Submitted:
28 June 2024
Posted:
28 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
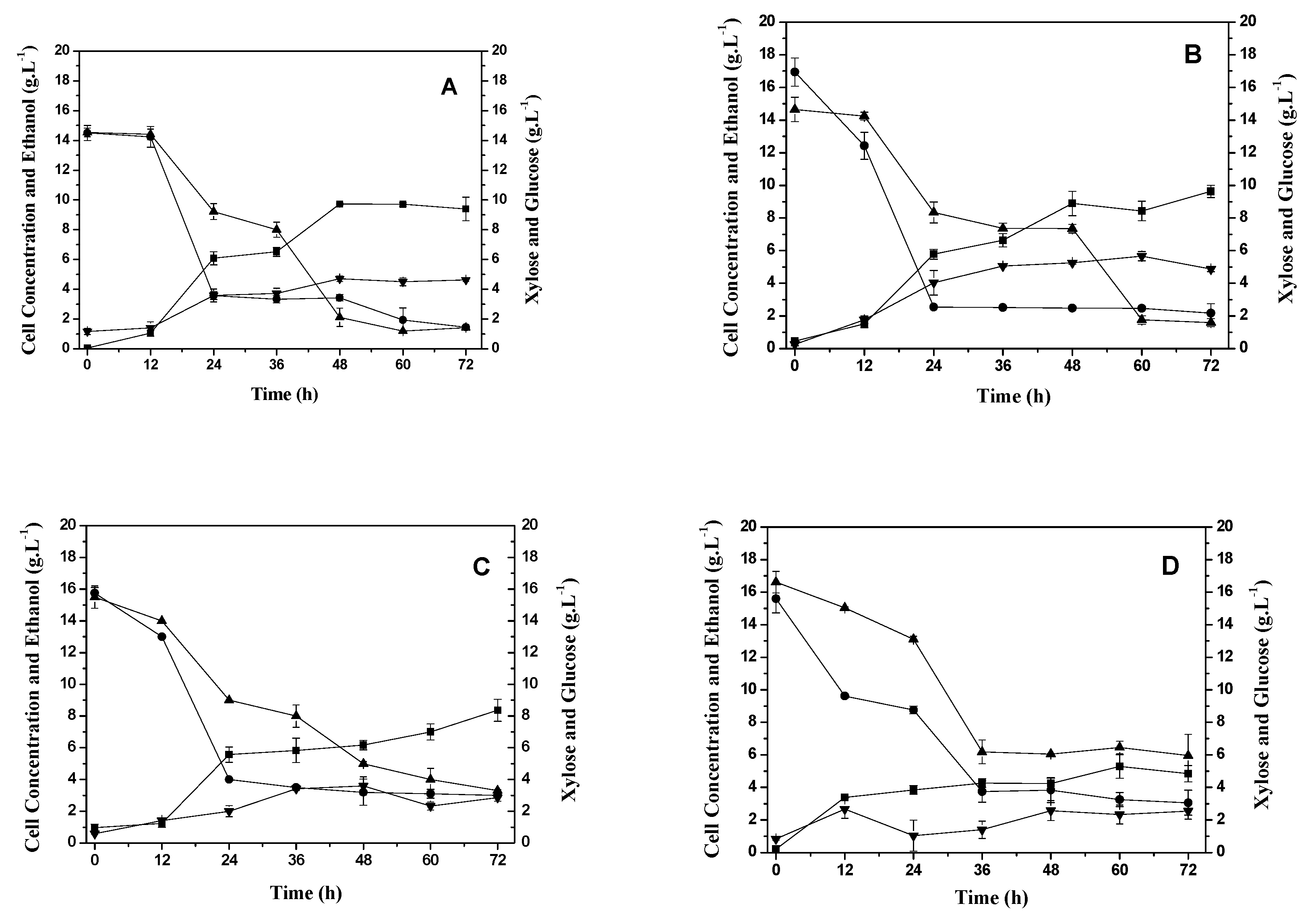
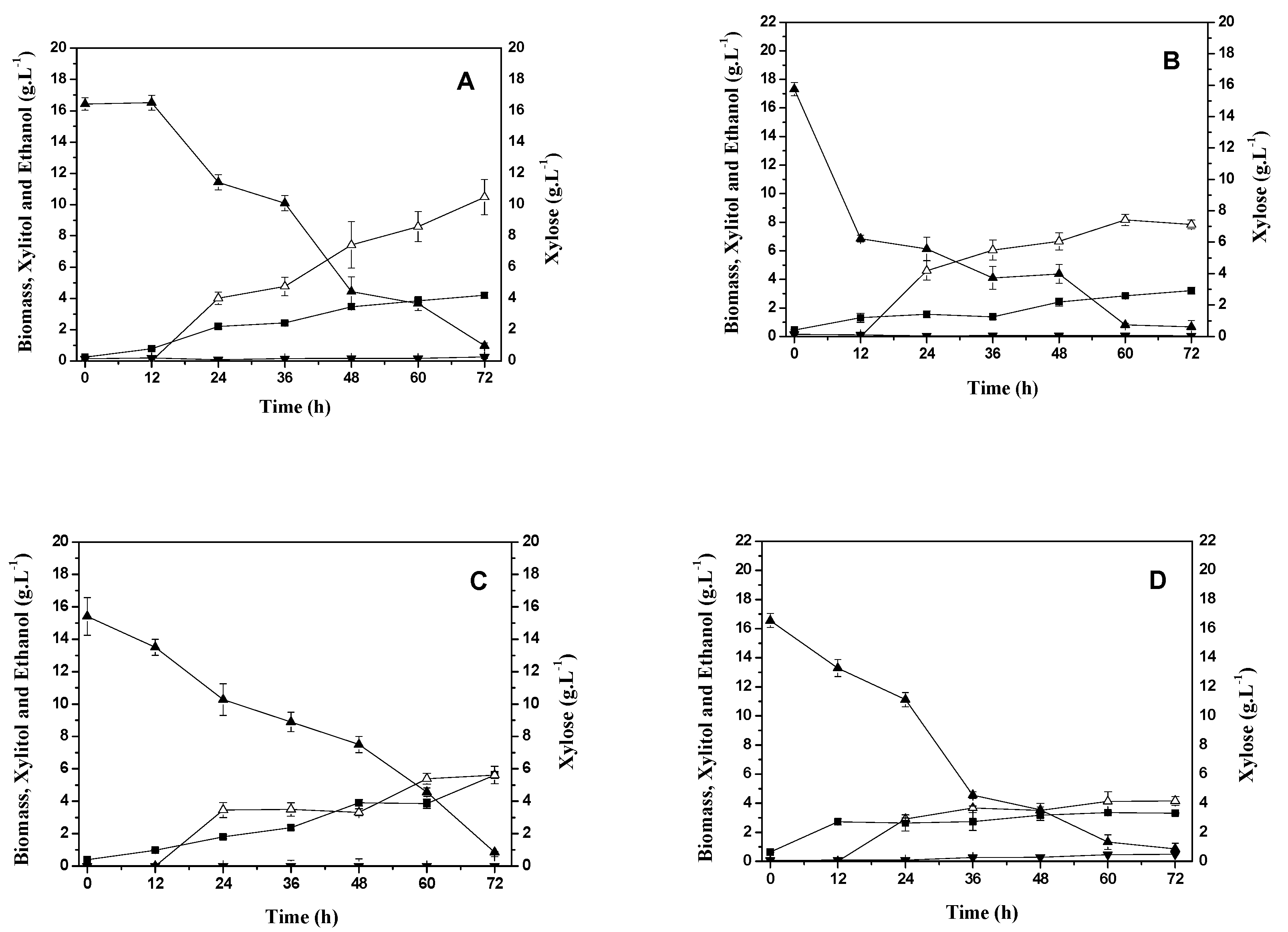
2.2. The production of xylitol and xylose reductase by Candida tropicalis.
2.3. Study of the production of xylose reductase enzyme in different aeration and fluid dynamic conditions
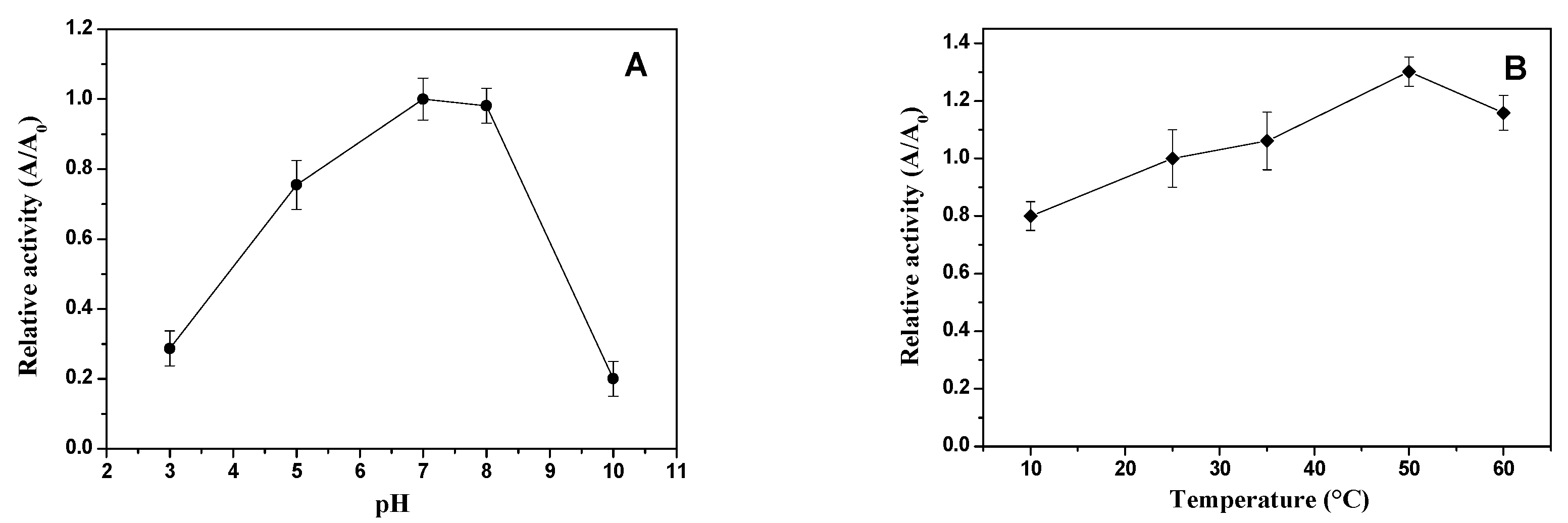
2.4. Characterization of xylose reductase enzyme produced using CABHM medium
3. Materials and Methods
