Introduction:
The most common and dangerous illness in the world, atherosclerotic disease greatly increases the morbidity and death rate worldwide, with 3.9 million people dying every year from its consequences. The accumulation of cholesterol and inflammatory cells on the artery walls causes atherosclerosis, which leads to narrowing of the arterial vessels, increased stiffness of their walls and decreased blood flow in the atherosclerotic arteries. Although death rate started to decline in the latest years in European countries, including those in Central and Eastern Europe, the incidence of atherosclerotic disease is rising, particularly in middle- and low-income countries, where urbanization and lifestyle changes led to the adoption of unhealthy dietary and behavioral habits. Atherosclerotic disease is a major contributor to heart failure, stroke, and myocardial infarction, significantly lowering the quality of life and life expectancy, with a significant burden on healthcare. Globally, controlling and preventing atherosclerotic disease is a top priority for public health.
Diagnosis of a signification coronary artery stenosis requires contrast opacification of the coronary vessels using a contrast material which is injected in the coronary ostium during invasive angiography. An alternative technique for visualization of coronary arteries is the non-invasive CT angiography, which uses intravenous injection of a contrast agent followed by a CT scan. However, AngioCT remains a diagnostic test [
1]. When AngioCT identifies a significant stenosis, the case is referred to the cath lab for percutaneous revascularization and stent implantation.
It is important to remember that not all the flow-limiting stenoses produce ischemia. Fractional flow reserve (FFR) is a technique frequently used in the cardiac catheterization laboratories for determination of the functional significance of a coronary lesion. It calculates the ratio between the maximum theoretical flow in a healthy coronary artery and the maximum achievable blood flow in an afflicted coronary artery. An FFR higher than 0.8 is regarded as normal, however an FFR of less than 0.75 to 0.80 is typically linked to myocardial ischemia (MI) [
2]. Using a pressure wire to assess the aortic pressure in the event of maximal myocardial hyperemia and the distal coronary pressure at a stenosis, this approach can be carried out during coronary angiography [
2]. Since FFR measurement offers an unbiased, quantitative evaluation of the functional severity of coronary stenoses, its application in cardiac catheterization laboratories has grown in frequency.
At the same time, not all the non-significant lesions are innocent. Some of them may carry inside them the so-called markers of vulnerability, that may expose them to an unpredictable evolution towards accelerated progression, rupture and development of acute coronary syndrome (ACS). The capacity of visual angiography to differentiate between stable lesions and those that potentially cause MI is limited, since these are not directly related with the degree of vessel narrowing. [
3] Therefore, additional imaging techniques like intravascular ultrasound (IVUS) or optical coherence tomography (OCT) are often utilized to provide more detailed information about plaque morphology and composition, helping to guide optimal treatment decisions.[
4]
A coronary atheromatous plaque becomes unstable when it has a large necrotic lipid core and a thin fibrous cap infiltrated by macrophages. This instability is often accompanied by active vascular remodeling, where the vessel diameter enlarges at the plaque site, and spotty calcifications are present within the plaque [
5]. The most commonly used intracoronary imaging methods for assessment of plaque vulnerability are intravascular ultrasound (IVUS) and optical coherence tomography. Due to its high resolution (10-15 µm), OCT can distinguish between the internal and external elastic laminae, as well as differentiate between the intima/media/adventitia layers, which is not possible with the lower resolution (100-150 µm) of intravascular ultrasound [
3]. Thin-Cap Fibroatheroma (TCFA) are plaques that are susceptible to rupture and have thin fibrous caps, with a cutoff of 70 µm, rich in macrophages overlying a large lipid-rich necrotic core. Ruptured plaques refer to areas within an arterial plaque that have developed a tear or rupture. This rupture allows the contents of the plaque, such as lipids and cellular debris, to encounter the bloodstream which leads to the formation of a thrombus. Unlike plaque rupture, plaque erosion is characterized by the presence of luminal thrombus and absence of the endothelium, with no evidence of disruption of the fibrous cap. Thanks to the high resolution of OCT, it is well-suited for identifying these high-risk plaques. [
3]
The aim of the present study was to assess the role of OCT and FFR, as adjunctive tools in the cathlab, for establishing the therapeutic decision in patients with multivascular disease in whom the results of angiography are inconclusive for the non-culprit vessel. Specifically, we aimed to investigate the impact of OCT and FFR on changing the therapeutic strategy in patients with bi or three-vessel coronary artery disease.
Methods
This is a single-center prospective randomized study investigating the role of FFR and OCT imaging in changing the therapeutic decision for non-culprit lesions in patients with acute coronary syndromes and multi-vessel CAD.
