1. Introduction
Depression (DEP) is a common mental disorder which manifests with anhedonia, insomnia, low affect, feeling of guilt, cognitive and social dysfunction which affect patient’s quality of life.
Schizophrenia (SCH) is a debilitating chronic mental disorder characterized by positive and negative symptoms, prone to relapses and comorbidities, cognitive decline and therefore poor prognosis. Both SCH and DEP are often stigmatized disorders, disorders of socioeconomic significance and a major public health problem [
1,
2,
3] . A science-based approach is needed in finding a strategy for treatment-resistant forms managing of both disorders. [
4,
5]
These are some of the reasons why in the last decades many efforts have been directed towards finding more about their etiology, pathophysiology and last but not least - a biomarker [
6,
7,
8,
9,
10] eligible for diagnostic use.
The term “connectivity” includes structural, functional and effective connectivity. Structural connectivity refers to the anatomical connections in the brain, while functional connectivity is time dependent, focused on finding deviations of statistical independence of spatially remote events, for example, by measuring correlation. Effective connectivity is a process used to describe the causal influence one region of the brain exerts over other brain regions. [
11,
12,
13]
The Default Mode Network (DMN), Central Executive Network (CEN) and Salience Network (SN) are large-scale networks in the human brain. While DMN’s main role is in internally focused processes, active in low-demand tasks, CEN could be considered “the opposite” of that - it has an important role in high-level cognitive functions, attention and complex activities. The SN is the one regulating the DMN and CEN [
14,
15,
16]. Each network has its main nodes: DMN - Medial Prefrontal Cortex (mPFC), Posterior Cingulate (PCC), Precuneus, Angular Gyrus (AG), Hippocampal formation. CEN is composed of Dorsolateral Prefrontal Cortex (DLPFC) and Posterior Parietal Cortex (PPC). SN is composed of the Anterior Insula (AI), Anterior Cingulate Cortex (ACC) and nodes in the amygdala, hypothalamus, ventral striatum, thalamus and brainstem nuclei [
17] . There is growing evidence of these networks' role in the pathophysiology of mental disorders, which are studied mostly using fMRI.
Schizophrenia and depression are the two main spectra of mental disorders, making them suitable for our objective which would be to be able to differentiate them using translational cross-validation of clinical assessment tools and fMRI.
Emerging data on the importance of brain networks and connectivity in DEP is growing, focusing on four main networks – Default Mode Network (DMN) , Affective Network (AN) , The Reward Network (RN) and The Cognitive Control Network (CCN).[
18] The suggested Triple Network Model in DEP has been studied, showing aberrant network connectivity [
19,
20], suggesting that patients suffering from major depression have an abnormal regulation of the switching between the internally focused (DMN) and the externally focused (CEN) network.
There are reported controversial results on brain networks activity and SCH. Some studies revealed decreased DMN functional connectivity [
21,
22,
23] , while others showed hyperconnectivity in the DMN [
24,
25]. Aberrant activity in the SN and therefore disrupted interactions within and across the DMN and CEN have been demonstrated in SCH patients [
26,
27] highlighting their importance SCH’s pathophysiology.
1.1. Current Road to Psychiatric Diagnoses
Current diagnostic practices in many medical fields leverage a multi-biomarker approach, enabling objective assessments and definitive diagnoses. In contrast, the field of psychiatry currently lacks a robust inventory of objective biomarkers, [
27] hindering the development of a more precise and data-driven diagnostic approach. This discrepancy persists despite significant advancements in neuroscience and technology. Addressing this gap is crucial for the advancement of psychiatric diagnosis and the implementation of more targeted treatment strategies.
