Submitted:
28 June 2024
Posted:
01 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
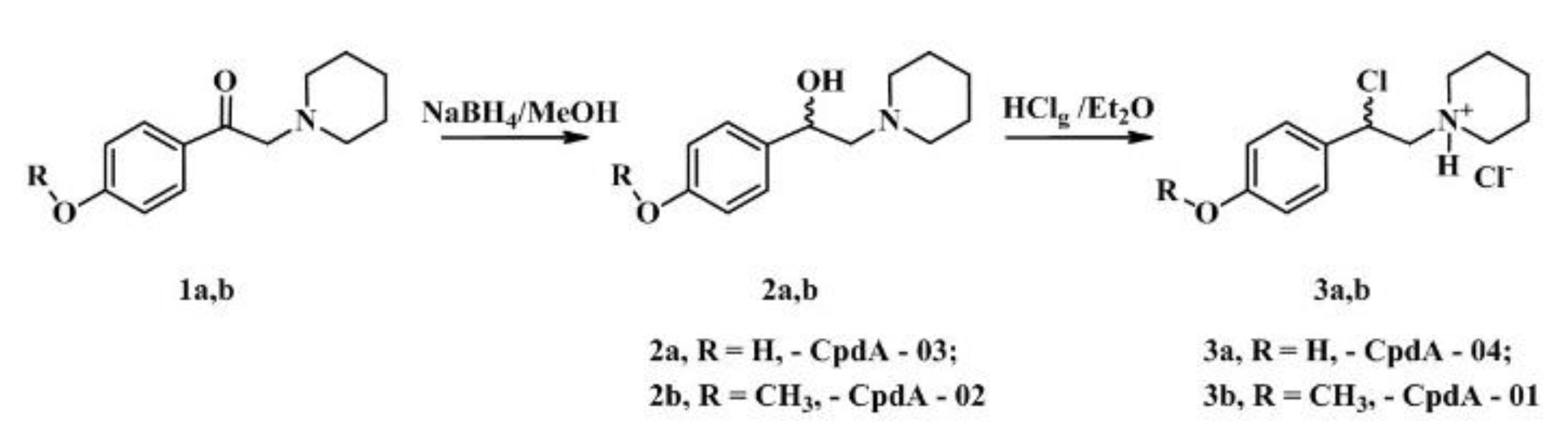
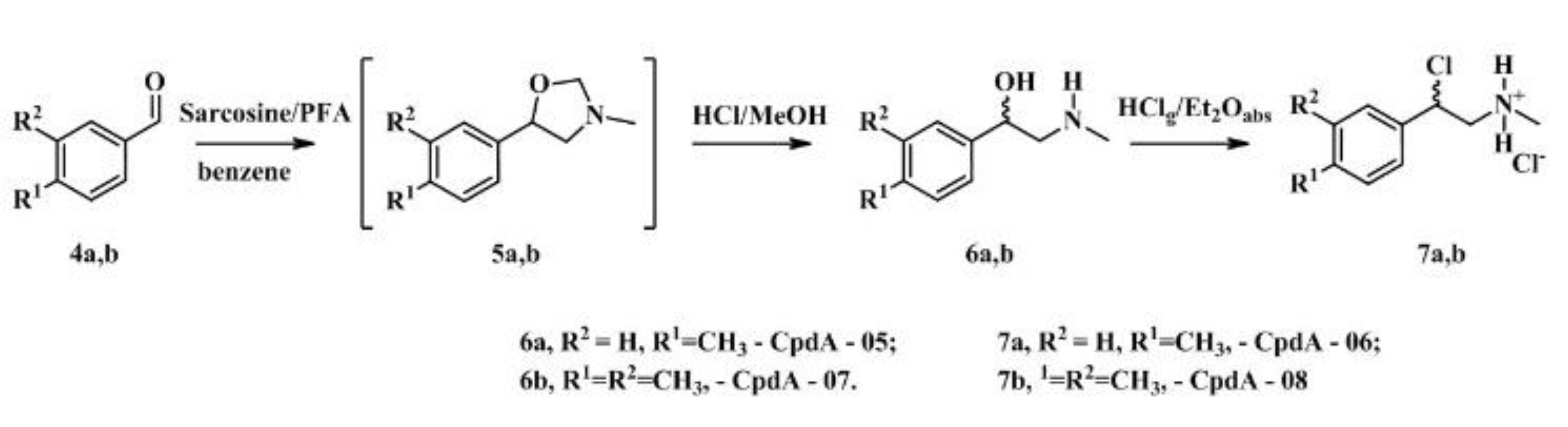
2.1. Synthesis of CpdA Chemical Derivatives
2.2. Screening of Cytotoxic Effects of Newly Synthesized CpdA Derivatives
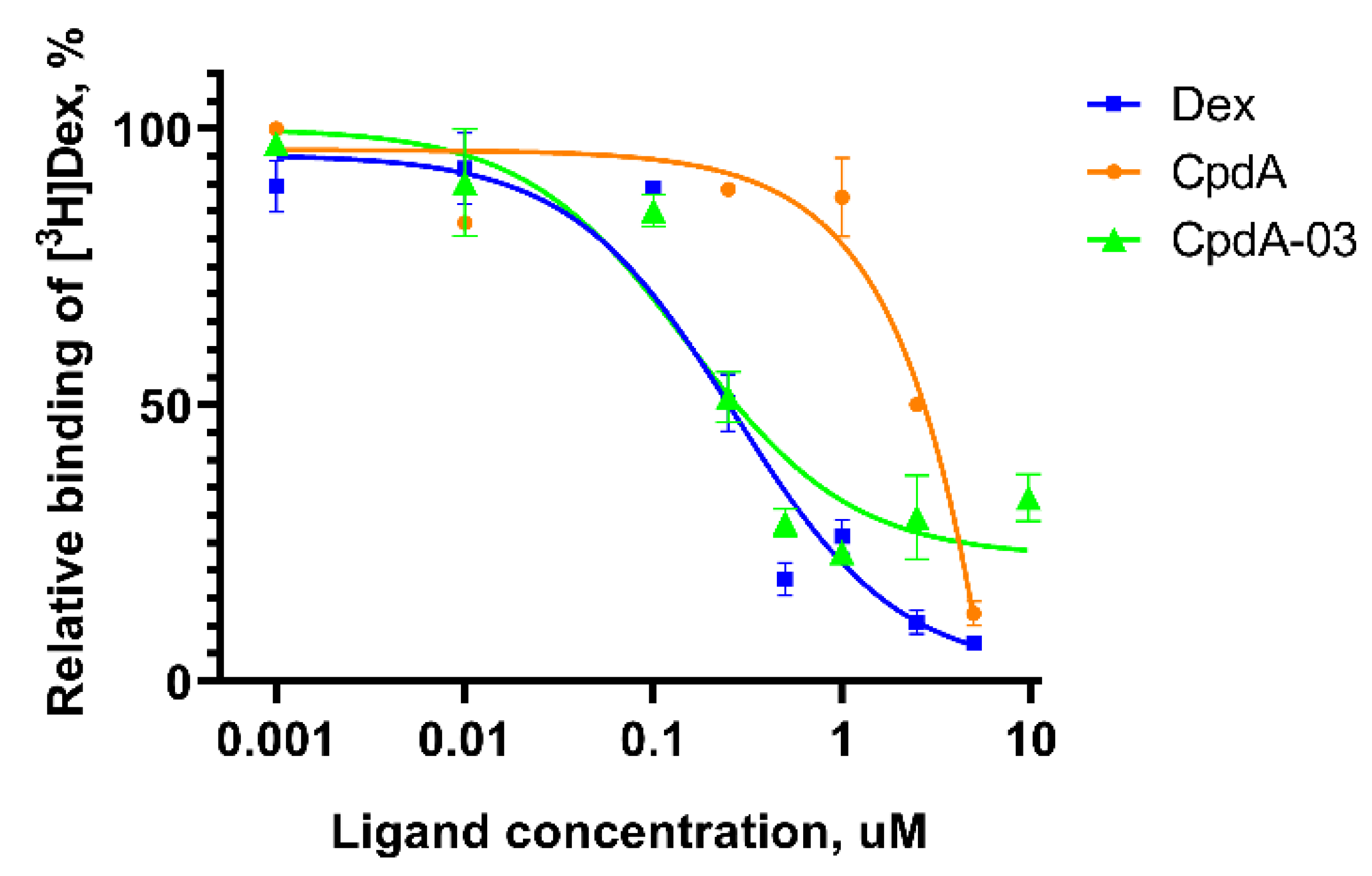
2.3. Affinity of CpdA-03 to GR
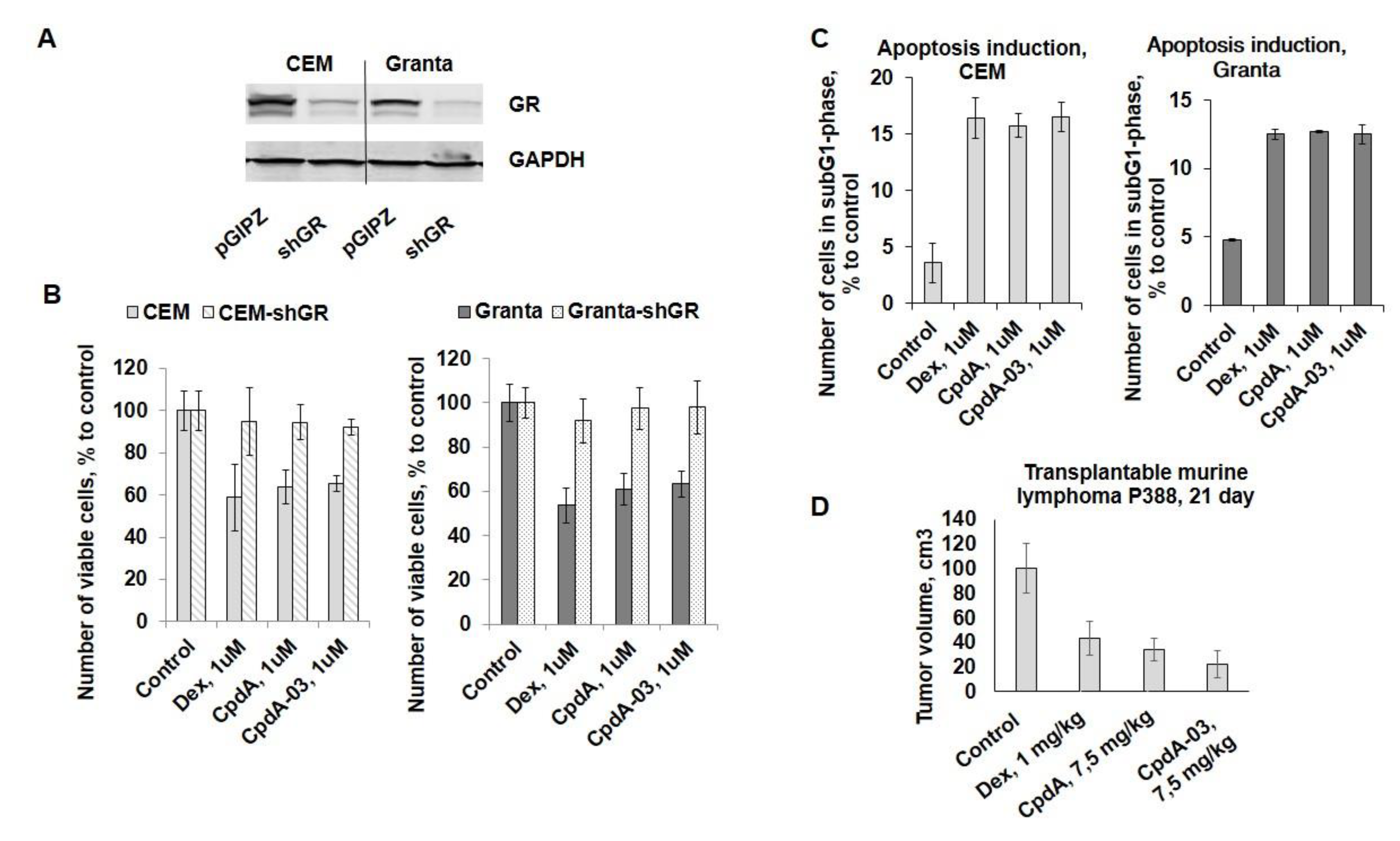
2.4. Anti-Cancer Effect of CpdA-03 In Vitro and In Vivo
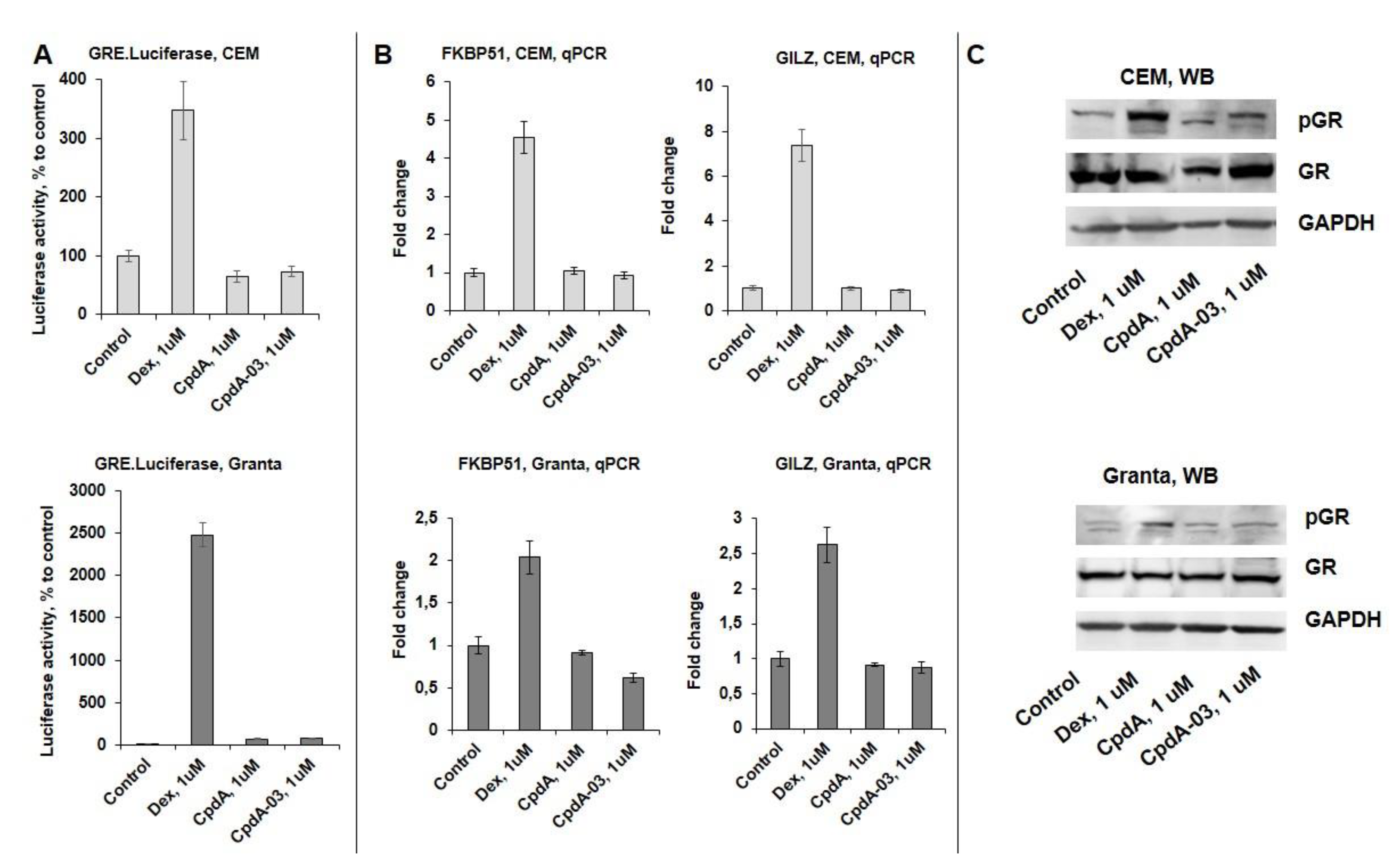
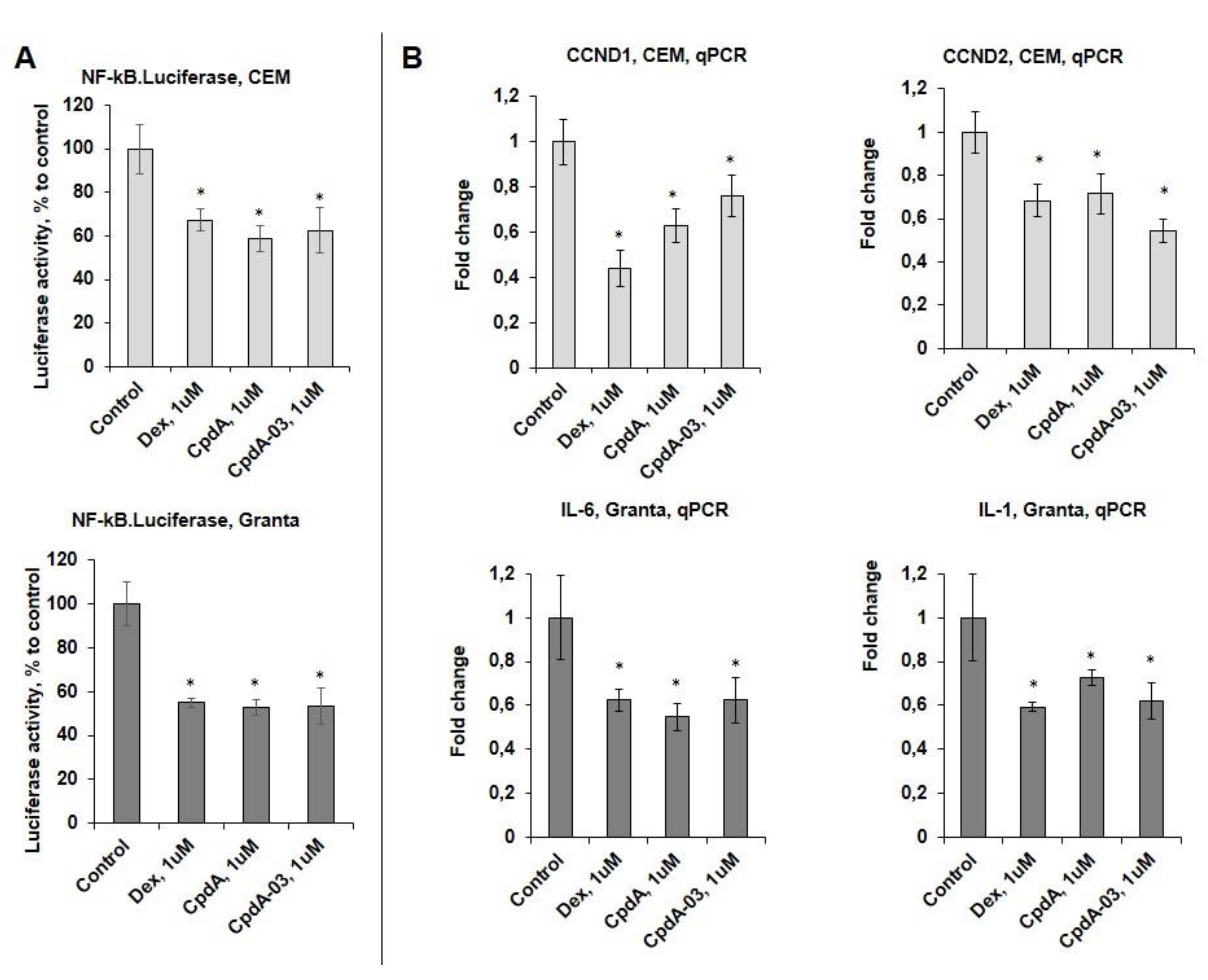
2.5. Effects of CpdA-03 on GR Functions
3. Discussion
4. Materials and Methods
4.1. Chemistry
4.1.1. General Information
4.1.2. Synthesis and Characterization of CpdA-01 – CpdA-04
4.1.3. Synthesis and Characterization of CpdA-05 – CpdA-08
4.2. Biology
4.2.1. Cell Cultures and Treatments
4.2.2. MTT Cytotoxicity Assay
4.2.3. Western Blot Analysis
4.2.4. Cell Viability
4.2.5. Distribution of Cells by Cell Cycle Phases
4.2.6. RNA Extraction and Q-PCR
4.2.7. Lentiviral Technology
4.2.8. Luciferase Assay
4.2.9. GR Binding Affinity Assay
4.2.10. Anti-cancer Study In Vivo
4.2.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Sacta, M.A.; Chinenov, Y.; Rogatsky, I. Glucocorticoid Signaling: An Update from a Genomic Perspective. Annu. Rev. Physiol. 2016, 78, 155–180. [Google Scholar] [CrossRef] [PubMed]
- Gulliver, L.S.M. Xenobiotics and the Glucocorticoid Receptor. Toxicol. Appl. Pharmacol. 2017, 319, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Zhidkova, E.M.; Lylova, E.S.; Grigoreva, D.D.; Kirsanov, K.I.; Osipova, A.V.; Kulikov, E.P.; Mertsalov, S.A.; Belitsky, G.A.; Budunova, I.; Yakubovskaya, M.G.; et al. Nutritional Sensor REDD1 in Cancer and Inflammation: Friend or Foe? Int. J. Mol. Sci. 2022, 23, 9686. [Google Scholar] [CrossRef] [PubMed]
- Lesovaya, E.A.; Chudakova, D.; Baida, G.; Zhidkova, E.M.; Kirsanov, K.I.; Yakubovskaya, M.G.; Budunova, I.V. The Long Winding Road to the Safer Glucocorticoid Receptor (GR) Targeting Therapies. Oncotarget 2022, 13, 408–424. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, M.C.; Sauders, R.H.; Schwartz, L.I.; Zannos, L.; Perez Santiago, E.; Dameshek, W. The Use of Adrenocorticotropic Hormone and Cortisone in the Treatment of Leukemia and Leukosarcoma. Blood 1951, 6, 804–823. [Google Scholar] [CrossRef] [PubMed]