3.1. Microorganism, material lignocellulosic and chemicals
3.2. Preparation of cashew apple bagasse hydrolysate
3.3. Batch fermentation for production of xylose reductase enzyme and xylitol
3.4. Influence of fluid dynamics and aeration on the production of the xylose reductase enzyme and xylitol
3.5. Extraction of XR enzyme
3.6. XR activity determination
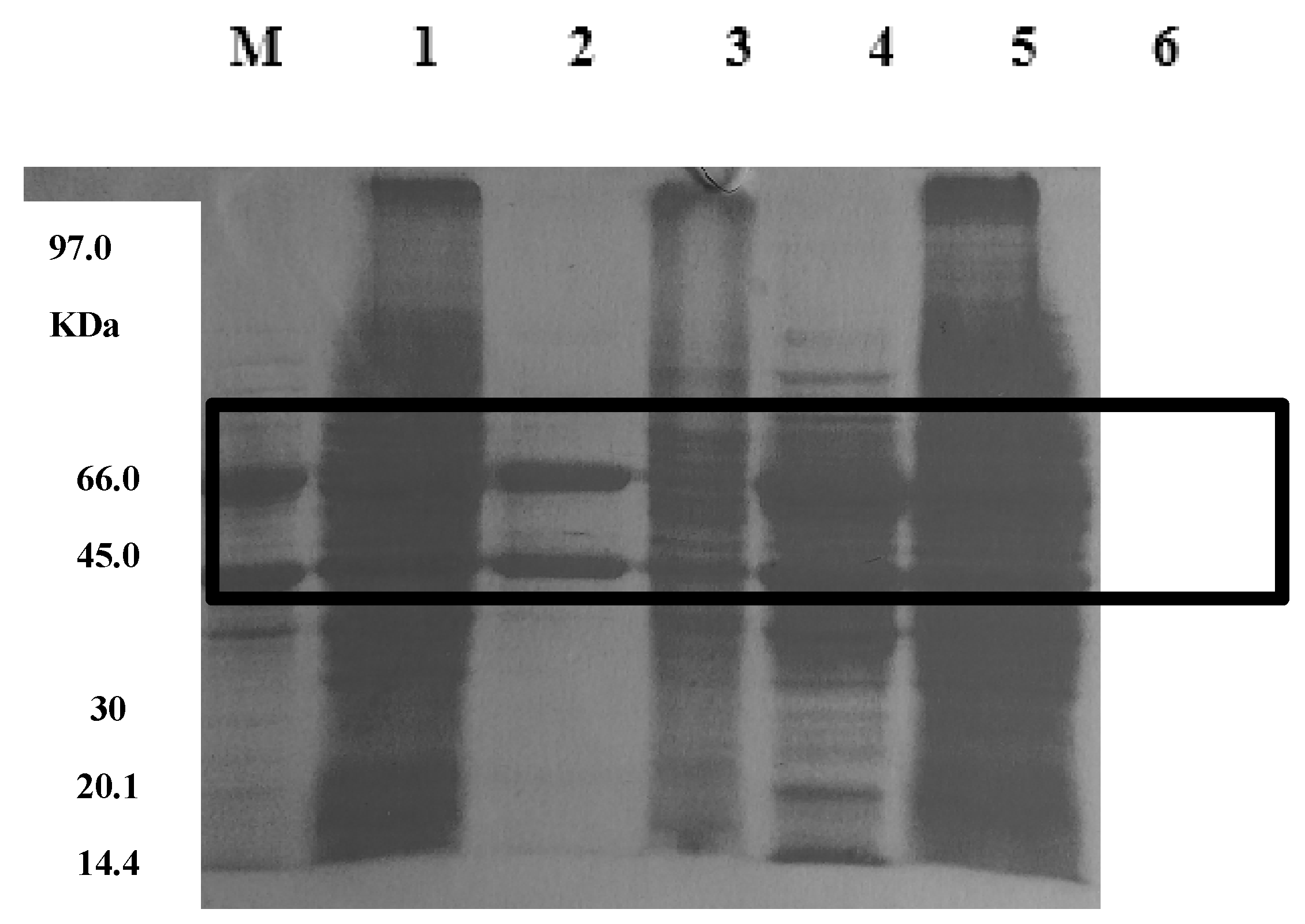
3.7. Electrophoresis and molecular mass determination
3.8. Analytical methods and Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Antonio, A.G.; Pierro, V.S.D.S.; Maia, L.C. Caries preventive effects of xylitol-based candies and lozenges: a systematic review. J. Public Health Dentistry 2011, 71, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Choi, B.K.; Kim, Y.J. The cariogenic characters of xylitol-resistant and xylitol-sensitive Streptococcus mutans in biofilm formation with salivary bacteria. Arch. Oral Biol. 2012, 57, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, T.L.; Silva Junior, I.J.; Macedo, G.R.; Rocha, M.V.P. Biotechnological production of xylitol from lignocellulosic wastes: a review. Process Biochem 2014, 49, 1779–1789. [Google Scholar] [CrossRef]
- Lugani, Y.; Sooch, B.S. Fermentative production of xylitol from a newly isolated xylose reductase producing Pseudomonas putida BSX-46. LWT 2020, 134, 109988. [Google Scholar] [CrossRef]
- Grand View Research, Inc. (2021) in https://www.prnewswire.com [accessed in March, 2022].
