1. Introduction
Arthropods play diverse ecological roles, offering ecosystem services or causing damage to agricultural systems [
1]. In agroecosystems, 18%–20% of global crop yield losses are attributed to herbivorous arthropods [
2]. Predators and parasitoids that prey on herbivores provide valuable ecosystem services for pest regulation in various crops [
3,
4]. Wild bees are crucial pollinators worldwide, significantly enhancing crop yields [
5,
6,
7]. Arthropods are closely linked to soil health and can indicate soil quality [
8].
Watermelon, an economically significant fruit of the Cucurbitaceae family [
9], is extensively cultivated in over 120 countries, with China being the largest producer [
10,
11,
12]. Watermelon cultivation requires adequate water, nutrients, and suitable temperatures to achieve high quality. Limited water resources often constrain the watermelon industry in arid and semi-arid regions [
13]. Gravel-sand mulching, a traditional agricultural practice in such areas, involves covering the soil with local sand, gravel monomers, or mixtures to enhance warming, water retention, and moisture conservation (
Figure 1) [
14,
15]. However, long-term gravel-sand mulching can decline soil fertility and disrupt soil microbial structure, increasing susceptibility to plant diseases [
13,
16]. This ancient drought-resistant technique is widely used in regions with low precipitation worldwide, including Colorado, Texas, and Montana, USA [
17], South Africa [
18], Montpellier, France [
19], Chamoson, Switzerland [
20], and around Lanzhou in Gansu Province, China [
21].
The cascade effect refers to changes in one subsystem of an ecosystem causing changes in others [
22], such as interactions between plants and herbivorous insects and the relationship between plant traits and soil microbial subsystems [
23]. Nitrogen is a limiting factor for insect growth, and excessive nitrogen can reduce the diversity of plant communities, increase aboveground biomass, and change the composition of plant communities to dominant species, affecting other parts of the food chain, such as insects [
24,
25,
26]. Research on cascade effects between insects and other soil elements has shown varied impacts, such as increased populations of invasive ant
Nylanderia fulva in calcium-fertilized land in the southeastern USA, independent of nitrogen, phosphorus, sodium, or potassium levels [
27]. Additionally, the cascade effect between soil and insects primarily focused on a single insect or group, and it was found that the survival rates of
Phoetaliotes nebrascensis adults were the highest under low nitrogen conditions. At the same time, the oviposition was unaffected by the nitrogen concentration of the plant host [
28]. The parasitism rate of wheat aphids by natural enemies increased under moderate nitrogen addition but decreased after excessive nitrogen application [
29].
Long-term agricultural activities can lead to soil degradation and loss of the ability to maintain ecological balance, resulting in a decline in biodiversity, particularly in arid and semi-arid regions [
30,
31]. However, few studies have explored how arthropod diversity changes interact with soil physicochemical properties variations in desert-steppe areas. Therefore, this study focuses on soil physicochemical properties and arthropod diversity in gravel-sand mulched watermelon fields in the desert steppe of Zhongwei City, Ningxia, China. Specifically, it addresses the following scientific questions: (1) How do soil physicochemical properties and arthropod diversity change over different watermelon planting years? (2) How does arthropod diversity respond to changes in soil physicochemical properties? By answering these questions, this study aims to provide insights into the development of dryland agriculture and biodiversity conservation in arid and semi-arid agricultural and pastoral areas.
2. Materials and Methods
2.1. Site Details and Field Experimental Design
The study was conducted in the gravel-sand mulching planting area of Xiangshan Township, Zhongwei City, Ningxia, China (104°50′0”E-105°30′0”E, 37°0′0”N-37°25′0”N). This region, located in the desert steppe of northwest China, is characterized by water scarcity, a fragile ecological environment, and low precipitation. The area experiences high evaporation, with vegetation coverage around 50%, predominantly herbaceous plants about 15 cm in height. Annual rainfall is <200 mm, while evaporation is >2000 mm. Gravel-sand mulching is a traditional dry farming practice here. Once a significant local industry, Watermelon was crucial in boosting farmers’ income and supporting socio-economic development. However, the lack of unified planning and management has led to extensive desertification and sand planting, particularly in the desert steppe of Zhongwei City, the primary production area for Ningxia’s gravel-sand mulching watermelon cultivation. To investigate the effects of varying durations of gravel-sand mulching on the local environment, areas with 1, 5, 10, and 21 years of watermelon cultivation were designated as treatment areas, labeled GPS-1Y, GPS-5Y, GPS-10Y, and GPS-21Y. Intact desert steppe and its transition zone adjacent to the compacted sand planting area were used as control areas, labeled STZ (within 3 km of the compacted sand land) and NG (more than 3 km from the compacted sand land).
2.2. Soil Collection and Physicochemical Properties Measurements
A random five-point sampling method was adopted in the monitoring area, with a minimum spacing of 150 m between sampling points. Mixed soil samples were collected from the 0–20 cm surface layer, ensuring the removal of sand and gravel mixtures before sampling. The samples were then analyzed for various properties, including total nitrogen (g/kg), total phosphorus (mg/kg), total potassium (mg/kg), alkali hydrolyzable nitrogen (mg/kg), available phosphorus (mg/kg), available potassium (mg/kg), organic matter (g/kg, and electrical conductivity (EC) as 10 indexes of leaching fluid conductivity us/cm, pH, and water content. Each index was measured in 10 replicates and categorized into soil nutrient indexes (total nitrogen [TN], total phosphorus [TP], total potassium [TK], alkali hydrolyzable nitrogen [AHN], available potassium [APP], available phosphorus [APK], and organic matter [OM]) and physical properties indexes (EC, pH, and water content [W]). Fresh soil samples were stored in aluminum boxes for moisture content determination. The soil samples were cleared of impurities, naturally air-dried, and passed through a 2 mm sieve before measuring the remaining soil indexes (
Table 1) [
32,
33].
2.3. Collection of Arthropods
In May, July, and September 2023, arthropod samples were collected during the spring, summer, and autumn seasons, corresponding to high arthropod activity periods. The random 5-point sampling method was used for field investigation and sampling [
34].
Trap method: Disposable plastic cups (diameter 7.5 cm, height 9 cm) were used as traps. Five sampling points were established in each monitoring area, serving as five replicates. Each replicate consisted of five traps spaced >5 m apart in a random 5-point arrangement. Thus, a total of 25 traps were set in each monitoring area. The cups were buried in the soil, flush with the ground surface, and filled with approximately 60 mL of an attractant solution of 33.33% ethylene glycol (ethylene glycol: water = 1:2) with 3% detergent. Arthropods trapped in the cups were collected every 10 days, and the traps were replaced simultaneously.
2.4. Statistical Analysis of Data
One-way analysis of variance (ANOVA) was performed to investigate differences in soil physicochemical properties and steppe insect diversity among GPS-1Y, GPS-5Y, GPS-10Y, GPS-21Y, STZ, and NG at the six study sites. Mantel test and the GAM analysis were conducted on four study sites with different tillage years, namely GPS-1Y, GPS-5Y, GPS-10Y, and GPS-21Y, to examine insect dynamics and soil factors.
The species and numbers of different arthropods were counted based on identification results. The diversity of arthropods in various study areas was analyzed using ecological methods, calculating the Margalef richness index (d), Simpson dominance index (λ), Shannon-Wiener diversity index (H), and Pielou evenness index (E) calculated according to the following formulas [
35,
36]:
S is the number of species, Pi is the proportion of the ith species to the total abundance, Ni is the abundance of the ith group, and N is the total abundance.
Fitting arthropod occurrence to soil factors: The GAM is a generalized linear model that uses a link function to establish the relationship between the mean of the response variable and the “smoothing” function of the explanatory variable. GAM can establish highly nonlinear and non-monotonic relationships between response and explanatory variables, making it widely used in ecological research. The optimal influencing factor was selected using the stepwise regression method, with the best model chosen based on the lowest Akaike Information Criterion and residual bias and the significance of the impact judged by the P value [
37,
38]. The general form of the formula is as follows:
In the formula, Y is the response variable, E(Y) is the expected value of the response variable Y, and g is a link function that relates E(Y) to a linear combination of the predictor (the independent or explanatory variable in the model that predicts or explains the change in the dependent variable), β0 is the intercept and is a constant, f1(X1), f2(X2),…., fk(Xk) are nonlinear smoothing functions also known as smooth terms, and they correspond to predictorsX1, X2,…., Xk.
GAM can best characterize the linear or nonlinear relationship between arthropod and soil variables using a nonparametric smoothing term. Therefore, based on the advantages of GAM in the nonlinear relationship between adaptive variables, GAM is expected to effectively simulate the relationship between soil variables and arthropods, providing reliable results [
39].