- Hachemi, Y.; Rapp, A.E.; Picke, A.-K.; Weidinger, G.; Ignatius, A.; Tuckermann, J. Molecular Mechanisms of Glucocorticoids on Skeleton and Bone Regeneration after Fracture. J. Mol. Endocrinol. 2018, 61, R75–R90. [Google Scholar] [CrossRef] [PubMed]
- Conaway, H.H.; Henning, P.; Lie, A.; Tuckermann, J.; Lerner, U.H. Activation of Dimeric Glucocorticoid Receptors in Osteoclast Progenitors Potentiates RANKL Induced Mature Osteoclast Bone Resorbing Activity. Bone 2016, 93, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Lesovaya, E.; Yemelyanov, A.; Swart, A.C.; Swart, P.; Haegeman, G.; Budunova, I. Discovery of Compound A--a Selective Activator of the Glucocorticoid Receptor with Anti-Inflammatory and Anti-Cancer Activity. Oncotarget 2015, 6, 30730–30744. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.K.; Wahli, W. A Trilogy of Glucocorticoid Receptor Actions. Proc. Natl. Acad. Sci. U. S. A. 2016, 113, 1115–1117. [Google Scholar] [CrossRef] [PubMed]
- Ratman, D.; Vanden Berghe, W.; Dejager, L.; Libert, C.; Tavernier, J.; Beck, I.M.; De Bosscher, K. How Glucocorticoid Receptors Modulate the Activity of Other Transcription Factors: A Scope beyond Tethering. Mol. Cell. Endocrinol. 2013, 380, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Langlais, D.; Couture, C.; Balsalobre, A.; Drouin, J. The Stat3/GR Interaction Code: Predictive Value of Direct/Indirect DNA Recruitment for Transcription Outcome. Mol. Cell 2012. [Google Scholar] [CrossRef] [PubMed]
- Hua, G.; Ganti, K.P.; Chambon, P. Glucocorticoid-Induced Tethered Transrepression Requires SUMOylation of GR and Formation of a SUMO-SMRT/NCoR1-HDAC3 Repressing Complex. Proc. Natl. Acad. Sci. U. S. A. 2016, 113, E635–43. [Google Scholar] [CrossRef] [PubMed]
- Baiula, M.; Bedini, A.; Baldi, J.; Cavet, M.E.; Govoni, P.; Spampinato, S. Mapracorat, a Selective Glucocorticoid Receptor Agonist, Causes Apoptosis of Eosinophils Infiltrating the Conjunctiva in Late-Phase Experimental Ocular Allergy. Drug Des. Devel. Ther. 2014, 8, 745–757. [Google Scholar] [CrossRef] [PubMed]