- Hongzhi, L.; Cheng, K.; Ge, J.; Ping, W. Statistical optimization of xylitol production from corncob hemicellulose hydrolysate by Candida tropicalis HDY-02. New Biotechnol. 2011, 28, 673–678. [Google Scholar]
- Albuquerque, T.L.; Gomes, S.D.L.; Marques, J.E.; Silva, I.J.D.; Rocha, M.V.P. Xylitol production from cashew apple bagasse by Kluyveromyces marxianus CCA510. Catal. Today 2015, 255, 33–40. [Google Scholar] [CrossRef]
- Dasgupta, D.; Ghosh, D.; Bandhu, S.; Agrawal, D.; Suman, S.K.; Adhikari, D.K. Purification, characterization and molecular docking study of NADPH dependent xylose reductase from thermotolerant Kluyveromyces sp. IIPE453. Process Biochem. 2016, 51(1), 124–133. [Google Scholar] [CrossRef]
- Jin, L.Q.; Yang, B.; Xu, W.; Chen, X.X.; Jia, D.X.; Liu, Z.Q.; Zheng, Y.G. , Immobilization of recombinant Escherichia coli whole cells harboring xylose reductase and glucose dehydrogenase for xylitol production from xylose mother liquor. Bioresour. Technol. 2019, 285, 121344. [Google Scholar] [CrossRef]
- Okamoto, K.; Kanawaku, R.; Masumoto, M.; Yanase, H. Efficient xylose fermentation by the brown rot fungus Neolentinus lepideus. Enz. Microb. Technol. 2012, 50(2), 96–100. [Google Scholar] [CrossRef]
- Suzuki, S.; Sugiyama, M.; Mihara, Y.; Hashiguchi, K.; Yokozeki, K. Novel enzymatic method for the production of xylitol from D-arabitol by Gluconobacter oxydans. Biosc. Biotechnol. Biochem. 2002, 66(12), 2614–2620. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.; Raghuwanshi, S.; Gupta, P.; Dutt, K.; Saxena, R.K. Fermentation behavior of osmophilic yeast Candida tropicalis isolated from the nectar of Hibiscus rosa sinensis flowers for xylitol production. Antonie van Leeuwenhoek 2012, 101(2), 393–402. [Google Scholar] [CrossRef] [PubMed]
- Rocha, M.V.P.; Rodrigues, T.H.S. , Albuquerque, T. L., Gonçalves, L.R.B.; Macedo, G.R. Evaluation of dilute acid pretreatment on cashew apple bagasse for ethanol and xylitol production. Chem. Eng. J. 2014, 243, 234–243. [Google Scholar] [CrossRef]
- Rafiqul, I.S.M.; Sakinah, A.M.M. Kinetic studies on acid hydrolysis of Meranti wood sawdust for xylose production. Chem Eng Sci. 2012, 71, 431–437. [Google Scholar] [CrossRef]
- Yokoyama, S.; Suzuki, T.; Kawai, K.; Horitsu, H.; Takamizawa, K. Purification, characterization and structure analysis of NADPH-dependent d-xylose reductases from Candida tropicalis. J. Ferment. Bioeng. 1995, 79(3), 217–223. [Google Scholar] [CrossRef]
- Rafiqul, I.S.M.; Sakinah, A.M.M.; Zularisam, A.W. Evaluation of sawdust hemicellulosic hydrolysate for bioproduction of xylitol by enzyme xylose reductase. Food Bioprod. Process. 2015, 94, 82–89. [Google Scholar] [CrossRef]
- Yablochkova, E.N.; Bolotnikova, O.I.; Mikhailova, N.P.; Nemova, N.N.; Ginak, A.I. The activity of xylose reductase and xylitol dehydrogenase in yeasts. Microbiology 2003, 72, 414–417. [Google Scholar] [CrossRef]