The aov() function in the stats package and the HSD.test() function in the agricolae package were used for one-way ANOVA analysis and Duncan multiple comparisons of the differences in soil physicochemical properties and diversity indexes in R language, respectively [
40]. In the Mantel test in R language, the Bray-Curtis and Euclidean distances between species were calculated using the mantel function of the linkET package, and the Mantel correlation between arthropods and soil was performed, along with Spearman correlation analysis between soil factors was performed [
41]. GAM analysis was performed using the mgcv package [
37].
Graphical visualization was done using the ggplot2 package, with data analysis and graphical output completed in R 4.3.1 [
42].
3. Results
3.1. Changes in Soil Physicochemical Properties
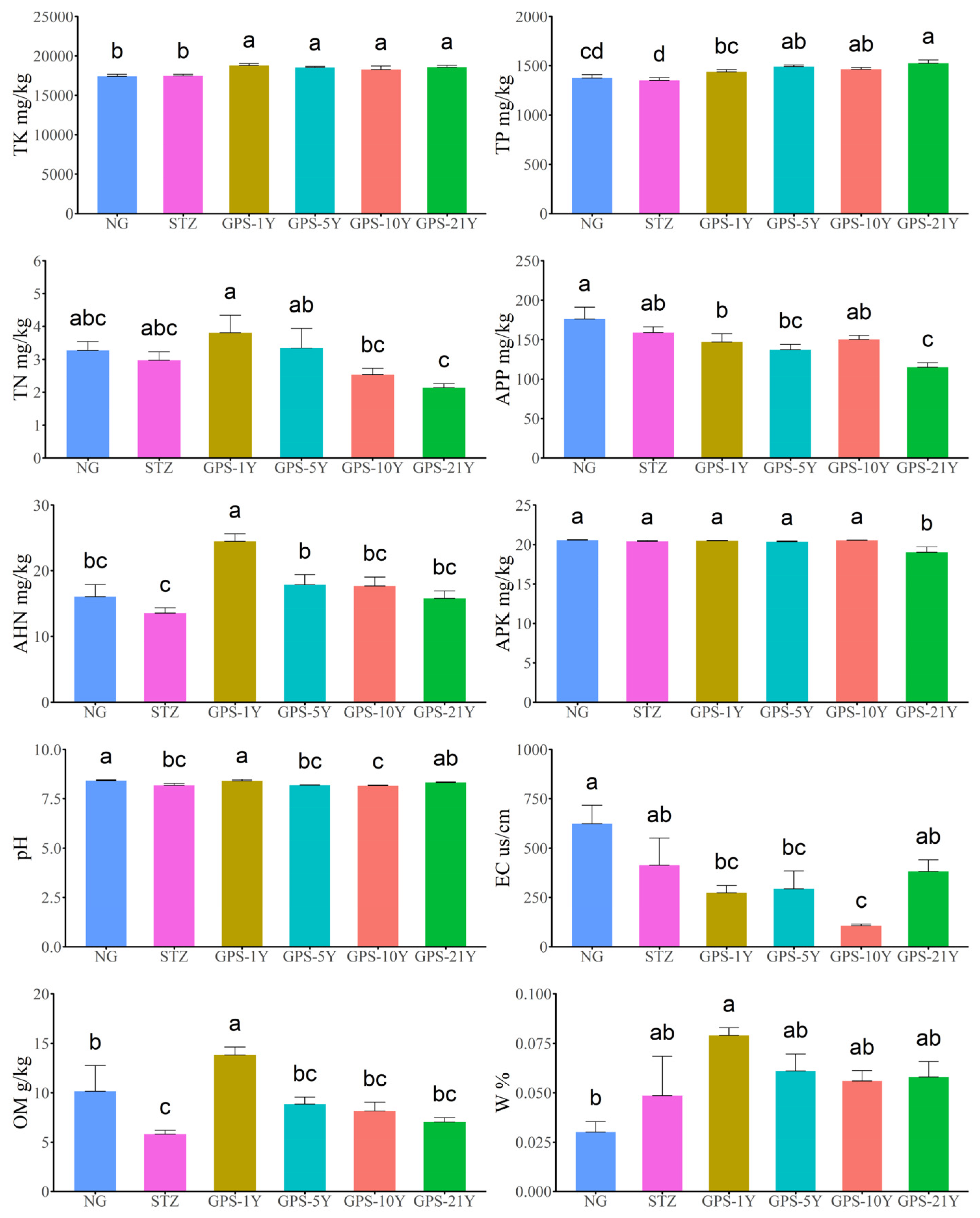
Generally, the changes in soil physicochemical properties of NG and STZ were consistent, with soil nutrients (TP, TK, TN, APP, AHN, APK, and OM) being significantly lower than those in the gravel-sand mulching melon fields with varying tillage years. Gravel-sand mulching plots with different tillage years maintained higher water content and lower salinization compared to the control groups NG and STZ. However, soil nutrients gradually decreased with increased years of planting.
In the control areas (NG and STZ), TK was significantly lower than in the gravel-sand mulching plots with different years of treatment (P < 0.05). TP in NG and STZ was significantly lower than in the gravel-sand mulching plots, and TP in GPS-1Y was significantly lower than in the other GPS treatments (P < 0.05). TN in GPS-1Y was significantly higher than in GPS-10Y and GPS-21Y, and TN in GPS-5Y was significantly higher than in GPS-21Y (P < 0.05). APP in NG was significantly higher than in GPS-1Y, GPS-5Y, and GPS-21Y, whereas APP in GPS-21Y was significantly lower than in STZ, GPS-1Y, and GPS-10Y (P < 0.05). AHN in GPS-1Y was significantly higher than in all other monitored areas, and AHN in STZ was significantly lower than in GPS-1Y and GPS-5Y (P < 0.05). APK in GPS-21Y was significantly the lowest among all monitored areas (P < 0.05). The pH in NG and GPS-1Y was significantly higher than in STZ, GPS-5Y, and GPS-10Y (P < 0.05). EC in NG was significantly higher than in GPS-1Y, GPS-5Y, and GPS-10Y, and EC in STZ and GPS-21Y was significantly higher than in GPS-10Y (P < 0.05). OM in GPS-1Y was significantly the highest among all monitored areas, and OM in NG was significantly higher than in STZ (P < 0.05). W in NG was the lowest among all monitored areas and was significantly lower than in GPS-1Y (P < 0.05) (
Figure 2).
3.2. Changes in Arthropod Diversity
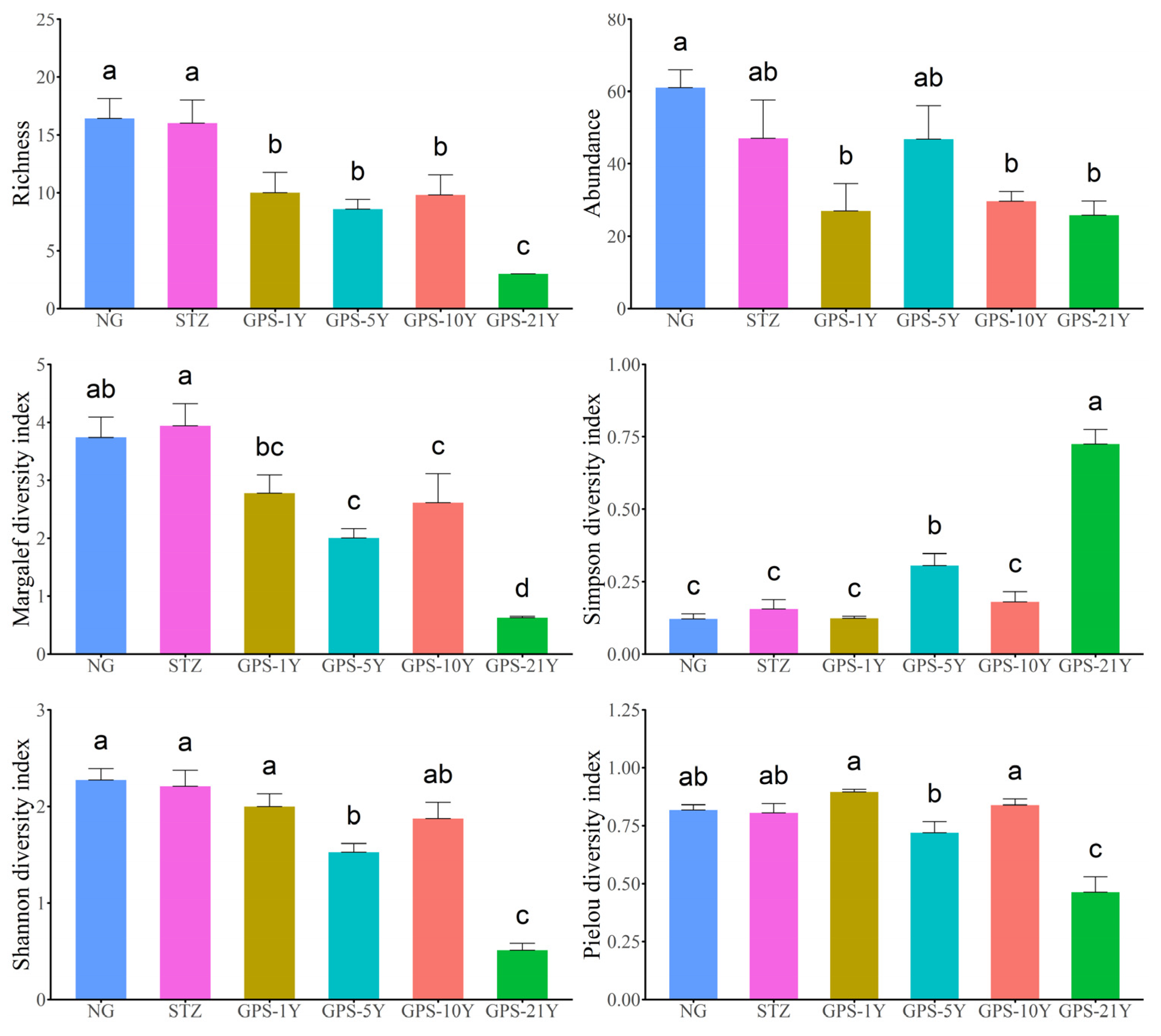
A total of 1,644 arthropods were sampled, representing 81 different species. Analysis of areas with varying years of planting revealed that species number, Margalef richness index, Shannon diversity index, and Pielou evenness index in GPS-21Y were significantly lower than those in the other three groups (
P < 0.05). These indices in all four treated groups were lower than those in the native steppe (NG) area. The number of individuals in GPS-5Y was significantly higher than in the other three treatment groups (
P < 0.05) but still lower than in the NG area. The Simpson dominance index in GPS-21Y was higher than in the other three treatment groups and the NG area (
Figure 3).