- De Bosscher, K.; Vanden Berghe, W.; Beck, I.M.E.; Van Molle, W.; Hennuyer, N.; Hapgood, J.; Libert, C.; Staels, B.; Louw, A.; Haegeman, G. A Fully Dissociated Compound of Plant Origin for Inflammatory Gene Repression. Proc. Natl. Acad. Sci. U. S. A. 2005, 102, 15827–15832. [Google Scholar] [CrossRef] [PubMed]
- Hua, G.; Zein, N.; Daubeuf, F.; Chambon, P. Glucocorticoid Receptor Modulators CpdX and CpdX-D3 Exhibit the Same in Vivo Antiinflammatory Activities as Synthetic Glucocorticoids. Proc. Natl. Acad. Sci. U. S. A. 2019, 116, 14191–14199. [Google Scholar] [CrossRef] [PubMed]
- Hua, G.; Zein, N.; Paulen, L.; Chambon, P. The Glucocorticoid Receptor Agonistic Modulators CpdX and CpdX-D3 Do Not Generate the Debilitating Effects of Synthetic Glucocorticoids. Proc. Natl. Acad. Sci. U. S. A. 2019, 116, 14200–14209. [Google Scholar] [CrossRef] [PubMed]
- Schäcke, H.; Schottelius, A.; Döcke, W.-D.; Strehlke, P.; Jaroch, S.; Schmees, N.; Rehwinkel, H.; Hennekes, H.; Asadullah, K. Dissociation of Transactivation from Transrepression by a Selective Glucocorticoid Receptor Agonist Leads to Separation of Therapeutic Effects from Side Effects. Proc. Natl. Acad. Sci. U. S. A. 2004, 101, 227–232. [Google Scholar] [CrossRef]
- Coghlan, M.J.; Jacobson, P.B.; Lane, B.; Nakane, M.; Lin, C.W.; Elmore, S.W.; Kym, P.R.; Luly, J.R.; Carter, G.W.; Turner, R.; et al. A Novel Antiinflammatory Maintains Glucocorticoid Efficacy with Reduced Side Effects. Mol. Endocrinol. Baltim. Md 2003, 17, 860–869. [Google Scholar] [CrossRef] [PubMed]
- van Lierop, M.-J.C.; Alkema, W.; Laskewitz, A.J.; Dijkema, R.; van der Maaden, H.M.; Smit, M.J.; Plate, R.; Conti, P.G.M.; Jans, C.G.J.M.; Timmers, C.M.; et al. Org 214007-0: A Novel Non-Steroidal Selective Glucocorticoid Receptor Modulator with Full Anti-Inflammatory Properties and Improved Therapeutic Index. PloS One 2012, 7, e48385. [Google Scholar] [CrossRef]