Study Population
Thirty-six patients with acute coronary syndrome defined as Unstable angina (UA), Non-ST-segment elevation myocardial infarction (NSTEMI) and ST-segment elevation myocardial infarction (STEMI) who underwent PCI at the Cardiology Clinic of the Târgu Mureș County Emergency Clinical Hospital between January 2022 and May 2024 were enrolled. The inclusion criteria comprised patients under 80 years of age presenting with acute coronary syndrome and multi-vessel disease (MVD), defined as one or more vessels affected by at least 60% stenosis, who demonstrated willingness to participate in the study. Exclusion criteria included patients with MVD necessitating coronary artery bypass grafting (CABG), those over 80 years of age, lesions situated in a grafted segment or vein graft, or instances where FFR and OCT were considered infeasible or hazardous due to patient anatomy. Randomization was determined by a computer-generated sequence assigning patients to either the FFR-guided (Group 1) revascularization or the FFR&OCT-guided (Group 2) revascularization. Lesions responsible for ACS underwent stenting in accordance with current guidelines. We evaluated the non-culprit lesions (not responsible for ACS) either using FFR alone for Group 1 or a combination of FFR and OCT for Group 2. In total, 18 patients were assigned to each group.
A UA/NSTEMI/STEMI was defined according to the standard criteria: STEMI was defined as a new ST-segment elevation ≥1 mm at the J-point in two or more contiguous leads accompanied by an elevation of troponin levels above the 99th percentile. NSTEMI was defined as a new ST-segment depression > 0.1 mm or T wave inversion of at least 0.3 mm in more than 2 contiguous leads accompanied by an elevation of troponin levels above the 99th percentile, and a UA was defined as a Canadian Cardiology Society ischemic discomfort class 3 or 4 accompanied by ST/T changes on electrocardiogram (ECG) but without ST segment elevation and without an increase in troponin levels.
Methodology
All patients were screened for the presence of risk factors (diabetes, smoker, dyslipidemia, hypertension, family history of heart disease, history of ACS and prior PCI). Diabetes mellitus was defined as patients having fasting plasma glucose ≥126 mg/dL and/or post-prandial plasma glucose ≥200 mg/dL and/or A1c ≥6.5% or a history of diabetes and/or taking medication for diabetes. Hypertension was defined as systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg and/or on antihypertensive treatment. Dyslipidemia was defined as a LDL > 100 mg/ dl and/or cholesterol > 200 mg/dl and/or triglycerides > 200 mg/dl and/or on hypolipidemic treatment. History of cardiovascular disease was defined as having a first-degree relative with atherosclerotic cardiovascular disease at an age younger than 55 years. Smoking was defined as either current ore before hospitalization.
Procedures
All patients underwent PCI of the culprit lesion responsible for ACS, FFR measurements and OCT imaging for non-culprit lesions were performed during the PCI procedure or during a staged procedure.
Urgent Coronary Angiography
Patients presenting with ACS were referred to our Cath Lab, where a 6 French sheath was inserted into either the radial, brachial, or femoral artery, and 7500 IU of heparin was administered before starting the procedure. Angiography imaging was performed using the Philips Azurion 7 B20 system (Veenpluis, Netherlands). All lesions deemed responsible for ACS were revascularized according to current guidelines. If one or more vessels had at least 60% stenosis and were suitable for FFR and OCT evaluation, the study terms were explained to the patient. After obtaining written consent, FFR measurements and OCT imaging were performed either in the same session or as a staged procedure. The decision for a staged procedure was based on either patient preference or the interventional cardiologist's recommendation. If a staged procedure was performed at a later date, the sheath was removed, hemostasis was achieved, and a new sheath was inserted at the start of the staged procedure, with 7500 IU of heparin administered.
FFR Measurements
FFR measurements of the non-culprit lesions were performed in both groups using the Abbott PressureWire X 0.014” (0.36 mm) x 175 cm (Coyol Alajuela, Costa Rica), positioned at the target lesion. FFR at baseline as well as Pd/Pa and RFR were recorded. After baseline recordings, adenosine was injected into the coronary artery (200 micrograms for the left coronary artery and 100 micrograms for the right coronary artery) and a second FFR measurement was obtained for each non culprit lesion.