The most widely used diagnostic instruments in psychiatry today are the Diagnostic and Statistical Manual of Mental Disorders (DSM) and The International Classification of Diseases (ICM) [
28,
29], primarily rely on clinical symptom presentation for diagnosis. These systems do not currently integrate pathophysiological mechanisms or disease etiology into diagnostic criteria Since clinical assessment is based on questionnaires and their interpretation which is dependent on the clinician's judgement, experience and the patients state of mind, it’s difficult to make an objective decision on the correct diagnosis or treatment plan due to the above-mentioned reasons and the lack of specific biomarkers. Due to the aforementioned mental diseases complexity, a nomothetic approach, consisting of psychological, neurobiological and psychopathological components is needed. [
3]
1.2. Translational Cross Validation of Task-Related fMRI and PDS: A Novel Approach
Task-related fMRI (tr-fMRI) registers signals from different brain regions by measuring blood oxygen level dependent (BOLD) changes in response to cognitive activities. In most studies these activities are diagnostically irrelevant and cannot be incorporated into the diagnostic approach. In such cases clinical assessment is done before and after imaging, creating a temporal gap which could result in a difference in emotional states. There are some limited efforts reported in the past to incorporate first-person introspective narratives into fMRI paradigm [
30,
31].
In a novel paradigm (Stoyanov et al.) real time ratings of patients are performed, using self-rating scales while simultaneously obtaining fMRI data. [
32]. Von Zerssen Paranoid-Depressive Scale was chosen in order to contrast different nosological groups. [
33,
34,
35,
36,
37]
The main challenge in the field of translational neuroimaging is twofold. On one hand the resting state connectivity findings are more consistent and stable across studied populations [
38], whereas the task-based fMRI methods and findings are very heterogenous and diverse. Those often include tasks and tests, specifically designed in laboratory control settings and have limited translation to clinical practice. On the other hand the use of functional MRI to measure resting state connectivity disturbances in each and every patient is an economic effort with inappropriate cost to benefit ratio. One possible resolution would be to test the underlying neural circuits with clinically relevant tasks and then to use them in clinical practice as externally validated assessment tools.
The aim of this scoping review is to gather and analyze existing data on the application of translational cross-validation using self-assessment scales in fMRI and effective connectivity, to assess the results of this convergent approach, to find whether specific or common neural circuits exist, which those circuits are and therefore to contribute to the long-standing debate in psychiatry.
2. Materials and Methods
2.1. Methods