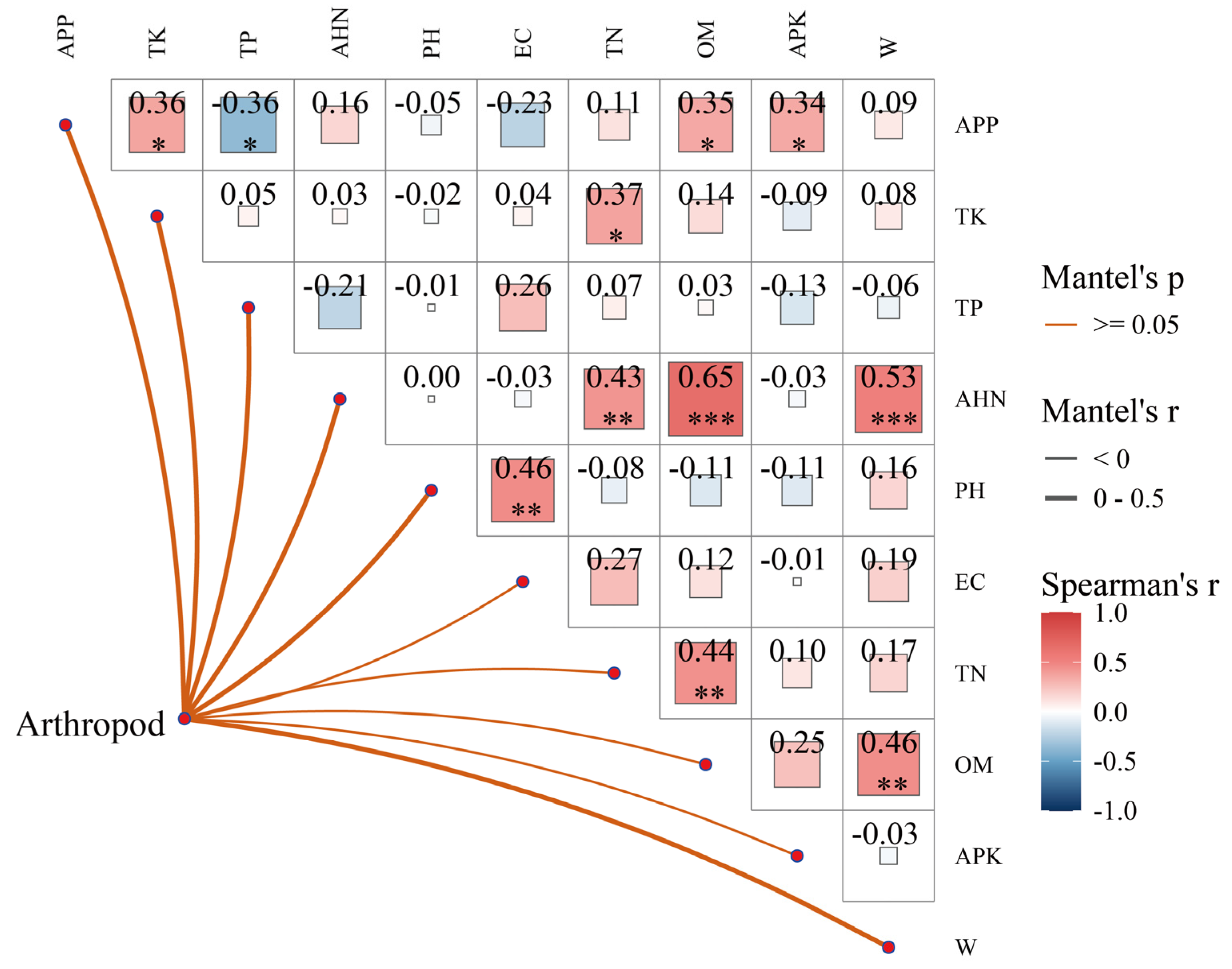
3.3. Mantel Test of Arthropod Community Changes and Soil Physicochemical Properties
Mantel test analysis showed no significant linear correlation between arthropods and soil factors. However, there was a significant correlation between soil factors, with APP positively correlated with APK, OM, and TK (
P < 0.05) and negatively correlated with TP (
P < 0.05). TK was positively correlated with TN (
P < 0.05). AHN was positively correlated with W (
P < 0.001), OM (
P < 0.001), and TN (
P < 0.01). pH was positively correlated with EC (
P < 0.01). TN was positively correlated with OM (
P < 0.01). OM was significantly positively correlated with W (
P < 0.01) (
Figure 4).
