1. Introduction
South Africa faces challenges ranging from high unemployment and poverty to the ongoing energy crisis and rising living costs [
1]. These negatively impact SA’s state of food security by making food expensive and inaccessible to many [
2]. An estimated 58 million people in SA are facing food insecurity, while almost 18.6 million children are stunted [
3]. This incident resulted in the DBE advocating for vegetable garden establishment at schools [
4]. The DBE is responsible for the National School Nutrition Program (NSNP). The NSNP has three pillars: (i) a feeding programme providing meals to learners, (ii) nutrition education promoting healthy lifestyles, and (iii) sustainable food production in schools, thereby promote food production and skills transfer to schools and communities aimed at food security [
5].
Vegetables in SA play a central role in addressing food security and providing nutritional supplements and requirements to people [
6,
7]. Beetroot is a biennial plant grown annually for its storage root [
6]. The roots are eaten grilled, boiled or roasted as a cooked vegetable or cold as a salad after cooking and adding oil and vinegar [
7]. Beetroot is a rich source of carbohydrates and protein. It also has high essential vitamins, minerals and micronutrients [
8]. The average beetroot consumption is approximately 57 794 tons per annum [
7]. This shows that SA is self-sufficient regarding beetroot production. The central producing regions are the North West, Gauteng, Mpumalanga, KwaZulu Natal and Western Cape [
7]. The area planted for Beetroot each year in SA is determined by climatic and economic factors, neglecting the soil factor.
Soil is a crucial component of any successful agricultural operation. It serves as a medium for plant growth and provides water and essential nutrients [
9]. Thus, it plays a critical role in the production of food. Nonetheless, soil is naturally different in space because of the way it is formed [
10]. The various climates, parent materials, topography and organisms across space interact in time to create specific types of soil [
11,
12]. Therefore, the cultivation of crops needs to be guided by considering the location-specific inherent soil capacity [
13]. Identification of the agricultural potential of soils is necessary for sustainable crop production [
14].
In SA, less attention has been paid to assessing the suitability of soils for agricultural production. Consequently, the need for reliable soil information to support agricultural decision-making has never been more significant [
15]. Knowledge of soils and their properties is indispensable for agricultural development; it opens opportunities for a more rational management of land resources [
16]. A soil survey describes the characteristics of soils, classifies, maps, and makes predictions about their behaviour [
17]. It involves grouping soils with respect to their spatial location, profile characteristics, and relationships to one another, suitability for various purposes and needs for types of management [
18]. Soil suitability assessment is estimating the potential of agricultural soil for specific use [
19,