The following bibliographic databases were searched from 2003 to May 2024: PubMed and Scopus. The electronic database search was supplemented by scanning relevant reviews.
The electronic database search was conducted in accordance with the PRISMA extension for Scoping Review (PRISMA-ScR) checklist.
2.2. Eligibility Criteria
In this scoping review we included original studies focused on translational cross-validation of clinical assessment scales and fMRI. For this purpose, two patient groups were chosen: depression and schizophrenia patients, all of which were adults.
Inclusion criteria: (1) Articles related to task-based fMRI or effective connectivity in depression and schizophrenia. (2) Articles published in the last 20 years (2004-2024). (3) Articles published in English or translated to English. (4) Articles published in scientific journals. (5) Articles including patient groups (depression and/or schizophrenia). (6) Articles including adult population. (7) Articles that are complete. (8) Original studies.
Exclusion criteria: (1) Articles that are not related to task-based fMRI or effective connecitivity in depression and schizophrenia. (2) Articles published more than 20 years ago. (3) Articles published in languages different than English or not translated to English. (4) Articles published in non-scientific journals. (5) Articles not including patient groups with depression and/or schizophrenia. (6) Articles including non-adult population. (7) Articles that are incomplete. (8) Systematic reviews
3. Results
3.1. Search Strategy
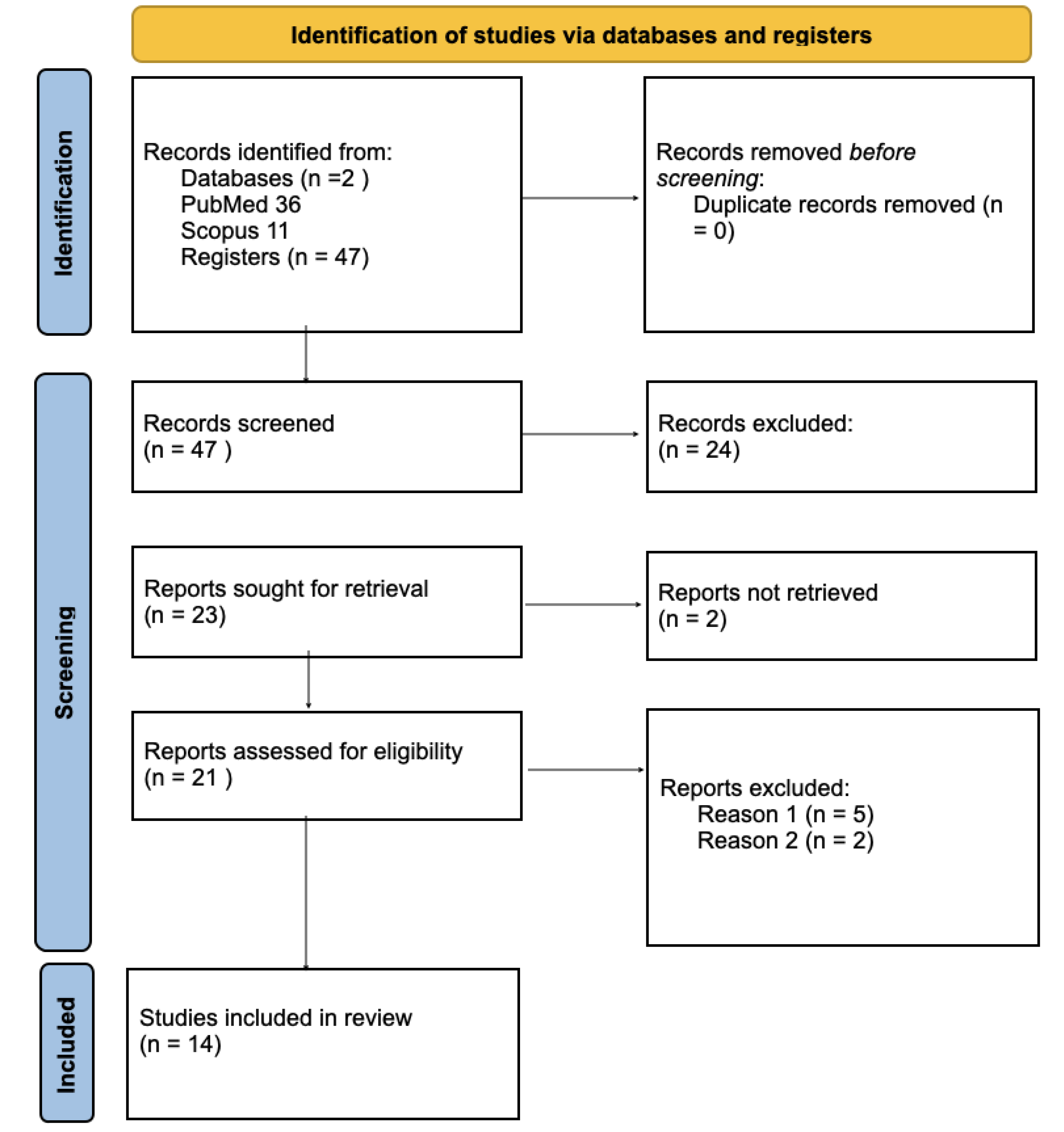
A scoping literature review was conducted in compliance with the inclusion and exclusion criteria in the electronic databases PubMed and Scopus. A total of 47 studies were identified.The following terms were used: independent component, psychiatric scale, paranoia scale, self-assessment scale, paranoid-depressive scale, clinical scale, task-based fMRI, effective connectivity, aberrant connectivity, depression and schizophrenia. The terms were identified from titles or abstracts of articles using Boolean operator to achieve accurate results. Search results abstracts were screened by two reviewers. In case of a disagreement the reviewers had a thorough discussion and drew a conclusion. Full text screening followed: 14 studies were included in this scoping review.
3.2. Figures, Tables and Schemes
Figure 1.
PRISMA 2020 flow diagram for new systematic reviews which included searches of databases and registers only.
Figure 1.
PRISMA 2020 flow diagram for new systematic reviews which included searches of databases and registers only.
Reason (1): Articles that are not related to task-based fMRI in depression and schizophrenia
Reason (2): Articles that are not related to effective connectivity in depression and schizophrenia
Reason (3): Articles not including patient groups with depression and/or schizophrenia.
3.4. Publication Year Distribution
Most of the studies were published in the last 10 years (n=11). Only 1 paper was published earlier than 2013. The interest in this topic is growing, but research is scarce with only 2 or less publications a year since 2013.