3.4. Responses of Arthropod Community Changes to Soil Physicochemical Properties
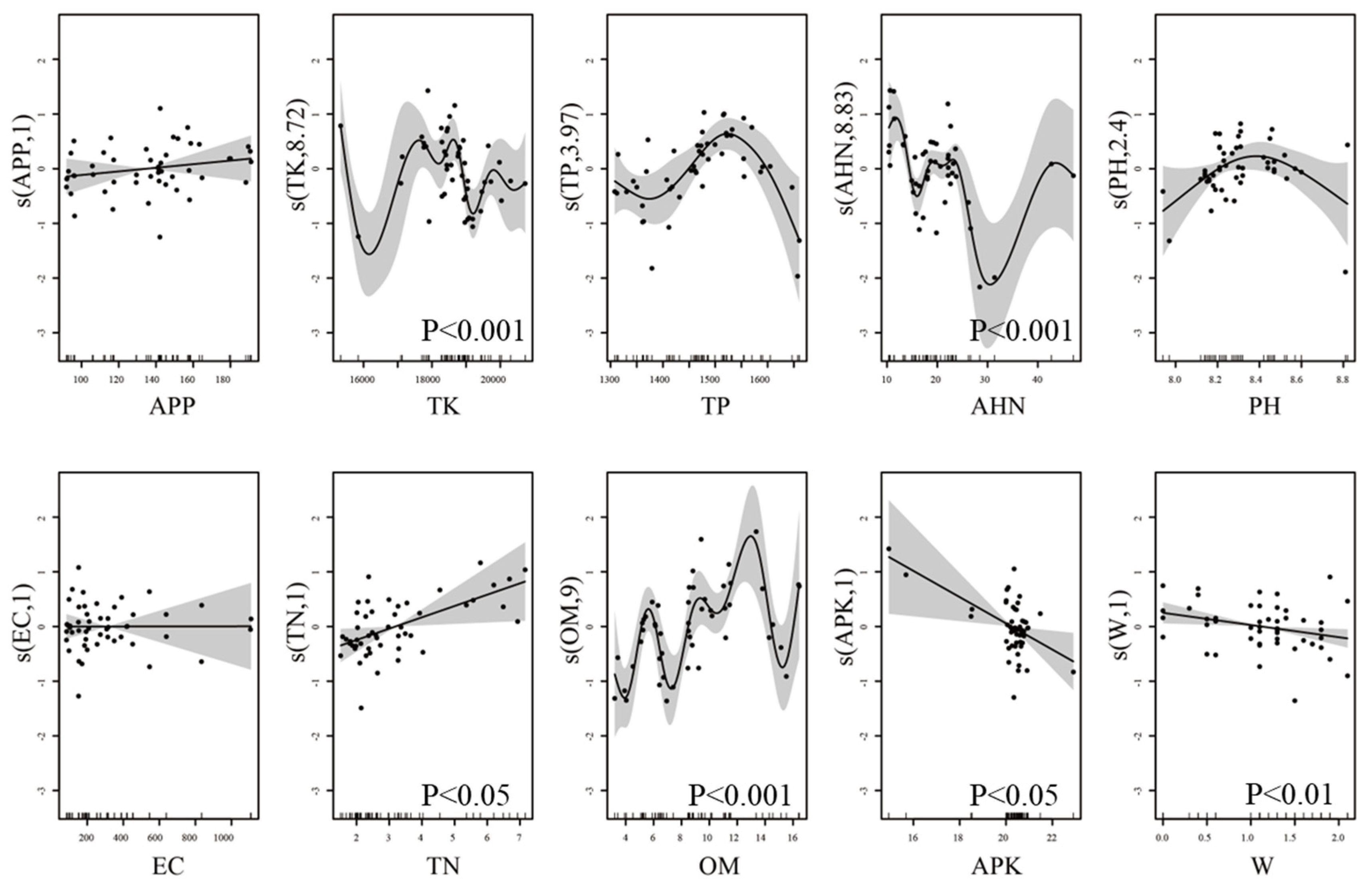
The results of the GAM showed that the number of grassland insects in areas with different sand compaction years was linearly correlated with APP, EC, TN, APK, and W; positively correlated with APP, EC, and TN; negatively correlated with APK and W; and significantly correlated with TN (P < 0.05), APK (P<0.05), and W (P<0.01). The number of grassland arthropods was nonlinearly correlated with TK, TP, AHN, pH, and OM, with AHN (P < 0.05), OM (P < 0.001), and TK (P < 0.001) being significant. When the TP value was lower than 1400 mg/kg or higher than 1550 mg/kg, the number of arthropods was negatively correlated with TP, while within the range of 1400 to 1550 mg/kg, TP was positively correlated with arthropod numbers. There was a positive correlation between the number of arthropods and a negative correlation when the pH was above 8.4 (
Figure 5).
4. Discussion
4.1. Changes in Soil Physicochemical Properties
It is undeniable that farmers who have practiced gravel-sand mulching for watermelon cultivation over extended periods have effectively maintained soil nutrients through field operations. The nutrient levels in these fields can be significantly higher than those in uncultivated grasslands [
43,
44]. Gravel-sand mulching fields can also maintain higher water content and lower salinization, likely due to fertilization and proper field management during long-term planting [
45].
N, P, and K are commonly used in evaluating soil nutrients, reflecting changes in soil nutrients from different perspectives. TP indicates the total storage of soil phosphorus and helps evaluate soil nitrogen availability. TN helps assess soil fertility and nitrogen supply capacity, while TK indicates soil potassium supply potential and reflects soil weathering [
33,
46]. In different years of cultivation (GPS-5Y, GPS-10Y, and GPS-21Y), TK and TP were significantly higher than in NG and STZ, suggesting that long-term planting and continuous fertilizer application increase soil potassium and phosphorus content [
47]. The TK content in GPS-1Y was significantly higher than in NG and STZ. In contrast, the TP content in GPS-1Y was not significantly different from NG. Still, it was significantly higher than in STZ, indicating that TP levels in areas with shorter cultivation periods remain consistent with uncultivated areas after fertilization and watering. There was no significant difference in TK between different planting years, suggesting that TK content is relatively stable in the pressed sand area. However, perennial planting can lead to the accumulation of TP [
48,
49]. TN was significantly higher in GPS-1Y than in GPS-10Y and GPS-21Y, and TN in GPS-5Y was significantly higher than in GPS-21Y. This may be due to faster decomposition of organic matter and fertilizers in the early planting stages (GPS-1Y and GPS-5Y), releasing more nitrogen, or it may indicate that perennial planting rapidly depletes TN, making it difficult to replenish soil fertility through fertilization alone [
50,
51].
APP was significantly higher in NG than GPS-1Y, GPS-5Y, and GPS-21Y, indicating higher potassium availability in uncultivated farmland. This could be due to more efficient organic matter decomposition and nutrient cycling in natural grassland ecosystems. AHN was significantly higher in GPS-1Y than in all other areas, indicating higher initial fertility in newly planted areas [
52]. AHN in STZ was significantly lower than in GPS-1Y and GPS-5Y, showing that agricultural practices can significantly improve soil fertility [
53]. In long-term cropping areas (GPS-21Y), phosphorus availability was significantly lower than in all other regions, suggesting that crop consumption exceeds the natural replenishment rate of phosphorus in the soil. This is consistent with research showing that long-term agricultural activities can deplete certain key soil nutrients [
54]. pH was significantly higher in NG and GPS-1Y than in STZ, GPS-5Y, and GPS-10Y, possibly due to soil acidification from agricultural activities and fertilizer use [
55]. EC in NG was significantly higher than in GPS-1Y, GPS-5Y, and GPS-10Y, while EC in STZ and GPS-21Y was significantly higher than in GPS-10Y. The high EC value in NG may result from natural salt accumulation, climate, geology, and other conditions [
56]. High EC values in STZ and GPS-21Y might be due to salt accumulation from long-term agricultural irrigation and fertilization [
57,
58]. OM in GPS-1Y was significantly higher than in other regions, possibly due to the initial application of large amounts of organic fertilizer or cover crop residues, increasing soil organic matter content [
59]. OM in NG was significantly higher than in STZ, reflecting organic matter accumulation in natural grassland soils due to natural vegetation decomposition and less human disturbance [
60,
61]. The water content in NG was significantly lower than in GPS-1Y, possibly due to the primary grassland’s soil structure and vegetation cover, which allows water to evaporate quickly. In contrast, GPS-1Y effectively maintained higher soil moisture due to gravel cover and water retention measures [
62].
4.2. Changes in the Diversity of Steppe Arthropods
Compared with the maintenance of soil physical and chemical properties in pressed sand melon fields, watermelon cultivation that destroys the original grassland habitat significantly reduces arthropod diversity by planting a single species and increases the vulnerability of the local desert steppe habitat. The number of species in NG and STZ was significantly higher than in the four continuous cultivation areas. The diversity index results for NG and STZ were generally consistent, indicating similar biodiversity levels at these sites. This similarity might be due to their habitat characteristics or exposure to similar environmental impacts. In contrast, the biodiversity in the watermelon cultivation areas with pressed sand gradually decreased with increasing years of planting, showing a trend toward species homogeneity. This may be due to the disturbance and habitat destruction caused by planting activities, highlighting the negative impact of such activities on biodiversity.
Regarding species number, Margalef richness index, Shannon diversity index, and Pielou evenness index, GPS-21Y showed significantly lower values than other planting areas of different years and the primary grassland (NG) areas. This suggests that biodiversity and ecosystem structure can be negatively affected over time and with prolonged planting, possibly due to soil erosion, habitat destruction, or other factors leading to ecosystem degradation [
63,
64]. On the other hand, the number of arthropod individuals in GPS-5Y was significantly higher than in other years’ planting areas. This might be because, after five years of planting, the ecosystem reached a stable state of biological community due to the storage of water resources and temperature in the planting area. However, the number of individuals was still lower than the original grassland (NG), possibly due to ecosystem changes caused by human intervention [
65,
66]. The Simpson concentration probability index of GPS-21Y was significantly higher than in other planting areas of different years and primary grassland (NG) areas. This suggests that after 21 years of watermelon cultivation, arthropod communities tend to congregate in a few species, possibly due to ecosystem degradation [
67]. These results support the idea that pressed sand cultivation impacts biodiversity and ecosystem structure. Long-term cultivation can lead to reduced biodiversity and ecosystem degradation [
68]. Medium-term (5 years) planting may promote an increase in the number of individuals but still not return to the level of the original ecosystem. These findings highlight the importance of preserving primary grasslands and avoiding over-cultivation to maintain biodiversity and ecosystem health.