20]. It matches the soil's inherent characteristics with the optimal crop requirements [
19]. As a result, it helps choose crops to be grown suitable to location-specific soil units for optimising crop productivity [
21]. The objective of this work was to (i) describe the characteristics of the soils and (ii) assess their suitability in various schools for Beetroot production.
2. Materials and Methods
2.1. Site Description
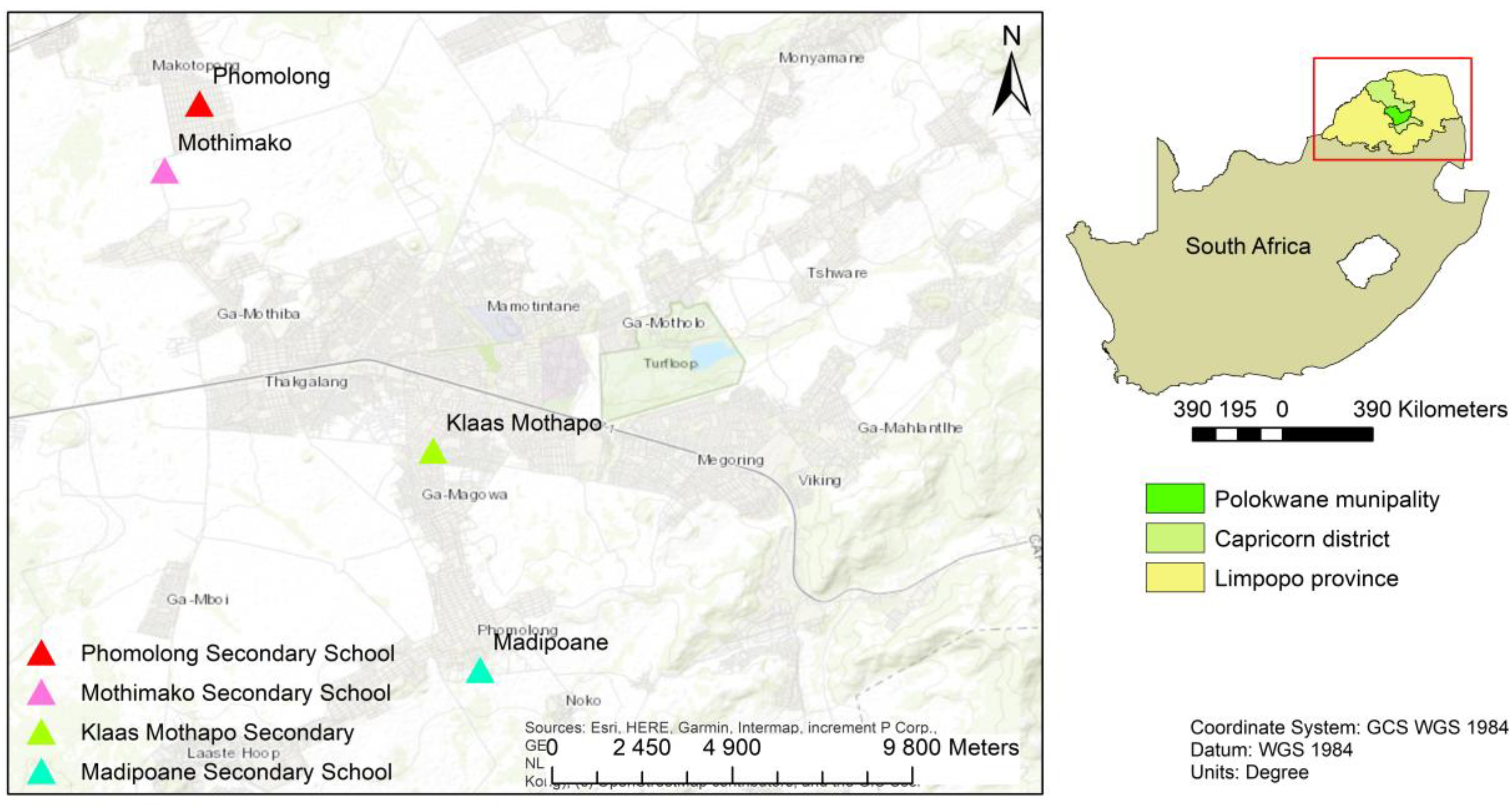
The research was conducted at Madipoane, Mothimako, Phomolong and Klaas Mothapo Secondary Schools of Kgakotlou Circuit in Capricorn District, Polokwane municipality in the Limpopo province of South Africa (
Figure 1). Capricorn district has a semi-arid climate, with the highest precipitation occurring during the summer months from October to March. The annual rainfall ranges from 600 mm to 800 mm [
22]. Mean annual temperatures range from 6°c to 29°c. All the selected schools' gardens are relatively flat. The altitude in all the gardens ranges from 1221 m to 1349 (
Table 1).
2.2. Soil Survey and Classification
A detailed soil survey was conducted in each garden to study the soils [
23]. Soil descriptions were based on the FAO guidelines [
24]. In each garden, a profile pit (1.5 m wide x 1.5 m long, depth was defined to the limiting layer) was dug using a pick and a spade to aid soil classification. For each soil profile, the following soil attributes and site characteristics were collected: top, sub and total depth; effective rooting depth; colour of the A and B horizon; soil permeability; slope percentage; surface cover type (rock, boulder, stone, gravel and grit). The soils were classified using SA taxonomy (SAT) [
25]. The soils were correlated with the World Reference Base for Soil Resources (WRB) [
18] using [
26].
Each profile pit was georeferenced using a portable handheld global positioning system (GPS) (Model Garmin 12 L). To assist soil mapping, the boundaries of each soil were established using an auger across the gardens and georeferenced using GPS.
2.3. Soil Sampling
Soil samples were collected from the dug profile pits. At each profile pit, soil samples were collected from the A horizon (top soil) and B horizon (sub soil) on three faces. Three soil samples were collected from each pit from the A horizon and three from the B horizon. In total, the soil profile had six samples for laboratory analysis. The samples were then labelled according to the school, profile pit number, and horizon and replicate number. From there, they were carefully handled and taken to the laboratory for preparation and analysis.
2.4. Soil Preparation and Analysis
Soil samples were air-dried, crushed and sieved before analysis. Particle size distribution of sand, silt and clay was determined using the hydrometer method [
27]. Soil organic carbon (OC) was determined using the Walkley and Black method [
28]. Soil boron (B), phosphorus (P), total nitrogen (N) and potassium (K) were determined using ICP-OES [
29]. Soil pH H
2O in 1:2.5 suspension of soil and water was determined using the pH meter [
29]. Soil EC was determined in 1:2.5 suspension of soil and water using an EC meter [
29].