3.4. Geographical Distribution of the Studies
Included studies have been conducted by researchers from Bulgaria – 43% of the articles (n=6), China (21%; n=3), 1 in California, 1 in Japan, 1 in Germany, 1 in France and 1 in Poland (each is 7%; n=1)
3.5. Findings
See
Table S1. In Supplementary materials.
4. Discussion
In this review, we analyzed studies focused on finding a data driven approach in the search for a valid tool which could be used to help differentiate and build a more accurate classification of psychiatric disorders. The proposed paradigm consists of cross-validation of clinical self-assessment scales and functional MRI performed concurrently. The established correspondence between brain signatures and clinical evaluation tests can be considered crucial in the evolution of neuropsychiatric research and its potential clinical applications.
On another note, we discuss the results of rs-fMRI studies in patient populations and their potential role in the explanation of their ethiopathogenesis.
We discuss the findings of 14 articles on the application of the aforementioned model and the use of fMRI in DEP and SCH. Although they’re not many, the articles on the topic could be planting a seed in the evolution of novel neuropsychiatric methods.
4.1. Schizophrenia
The results were variable with significant activations of right angular gyrus, left posterior cingulate and precuneus, right transverse temporal gyrus [
36], the right superior parietal lobule, angular gyrus, planum temporale and thalamus [
16]. Before commenting on these results we should add to this discussion the ICA finding of an association of paranoid-specific stimuli with frontal brain areas – disturbed sensory-motor networks and DLPFC, VLPFC and OFC [
39], while a resting state fMRI ICA indicated decreased positive network FC between both sides of the FPN and the language network and a decrease in negative network FC between the right FPN and the DAN [
40].
Furthermore, IPL and DLPFC together with Planum Temporale (PT) and Broca’s area are a part of the heteromodal association cortex (HAC). HAC has an important role in higher cognitive functions (attention, language, memory, conscious thought) and its impairment could explain the occurrence of SCH symptoms as disorganized thoughts, auditory hallucinations, cognitive deficits, psychomotor poverty and other typical symptoms. It has been hypothesized that the dysfunction specifically in IPL in SCH would cause a disruption of its functions-attention, language and social cognition, while the abnormality of planum temporale could be responsible for language disorders, auditory hallucinations, reality-distortion syndrome, disorganization syndrome [
41]. Few studies have found a decrease in GMV in SCH in the HAC [
42,
43].
SCH patients demonstrated negative effective connectivity from the anterior precuneus (aPRC) to the lateral OFC, differentiating them from HC and MDD patients [
44]. In this article Kandilarova et al. Suggested that the inhibitory effect of the DMN on the OFC, OFC being a part of the reward processing system, can explain some of the negative symptoms in SCH – anhedonia, apathy, avolition and social withdrawal [
44,
45].
One search result also reports data on reduced FC within DMN (in the right anterior paracingulate cortex) and SN (in the striatum) in SCH, supported by previous studies with similar findings [
22,
46,
47,
48,
49], as some of them suggest possible explanations for the clinical manifestation of schizophrenia (negative, positive symptoms, altered cognition).
SCH patients were differentiated from HC using AI → AMY and AMY → SPL connections [
50] and another study showed SN dysfunction (reduced SN connectivity in the pallidum) in schizophrenia patients causing depressed moods which disrupt patients' quality of life [
51]. All these findings sway us into focusing on the Salience Network (SN) and its dysconnectivity in the schizophrenia. SN’s role as a dynamic switch between the DMN and CEN is key in understanding the connection between fMRI findings and clinical manifestation. That goes on to say that a disrupted SN (ex. AI, dACC) connectivity would lead to abnormal activity of the other two networks, hence causing relevant symptoms. Stoyanov et al. [
16] suggested that there is a common neural network in MDD and SCH that is disrupted, and the clinical syndrome depends on the direction of inhibition-in SCH that would be from the DLPFC to the AI, reflecting in paranoid symptoms.