4.3. Responses of Steppe Arthropod Diversity to Changes in Soil Physicochemical Properties
Mantel test analysis showed no significant linear correlation between arthropod community structure and soil factors. This test examined the linear correlation between the arthropod community structure and soil physicochemical properties matrices [
69]. APP, an essential source of plant nutrients for assessing soil potassium supply capacity, was positively correlated with other nutrients such as OM and TK, indicating that fertile soils are generally nutrient-rich [
70]. The negative correlation between APP and TP might be due to the differing chemical behaviors of phosphorus in soil. For instance, Baribault et al. found a significant negative correlation between soil N and P when studying tropical tree growth limiting factors [
71]. However, Bhattacharyya et al. found that soil K was significantly negatively correlated with some soil nutrients after 30 years of studying fertilization in the Himalayas [
72]. The positive correlation between total potassium and total nitrogen suggests these nutrients may accumulate in the soil through common sources (e.g., organic matter decomposition) or soil management practices (e.g., fertilization). [
73,
74]. AHN was positively correlated with water content and organic matter, indicating that soils with high organic matter tend to have higher nitrogen content and better water retention capacity, which is essential for plant and microbial activities [
75]. The positive correlation between soil pH and electrical conductivity showed that higher pH values may be associated with higher salt content. Conductivity, as an indicator of salt concentration, reflects soil salinity status and the degree of salinization [
76]. The positive correlation between total nitrogen and organic matter further confirmed that organic matter decomposition is a primary source of soil nitrogen. There was a significant positive correlation between organic matter and water content, supporting that soils rich in organic matter generally have better water retention capacity, essential for maintaining the health and stability of soil biomes [
77].
GAM found that the relationship between arthropod communities and soil factors may not be simply linear. Simple linear analysis does not capture complex relationships because the soil environment is highly heterogeneous. Different soil factors affect arthropods through various pathways and mechanisms, making it difficult for a single linear correlation analysis to reveal these complex ecological relationships [
78] entirely. In this study, soil TN was significantly positively correlated with the number of arthropods. Nitrogen is a limiting factor for arthropod growth and development, and nitrogen-mediated changes in plant community composition may have cascading effects on arthropods [
26]. For example, low nitrogen fertilizer can increase flower yield [
79], leading to more pollinator visits per plant and higher pollinator diversity[
80]. Haddad et al. (2000) showed that herbivorous and saprophytic insect abundance was significantly positively correlated with nitrogen addition rates in grasslands over 14 years [
81].
APK and W were significantly negatively correlated with arthropod numbers in this study. High levels of APK can cause nutrient imbalances that negatively affect plant health, reducing food resources for arthropods. Nutrient imbalances lead to impaired plant health, and E. Fenn et al. (1998) in North America studied the effects of nitrogen excess on ecosystems and found that watershed nitrogen excess is harmful because plant/soil nutrient relationships are disrupted, soil acidification and aluminum mobility increase and long-term nitrogen input forests may lead to decreased productivity and increased mortality [
82]. Excess soil moisture can lead to soil hypoxia, inhibiting plant growth and reducing arthropod food sources. It can also promote arthropod pathogens, decreasing arthropod populations [
83,
84].
Soil AHN, OM, and TK were significantly nonlinearly correlated with arthropod numbers, showing a threshold effect. AHN affects soil nitrogen supply capacity, directly impacting plant growth and health. Fluctuations in AHN can lead to complex ecological responses. Related studies generally found that arthropod diversity initially increased after short-term nitrogen treatment, then showed almost no further increase [
85,
86]. This may be due to the rise in short-term nitrogen content in leaves, which attracts exotic insects. However, the diversity of some species, such as
Cicadellidae [
87] and
Collembola [
88], showed a downward trend after nitrogen application. Öckinger et al. (2006) demonstrated that species numbers decreased after nitrogen application. Still, nutrient-dependent species tended to increase, while nutrient-scarce-dependent species decreased, indicating that atmospheric nitrogen deposition negatively impacts species composition [
89]. The effects of TK on plants are complex and may vary at different concentrations, reflected in the nonlinear relationship between arthropods and soil factors [
90]. OM is a vital soil fertility indicator, affecting soil structure and nutrient supply. The nonlinear relationship of its effects on arthropods may reflect complex ecological feedback mechanisms, with fluctuating organic matter promoting soil health and plant growth and fluctuating arthropod populations [
91].
Combining the Mantel Test and GAM analysis provides a more comprehensive understanding of the complex relationship between soil factors and arthropod or insect populations. The Mantel Test’s failure to detect significant linear correlations suggests these relationships may not be linear. GAM analysis revealed the complex linear and nonlinear effects of soil factors on arthropod populations, reflecting the cascading effects of soil factors on arthropods.
5. Conclusions
1. Compared to the intact desert steppe (control areas NG and STZ), the manually intervened gravel-sand mulching watermelon plots (GPS-1Y, GPS-5Y, GPS-10Y, and GPS-21Y) maintained the physical and chemical properties of the soil. However, soil nutrients decreased in the gravel-sand mulching watermelon plots with different cultivation years (TN, APP, AHN, APK, and OM). At the same time, salinity and alkalinization (EC and pH) increased with the increase in watermelon planting years.
2. The arthropod diversity in the manually intervened gravel-sand mulching fields was significantly lower compared to the intact desert steppe, and it decreased year by year with the increase in planting years. Continuous cultivation for 21 years (GPS-21Y) showed the lowest arthropod diversity, characterized by a single dominant species and the most unstable arthropod community.
3. Through matrix linear analysis and the Mantel test, it was found that the response of steppe arthropods to the soil physicochemical properties in the gravel and mulching watermelon fields was insignificant. Additionally, the correlation between soil factors in the manually intervened gravel-sand mulching watermelon plots varied, with both positive and negative correlations observed—the most significant and numerous correlations involved AHN, APP, and OM.
4. The GAM analysis indicated that the number of arthropods in the manually intervened gravel-sand mulching plots was significantly and linearly correlated with TN, APK, and W, positively correlated with TN, and negatively correlated with APK or W. The occurrence of arthropods was significantly nonlinearly correlated with TK, AHN, and OM, with the response to changes in OM being the most sensitive.
Author Contributions
S.W. and L.B. conceived and designed research. H.Z., Z.C., W.S., Y.W. and R.Z. conducted the experiments; H.Z. and S.W. wrote the manuscript. All authors read and approved the manuscript.
Funding
This work was supported by the National Natural Science Foundations of China (32160344), National Key R & D Program of China (2022YFD1401104), the Ningxia Province Sci-Tech Innovation Demonstration Program of High-Quality Agricultural Development and Ecological Conservation (NGSB-2021-14-05) and the National Science & Technology Fundamental Resources Investigation Program of China (2019FY100403).
Data Availability Statement
Data will be made available on request.
Acknowledgments
Thanks to the local farmers for allowing our team to do research in their fields. We sincerely thank anonymous reviewers for helpful suggestions on an earlier draft of this manuscript. We also express gratitude to the editors for their guidance and support throughout the publication process.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Flores-Gutierrez, A.M.; Mora, F.; Avila-Cabadilla, L.D.; Boege, K.; del-Val, E. Assessing the Cascading Effects of Management and Landscape on the Arthropod Guilds Occurring in Papaya Plantations. Agriculture, Ecosystems & Environment 2020, 293, 106836. [Google Scholar] [CrossRef]
- Oerke, E.-C. Crop Losses to Pests. The Journal of Agricultural Science 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Isaacs, R.; Tuell, J.; Fiedler, A.; Gardiner, M.; Landis, D. Maximizing Arthropod-Mediated Ecosystem Services in Agricultural Landscapes: The Role of Native Plants. Frontiers in Ecology and the Environment 2009, 7, 196–203. [Google Scholar] [CrossRef]
- Zhang, W.; Ricketts, T.H.; Kremen, C.; Carney, K.; Swinton, S.M. Ecosystem Services and Dis-Services to Agriculture. Ecological Economics 2007, 64, 253–260. [Google Scholar] [CrossRef]
- Shi, X.; Ma, C.; Gustave, W.; Orr, M.; Sritongchuay, T.; Yuan, Z.; Wang, M.; Zhang, X.; Zhou, Q.; Huang, Y.; et al. Effects of Arsenic and Selenium Pollution on Wild Bee Communities in the Agricultural Landscapes. Science of The Total Environment 2024, 907, 168052. [Google Scholar] [CrossRef] [PubMed]
- Pirk, C.W.W.; Crewe, R.M.; Moritz, R.F.A. Risks and Benefits of the Biological Interface between Managed and Wild Bee Pollinators. Functional Ecology 2017, 31, 47–55. [Google Scholar] [CrossRef]
- Liu, R.; Chen, D.; Luo, S.; Xu, S.; Xu, H.; Shi, X.; Zou, Y. Quantifying Pollination Efficiency of Flower-Visiting Insects and Its Application in Estimating Pollination Services for Common Buckwheat. Agriculture, Ecosystems & Environment 2020, 301, 107011. [Google Scholar] [CrossRef]
- Menta, C.; Remelli, S. Soil Health and Arthropods: From Complex System to Worthwhile Investigation. Insects 2020, 11, 54. [Google Scholar] [CrossRef]
- Baloglu, M.C.; Ulu, F.; Altunoglu, Y.C.; Pekol, S.; Alagoz, G.; Ese, O. Identification, Molecular Characterization and Expression Analysis of RPL24 Genes in Three Cucurbitaceae Family Members: Cucumber, Melon and Watermelon. Biotechnology & Biotechnological Equipment 2015, 29, 1024–1034. [Google Scholar] [CrossRef]
- Wijesinghe, S.A.E.C.; Evans, L.J.; Kirkland, L.; Rader, R. A Global Review of Watermelon Pollination Biology and Ecology: The Increasing Importance of Seedless Cultivars. Scientia Horticulturae 2020, 271, 109493. [Google Scholar] [CrossRef]
- Fehér, T. 21 - Watermelon: Citrullus Lanatus (Thunb.) Matsum. & Nakai. In Genetic Improvement of Vegetable Crops; Kalloo, G., Bergh, B.O., Eds.; Pergamon: Amsterdam, 1993; ISBN 978-0-08-040826-2. [Google Scholar]
- Adeoye, I.B.; Olajide-Taiwo, F.B.; Adebisi-Adelani, O.; Usman, J.M.; Badmus, M.A. Economic Analysis of Watermelon Based Production System in Oyo State Nigeria. 2011.