2.5. Land Suitability Assessment for Beetroot Production
We used the principles and concepts of the FAO Framework for Land Evaluation [
19] and the FAO guidelines for land evaluation for rainfed agriculture [
30] modified by [
31] and [
21] to assess the suitability of the soils for Beetroot production. Ten important soil parameters i.e. soil depth, soil texture, soil reaction (pH), soil organic carbon (SOC), EC, nitrogen (N), potassium (K), phosphorus (P), and Boron (B) for Beetroot growth and yielding [
6,
7] were used in the suitability assessment. Rainfall, temperature, and slope were homogenous across the schools and thus excluded from the evaluation. The soil suitability was determined for four suitability classes: highly suitable (S1), moderately suitable (S2), marginally suitable (S3) and not suitable (NS) and suborder (indicating limitations) using the maximum limitation method [
19,
21,
30,
31].
2.6. Soil and Suitability Mapping
We used soil surveys and laboratory data to develop soil mapping units in the vegetable gardens. The units were spatially explicitly mapped by developing polygons utilising Google Earth Pro (Google earth, 2024, Keyhole, Inc., Mountain View, CA, USA) and ArcGIS 10.8.1 (ESRI, Redlands, CA, USA) software following the method outlined by [
32]. We adopted the procedure outlined by [
32,
33] to develop the suitability maps of the gardens for Beetroot production.
3. Results
3.1. Morphological Characteristics of the Soils
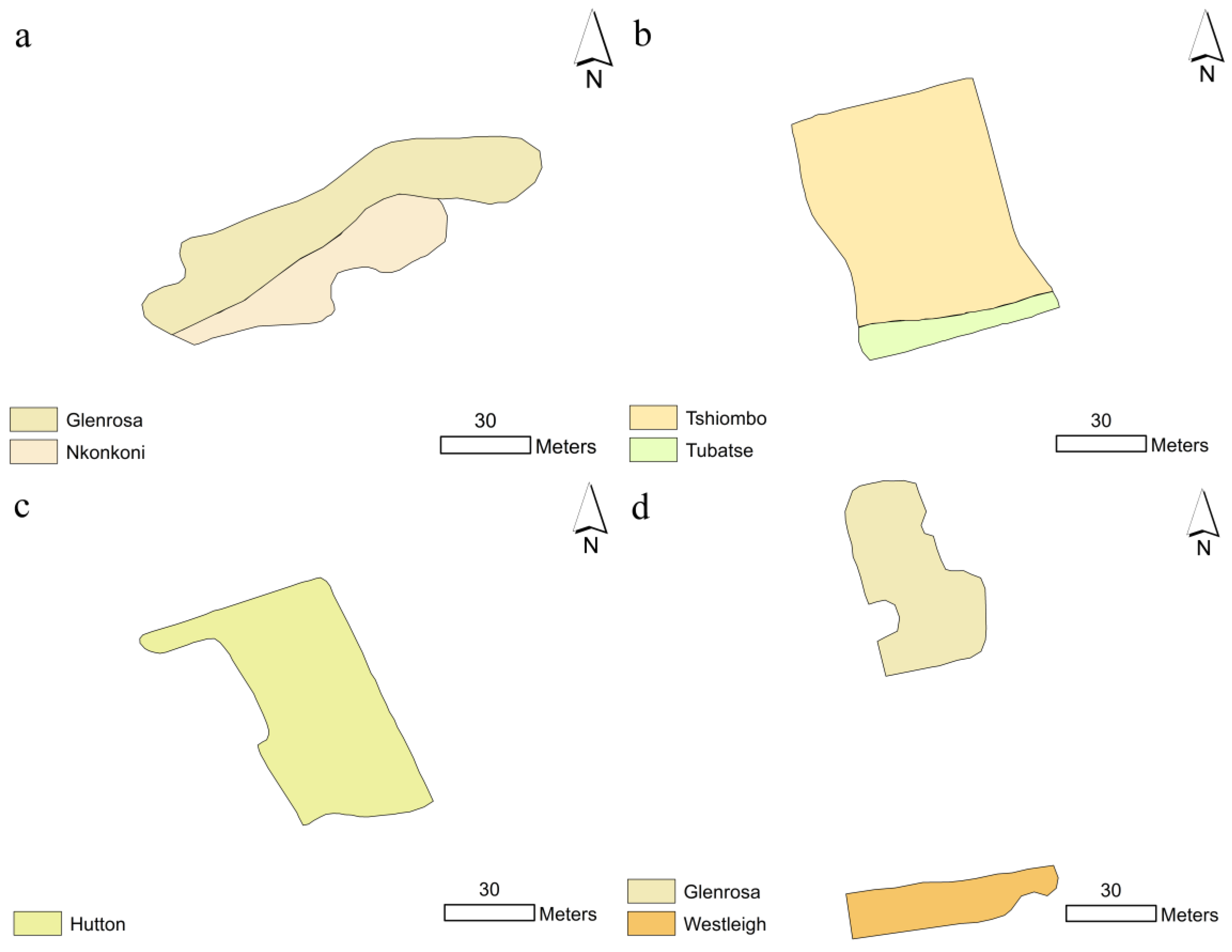
Morphological characteristics of the soils in vegetable gardens of Secondary Schools in the Kgakotlou circuit are shown in
Table 2. Two soils were found at Klaas Mothapo Secondary School: Nkonkoni (Cambisol) and Glenrosa (Leptosol). An Orthic A horizon marks both soils. They are distinguishable by the subsoil soil horizon, with Nkonkoni as red apedal B/Lithic and Glenrosa as Lithic B. The presence of Lithic B makes Glenrosa a shallow soil, whereas Nkonkoni is a deeper soil. The colour varies from 2.5YR 3/3 dark reddish brown to 2.5YR 4/3 reddish brown on their topsoils. The subsoils have the same colour as dark reddish brown. The soils were well drained. The area covered by Glenrosa is 1103 m
2, while Nkonkoni is 568 m
2 out of the total vegetable garden area (1671 m
2) (
Figure 2a).