OCT Imaging
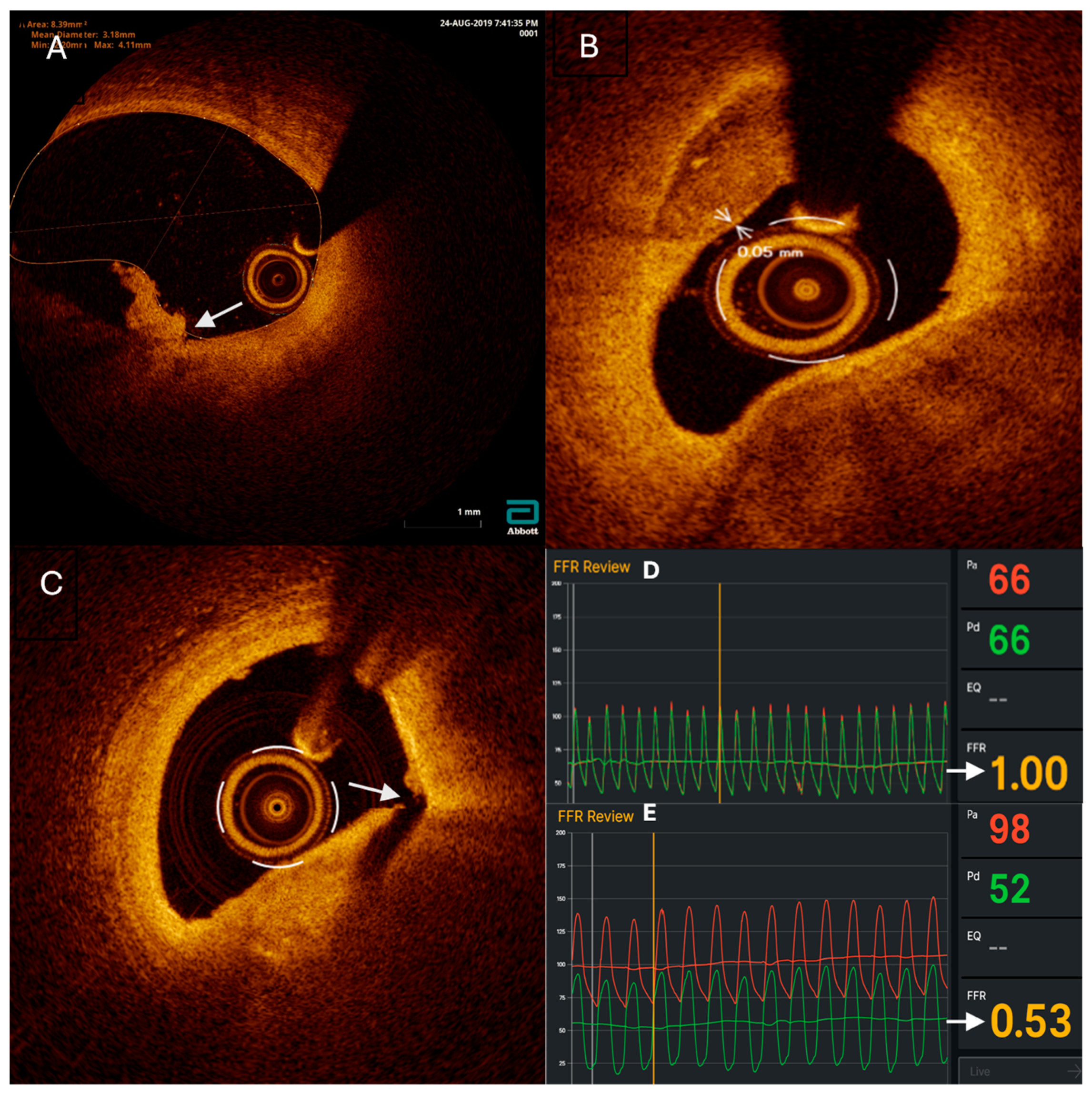
OCT imaging was performed, only in the FFR and OCT group, using the Abbott Dragonfly OpStar Imaging Catheter (1.78 mm/0.36 mm/135 cm) (Westford, USA). Upon reaching the target lesion, the catheter was flushed with saline solution followed by injection of contrast media, and OCT pullback was performed. The recorded images were analyzed using Abbott’s OPTIS™ Integrated Next Imaging System (Westford, USA) to assess signs of vulnerability, luminal stenosis percentage, lesion length, proximal lesion diameter, and distal lesion diameter. Vulnerable plaques (VP) were defined as thin-cap fibroatheroma (TCFA) with a cap thickness of 0.075 mm or less, plaque rupture (PR), or plaque erosion. OCT imaging was performed after stent placement, and stent optimization was conducted based on the findings if malapposition was observed.
The Decision for Revascularization
For the FFR group, revascularization decisions were based solely on FFR measurements. If the FFR was >0.8, the procedure concluded, and the patient received maximal medical treatment. If the FFR was ≤0.8, a stent was placed.