- Zhang, M.; Puri, A.K.; Wang, Z.; Singh, S.; Permaul, K. A unique xylose reductase from Thermomyces lanuginosus: Effect of lignocellulosic substrates and inhibitors and applicability in lignocellulosic bioconversion. Bioresour. Technol. 2019, 281, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Mayr, P.; Brüggler, K.; Kulbe, K.D.; Nidetzky, B. D-Xylose metabolism by Candida intermedia: isolation and characterization of two forms of aldose reductase with different coenzyme specificities. J. Chromatogr. B. Biomed. Sci. Appl. 2000, 737(1–2), 195-202. [CrossRef]
- Marques Junior, J.E.; Rocha, M.V.P. Development of a purification process via crystallization of xylitol produced for bioprocess using a hemicellulosic hydrolysate from the cashew apple bagasse as feedstock. Bioprocess Biosyst. Eng. 2021, 44, 713–725. [Google Scholar] [CrossRef]
- Silva, J.S.; Mendes, J.S.; Correia, J.A.C.; Rocha, M.V.P.; Micoli, L. Cashew apple bagasse as new feedstock for the hydrogen production using dark fermentation process. J. Biotechnol. 2018, 286, 71–78. [Google Scholar] [CrossRef]
- Rodrigues, T.H.S.; De Barros, E.M.; De Sá Brígido, J.; Da Silva, W.M. , Rocha, M.V.P.; Gonçalves, L.R.B. The Bioconversion of Pretreated Cashew Apple Bagasse into Ethanol by SHF and SSF Processes. Appl. Biochem. Biotechnol. 2016, 178, 1167–1183. [Google Scholar] [CrossRef]
- Marques Junior, J.E.; de Queiroz, L.P.; Albuquerque, T.L.; Zampieri, D.S.; Melo, V.M.M.; Rocha, M.V.P. Lactic acid production from cashew apple bagasse, an agro-industrial waste, and its application in the enzymatic synthesis of polylactic acid, Biocatal. Agric. Biotechnol. 2024, 56, 102987. [Google Scholar] [CrossRef]
- Serpa, J.F.; Silva, J.S.; Reis, C.L.B.; Micoli, L.; Silva, L.M.A.; Canuto, K.M.; de Macedo, C.A.; Rocha, M.V.P. Extraction and characterization of lignins from cashew apple bagasse obtained by different treatments. Biomass Bioenergy 2020, 141, 105728. [Google Scholar] [CrossRef]
- de Arruda, P.V.; Rodrigues, R. de C.; da Silva, D.D.V.; Felipe, M. das G.A. Evaluation of hexose and pentose in pre-cultivation of Candida guilliermondii on the key enzymes for xylitol production in sugarcane hemicellulosic hydrolysate. Biodegradation 2011, 22(4), 815-822. [CrossRef]
- Yablochkova, E.N.; Bolotnikova, O.I.; Mikhailova, N.P.; Nemova, N.N.; Ginak, A.I. The activity of key enzymes in xylose-assimilating yeasts at different rates of oxygen transfer to the fermentation medium. Microbiology 2004, 73, 129–133. [Google Scholar] [CrossRef]
- Kastner, J.R.; Eiteman, M.A.; Lee, S.A. Glucose repression of xylitol production in Candida tropicalis mixed-sugar fermentations. Biotechnol. Lett. 2001, 23, 1663–1667. [Google Scholar] [CrossRef]
- Cortez, E.V.; Pessoa-Jr, A.; Felipe, M.G.A.; Roberto, I.C.; Vitolo, M. Characterization of xylose reductase extracted by CTAB-reversed micelles from Candida guilliermondii homogenate. Braz. J. Pharm. Sci., 2006, 42, 251–257. [Google Scholar] [CrossRef]