At Madipoane Secondary School, we found two soils (Tshiombo and Tubatse) covering the vegetable garden area. The soils are broadly known as Acrisols. They are marked by an Orthic A horizon. Tshiombo was distinguished by Neocutanic B underlain by unspecified material showing signs of wetness in the subsoil. Whereas Tubatse was distinguished by Neocutanic B underlain by Lithic material. Both soils were deep. Tshiombo had a similar colour (5YR 5/6 Yellowish red) in the topsoil and subsoil. Tubatse was marked by a 2.5YR 3/3 dark reddish brown colour in the topsoil and 5YR 4/3 reddish brown in the subsoil. Out of the vegetable garden's total area (750.4 m
2), 672 m
2 and 78.4 m
2 was covered by Tshiombo and Tubatse respectively (
Figure 2b).
We found only one soil type at Mothimako Secondary School. Hutton (Cambisol) soil was distinguishable by a red Orthic A horizon underlain by a red apedal B horizon. The top horizon of the soil was 7.7 cm, while the subsoil was 53 cm. Hutton soil covered 3190 m
2, the total area of the vegetable garden (
Figure 2c).
We found two soils at Phomolong Secondary School, namely Glenrosa and Westleigh soil. Westleigh soil is chiefly known as Plinthosol, while Glenrosa is known as Leptosol. The two soils have the same Orthic A horizon. They are distinguished by the subsoil, with Glenrosa having a Lithic B and Westleigh having a soft plinthic B. The colour of Glenrosa was 5YR Reddish brown in the top soil and subsoil. Westleigh soil was marked by a 7.5YR 5/3 Brown colour. Westleigh and Glenrosa covered 368 m
2 and 578 m
2 respectively, with total area of the vegetable garden being 946 m
2 (
Figure 2d).
3.2. Physical and Chemical Characteristics of the Soils
The chemical and physical characteristics of the soils are shown in
Table 3. Almost all the soils had a pH above 7.0, indicating alkaline conditions, except for Westleigh soil at Phomolong Secondary School with a pH of 5.95. The EC content of the soils varied from 4.64 us/cm to 221.6 us/cm. The lowest EC content was observed at Phomolong under Westleigh soil, while the highest was at Mothimako in Hutton soil. The OC content of the soils varied from 0.05% to 0.81%. The lowest content was found in Nkonkoni and Glenrosa soil at KlaasMothapo and Phomolong Secondary School. The N content of the soils varied from 8.90 mg/kg to 25.97 mg/kg. Both soils at Phomolong had low N content. The highest N content was at Madipoane in Tshiombo soil, followed by Glenrosa at Klaas Mothapo. The K content of the soils ranged from 149 mg/kg to 351.33 mg/kg. The low P content was found in Tubatse soil at Madipoane while the highest was at Phomolong in Glenrosa soil. The K content of the soils ranged from 30.18 mg/kg to 94.57 mg/kg. There was a slight difference in the K content of soils except for Nkonkoni, Tshiombo, Westleigh and Glenrosa at Phomolong Secondary School. Boron content of the soils ranged from 245.33 mg/kg to 543.67 mg/kg. The highest B content was found at Phomolong under Glenrosa soil, while the lowest was found at Madipoane under Tubatse soil. Soil texture was slightly different. Except for Glenrosa soil with Sandy loam texture, the textures of the soils varied sand and loamy sand in all the schools.