For the FFR + OCT group, if the FFR was >0.8, the revascularization decision was based on OCT findings. If there were no vulnerable plaques (VP), the procedure concluded, and the patient received maximal medical treatment. If OCT imaging indicated VP, then the patient underwent revascularization. If the FFR was ≤0.8, the patient underwent revascularization regardless of OCT findings.
Postrevascularization Procedures
After the procedure ended the arterial sheath was removed and hemostasis was performed either whit a hemostasis device for the radial artery or manual hemostasis for the brachial and femoral artery completed with a compression bandage. The patients mean hospitalization days were between 6 and 10 days. The patient was properly hydrated to prevent contrast media renal toxicity. Standard treatment for acute coronary syndrome was administered as well as personalized treatment depending on each patient’s cholesterol, LDL, HDL, triglycerides levels.
Statistical Analysis
Graph Pad Prism 9.0 software (GraphPad Software Inc., San Diego, USA) was used for statistical analysis. Prior to statistical analysis, all data were checked for normality. The results were expressed as numbers and percentage. Statistical significance, expressed as p, was set at 0.05
Ethics
The study has been carried out in accordance with the code of Ethics of the World Medical Association (Declaration of Helsinki), all patients gave written informed consent, and the study protocol was approved by the ethics committee of the hospital.
Results
The clinical baseline characteristics of the study population are listed in
Table 1.
Plaque characteristics of non-culprit lesions
The number of non-culprit lesions was comparable between the two groups, with 23 lesions in the FFR group and 30 in the FFR and OCT group. Statistical analysis revealed no significant differences between the groups in terms of lesion location, balloon predilatation, location of predilatation, and total number of stent placements (
Table 2). However, in the FFR group, only one case required stent optimization, whereas in the FFR and OCT group, OCT imaging indicated that 8 (44.44%) cases needed stent optimization, two in the right coronary artery, three in the anterior descending artery and two in the circumflex artery. This difference was statistically significant (p = 0.0178; Relative Risk = 0.1765; 95% CI: 0.03113 to 0.7305).