- Ripa, L.; Edman, K.; Dearman, M.; Edenro, G.; Hendrickx, R.; Ullah, V.; Chang, H.-F.; Lepistö, M.; Chapman, D.; Geschwindner, S.; et al. Discovery of a Novel Oral Glucocorticoid Receptor Modulator (AZD9567) with Improved Side Effect Profile. J. Med. Chem. 2018, 61, 1785–1799. [Google Scholar] [CrossRef]
- Schäcke, H.; Zollner, T.M.; Döcke, W.D.; Rehwinkel, H.; Jaroch, S.; Skuballa, W.; Neuhaus, R.; May, E.; Zügel, U.; Asadullah, K. Characterization of ZK 245186, a Novel, Selective Glucocorticoid Receptor Agonist for the Topical Treatment of Inflammatory Skin Diseases. Br. J. Pharmacol. 2009, 158, 1088–1103. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Du, S.; Tunca, C.; Braden, T.; Long, K.R.; Lee, J.; Webb, E.G.; Dietz, J.D.; Hummert, S.; Rouw, S.; et al. The Antagonists but Not Partial Agonists of Glucocorticoid Receptor Ligands Show Substantial Side Effect Dissociation. Endocrinology 2011, 152, 3123–3134. [Google Scholar] [CrossRef] [PubMed]
- Swart, P.; Swart, A.C.; Louw, A.; van der Merwe, K.J. Biological Activities of the Shrub Salsola Tuberculatiformis Botsch.: Contraceptive or Stress Alleviator? BioEssays News Rev. Mol. Cell. Dev. Biol. 2003, 25, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Lesovaya, E.; Yemelyanov, A.; Kirsanov, K.; Popa, A.; Belitsky, G.; Yakubovskaya, M.; Gordon, L.I.; Rosen, S.T.; Budunova, I. Combination of a Selective Activator of the Glucocorticoid Receptor Compound A with a Proteasome Inhibitor as a Novel Strategy for Chemotherapy of Hematologic Malignancies. Cell Cycle Georget. Tex 2013, 12, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Louw, A.; Swart, P.; de Kock, S.S.; van der Merwe, K.J. Mechanism for the Stabilization in Vivo of the Aziridine Precursor --(4-Acetoxyphenyl)-2-Chloro-N-Methyl-Ethylammonium Chloride by Serum Proteins. Biochem. Pharmacol. 1997, 53, 189–197. [Google Scholar] [CrossRef] [PubMed]
- IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man: Some Aziridines, N-, S- & O-Mustards and Selenium. IARC Monogr. Eval. Carcinog. Risk Chem. Man 1975, 9, 1–268.
- Stohs, S.J. Safety, Efficacy, and Mechanistic Studies Regarding Citrus Aurantium (Bitter Orange) Extract and p-Synephrine. Phytother. Res. PTR 2017, 31, 1463–1474. [Google Scholar] [CrossRef] [PubMed]