- Kim, J.S.; Park, J.B.; Jang, S.W.; Ha, S.J. Enhanced xylitol production by mutant Kluyveromyces marxianus 36907 – FMEL1 due to improved xylose reductase activity. Appl. Biochem. Biotechnol. 2015, 176(7), 1975–1984. [Google Scholar] [CrossRef] [PubMed]
- Rafiqul, I.S.M.; Sakinah, A.M.M. Production of xylose reductase from adapted Candida tropicalis grown in sawdust hydrolysate. Biocatal. Agric. Biotechnol. 2014, 3(4), 227–235. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, L. , Wang, D.; Gao, X.; Hong, J. Identification of a xylose reductase gene in the xylose metabolic pathway of Kluyveromyces marxianus NBRC1777. J. Ind. Microbiol. Biotechnol. 2011, 38(12), 2001–2010. [Google Scholar] [CrossRef]
- Azizan, A.; Büchs, J. Three-dimensional (3D) evaluation of liquid distribution in shake flask using an optical fluorescence technique. J. Biol. Eng. 2017, 11, 28. [Google Scholar] [CrossRef]
- Nidetzky, B.; Brüggler, K.; Kratzer, R.; Mayr, P. Multiple forms of xylose reductase in Candida intermedia: comparison of their functional properties using quantitative structure–activity relationships, steady-state kinetic analysis, and pH studies. J. Agric. Food Chem. 2003, 51(27), 7930–7935. [Google Scholar] [CrossRef]
- Lee, J.K.; Koo, B.S.; Kim, S.Y. Cloning and characterization of the xyl1 gene, encoding an NADH-Preferring xylose reductase from Candida parapsilosis, and its functional expression in Candida tropicalis. Appl. Environ. Microbiol. 2003, 69(10), 6179–6188. [Google Scholar] [CrossRef] [PubMed]
- Woodyer, R.; Simurdiak, M.; van der Donk, W.A.; Zhao, H. Heterologous expression, purification and characterization of a highly active xylose redutase from Neurospora crassa. Appl. Biochem. Microbiol. 2005, 71(3), 1642–1647. [Google Scholar] [CrossRef]
- Verduyn, C.; Kleef, R.V.; Frank, J.; Schreuder, H.; Van Dijken, J.P.; Scheffers, W.A. Properties of the NAD(P)H- dependent xylose reductase from the xylose-fermenting yeast Pichia stipitis. Biochem. J. 1985, 226(3), 669–677. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Luo, H.; Tian, J.; Turunen, O.; Huang, H.; Shi, P.; Hua, H.; Wang, C.; Wang, S.; Yao, B. Thermostability improvement of a Streptomyces xylanase by introducing proline and glutamic acid residues. Appl. Environ. Microb. 2014, 80, 2158–2165. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, R.; Sharma, N.; Bhalla, T.C. Computational analysis of amino acid sequences in relation to thermostability of interspecific nitrile degrading enzyme (amidase) from various thermophiles/hyperthermophiles. Sci. Rep. 2012, 1, 1–7. [Google Scholar] [CrossRef]
- Ho, N.W.Y. Purification, characterization and amino terminal sequence of xylose reductase from Candida shehatae. Enzyme Microb. Technol. 1990, 12(1), 33-39 (1990. [CrossRef]