Studies on the DMN have shown variable results, but there is consistent evidence of its disrupted function in SCH [
52,
53], moreover-data from different studies over the years has shown dysfunctional activation patterns in the DMN, which are consistent with our findings, with a tendency of hyperactivation during rest and reduced deactivation during tasks [
16,
54,
55]. This emphasizes the significance of task-performing fMRI, especially when the conducted task is related to the process of clinical diagnosis for ex. via clinical scales, with the idea of activating, or, we could say “to provoke” the engaged in the diagnostic process regions, and compare the results of both methods, which if consistent, create a combination of subjective (clinical interview) and objective(neuroimaging) data, helping the clinician to establish a more accurate diagnosis.
In support of these results on the importance of the discussed networks in schizophrenia is data from another article from our search, reporting reduced FC within DMN (in the right anterior paracingulate cortex) and SN (in the striatum). [
46].
The findings regarding the amygdala (AMY) should not be ignored, since it's role in mental disorders has been an ongoing topic in neuropsychiatric research. In SCH, there is additional data showing functional abnormalities as decrease in activation and altered connectivity [
56,
57]. These changes result in symptomatic manifestations: emotional dysregulation, blunted affect and social withdrawal.
A study on high and low suicide risk SCH patients found hyperconnectivity from PCC to MPFC and hypoconnectivity from MPFC to PCC (which only existed in HSR group) compared to HC [
58]. This could be linked with the occurrence of executive function deficits, ideas of reference and cognitive bias and their relation to higher suicide risk in SCH patients.
One of our results showed that the combination of rs-fMRI with questions from clinical scales across different patient groups, including SCH, revealed information on symptom severity by measuring connectivity [
59]. They included anhedonia models which found elements of the reward circuit of Williams, multiple DM nodes, SN, cingulo-opercular task control, frontoparietal task control and visual networks.
Disruption in brain networks, especially the SN was a common finding in another study reporting of enhanced connectivity between the SN and the right Inferior Temporal Gyrus (ITG) and MTG, but negative FC between the SN and the left caudate and hyperconnectivity between the SN and the right paracentral gyrus. [
58]. Since the ITG and MTG have been referred to as task-negative brain regions, their hyperconnectivity with the SN (task-positive) during resting fMRI is considered abnormal and is not found in HC. The caudate is involved in salience processing, therefore we would expect positive FC to the SN, which was not the case in SCH.
4.2. Depression
In depression, as in SCH, we found significant data related to brain networks and again-especially the ones constituting the triple-brain network model. A study performing task-based fMRI and rsfMRI uncovered that the connections involved in DEP are mainly the AI, OFC and HPC. Although no significant clusters of activations were found on the task-based fMRI in DEP, noteworthy is the reduced connectivity in rsfMRI from AI to the DLPFC [
14] , which corresponds with the results of other studies [
15], and yet another one of our results showing negative FC between the SN and right ITG and MTG [
60] , both performing resting-state fMRI. The recurrence of these regions in different studies [
14,
60,
61,
62] expose a possible connection between the etiopathophysiology of MDD and the SN. An article on anhedonia used models that also found nodes in the SN, DMN and cingula-opercular task control network (COTCN), FPN task control network and visual networks. Data on anhedonia is extremely valuable in DEP as it is one of its main symptoms [
59]. Although the SN was already discussed in this review, we should emphasize its role on emotional cognitive functions and social communication, which, when disrupted, cause symptoms seen in people suffering from this depressive disorder.
DLPFC is one of the regions constituting the CEN. The occurrence of negative FC between AI and DLPFC during fMRI supports the hypothesis that depressive symptoms are caused by dysfunction in the Triple Brain Network Model (TBNM). CEN’s functions are mainly goal-oriented behavior, cognitive and executive tasks and working memory, which are clearly impeded in DEP.This is supported by the already mentioned results from the study on MLM with findings of SN and FPN nodes in anhedonia models [
59].
Stoyanov et al. [
14] also reported self-inhibitory connection of the AG (main component of the DMN) - again giving plausible explanation for the clinical manifestation of depression.
One of the results, investigating the transdiagnostic effects of electroconvulsive therapy (ECT) on brain networks in schizophrenia (SCH) and major depressive disorder (MDD), found a correspondence between clinical improvement and ECT's impact on large-scale brain networks. While the study focused on therapeutic applications, it also highlights the potential role of these networks in the underlying mechanisms of symptom presentation in these mental illnesses [
63].