- Dodonova, S.A.; Zhidkova, E.M.; Kryukov, A.A.; Valiev, T.T.; Kirsanov, K.I.; Kulikov, E.P.; Budunova, I.V.; Yakubovskaya, M.G.; Lesovaya, E.A. Synephrine and Its Derivative Compound A: Common and Specific Biological Effects. Int. J. Mol. Sci. 2023, 24, 17537. [Google Scholar] [CrossRef] [PubMed]
- Moshkin, V.S.; Sosnovskikh, V.Ya. A Simple Two-Step Synthesis of 2-(Alkylamino)-1-Arylethanols, Including Racemic Adrenaline, from Aromatic Aldehydes via 5-Aryloxazolidines. Tetrahedron Lett. 2013, 54, 5869–5872. [Google Scholar] [CrossRef]
- Brandon, D.D.; Kendall, J.W.; Alman, K.; Tower, P.; Loriaux, D.L. Inhibition of Dexamethasone Binding to Human Glucocorticoid Receptor by New World Primate Cell Extracts. Steroids 1995, 60, 463–466. [Google Scholar] [CrossRef] [PubMed]
- Yemelyanov, A.; Czwornog, J.; Gera, L.; Joshi, S.; Chatterton, R.T.; Budunova, I. Novel Steroid Receptor Phyto-Modulator Compound a Inhibits Growth and Survival of Prostate Cancer Cells. Cancer Res. 2008, 68, 4763–4773. [Google Scholar] [CrossRef] [PubMed]
- Ronacher, K.; Hadley, K.; Avenant, C.; Stubsrud, E.; Simons, S.S.; Louw, A.; Hapgood, J.P. Ligand-Selective Transactivation and Transrepression via the Glucocorticoid Receptor: Role of Cofactor Interaction. Mol. Cell. Endocrinol. 2009, 299, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Lesovaya, E.A.; Yemelyanov, A.Y.; Kirsanov, K.I.; Yakubovskaya, M.G.; Budunova, I. V Antitumor Effect of Non-Steroid Glucocorticoid Receptor Ligand CpdA on Leukemia Cell Lines CEM and K562. Biochem. Biokhimiia 2011, 76, 1242–1252. [Google Scholar] [CrossRef] [PubMed]
- Robak, T.; Szmigielska, A. Dexamethasone Does Not Enhance Antileukemic Activity of Cladribine in Mice with Leukemias L1210 and P388. Neoplasma 2000, 47, 168–171. [Google Scholar] [PubMed]
- Trafalis, D.T.P.; Chrysogelou, E.; Dalezis, P.; Geromichalos, G.; Kontos, M.; Andreadis, C.; Ziras, N.; Koutsilieris, M.; Athanassiou, A.E.; Pangalis, G.A.; et al. Octreotide Neutralizes Dexamethasone Antitumor Actions on P388 Murine Lymphocytic Leukemia in Vivo. J. BUON Off. J. Balk. Union Oncol. 10, 89–94.