Impact of OCT on Decision-Making
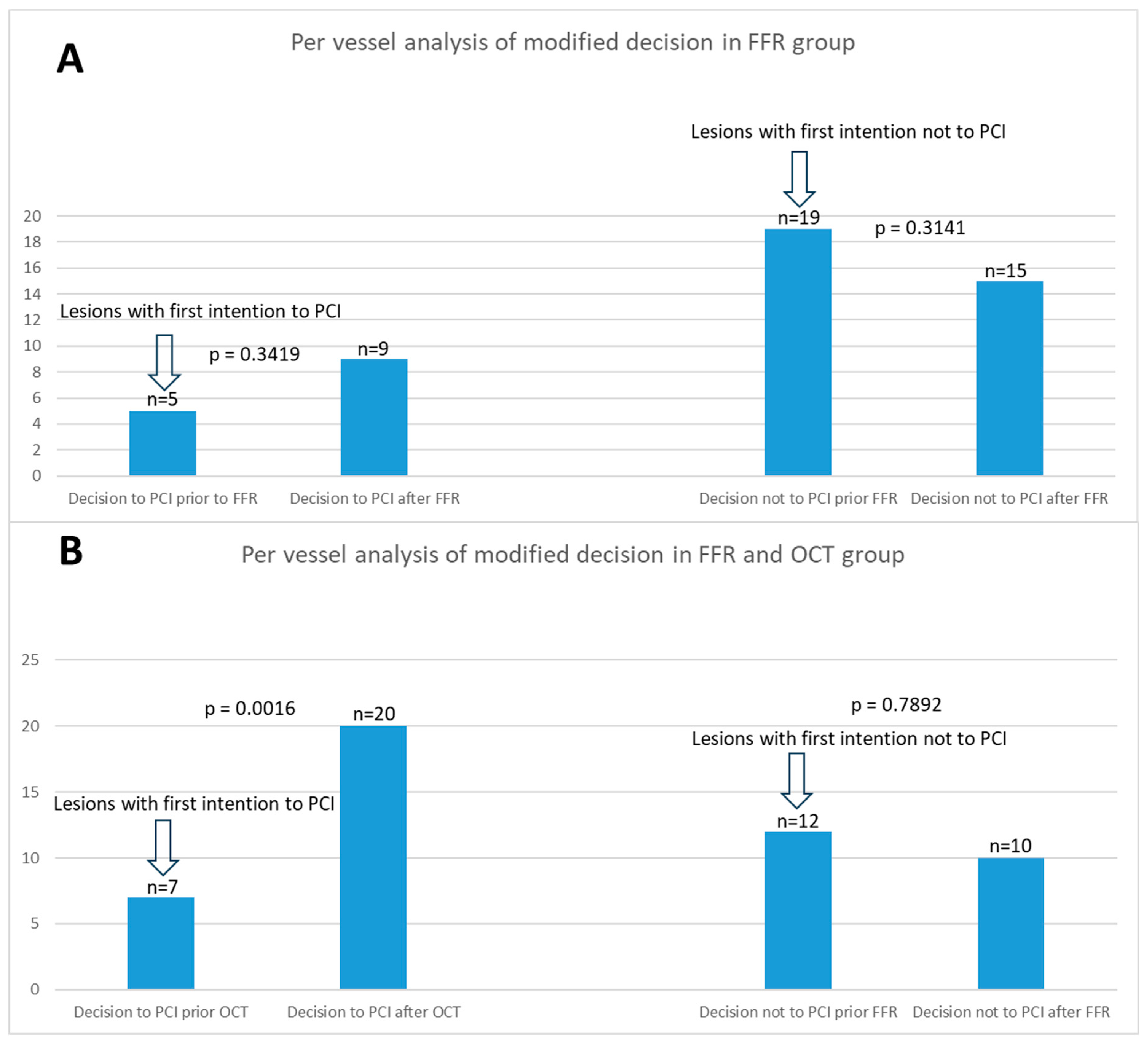
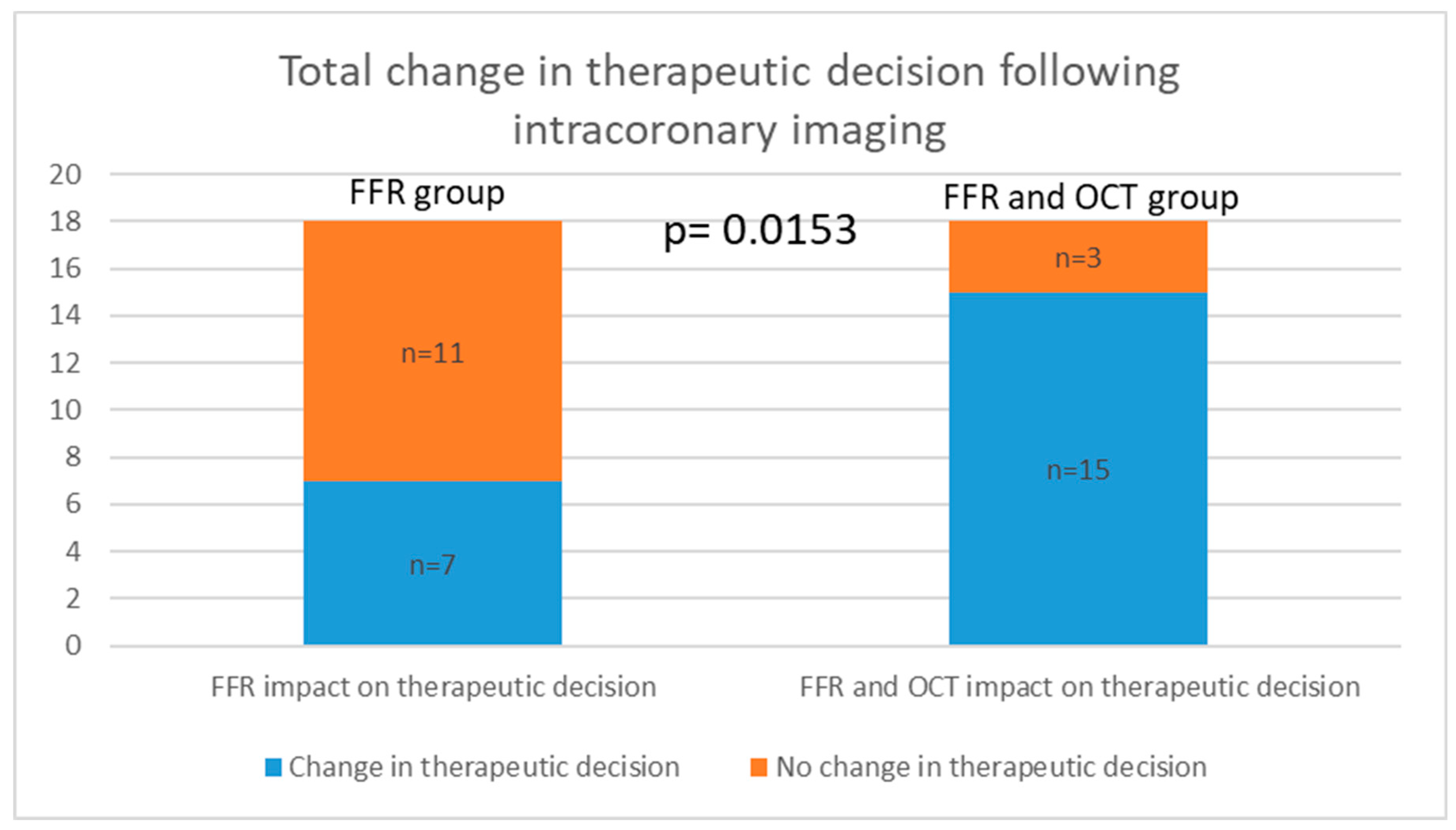
There was no statistically significant difference between the two groups in the decision to stent based on FFR alone, with both groups having 4 cases each. Additionally, there was no statistical significance between the groups when analyzing the decision not to place a stent based on FFR findings. However, when considering OCT imaging, the medical decision was altered in 11 cases where FFR measurements were above 0.8, but the lesions were characterized as VP. Analyzing the total change in the decision to stent, 4 cases in the FFR group and 15 cases in the FFR and OCT group (4 based on FFR and 11 on OCT) revealed a statistically significant difference (p = 0.0006; Relative Risk = 0.2556; 95% CI: 0.1013 to 0.5603). When analyzing the change in the total decision both to stent and not to stent, we observed a statistically significant difference, with Group 1 having 7 cases and Group 2 having 15 cases (p = 0.0153; Relative Risk = 0.4050; 95% CI: 0.2004 to 0.7698) (
Table 3).
The distribution and characteristics of lesions in the 11 cases where OCT imaging led to a modified clinical decision. The lesions were located 4 in the right coronary artery, 5 in the left anterior descending artery, and 4 in the circumflex artery. The vulnerability criteria revealed that thin-cap fibroatheroma was present in 11 of the lesions. Plaque rupture and plaque erosion were each observed in 4 lesions (
Table 4).
The fractional flow reserve measurements taken after adenosine administration were recorded for each artery. The mean FFR value for the RCA was 0.88 with a standard deviation of 0.07, for the ADA it was 0.85 with an SD of 0.04, and for the ACX it was 0.89 with an SD of 0.08.
Per vessel distribution of analysis of modified decision in both groups is illustrated in
Figure 1. The figure shows a comparative analysis of the comprehensive change in treatment strategies based on FFR and OCT across both groups.
Figure 2. summarizes the overall shift in therapeutic decisions across both groups.
Discussions:
Our study aimed to evaluate the impact of optical coherence tomography on decision-making in patients undergoing coronary revascularization based on fractional flow reserve assessments. The results provide insights into the clinical and procedural implications of incorporating OCT alongside FFR in the management of non-culprit lesions in acute coronary syndrome.
Integration of OCT in Clinical Decision-Making and Its Impact on Patient Outcomes
This study identified an important difference between the two groups in terms of stent optimization. In the FFR group, only one case required stent optimization, whereas OCT imaging indicated that eight cases required such optimization in the FFR and OCT group, distributed across various coronary arteries, and this difference was statistically significant (p = 0.0178).
Fractional Flow Reserve has been extensively validated in numerous clinical studies as a dependable index for guiding decisions on coronary revascularization in the catheterization laboratory. Several pivotal studies have underscored the reliability of FFR in clinical settings, demonstrating its effectiveness and safety in determining whether to defer or proceed with coronary interventions based on the severity of ischemia [
6,
7,
8,
9].
Numerous studies have validated optical coherence tomography for its efficacy in detecting and characterizing vulnerable plaques, particularly thin-cap fibroatheromas, which are associated with heightened cardiovascular risk [
10,
11,
12,
13].
The integration of OCT significantly influenced clinical decision-making in our study. While there was no significant difference between the groups in the decision to stent based solely on FFR values, OCT imaging led to modifications in medical decisions in 11 cases where FFR values were above 0.8 but lesions were characterized as vulnerable plaques. This shift resulted in a higher number of cases requiring stent when OCT was added to FFR, as evidenced by the significant difference in total decision changes (p = 0.0006). Furthermore, when considering the decision change in both directions (from no stent to stent and from stent to no stent), a significant differences were observed between the groups, underscoring OCT's impact on treatment strategies (p = 0.0153).