4.2. Differentiation of SCH and DEP Using fMRI and PDS
A part of our results discussed the differentiation between SCH and DEP using task-based fMRI. Key findings have been already mentioned and analyzed, but here we must highlight those relevant to distinguishing between the two mental disorders, using translational cross-validation.
By using translational methodology - the PDS and fMRI with ICA, the following results were obtained:
Positive pattern in the Parietal Cortex (PC), PRC, Inferior Occpital Cortex (IOC), Th (Thalamus), Inferior cingulate gyrus (ICG) and the Postcentral Gyrus (PG), corresponding to positive loading for Depression Scale (DS) and Diagnostic Neutral (DN) and negative loadings for the Paranoid Scale (PS) [
37]. Knowing the role of each of these regions in the human brain, it’s not difficult to connect these results with clinical manifestation. PC is responsible for initial processing, integrating and interpreting sensory information, visuospatial processing, spatial orientation and navigation. Here we also have located the AG and the PRC, which, as mentioned – are a major part of the DMN, whilst the Posterior Parietal Cortex (PCC) is a part of the CEN. These structures were discussed earlier. Most of the mentioned in this component elements are participating in vision, sensations and attention. These regions being positive in PS and DS could explain some of the symptoms in both disorders, but also be related to the Triple Network Model. The positive patterns found in the same study in C2 were corresponding to positive loading for DS and PS but not DN. The positive regions in this component were those participating in auditory functions: central operculum (auditory and language processing, motor control), superior temporal gyrus (integration of auditory information with other stimuli) , and left hippocampus (verbal and episodic memory). These results can explain auditory hallucinations in SCH (which could be seen in psychotic depression), difficulties in processing auditory stimuli and disrupted memory. The last component in this study was negative only for the DS – positive patterns were found in the lingual gyrus, PRC and insula – again corresponding to abnormal memory functions and also self-referential processing.
We mentioned the discovered activations in the right AG, left PRC, left PCC, right TTG [
35] and here we must add that those results were acquired in response to PS. Connecting these regions with cognitive functions (ex. social and conceptual processing), autobiographical memory processing – all of them leading us to a possible genesis of negative symptoms in SCH [
64,
65]. Dysfunctions in focal attention can be related to activations found in the AI [
66]. When discussing positive symptoms and more specifically the internal and external auditory hallucinations seen in SCH we can focus our attention on the activations found in Heschl’s gyrus for the PS – a region connected to efficient auditory and speech cues processing , as well as the Inner Voice (or the internal dialogue with oneself) [
67] . In MDD this article reported significant activations in the left MCG and right STG, regions considered important in the context of connectivity in MDD, their connections to the DMN or activity in the SN and these networks disruptions in DEP, all of which were commented on and thoroughly discussed previously in our review [
59,
66].
Both frontal motor/language and parietal regions are reported in both conditions [
39], their involvement ment in SCH and DEP was discussed in detail in our review and data from different sources confirm their importance. [
41,
69]. SCH was differentiated in one component, located in the PFC (DLPFC,VLPFC and OFC) - , while 2 components were modulated by the DS condition: PCC, PRC, Occipital ares, PHG, each of these areas has already been analyzed in the context of our topic earlier in the discussion.
5. Conclusions
Even a cursory analysis of our results can highlight that research on connectivity is the prevailing trend, whilst studies task-based approaches are deficient, but promising. The results of our review reveal significant correlations between activation patterns in functionally defined brain regions and their underlying functional connectivity. This convergence suggests a potential neural signature for psychiatric disorders. These findings provide a foundation for the development of a diagnostic model that leverages objective connectivity biomarkers, alongside traditional subjective assessments, for a more comprehensive approach to psychiatric diagnosis. Such a model, aligned with the objective biomarker approach prevalent in other medical fields, could facilitate the creation of a more robust classification system for nosological entities.
Our proposition to address the gap between classical psychiatric diagnostic methods and biological ones is by endorsing the research and future implementation of translational cross-validation of clinical assessment instruments and fMRI. To substantiate these findings and enhance generalizability, further investigations employing this model with amplified sample sizes are warranted. Additionally, rigorous replication efforts and standardized protocols are crucial to ensure robustness and facilitate cross-study comparisons.