- Wilson, D.K., Kavanagh, K.L., Klimacek, M.; Nidetzky, B. The xylose reductase (AKR2B5) structure: homology and divergence from other aldo-keto reductase and opportunities for protein engineering. Chem. Biol. Interact. 2003, 143-144, 515-521. [CrossRef]
- Correia, J.A.C.; Silva, J.S.; Gonçalves, L.R.B.; Rocha, M.V.P. Different design configurations of simultaneous saccharification and fermentation to enhance ethanol production from cashew apple bagasse pretreated with alkaline hydrogen peroxide applying the biorefinery concept. Biomass Convers. Biorefin. 2022, 12, 2767–2780. [Google Scholar] [CrossRef]
- Albuquerque, T.L.; Gomes, S.D.L.; da Silva Junior, I.J.; Gonçalves, L.R.B.; Rocha, M.V.P. Xylitol production by different yeasts: Kinetic study and biosynthesis from cashew apple bagasse hydrolysate. Can. J. Chem. Eng. 2023, 101(7), 3668–3679. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of proteins utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
| Composition (g.L-1) |
Hydrolysate | ||
|---|---|---|---|
| CABH | CABHM | CABHM* | |
| Glucose | 28.57 | 24.71 | 22.70 |
| Xylose | 18.30 | 17.28 | 15.17 |
| Arabinose | 11.74 | 11.05 | 11.03 |
| Cellobiose | 6.79 | 4.38 | 3.69 |
| Acetic acid | 1.64 | 1.21 | 1.19 |
| Formic acid | 0.90 | 0.26 | 0.26 |
| Furfural | ND | ND | ND |
| Hydroxymethyl Furfural (HMF) | ND | ND | ND |
| Temperature (°C) |
Biomass (g.L-1) | Xylose remaining (g.L-1) | P1max (g.L-1) |
YP1/S1 (g.g-1) |
QP1 (g.h-1.L-1) |
|---|---|---|---|---|---|
| 25 | 4.2 ± 0.1 | 0.97 ± 0.2 | 10.47 ± 1.0 | 0.68 | 0.15 |
| 30 | 3.1 ± 0.0 | 0.67 ± 0.2 | 9.26 ± 0.1 | 0.56 | 0.13 |
| 35 | 5.6 ± 0.1 | 0.85 ± 0.1 | 5.62 ± 0.3 | 0.39 | 0.08 |
| 40 | 3.3 ± 0.3 | 0.87 ± 0.1 | 4.15 ± 0.2 | 0.26 | 0.06 |
| Temperature (°C) | FORMULATED MEDIUM | CABHM | ||||
|---|---|---|---|---|---|---|
| Enzymatic Activity (U.mLExtract-1) | Enzymatic Activity (U.gCells-1) |
Enzymatic Activity (U.mgProtein-1) |
Enzymatic Activity (U.mLExtract-1) | Enzymatic Activity (U.gCells-1) |
Enzymatic Activity (U.mgProtein-1) |
|
| 25 | 0.365 | 0.73 | 0.076 | 0.265 | 0.530 | 0.071 |
| 30 | 0.297 | 0.59 | 0.076 | 0.181 | 0.362 | 0.041 |
| 35 | 0.238 | 0.45 | 0.062 | 0.111 | 0.222 | 0.036 |
| 40 | 0.189 | 0.38 | 0.057 | 0.033 | 0.066 | 0.06 |
| Experiment Volume (Flask) |
Headspace of air (mL) | Aeration Condition | Cell conc. (g/L) |
XR Activity (U/mL)(i) | Xylitol conc. (g/L) |
Ethanol conc.(g/L) |
|---|---|---|---|---|---|---|
| 01 100 mL (250 mL – Erlenmeyer) |
150 mL | Semiaerobic | 6.05 ± 0.70 | 0.272 ± 0.04 (2.0 U/g cells) |
(i)NP | 4.2 ± 0.2 |
| 02 300 mL (1000 mL – Erlenmeyer) |
700 mL | Aerobic | 2.35 ± 0.19 | 0.320 ± 0.06 (2.35 U/g cells) |
2.5 ± 0.3 | 3.8 ± 0.3 |
| 03 1000 mL (2000 mL – Erlenmeyer) |
1000 mL | Microaerobic | 2.07 ± 0.25 | 1.530 ± 0.18 (4 U/g cells) |
8.3 ± 1.1 | ± 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





