozano, O.; López-Gutiérrez, B.N.; Zepeda-Hernández, Z.K. Palm Sunday in central Mexico: among sellers, palms and syncretism. J. Ethnobiol. Ethnomed. 2023, 19, 22. [Google Scholar] [CrossRef] [PubMed]
- Ticktin, T. The ecological implications of harvesting non-timber forest products. J. Appl. Ecol. 2024, 41, 11–21. [Google Scholar] [CrossRef]
- Chapagain, D.J.; Meilby, H.; Baniya, C.B.; Budha-Magar, S.; Ghimire, S.K. Illegal harvesting and livestock grazing threaten the endangered orchid Dactylorhiza hatagirea (D. Don) Soó in Nepalese Himalaya. Ecol. Evol. 2021, 11, 6672–6687. [Google Scholar] [CrossRef]
- Chan, K.M.; Pringle, R.M.; Ranganathan, J.A.I.; Boggs, C.L.; Chan, Y.L.; Ehrlich, P.R.; Macmynowski, D.P. When agendas collide: human welfare and biological conservation. Conserv. Biol. 2007, 21, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.G. Ethical pluralism, pragmatism, and sustainability in conservation practice. Biol. Conserv. 2011, 144, 958–965. [Google Scholar] [CrossRef]
- Douglas, J. (University of Hawaiʻi, Mānoa, USA); Hernández-Apolinar, M. (Universidad Nacional Autónoma de Mexico, Mexico City, Mexico); Ticktin, T. (University of Hawaiʻi, Mānoa, USA). Personal communication, 2024.
- Thiers, B. Index Herbariorum: A global directory of public herbaria and associated staff. New York Botanical Garden’s Virtual Herbarium. http:// sweetgum.nybg.org/science/ih/ (accessed on April 2024).
- R Core Team (2023). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2023. URL https://www.R-project.org/https://statistica.software.informer.com/10.
- StatSoft. Statistica 10.0. TIBCO Software Inc. 2010. https://statistica.software.informer.com/10.0/https://statistica.software.informer.com/10.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).