Importance of Plaque Vulnerability Assessment
Our outcomes were consistent with findings by Jan-Quinten Mol et al., who investigated patients presenting with acute coronary syndrome and FFR-negative non-culprit lesions. Their research underscored that the presence of high-risk plaques in such lesions significantly correlates with poorer clinical outcomes, particularly an increased incidence of unplanned revascularizations [
14]. This correlation highlights the critical importance of plaque vulnerability assessment in clinical decision-making. Our study supports the notion that integrating optical coherence tomography alongside Fractional Flow Reserve measurements provides valuable insights into lesion characteristics beyond ischemic severity alone. By identifying high-risk features such as thin-cap fibroatheromas, plaque rupture, or erosion, OCT enables more precise risk stratification and guides optimal therapeutic strategies. Furthermore, our findings emphasize the potential of OCT to influence clinical management decisions by identifying lesions that may benefit from more aggressive therapeutic approaches. This approach aims to reduce adverse clinical events and enhance overall patient outcomes in the context of coronary artery disease.
Furthermore, our study corroborates the findings of the LightLab Initiative, which demonstrated that OCT workflow influenced lesion assessment and PCI decision-making in 86% of cases, primarily affecting pre-PCI lesion evaluation and treatment strategy planning, leading to a more aggressive approach. Post-PCI OCT modified stent optimization decisions in 31% of cases, compared to our study, where stent optimization was performed in 44.44% of cases. This underscores the value of OCT-based decision-making in identifying high-risk plaques. Integrating OCT into routine clinical practice offers significant benefits in optimizing PCI strategies and ultimately improving patient care in the management of coronary artery disease [
15].
Study Limitations:
This study has several limitations. First, there is a potential for over-stenting due to the use of OCT in guiding PCI. OCT, while providing detailed insights into plaque morphology and lesion characteristics, may lead to more aggressive treatment decisions, such as additional stent placements, based on findings that may not necessarily translate into clinical benefit over the long term. Secondly, there are no long-term follow-up data in our study. Without extended monitoring, we cannot assess the sustained effectiveness or potential complications associated with OCT-guided interventions. This absence of follow-up limits our ability to evaluate the true impact of OCT on patient outcomes beyond the immediate post-procedural period.
Conclusions
Our findings demonstrate that OCT, when used in conjunction with FFR, enhances the precision of coronary revascularization decisions by identifying and characterizing vulnerable plaques that may not be detected by functional assessments alone. This approach represents a paradigm shift in coronary artery disease risk stratification, potentially leading to improved patient outcomes through targeted interventions. Future studies should further explore the long-term clinical benefits and cost-effectiveness of integrating OCT into routine clinical practice.
These insights contribute to the evolving landscape of interventional cardiology, where precise lesion characterization plays an increasingly pivotal role in optimizing therapeutic strategies for ACS patients.
Acknowledgment funding
This work was supported by the University of Medicine, Pharmacy, Science and Technology ,, George Emil Palade’’ of Targu Mures Research Grant number 510/14/17.01.2022”
References
- Mátyás, Botond-Barna, Emanuel Blîndu, Nóra Rat, István Kovács, Corneliu-Florin Buicu, and Theodora Benedek. "A Race Against Time: Coronary Computed Tomography Angiography Discovers a Highly Inflamed Plaque in 49-Year-Old Right Before STEMI." Journal of Cardiology and Cardiovascular Therapy 10, no. 3 (2023): 123-130. [CrossRef]
- Kennedy MW, Fabris E, Ijsselmuiden AJ, Nef H, Reith S, Escaned J, Alfonso F, van Royen N, Wojakowski W, Witkowski A, Indolfi C, Ottervanger JP, Suryapranata H, Kedhi E. Combined optical coherence tomography morphologic and fractional flow reserve hemodynamic assessment of non- culprit lesions to better predict adverse event outcomes in diabetes mellitus patients: COMBINE (OCT-FFR) prospective study. Rationale and design. Cardiovasc Diabetol. 2016 Oct 10;15(1):144. PMID: 27724869; PMCID: PMC5057218. [CrossRef]
- Elvin Kedhi, Balazs Berta, Tomasz Roleder, Renicus S Hermanides, Enrico Fabris, Alexander J J IJsselmuiden, Floris Kauer, Fernando Alfonso, Clemens von Birgelen, Javier Escaned, Cyril Camaro, Mark W Kennedy, Bruno Pereira, Michael Magro, Holger Nef, Sebastian Reith, Arif Al Nooryani, Fernando Rivero, Krzysztof Malinowski, Giuseppe De Luca, Hector Garcia Garcia, Juan F Granada, Wojciech Wojakowski, Thin-cap fibroatheroma predicts clinical events in diabetic patients with normal fractional flow reserve: The COMBINE OCT–FFR trial, European Heart Journal, Volume 42, Issue 45, 1 December 2021, Pages 4671–4679. [CrossRef]
- Sinclair, H, Bourantas, C, Bagnall, A. et al. OCT for the Identification of Vulnerable Plaque in Acute Coronary Syndrome. J Am Coll Cardiol Img. 2015 Feb, 8 (2) 198–209. [CrossRef]
- Blîndu, Emanuel, Imre Benedek, Ioana-Patricia Rodean, Vasile-Bogdan Halațiu, Nora Raț, Constantin Țolescu, Theofana Mihăilă, Aurelian Roșca, Botond-Barna Mátyás, Evelin Szabó, Renáta Gerculy, Dan Păsăroiu, Florin Buicu, and Theodora Benedek. "Regional Differences in the Level of Inflammation Between the Right and Left Coronary Arteries – a Coronary Computed Tomography Angiography Study of Epicardial Fat Attenuation Index in Four Scenarios of Cardiovascular Emergencies." Journal of Cardiology and Cardiovascular Therapy 11, no. 2 (2023): 45-54. [CrossRef]
- Bech, G. J., De Bruyne, B., Pijls, N. H., de Muinck, E. D., Hoorntje, J. C., Escaned, J., ... & Van Gelder, B. (2001). Fractional flow reserve to determine the appropriateness of angioplasty in moderate coronary stenosis: A randomized trial. Circulation, 103(24), 2928-2934.