- Zhou, W.; Zhou, X.; Cai, L.; Jiang, Q.; Zhang, R. Temporal and Habitat Dynamics of Soil Fungal Diversity in Gravel-Sand Mulching Watermelon Fields in the Semi-Arid Loess Plateau of China. Microbiology Spectrum 2023, 11, e03150–22. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xie, Z.; Malhi, S.S.; Vera, C.L.; Zhang, Y.; Guo, Z. Effects of Gravel–Sand Mulch, Plastic Mulch and Ridge and Furrow Rainfall Harvesting System Combinations on Water Use Efficiency, Soil Temperature and Watermelon Yield in a Semi-Arid Loess Plateau of Northwestern China. Agricultural Water Management 2011, 101, 88–92. [Google Scholar] [CrossRef]
- Hao, H.; Zhao, X.; Wang, Y.; Zhang, Y.; Xie, Z.; Guo, Z.; Wang, R. Effects of Gravel-Sand Mulching on Soil Bacterial Community and Metabolic Capability in the Semi-Arid Loess Plateau, China. World J Microbiol Biotechnol 2017, 33, 209. [Google Scholar] [CrossRef]
- Qiu, Y.; Wang, Y.; Xie, Z. Long-Term Gravel–Sand Mulch Affects Soil Physicochemical Properties, Microbial Biomass and Enzyme Activities in the Semi-Arid Loess Plateau of North-Western China. Acta Agriculturae Scandinavica, Section B — Soil & Plant Science 2014, 64, 294–303. [Google Scholar] [CrossRef]
- Li, X.-Y. Effects of Gravel and Sand Mulches on Dew Deposition in the Semiarid Region of China. Journal of Hydrology 2002, 260, 151–160. [Google Scholar] [CrossRef]
- Adams, J.E. Influence of Mulches on Runoff, Erosion, and Soil Moisture Depletion. Soil Science Soc of Amer J 1966, 30, 110–114. [Google Scholar] [CrossRef]
- Lamb, J.; Chapman, J.E. Effect of Surface Stones on Erosion, Evaporation, Soil Temperature, and Soil Moisture. 1943.
- Nachtergaele, J.; Poesen, J.; van Wesemael, B. Gravel Mulching in Vineyards of Southern Switzerland. Soil and Tillage Research 1998, 46, 51–59. [Google Scholar] [CrossRef]
- Li, X.-Y.; Gong, J.-D.; Wei, X.-H. In-Situ Rainwater Harvesting and Gravel Mulch Combination for Corn Production in the Dry Semi-Arid Region of China. Journal of Arid Environments 2000, 46, 371–382. [Google Scholar] [CrossRef]
- Wookey, P.A.; Aerts, R.; Bardgett, R.D.; Baptist, F.; Bråthen, K.A.; Cornelissen, J.H.C.; Gough, L.; Hartley, I.P.; Hopkins, D.W.; Lavorel, S.; et al. Ecosystem Feedbacks and Cascade Processes: Understanding Their Role in the Responses of Arctic and Alpine Ecosystems to Environmental Change. Global Change Biology 2009, 15, 1153–1172. [Google Scholar] [CrossRef]
- Wardle, D.A.; Bardgett, R.D.; Klironomos, J.N.; Setälä, H.; Van Der Putten, W.H.; Wall, D.H. Ecological Linkages Between Aboveground and Belowground Biota. Science 2004, 304, 1629–1633. [Google Scholar] [CrossRef]
- Clark, C.M.; Tilman, D. Loss of Plant Species after Chronic Low-Level Nitrogen Deposition to Prairie Grasslands. Nature 2008, 451, 712–715. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Dong, S.; DiTommaso, A.; Xiao, J.; Lu, W.; Zhi, Y. Nitrogen Deposition Shifts Grassland Communities Through Directly Increasing Dominance of Graminoids: A 3-Year Case Study From the Qinghai-Tibetan Plateau. Front. Plant Sci. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Song, M.-H.; Zheng, L.-L.; Yin, T.-F.; Zhang, X.-Z.; Yu, F.-H.; Cornelissen, J.H.C. Changes in Quantity Rather than Palatability of Alpine Meadow Species Induce Cascading Effects of Long-Term Nitrogen Fertilization on Phytophagous Insect Abundance. Journal of Vegetation Science 2018, 29, 867–876. [Google Scholar] [CrossRef]
- Reihart, R.W.; Angelos, K.P.; Gawkins, K.M.; Hurst, S.E.; Montelongo, D.C.; Laws, A.N.; Pennings, S.C.; Prather, C.M. Crazy Ants Craving Calcium: Macronutrients and Micronutrients Can Limit and Stress an Invaded Grassland Brown Food Web. Ecology 2021, 102, e03263. [Google Scholar] [CrossRef]
- Joern, A.; Behmer, S.T. Impact of Diet Quality on Demographic Attributes in Adult Grasshoppers and the Nitrogen Limitation Hypothesis. Ecological Entomology 1998, 23, 174–184. [Google Scholar] [CrossRef]
- Wang, L.; Cui, H.; Chang, X.; Zhu, M.; Zhao, Z. Increased Nitrogen Fertilization Inhibits the Biocontrol Activity Promoted by the Intercropping Partner Plant. Insect Science 2021, 28, 1179–1190. [Google Scholar] [CrossRef]
- Fengrui, L.; Songling, Z.; Geballe, G.T. Water Use Patterns and Agronomic Performance for Some Cropping Systems with and without Fallow Crops in a Semi-Arid Environment of Northwest China. Agriculture, Ecosystems & Environment 2000, 79, 129–142. [Google Scholar] [CrossRef]
- Stanturf, J.A. Chapter 5 - Landscape Degradation and Restoration. In Soils and Landscape Restoration; Stanturf, J.A., Callaham, M.A., Eds.; Academic Press, 2021; pp. 125–159 ISBN 978-0-12-813193-0.