- Saksida, T.; Vujicic, M.; Nikolic, I.; Stojanovic, I.; Haegeman, G.; Stosic-Grujicic, S. Compound A, a Selective Glucocorticoid Receptor Agonist, Inhibits Immunoinflammatory Diabetes, Induced by Multiple Low Doses of Streptozotocin in Mice. Br. J. Pharmacol. 2014, 171, 5898–5909. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Mirzoeva, S.; Readhead, B.; Dudley, J.T.; Budunova, I. PI3K Inhibitors Protect against Glucocorticoid-Induced Skin Atrophy. EBioMedicine 2019, 41, 526–537. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Baban, B.; Isales, C.M.; Shi, X.-M. Role of Glucocorticoid-Induced Leucine Zipper (GILZ) in Inflammatory Bone Loss. PloS One 2017, 12, e0181133. [Google Scholar] [CrossRef]
- Fan, H.; Kao, W.; Yang, Y.H.; Gu, R.; Harris, J.; Fingerle-Rowson, G.; Bucala, R.; Ngo, D.; Beaulieu, E.; Morand, E.F. Macrophage Migration Inhibitory Factor Inhibits the Antiinflammatory Effects of Glucocorticoids via Glucocorticoid-Induced Leucine Zipper. Arthritis Rheumatol. Hoboken NJ 2014, 66, 2059–2070. [Google Scholar] [CrossRef] [PubMed]
- Tonsing-Carter, E.; Hernandez, K.M.; Kim, C.R.; Harkless, R. V; Oh, A.; Bowie, K.R.; West-Szymanski, D.C.; Betancourt-Ponce, M.A.; Green, B.D.; Lastra, R.R.; et al. Glucocorticoid Receptor Modulation Decreases ER-Positive Breast Cancer Cell Proliferation and Suppresses Wild-Type and Mutant ER Chromatin Association. Breast Cancer Res. BCR 2019, 21, 82. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Hua, C.; Geng, Y.; Chen, Q.; Niu, L.; Tao, S.; Ni, Y.; Zhao, R. Chronic Dexamethasone Exposure Activates the TLR4-Mediated Inflammation Pathway and Induces Epithelial Apoptosis in the Goat Colon. Biochem. Biophys. Res. Commun. 2019, 518, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Lesovaya, E.; Agarwal, S.; Readhead, B.; Vinokour, E.; Baida, G.; Bhalla, P.; Kirsanov, K.; Yakubovskaya, M.; Platanias, L.C.; Dudley, J.T.; et al. Rapamycin Modulates Glucocorticoid Receptor Function, Blocks Atrophogene REDD1, and Protects Skin from Steroid Atrophy. J. Invest. Dermatol. 2018, 138, 1935–1944. [Google Scholar] [CrossRef] [PubMed]
- Baida, G.; Bhalla, P.; Kirsanov, K.; Lesovaya, E.; Yakubovskaya, M.; Yuen, K.; Guo, S.; Lavker, R.M.; Readhead, B.; Dudley, J.T.; et al. REDD1 Functions at the Crossroads between the Therapeutic and Adverse Effects of Topical Glucocorticoids. EMBO Mol. Med. 2015, 7, 42–58. [Google Scholar] [CrossRef] [PubMed]