3.3. Soil Suitability Classification for Beetroot Production
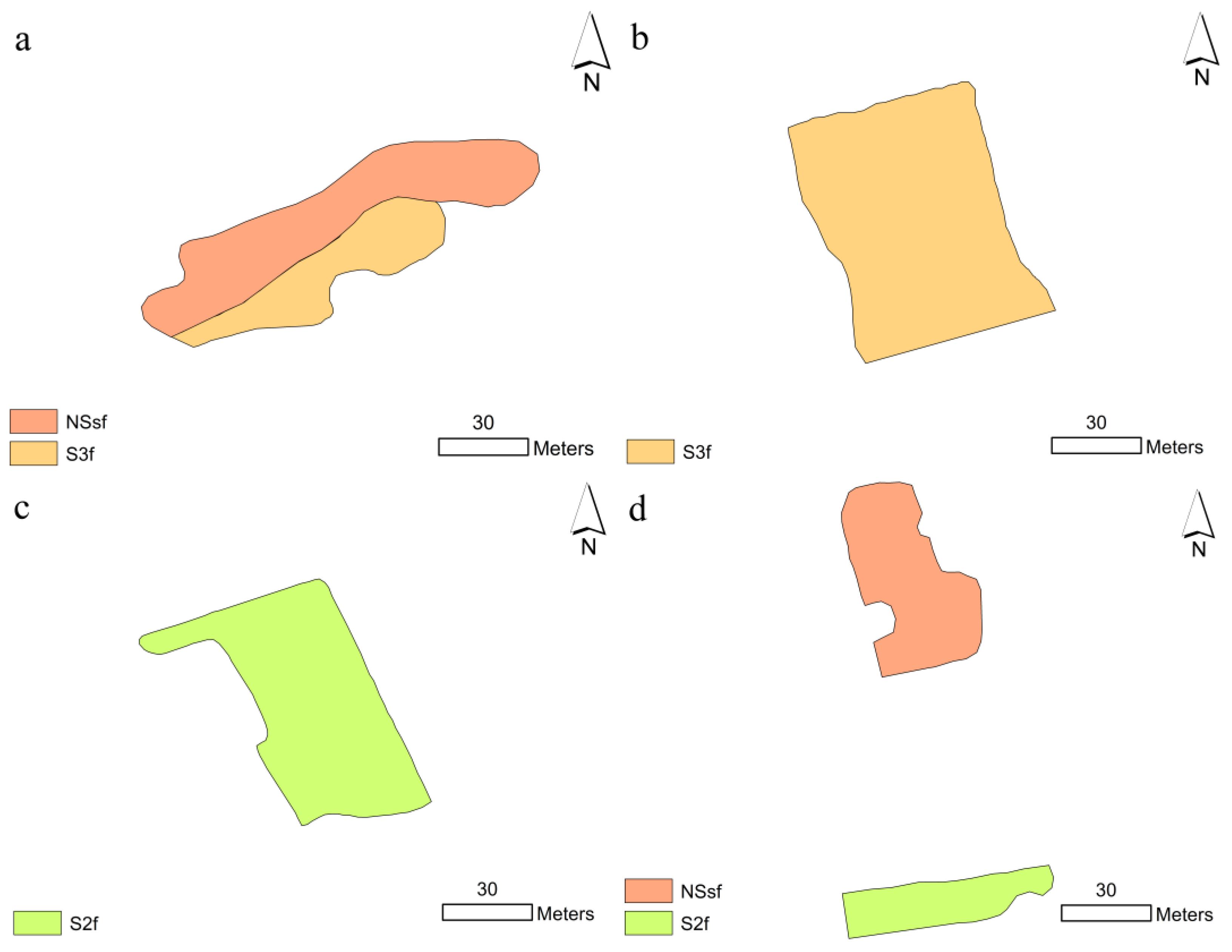
Soil suitability assessment revealed varied suitability classes for Beetroot production in the Kgakotlou (
Figure 3). At Klaas Mothapo Secondary School, the portion of the vegetable garden underlain by Glenrosa soil was not suitable (NS) for Beetroot production (
Figure 3a). A shallow depth, and high EC and pH limited the soil. In the same school, the portion belonging to Nkonkoni soil was marginally suitable (S3), limited by high pH and EC. Similarly, the garden at Madipoane Secondary School was marginally suitable under Hutton (S3) (
Figure 3a). All the soils in the school were limited by a high pH and EC (
Figure 3b). The vegetable garden area at Mothimako Secondary School was moderately suitable (S2) for Beetroot production, limited by a slightly high pH (
Figure 3c). The portion of the Phomolong Secondary School Garden was unsuitable (NS) for beetroot production limited by a shallow depth, high pH and EC (
Figure 3d). On the other side of the school, there was a moderately suitable (S2) portion for Beetroot production limited by a low pH (
Figure 3c).