- Wang, T.; Ren, W.; Yang, F.; Niu, L.; Li, Z.; Zhang, M. Effects of Different Tillage and Residue Retention Measures on Silage Maize Yield and Quality and Soil Phosphorus in Karst Areas. Agronomy 2023, 13, 2306. [Google Scholar] [CrossRef]
- Wu, Y.; Yu, Y. Soil Carbon, Nitrogen and Phosphorus Fractions and Response to Microorganisms and Mineral Elements in Zanthoxylum Planispinum ‘Dintanensis’ Plantations at Different Altitudes. Agronomy 2023, 13, 558. [Google Scholar] [CrossRef]
- Wei, S.; Huang, W.; Zhu, M.; Gao, L.; Wang, Y.; Zhang, R.; Li, Z.; Zhao, Z. The Asymmetric Responses of Carabid Beetles to Steppe Fragmentation in Northwest China. Global Ecology and Conservation 2020, 23, e01058. [Google Scholar] [CrossRef]
- MacDonald, Z.G.; Nielsen, S.E.; Acorn, J.H. Negative Relationships between Species Richness and Evenness Render Common Diversity Indices Inadequate for Assessing Long-Term Trends in Butterfly Diversity. Biodivers Conserv 2017, 26, 617–629. [Google Scholar] [CrossRef]
- Zhao, **g**g; Gong, L. ; Chen, **n Relationship between Ecological Stoichiometry and Plant Community Diversity in the Upper Reaches of Tarim River, Northwestern China. J. Arid Land 2020, 12, 227–238. [Google Scholar] [CrossRef]
- Lehmann, A.; Overton, J.M.; Leathwick, J.R. GRASP: Generalized Regression Analysis and Spatial Prediction. Ecological Modelling 2002, 157, 189–207. [Google Scholar] [CrossRef]
- Gomez-Rubio, V. Generalized Additive Models: An Introduction with R. Journal of Statistical Software 2018, 86, 1–5. [Google Scholar] [CrossRef]
- Liu, X.; Wang, **npu; Bai, M.; Shaw, J.J. Decrease in Carabid Beetles in Grasslands of Northwestern China: Further Evidence of Insect Biodiversity Loss. Insects 2022, 13, 35. [Google Scholar] [CrossRef]
- Fromont, C.; Riegler, M.; Cook, J.M. Relative Abundance and Strain Diversity in the Bacterial Endosymbiont Community of a Sap-Feeding Insect Across Its Native and Introduced Geographic Range. Microb Ecol 2017, 74, 722–734. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Lu, M.; Xu, B.; Chen, H.; Li, J.; Zhu, Z.; Yu, M.; Zheng, J.; Peng, P.; Wu, S. Multiple Perspectives Reveal the Gut Toxicity of Polystyrene Microplastics on Eisenia Fetida: Insights into Community Signatures of Gut Bacteria and Their Translocation. Science of The Total Environment 2022, 838, 156352. [Google Scholar] [CrossRef]
- Grunsky, E.C. R: A Data Analysis and Statistical Programming Environment–an Emerging Tool for the Geosciences. Computers & Geosciences 2002, 28, 1219–1222. [Google Scholar] [CrossRef]
- Cui, X.; Wang, J.; Zhang, L.; Chen, T.; Zhuge, Y.; Dong, Y. Soil Nutrient Retention and Yield Effect of Nitrogen, Phosphorus Synergists on Wheat/Maize Rotation in Brown Soil. Agronomy 2022, 12, 2445. [Google Scholar] [CrossRef]
- Zheng, X.; Wei, L.; Lv, W.; Zhang, H.; Zhang, Y.; Zhang, H.; Zhang, H.; Zhu, Z.; Ge, T.; Zhang, W. Long-Term Bioorganic and Organic Fertilization Improved Soil Quality and Multifunctionality under Continuous Cropping in Watermelon. Agriculture, Ecosystems & Environment 2024, 359, 108721. [Google Scholar] [CrossRef]
- Weyers, S.L.; Archer, D.W.; Johnson, J.M.F.; Wilts, A.R. Management Drives Differences in Nutrient Dynamics in Conventional and Organic Four-Year Crop Rotation Systems. Agronomy 2020, 10, 764. [Google Scholar] [CrossRef]
- Rodríguez-Berbel, N.; Soria, R.; Villafuerte, A.B.; Ortega, R.; Miralles, I. Short-Term Dynamics of Bacterial Community Structure in Restored Abandoned Agricultural Soils under Semi-Arid Conditions. Agronomy 2023, 13, 86. [Google Scholar] [CrossRef]
- Belay, A.; Claassens, A.; Wehner, F. Effect of Direct Nitrogen and Potassium and Residual Phosphorus Fertilizers on Soil Chemical Properties, Microbial Components and Maize Yield under Long-Term Crop Rotation. Biol Fertil Soils 2002, 35, 420–427. [Google Scholar] [CrossRef]
- Crews, T.E.; Brookes, P.C. Changes in Soil Phosphorus Forms through Time in Perennial versus Annual Agroecosystems. Agriculture, Ecosystems & Environment 2014, 184, 168–181. [Google Scholar] [CrossRef]
- Assmann, J.M.; Martins, A.P.; Anghinoni, I.; de Oliveira Denardin, L.G.; de Holanda Nichel, G.; de Andrade Costa, S.E.V.G.; Pereira e Silva, R.A.; Balerini, F.; de Faccio Carvalho, P.C.; Franzluebbers, A.J. Phosphorus and Potassium Cycling in a Long-Term No-till Integrated Soybean-Beef Cattle Production System under Different Grazing Intensities Insubtropics. Nutr Cycl Agroecosyst 2017, 108, 21–33. [Google Scholar] [CrossRef]
- Riggs, C.E.; Hobbie, S.E. Mechanisms Driving the Soil Organic Matter Decomposition Response to Nitrogen Enrichment in Grassland Soils. Soil Biology and Biochemistry 2016, 99, 54–65. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Mirabelli, C.; Cardarelli, M. Nitrogen-use Efficiency Traits of Mini-watermelon in Response to Grafting and Nitrogen-fertilization Doses. Z. Pflanzenernähr. Bodenk. 2011, 174, 933–941. [Google Scholar] [CrossRef]
- Xu, S.; Li, **; Sayer, E.J.; Zhang, B.; Wang, **g; Qiao, C.; Peng, Z.; Diao, L.; Chi, Y.; Liu, W.; et al. Initial Soil Organic Matter Content Influences the Storage and Turnover of Litter, Root and Soil Carbon in Grasslands. Ecosystems 2018, 21, 1377–1389. [Google Scholar] [CrossRef]
- Chen, B.; Yang, H.; Song, W.; Liu, C.; Xu, J.; Zhao, W.; Zhou, Z. Effect of N Fertilization Rate on Soil Alkali-Hydrolyzable N, Subtending Leaf N Concentration, Fiber Yield, and Quality of Cotton. The Crop Journal 2016, 4, 323–330. [Google Scholar] [CrossRef]
- Xu, C.; Chen, Y.; Zang, Q.; Li, Y.; Zhao, J.; Lu, X.; Jiang, M.; Zhuang, H.; Huang, L. The Effects of Cultivation Patterns and Nitrogen Levels on Fertility and Bacterial Community Characteristics of Surface and Subsurface Soil. Front. Microbiol. 2023, 14. [Google Scholar] [CrossRef]
- Bai, Z.; Caspari, T.; Gonzalez, M.R.; Batjes, N.H.; Mäder, P.; Bünemann, E.K.; de Goede, R.; Brussaard, L.; Xu, M.; Ferreira, C.S.S.; et al. Effects of Agricultural Management Practices on Soil Quality: A Review of Long-Term Experiments for Europe and China. Agriculture, Ecosystems & Environment 2018, 265, 1–7. [Google Scholar] [CrossRef]
- Kudrevatykh, I.Y.; Kalinin, P.I.; Mitenko, G.V. The Effect of Changes Vegetation Cover on the Chemical Properties of Steppe Soils during Climate Aridization. Plant Soil 2023, 491, 265–284. [Google Scholar] [CrossRef]
- Yan, F.; Zhang, F.; Fan, J.; Hou, X.; Bai, W.; Liu, X.; Wang, Y.; Pan, X. Optimization of Irrigation and Nitrogen Fertilization Increases Ash Salt Accumulation and Ions Absorption of Drip-Fertigated Sugar Beet in Saline-Alkali Soils. Field Crops Research 2021, 271, 108247. [Google Scholar] [CrossRef]
- Chen, H.; Ren, A.; Hu, Z.; Jia, C.; Wang, J. Effects of Irrigation and Fertilization on Soil Salt Migration, Yield, and Water Use Efficiency of Winter Wheat in the Yellow River Delta. Crop Science 2022, 62, 1584–1602. [Google Scholar] [CrossRef]
- van Wesemael, B.; Chartin, C.; Wiesmeier, M.; von Lützow, M.; Hobley, E.; Carnol, M.; Krüger, I.; Campion, M.; Roisin, C.; Hennart, S.; et al. An Indicator for Organic Matter Dynamics in Temperate Agricultural Soils. Agriculture, Ecosystems & Environment 2019, 274, 62–75. [Google Scholar] [CrossRef]