- De Bosscher, K.; Beck, I.M.; Haegeman, G. Classic Glucocorticoids versus Non-Steroidal Glucocorticoid Receptor Modulators: Survival of the Fittest Regulator of the Immune System? Brain. Behav. Immun. 2010, 24, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- U, M.; Shen, L.; Oshida, T.; Miyauchi, J.; Yamada, M.; Miyashita, T. Identification of Novel Direct Transcriptional Targets of Glucocorticoid Receptor. Leukemia 2004, 18, 1850–1856. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lou, Z.; Wang, L. The Role of FKBP5 in Cancer Aetiology and Chemoresistance. Br. J. Cancer 2011, 104, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Pei, H.; Li, L.; Fridley, B.L.; Jenkins, G.D.; Kalari, K.R.; Lingle, W.; Petersen, G.; Lou, Z.; Wang, L. FKBP51 Affects Cancer Cell Response to Chemotherapy by Negatively Regulating Akt. Cancer Cell 2009, 16, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Bähn, S.; Tillack, A.; Imm, S.; Mevius, K.; Michalik, D.; Hollmann, D.; Neubert, L.; Beller, M. Ruthenium--catalyzed Selective Monoamination of Vicinal Diols. ChemSusChem 2009, 2, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Yemelyanov, A.; Czwornog, J.; Chebotaev, D.; Karseladze, A.; Kulevitch, E.; Yang, X.; Budunova, I. Tumor Suppressor Activity of Glucocorticoid Receptor in the Prostate. Oncogene 2007, 26, 1885–1896. [Google Scholar] [CrossRef] [PubMed]
- Workman, P.; Aboagye, E.O.; Balkwill, F.; Balmain, A.; Bruder, G.; Chaplin, D.J.; Double, J.A.; Everitt, J.; Farningham, D.A.H.; Glennie, M.J.; et al. Guidelines for the Welfare and Use of Animals in Cancer Research. Br. J. Cancer 2010, 102, 1555–1577. [Google Scholar] [CrossRef] [PubMed]
| Compound | IC50, uМ, CEM | IC50, uМ, Granta | IC50, uМ, K562 |
|---|---|---|---|
| Dex | 1,35±0,48 | 3,55±0,69 | 8,87±0,91 |
| CpdA | 1,36±0,27 | 5,56±0,27 | 14,61±1,27 |
| CpdA-01 | 15,33±2,44 | 19,55±2,85 | 25,04±3,49 |
| CpdA-02 | 18,34±2,71 | 26,54±1,84 | 38,74±5,92 |
| CpdA-03 | 3,26±0,22 | 5,78±0,63 | 18,81±1,62 |
| CpdA-04 | 24,33±3,34 | 28,65±3,50 | 34,41±3,63 |
| CpdA-05 | 9,67±0,51 | 11,71±2,01 | 25,78±3,17 |
| CpdA-06 | 9,13±1,78 | 19,25±1,79 | 33,91±5,17 |
| CpdA-07 | 18,61±1,62 | 20,61±2,02 | 39,87±3,68 |
| CpdA-08 | 27,93±3,94 | 19,95±2,56 | 47,38±5,47 |
| Compound | Structure | Characterization |
|---|---|---|
| 1-(2-chloro-2-(4-methoxyphenyl)ethyl)piperidin-1-ium chloride (CpdA-01) |
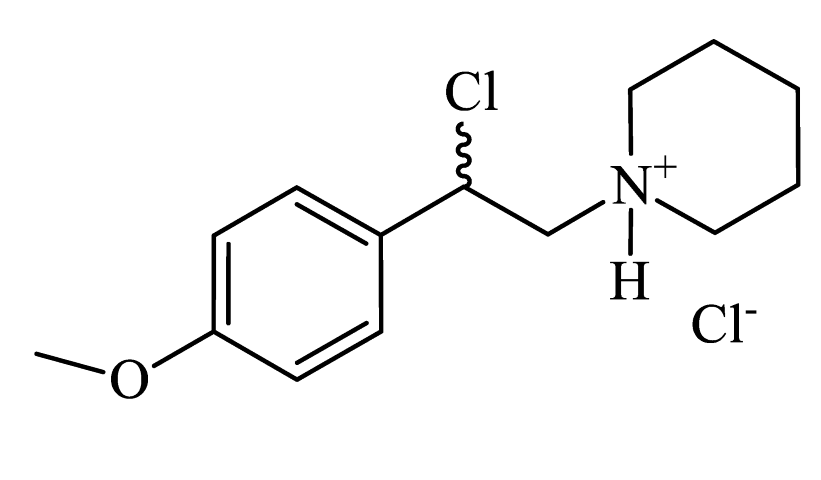 |
Colorless powder, 99% yield (858 mg). 1H NMR (300 MHz, DMSO-d6): δ = 1.35-1.42 (m, 1H, CH2); 1.65-1.79 (m, 5H, CH2); 2.99-3.01 (m, 2H, CH2); 3.37-3.60 (m, 4H, CH2); 3.74 (s, 3H, OCH3); 6.28-6.30 (m, 1H, CH); 6.95-6.98 (m, 2H, Harom); 7.36-7.40 (m, 2H, Harom); 10.61 (s, 1H, NH). MS (EI): m/z (%) = 253 [M]+. HRMS (ESI-TOF) m/z: [M + H]+ Calculated for C14H20ClNO: 254.1306; found: 254.1317. |
| 1-(4-methoxyphenyl)-2-(piperidin-1-yl)ethanol (CpdA-02) |
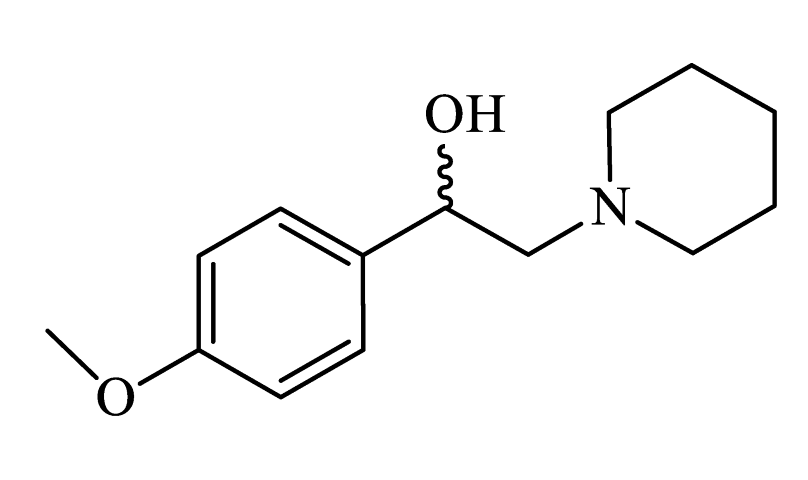 |
Colorless powder, 71% yield (500 mg). 1H NMR (300 MHz, CDCl3) δ = 1.49 (dd, J = 11.3, 5.6 Hz, 2H, CH2); 1.61-1.67 (m, 4H, CH2); 2.37-2.45 (m, 4H, CH2); 2.70-2.76 (m, 2H, CH2); 3.81 (s, 3H, OCH3); 4.69 (dd, J = 9.8, 4.3 Hz, 1H, CH); 6.89 (d, J = 8.6 Hz, 2H, Harom); 7.26-7.32 (m, 2H, Harom). Anal. Calculated for C14H21NO2: C, 71.38; H, 9.00; N, 5.85. Found: C, 71.46; H, 8.99; N, 5.95. Lit. [S1]. |