4. Discussion
We generally found moderate, marginal and unsuitable soils for Beetroot production in the Kgakotlou circuit schools. Hutton soil occupying the total vegetable garden area at Mothimako was moderately suitable (S2) for beetroot production. Likewise, Westleigh soil which was occupying 39% at Phomolong was moderately suitable (S2). At Klass Mothapo Secondary School, 34% on Nkonkoni was marginally suitable, while 66% under Glenrosa soil was not suitable for beetroot production. Like Nkonkoni soil at Klaas Mothapo, all the soils (Tshiombo and Tubatse) at Madipoane Secondary School were marginally suitable for beetroot production. Glenrosa soil at Phomolong occupying 61% of the vegetable garden area was not suitable for beetroot production.
The limitations associated with Glenrosa (Leptosol) soil at Klaas Mothapo and Phomolong include a shallow depth, pH and EC. Soil depth determines rooting, moisture and nutrient storage, mineral reserve, anchorage, and various plant growth conditions [
34]. Glenrosa soil has a restrictive limiting layer (Lithic B) that impedes water and air movement through the soil or restricts roots or otherwise provides an unfavorable root environment [
35]. The roots of the plant will remain confined to a small volume of soil that cannot provide adequate anchorage, water and nutrients [
35,
36]. Nutrient and water absorption capacity of the restricted roots is also low. Shallow soils cannot store enough water to support the plants, and the soils are droughty in the dry season [
36]. Glenrosa soils have excessive internal drainage naturally (in our case it is evident by low clay and OC contents) which can cause drought despite the soil receiving sufficient water either from rain or irrigation [
35]. The Glenrosa soils are at a low stage of weathering and development, and most of the ions are held in the form of primary minerals [
37]. Subsequently, they have low exchangeable cations and exchangeable capacity to sustain crop production. As such, Beetroot grown on this soil will suffer from a lack of water and nutrients, and restricted root growth will affect the anchorage of the crop.
All the soils in this study, their suitability was affected by EC. Soil salinity is simply the salt content in the soil. The significant limitations of salt-affected soils include their low osmotic potential due to the high soluble salt contents and low contents of micronutrients such as Fe, Mn, Zn, Cu and B, which is vital for Beetroot [
6,
38]. A continuous osmotic phase inhibits water uptake by plants due to osmotic pressure of saline soil solution lowering its potential energy; and a slower ionic phase when the accumulation of specific ions in the plant over a period leads to ion toxicity or ion imbalance [
38].
Westleigh soil suitability was limited by a low pH, indicating acidic conditions. Soil acidity affects emergence, survival, root growth, and microbial growth [
39]. Under acidic conditions, aluminium and manganese become more soluble and toxic in most soils, and deficiencies of essential plant nutrients such as P, Ca, K, Mg, and Mo occur [
39]. Increased solubility of toxic metals (Al and Mn) in soil restricts the plant’s access to water and nutrients, thereby causing severe injury to roots, a reduction in crop yield, and an increase in plant susceptibility to pathogens.
A soft plinthic material characterises Westleigh soil in the subsoil. Soft plinthite is dense and obstructs deep percolation of water and penetration of plant roots [
35]. Even though plinthite is initially formed below the soil surface and remains soft, it is often exposed to the surface through erosion. It hardens irreversibility to iron pan making the land quite unusable for agriculture [
40,
41]. In addition to the limitation imposed by salinity on Tshiombo and Tubatse. These soils naturally have poor chemical properties. Levels of plant nutrients are low, and aluminium toxicity and P-sorption have substantial limitations [
35].
5. Conclusions
This study was undertaken to describe the characteristics of the soils and assess their suitability for Beetroot production in various schools in the Kgakotlou circuit. We found that Klaas Mothapo Secondary School Garden was characterised by Nkonkoni and Glenrosa soil. Tshiombo and Tubatse soils were found at Madipoane Secondary School. Mothimako Secondary School Garden was marked by Hutton soil. At Phomolong Secondary School we found Glenrosa and Westleigh soils. The soils in the study were mostly loamy sand, with some having sandy loam and sand texture. The soils varied highly in terms of their chemical properties.
Soil suitability assessment revealed that Hutton soil occupying the total vegetable garden area at Mothimako was moderately suitable (S2) for beetroot production. Likewise, Westleigh soil which was occupying 39% at Phomolong was moderately suitable (S2). At Klaas Mothapo Secondary School, 34% on Nkonkoni was marginally suitable, while 66% under Glenrosa soil was not suitable for beetroot pro-duction. Like Nkonkoni soil at Klaas Mothapo, all the soils (Tshiombo and Tubatse) at Madipoane Secondary School were marginally suitable for beetroot production. Glenrosa soil at Phomolong occupying 61% of the garden was not suitable for beetroot production. The major limitations in this work were soil depth, EC and pH.