- Tonino, P. A., De Bruyne, B., Pijls, N. H., Siebert, U., Ikeno, F., van’ t Veer, M., ... & Fearon, W. F. (2009). Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. New England Journal of Medicine, 360(3), 213-224.
- De Bruyne, B., Pijls, N. H., Kalesan, B., Barbato, E., Tonino, P. A., Piroth, Z., ... & van ‘t Veer, M. (2012). Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. New England Journal of Medicine, 367(11), 991-1001.
- Davies, J. E., Sen, S., Dehbi, H. M., Al-Lamee, R., Petraco, R., Nijjer, S. S., ... & Mayet, J. (2017). Use of the instantaneous wave-free ratio or fractional flow reserve in PCI. New England Journal of Medicine, 376(19), 1824-1834. [CrossRef]
- Jang, I. K., Bouma, B. E., Kang, D. H., Park, S. J., Park, S. W., Seung, K. B., ... & Yoon, M. H. (2010). Visualization of coronary atherosclerotic plaques in patients using optical coherence tomography: Comparison with intravascular ultrasound. Journal of the American College of Cardiology: Cardiovascular Imaging, 3(3), 317-327.
- Stone, G. W., Maehara, A., Lansky, A. J., de Bruyne, B., Cristea, E., Mintz, G. S., ... & Mehran, R. (2011). A prospective natural-history study of coronary atherosclerosis. New England Journal of Medicine, 364(3), 226-235. [CrossRef]
- Tearney, G. J., Regar, E., Akasaka, T., Adriaenssens, T., Barlis, P., Bezerra, H. G., ... & Prati, F. (2019). Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: A report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. JACC Cardiovascular Imaging, 12(3), 458-472.
- Prati, F., Regar, E., Mintz, G. S., Arbustini, E., Di Mario, C., Jang, I. K., ... & Guagliumi, G. (2011). Expert review document on methodology, terminology, and clinical applications of optical coherence tomography: Physical principles, methodology of image acquisition, and clinical application for assessment of coronary arteries and atherosclerosis. European Heart Journal, 32(3), 334-342. [CrossRef]
- Mol JQ, Volleberg RHJA, Belkacemi A, Hermanides RS, Meuwissen M, Protopopov AV, Laanmets P, Krestyaninov OV, Dennert R, Oemrawsingh RM, van Kuijk JP, Arkenbout K, van der Heijden DJ, Rasoul S, Lipsic E, Rodwell L, Camaro C, Damman P, Roleder T, Kedhi E, van Leeuwen MAH, van Geuns RM, van Royen N. Fractional Flow Reserve-Negative High-Risk Plaques and Clinical Outcomes After Myocardial Infarction. JAMA Cardiol. 2023 Nov 1;8(11):1013-1021. PMID: 37703036; PMCID: PMC10500430. [CrossRef]
- Bergmark B, Dallan LAP, Pereira GTR, Kuder JF, Murphy SA, Buccola J, Wollmuth J, Lopez J, Spinelli J, Meinen J, West NEJ, Croce K; LightLab Initiative Investigators. Decision-Making During Percutaneous Coronary Intervention Guided by Optical Coherence Tomography: Insights From the LightLab Initiative. Circ Cardiovasc Interv. 2022 Nov;15(11):872-881. Epub 2022 Nov 15. PMID: 36378739; PMCID: PMC9648988. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).