| 4-(1-hydroxy-2-(piperidin-1-yl)ethyl)phenol (CpdA-03) |
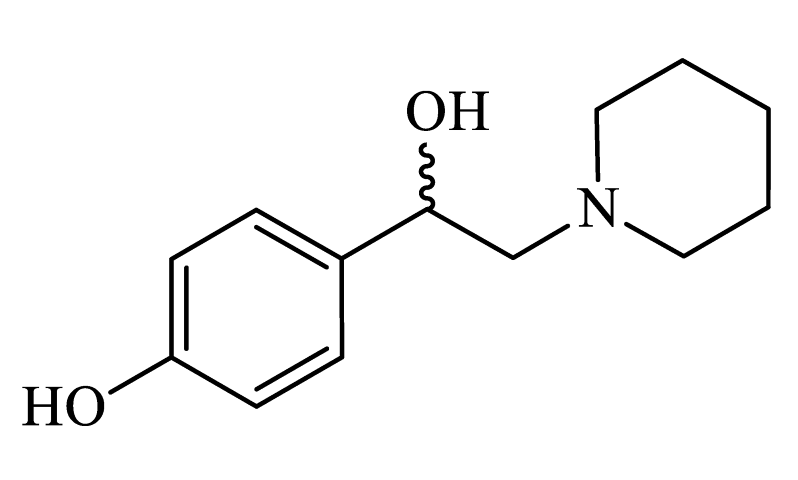 |
Colorless powder, 33% yield (219 mg). 1H NMR (300 MHz, DMSO-d6): δ = 1.32-1.39 (m, 2H, CH2); 1.46-1.47 (m, 4H, CH2); 2.24-2.44 (m, 6H, CH2); 4.54-4.56 (m, 1H, CH); 6.66-6.69 (m, 2H, Harom); 7.09-7.12 (m, 2H, Harom); 9.18 (s, 1H, OH). Anal. Calculated for C13H19NO2: C, 70.45; H, 8.65; N 6.20, Found: C, 70.56; H, 8.65; N, 6.33. Lit. [S2]. |
| 1-(2-chloro-2-(4-hydroxyphenyl)ethyl)piperidin-1-ium chloride (CpdA-04) |
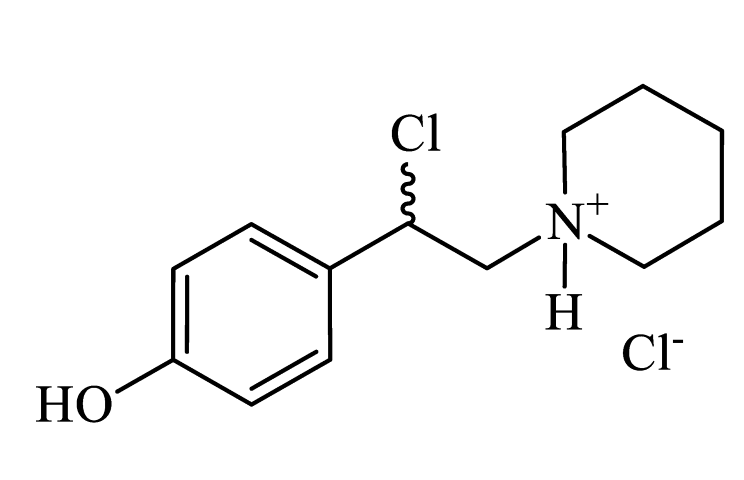 |
Colorless powder, 99% yield (817 mg). 1H NMR (300 MHz, DMSO-d6): δ = 1.33-1.49 (m, 1H, CH2); 1.65-1.81 (m, 5H, CH2); 2.94-3.11 (m, 2H, CH2); 3.36-3.839 (m, 4H, CH2); 6.37-6.41 (m, 1H, CH); 7.31-7.35 (m, 2H, Harom); 7.55-7.68 (m, 2H, Harom); 10.75 (s, 1H, NH). MS (EI): m/z (%) = 203 [M-HCl]+. |
| 1-(4-methoxyphenyl)-2-(methylamino)ethanol (CpdA-05) |
 |
Colorless powder, 33% yield (60 mg). 1H NMR (300 MHz, DMSO-d6): δ = 2.29 (s, 3H, CH3); 2.50-2.56 (m, 2H, CH2); 3.73 (s, 3H, OCH3); 4.53-4.59 (m, 1H, CH); 6.87 (d, J = 8.40 Hz, 2H, Harom); 7.24 (d, J = 8.40 Hz, 2H, Harom). MS (EI): m/z (%) = 181 [M]+. Anal. Calculated for C10H15NO2: C, 66.13; H, 8.42; N, 7.35. Found: C, 66.27; H, 8.34; N, 7.73. Lit. [S3]. |
| 2-chloro-2-(4-methoxyphenyl)-N-methylethanaminium chloride (CpdA-06) |
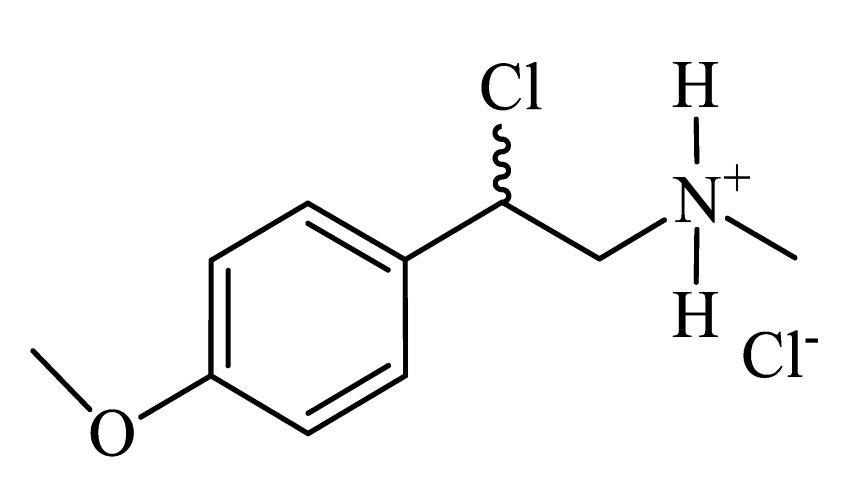 |
Colorless powder, 33% yield (233 mg). 1H NMR (300 MHz, DMSO-d6): δ = 2.63 (s, 3H, CH3); 3.76 (s, 5H, OCH3+CH2); 6.13-6.19 (m, 1H, CH); 7.00 (d, J = 8.06 Hz, 2H, Harom); 7.41 (d, J = 8.06 Hz, 2H, Harom); 9.29 (s, 2H, NH). MS (EI): m/z (%) = 199 [M]+. |
| 1-(3,4-dimethoxyphenyl)-2-(methylamino)ethanol (CpdA-07) |
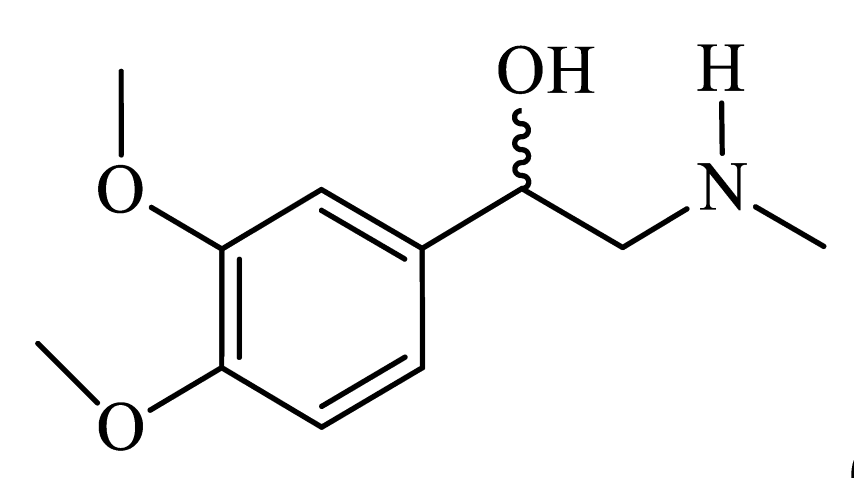 |
Colorless powder, 63% yield (133 mg). 1H NMR (300 MHz, CDCl3) δ = 2.44 (s, 3H, CH3); 2.66-2.80 (m, 2H, CH2); 2.88 (s, 2H, OH+NH); 3.86 (s, 3H, OCH3); 3.88 (s, 3H, OCH3);4.67-4.72 (dd, J = 8.4, 4.3 Hz, 1H, CH); 6.84 (s, 1H, Harom); 6.86-6.87 (m, 1H, Harom); 6.93-6.94 (m, 1H, Harom). MS (EI): m/z (%) 211 [M]+. HRMS (ESI-TOF) m/z: [M + H]+. Calculated for C11H17NO3: 212.1281; found: 212.1275. Lit. [S3]. |
| 2-chloro-2-(3,4-dimethoxyphenyl)-N-methylethanaminium chloride (CpdA-08) |
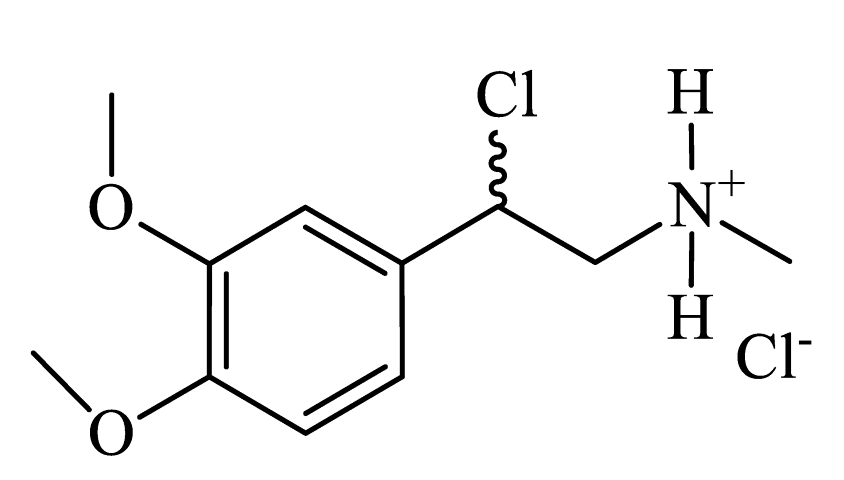 |
Colorless powder, 33% yield (262 mg). 1H NMR (300 MHz, DMSO-d6) δ = 2.62 (s, 3H, CH3); 3.74 (s, 4H, CH+OCH3); 3.76 (s, 4H, CH+OCH3); 6.12-6.16 (m, 1H, CH); 6.98 (s, 2H, Harom); 7.06 (s, 1H, Harom); 9.21 (s, 2H, NH). MS (EI): m/z (%) = 198 [M - MeO]+. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