The areas occupied by Glenrosa at Klaas Mothapo and Phomolong Secondary School should not be used for cultivation of Beetroot. Application of lime should be done on soil Westleigh to increase the soil suitability for Beetroot, while on the other soils gypsum should be applied. The EC can be managed through adequate irrigation, leaching and draining.
Author Contributions
Conceptualisation, A.S.D., S.M.S. and R.S.M.; methodology, A.S.D. and S.M.S.; software, S.M.S.; investigation, R.S.M.; writing—original draft preparation, A.S.D and R.S.M; writing—review and editing, S.M.S. and A.S.D.; supervision, A.S.D. and S.M.S. All authors have read and agreed to the published version of the manuscript.”.
Funding
This research received no external funding.
Acknowledgments
We would like to extend our heartfelt gratitude to the University of Limpopo 2023 Experiential Learning (SEPA030) students for assisting us with soil survey, classification, mapping, and sampling. We also wish to thank the principals and teachers at Klaas Mothapo, Phomolong, Madipoane and Mothimako Secondary Schools.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Maluleke, R. Assessing Food Inadequacy and Hunger in South Africa in 2021 using the General Household Survey (GHS). Statistics South Africa, Private Bag X44, Pretoria 0001, 2023.
- Focus on Food Inadequacy and hunger in South Africa 2021. Available online: https://www.statssa.gov.za/?p=16235 (accessed on 25 April 2024).
- The Crisis of Hunger in South Africa Requires Urgent Attention. Available online: https://www.oxfam.org.za/the-crisis-of-hunger-in-south-africa-requires-urgent-attention/#:~:text=As%20the%20world%20gears%20up,of%20stunted%20children%20in%20Africa (accessed 04 June 2024).
- Laurie, S.M.; Mieke, F.; Mamokele, M.M. Assessment of Food Gardens as Nutrition Tool in Primary Schools in South Africa. SAJCN 2017, 30(4), 80-86.
- Department of Education. National guidelines for the implementation, monitoring and reporting of the National School Nutrition Programme. Pretoria: South African Department of Education, 2008.
- Vegetables – Agribook Digital. Available online: https://www.agribook.co.za/vegetables/ (accessed on 28 April 2024).
- Directorate of Statistics and Economic Analysis, Private Bag X246, Pretoria 001, 2021. Available online: https://www.darlld.gov.za (accessed on 04 June 2024).
- Clifford, T.; Howatson, G.; West, D. J.; Stevenson, E. J. The potential benefits of red beetroot supplementation in health and disease. Nutrients. 2015, 7(4), 2801-2822.
- Smith, S.; Gallaher, C.M. Soil and Agriculture. In Book Encyclopedia of Food and Agricultural Ethics, 2nd ed.; Kaplan, D.M., Thompson, P.B. Eds.; Springer Nature B.V.: Van Godewijckstraat 30, 3311 GX Dordreccht, The Netherlands, 2019.
- Swafo, S. M.; Dlamini, P. E. Utilisation of Intrinsic and Extrinsic Soil Information to Derive Soil Nutrient Management Zones for Banana Production in a Smallholder Farm. Land, 2023, 12(9), 1651.
- Jenny, H. Factors of Soil Formation. MacGraw-Hill, 1941.
- Parikh, S.J. Soil: The Foundation of Agriculture. Available online: https://www.nature.com/scitable/knowledge/library/soil-the-foundation-of-agriculture-84224268/ (accessed on 06 June 2024).
- Choudhury, B. U.; Fiyaz, A. R.; Mohapatra, K. P.; Ngachan, S. V. Impact of land use systems, agro-physical parameters and altitudinal variation in soil organic carbon content of Northeastern Himalayan Region of India. Land Degrad Dev. 2016, 27(4), 1163-1174.
- AbdelRahman, M.A.E.; Natarajan, A.; Hegde, R. Assessment of land suitability and capability by integrating remote sensing and GIS for agriculture in Chamarajanagar district, Karnataka, India. Egypt. J. Remote Sens. Space Sci. 2016, 19, 125–141.
- Manderson, A. and Palmer, A. Soil information for agricultural decision making: a New Zealand perspective. Soil Use Manage. 2006, 22(4): 393-400.
- Landon, J.R. Booker Tropical Soil Manual: A Handbook for Soil Survey and Agricultural Land Evaluation in the Tropics and Subtropics. Routledge. 2014.
- Brewer, J. What are soil map units and web soil surveys? Maryland soil survey work planning conference. United States Department of Agriculture, Natural Resources Conservation Service. 2011.
- IUSS Working Group WRB. World Reference Base for Soil Resources. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; Inter-national Union of Soil Sciences (IUSS): Vienna, Austria, 2022.