- Peñuelas, J.; Poulter, B.; Sardans, J.; Ciais, P.; van der Velde, M.; Bopp, L.; Boucher, O.; Godderis, Y.; Hinsinger, P.; Llusia, J.; et al. Human-Induced Nitrogen–Phosphorus Imbalances Alter Natural and Managed Ecosystems across the Globe. Nat Commun 2013, 4, 2934. [Google Scholar] [CrossRef] [PubMed]
- Simpson, M.; McLenaghen, R.D.; Chirino-Valle, I.; Condron, L.M. Effects of Long-Term Grassland Management on the Chemical Nature and Bioavailability of Soil Phosphorus. Biol Fertil Soils 2012, 48, 607–611. [Google Scholar] [CrossRef]
- Han, J.; **e, J.; Zhang, Y. Potential Role of Feldspathic Sandstone as a Natural Water Retaining Agent in Mu Us Sandy Land, Northwest China. Chin. Geogr. Sci. 2012, 22, 550–555. [Google Scholar] [CrossRef]
- Uchida, K.; Takahashi, S.; Shinohara, T.; Ushimaru, A. Threatened Herbivorous Insects Maintained by Long-Term Traditional Management Practices in Semi-Natural Grasslands. Agriculture, Ecosystems & Environment 2016, 221, 156–162. [Google Scholar] [CrossRef]
- Parker, M.; Mac Nally, R. Habitat Loss and the Habitat Fragmentation Threshold: An Experimental Evaluation of Impacts on Richness and Total Abundances Using Grassland Invertebrates. Biological Conservation 2002, 105, 217–229. [Google Scholar] [CrossRef]
- Cloudsley-Thompson, J. Thermal and Water Relations of Desert Beetles. Naturwissenschaften 2001, 88, 447–460. [Google Scholar] [CrossRef] [PubMed]
- Rivnay, E. The Influence of Man on Insect Ecology in Arid Zones. Annu. Rev. Entomol. 1964, 9, 41–62. [Google Scholar] [CrossRef]
- Collinge, S.K. Effects of Grassland Fragmentation on Insect Species Loss, Colonization, and Movement Patterns. Ecology 2000, 81, 2211–2226. [Google Scholar] [CrossRef]
- Moebius-Clune, B.N.; van Es, H.M.; Idowu, O.J.; Schindelbeck, R.R.; Kimetu, J.M.; Ngoze, S.; Lehmann, J.; Kinyangi, J.M. Long-Term Soil Quality Degradation along a Cultivation Chronosequence in Western Kenya. Agriculture, Ecosystems & Environment 2011, 141, 86–99. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, Z.; Liu, P.; Zhou, L.; Tan, S.; Kuang, S. Arbuscular Mycorrhizal Fungi Diversity in Sophora Japonica Rhizosphere at Different Altitudes and Lithologies. Journal of Fungi 2024, 10, 340. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Nayak, A.K.; Thilagam, V.K.; Chatterjee, D.; Shahid, M.; Tripathi, R.; Mohanty, S.; Kumar, A.; Lal, B.; Gautam, P.; et al. Measuring Potassium Fractions Is Not Sufficient to Assess the Long-Term Impact of Fertilization and Manuring on Soil’s Potassium Supplying Capacity. J Soils Sediments 2018, 18, 1806–1820. [Google Scholar] [CrossRef]
- Baribault, T.W.; Kobe, R.K.; Finley, A.O. Tropical Tree Growth Is Correlated with Soil Phosphorus, Potassium, and Calcium, Though Not for Legumes. Ecological Monographs 2012, 82, 189–203. [Google Scholar] [CrossRef]
- Bhattacharyya, R.; Prakash, V.; Kundu, S.; Srivastva, A.K.; Gupta, H.S. Soil Properties and Their Relationships with Crop Productivity after 30 Years of Different Fertilization in the Indian Himalayas. Archives of Agronomy and Soil Science 2009, 55, 641–661. [Google Scholar] [CrossRef]
- Smith, O.L. An Analytical Model of the Decomposition of Soil Organic Matter. Soil Biology and Biochemistry 1979, 11, 585–606. [Google Scholar] [CrossRef]
- Turkington, R.; John, E.; Krebs, C.J.; Dale, M.R.T.; Nams, V.O.; Boonstra, R.; Boutin, S.; Martin, K.; Sinclair, A.R.E.; Smith, J.N.M. The Effects of NPK Fertilization for Nine Years on Boreal Forest Vegetation in Northwestern Canada. Journal of Vegetation Science 1998, 9, 333–346. [Google Scholar] [CrossRef]
- Cheng, X.; Zhou, T.; Liu, S.; Sun, **aobo; Zhou, Y.; Xu, L.; **e, B.; Ying, J.; Shi, Y. Effects of Climate on Variation of Soil Organic Carbon and Alkali-Hydrolyzed Nitrogen in Subtropical Forests: A Case Study of Zhejiang Province, China. Forests 2023, 14, 914. [Google Scholar] [CrossRef]
- Ondrasek, G.; Rengel, Z. Environmental Salinization Processes: Detection, Implications & Solutions. Science of The Total Environment 2021, 754, 142432. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, C.P.; Tripathi, S.K.; Singh, K.P. Soil Organic Matter and Water-Stable Aggregates under Different Tillage and Residue Conditions in a Tropical Dryland Agroecosystem. Applied Soil Ecology 2001, 16, 229–241. [Google Scholar] [CrossRef]
- Roth, N.; Hacker, H.H.; Heidrich, L.; Friess, N.; García-Barros, E.; Habel, J.C.; Thorn, S.; Müller, J. Host Specificity and Species Colouration Mediate the Regional Decline of Nocturnal Moths in Central European Forests. Ecography 2021, 44, 941–952. [Google Scholar] [CrossRef]
- Hoover, S.E.R.; Ladley, J.J.; Shchepetkina, A.A.; Tisch, M.; Gieseg, S.P.; Tylianakis, J.M. Warming, CO2, and Nitrogen Deposition Interactively Affect a Plant-Pollinator Mutualism. Ecology Letters 2012, 15, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Burkle, L.A.; Irwin, R.E. Beyond Biomass: Measuring the Effects of Community-level Nitrogen Enrichment on Floral Traits, Pollinator Visitation and Plant Reproduction. Journal of Ecology 2010, 98, 705–717. [Google Scholar] [CrossRef]
- Haddad, N.M.; Haarstad, J.; Tilman, D. The Effects of Long-Term Nitrogen Loading on Grassland Insect Communities. Oecologia 2000, 124, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Fenn, M.E.; Poth, M.A.; Aber, J.D.; Baron, J.S.; Bormann, B.T.; Johnson, D.W.; Lemly, A.D.; McNulty, S.G.; Ryan, D.F.; Stottlemyer, R. Nitrogen Excess in North American Ecosystems: Predisposing Factors, Ecosystem Responses, and Management Strategies. Ecological Applications 1998, 8, 706–733. [Google Scholar] [CrossRef]
- Buxton, P.A. Terrestrial Insects and the Humidity of the Environment. Biological Reviews 1932, 7, 275–320. [Google Scholar] [CrossRef]
- Crawford, R.M.M.; Braendle, R. Oxygen Deprivation Stress in a Changing Environment. Journal of Experimental Botany 1996, 47, 145–159. [Google Scholar] [CrossRef]
- Hurd, L.E.; Wolf, L.L. Stability in Relation to Nutrient Enrichment in Arthropod Consumers of Old-Field Successional Ecosystems. Ecological Monographs 1974, 44, 465–482. [Google Scholar] [CrossRef]
- Kirchner, T.B. The Effects of Resource Enrichment on the Diversity of Plants and Arthropods in a Shortgrass Prairie. Ecology 1977, 1334–1344. [Google Scholar]
- Prestidge, R.A. The Influence of Nitrogenous Fertilizer on the Grassland Auchenorrhyncha (Homoptera). Journal of Applied Ecology 1982, 19, 735–749. [Google Scholar] [CrossRef]
- Song, L.; Liu, J.; Yan, X.; Chang, L.; Wu, D. Euedaphic and Hemiedaphic Collembola Suffer Larger Damages than Epedaphic Species to Nitrogen Input. Environmental Pollution 2016, 208, 413–415. [Google Scholar] [CrossRef] [PubMed]
- Öckinger, E.; Hammarstedt, O.; Nilsson, S.G.; Smith, H.G. The Relationship between Local Extinctions of Grassland Butterflies and Increased Soil Nitrogen Levels. Biological Conservation 2006, 128, 564–573. [Google Scholar] [CrossRef]
- Li, T.; Wang, H.; Zhou, Z.; Chen, X.; Zhou, J. A New Grading System for Plant-Available Potassium Using Exhaustive Cropping Techniques Combined with Chemical Analyses of Soils. Sci Rep 2016, 6, 37327. [Google Scholar] [CrossRef]
- Oldfield, E.E.; Wood, S.A.; Bradford, M.A. Direct Evidence Using a Controlled Greenhouse Study for Threshold Effects of Soil Organic Matter on Crop Growth. Ecological Applications 2020, 30, e02073. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).