- Food and Agriculture Organizations of the United States, FAO. A Framework for land evaluation. In Soils Bulletin 3; FAO: Rome, Italy, 1976; Volume 72.
- Mandal, S.; Choudhury, B. U.; Satpati, L. Soil site suitability analysis using geo-statistical and visualisation techniques for selected winter crops in Sagar Island, India. Appl Geogr, 2020; 122, 102249.
- Naidu, L.G.K.; Ramamurthy, V.; Challa, O.; Hegde, R.; Krishnan, P. Manual on Soil-Site Suitability Criteria for Major Crops; NBSS publication 129; NBSS: Nagpur, India, 2006.
- Patric, D. and Schaab, G. Ground Truthing: Studying Peri-Urbanization and Land-Based Livelihoods in Mankweng and its Environs (Limpopo, South Africa). The International Archives of Photogrammetry. Remote Sens. Spatial Info. Sci. 2023, 48, 241-248.
- United States. Department of Agriculture. Soil Survey Division, and United states. Division of Soil Survey. In Soil Survey Manual (No. 18); US Department of Agriculture: Washington, DC, USA, 1993.
- Jahn, R., Blume, H.P., Asio, V.B., Spaargaren, O. and Schad, P. Guidelines for soil description. FAO. 2006.
- Soil Classification Working Group. Soil Classification: A Natural and Anthropogenic System for South Africa; Agricultural Research Council, Institute for Soil, Climate and Water (ARC-ISCW): Pretoria, South Africa, 2018.
- van Huyssteen, C. Relating the South African soil taxonomy to the World Reference Base for soil resources (p. 134). UJ Press. 2020.
- Bouyoucos, G.J. Hydrometer Method improved for making particle size analysis of soils. Agron. J. 1962, 54, 464–465.
- Walkley, A.; Black, I.A. An Examination of Different Method for Determining Soil Organic Matter and a Proposed Modification of the Chromic Acid Titration Method. J. Soil Sci. 1934, 37, 29–37.
- Manson, A.D.; Bainbridge, S.H.; Thibaud, G.R. Methods Used for the Analysis of Soils and Plant Material by Analytical Services at Cedara; KwaZulu-Natal Department of Agriculture and Rural Development: KwaDukuza, South Africa, 2020.
- Food and Agriculture Organizations of the United States, FAO. Guidelines in land evaluation for rain fed agriculture. In Soils Bulletin; Food and agricultural organisation: Rome, Italy, 1983; Volume 52, pp. 210–237.
- Sys, C.; Van Ranst, E.; Debaveye, J.; Beenaert, F. Land evaluation part III. Crop Requir. 1993, 1, 3.
- Swafo, S.M. and Dlamini, P.E.. Unlocking the land capability and soil suitability of Makuleke Farm for sustainable banana production. Sustainability, 2022, 15(1), p.453.
- Ziadat, F.M., 2007. Land suitability classification using different sources of information: Soil maps and predicted soil attributes in Jordan. Geoderma. 2007, 140(1-2), pp.73-80.
- Minasny, B.; Stockmann, U.; Hartemink, A.E.; McBratney, A.B. Measuring and Modelling Soil Depth Functions. In: Hartemink, A., Minasny, B. (eds) Digital Soil Morphometrics. Progress in Soil Science. Springer Cham, 2016. Available online . [CrossRef]
- Driessen, P.; Deckers, J.; Spaargaren, O.; Nachtegaele, F. Lecture notes on the major soils of the world. World Soil Resources Repots, 2000, 94, vi+-334.
- Osman, K.T. Shallow Soils. In: Management of Soil Problems. Springer, Cham, 2018.Availabe online: . [CrossRef]
- Bedadi, B.; Beyene, S.; Erkossa, T.; Fekadu, E. Soil Management. In Book The Soils of Ethiopia, World Book Series; Springer Nature: Bern, Switzerland, 2023.
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Ann Rev Plant Biol, 2008, 59, 651–681.
- Wang, J.; Raman, H.; Zhang, G.; Mendham N.; Zou, M. Aluminium tolerance in barely (Hordeum vulgare L.): physiological mechanisms, genetics and screening methods. J Zhejiang Univ Sci. 2006, 7, 769–787.
- Esu, I.E. Soil characterisation, classification and survey. HEBN Publishers, 2010.
- Sivarajasingham, S.; Alexander, L.T.; J. G. Cady, G.; Cline. M.G. "Laterite." Adv. Agron., 1962, 14, 1-60.
- Yadav, D.S., Jaiswal, B., Gautam, M., Agrawal, M. (2020). Soil Acidification and its Impact on Plants. In: Singh, P., Singh, S.K., Prasad, S.M. (eds) Plant Responses to Soil Pollution. Springer, Singapore, 2020. Available online . [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).