6. Limitations
The study is focused on a very specific problem, pre-determined by critical methodological considerations and earlier advances in the field. This may have produced certain bias in literature selection. Moreover, literature in the field is limited, which made the authors include some titles which have less in common with the theme of the review than others. Furthermore, the heterogeneity of the data and the atlases used may have an influence on the results, leading to different regions being listed, while in fact, they are the same anatomically, confounding the outcome of the review.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Table S1: Table 1.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available upon reasonable request from the authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Greenberg, P.E.; Fournier, A.-A.; Sisitsky, T.; Simes, M.; Berman, R.; Koenigsberg, S.H.; Kessler, R.C. The Economic Burden of Adults with Major Depressive Disorder in the United States (2010 and 2018). PharmacoEconomics 2021, 39, 653–665. [Google Scholar] [CrossRef] [PubMed]
- Jacob, KS. Depression: a major public health problem in need of a multi-sectoral response. Indian J Med Res. 2012 Oct;136(4):537-9. PMCID: PMC3516018. [PubMed]
- Evans, M. . Schizophrenia as a Public Health Problem, in: Evans, M. (Ed.), Peer Support Services Reaching People with Schizophrenia: Considerations for Research and Practice. Springer Nature Switzerland, 2023, Cham, pp. 1–20. [CrossRef]
- Potkin, S.G.; Kane, J.M.; Correll, C.U.; Lindenmayer, J.-P.; Agid, O.; Marder, S.R.; Olfson, M.; Howes, O.D. The neurobiology of treatment-resistant schizophrenia: paths to antipsychotic resistance and a roadmap for future research. Schizophrenia 2020, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Voineskos, D.; Daskalakis, Z.J.; Blumberger, D.M. Management of Treatment-Resistant Depression: Challenges and Strategies. Neuropsychiatr. Dis. Treat. 2020, 16, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Stoyanov, D.; Khorev, V.; Paunova, R.; Kandilarova, S.; Kurkin, S.; Calhoun, V.D. Group independent components underpin responses to items from a depression scale. Acta Neuropsychiatr. 2023, 36, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Abi-Dargham, A.; Moeller, S.J.; Ali, F.; DeLorenzo, C.; Domschke, K.; Horga, G.; Jutla, A.; Kotov, R.; Paulus, M.P.; Rubio, J.M.; et al. Candidate biomarkers in psychiatric disorders: state of the field. World Psychiatry 2023, 22, 236–262. [Google Scholar] [CrossRef] [PubMed]
- García-Gutiérrez, M.S.; Navarrete, F.; Sala, F.; Gasparyan, A.; Austrich-Olivares, A.; Manzanares, J. Biomarkers in Psychiatry: Concept, Definition, Types and Relevance to the Clinical Reality. Front. Psychiatry 2020, 11, 432. [Google Scholar] [CrossRef] [PubMed]
- Glannon, W. Biomarkers in Psychiatric Disorders. Camb. Q. Heal. Ethic- 2022, 31, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, T. Biomarkers in psychiatry: a clinician's viewpoint. Br. Med Bull. 2020, 135, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Friston, K.J. Functional and Effective Connectivity: A Review. Brain Connect. 2011, 1, 13–36. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M. The future of FMRI connectivity. NeuroImage 2012, 62, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Sporns, O. Structure and function of complex brain networks. Dialog- Clin. Neurosci. 2013, 15, 247–262. [Google Scholar] [CrossRef] [PubMed]
- Heinonen, J.; Numminen, J.; Hlushchuk, Y.; Antell, H.; Taatila, V.; Suomala, J. Default Mode and Executive Networks Areas: Association with the Serial Order in Divergent Thinking. PLOS ONE 2016, 11, e0162234. [Google Scholar] [CrossRef] [PubMed]
- Kandilarova, S.; Stoyanov, D.; Kostianev, S.; Specht, K. Altered Resting State Effective Connectivity of Anterior Insula in Depression. Front. Psychiatry 2018, 9, 83. [Google Scholar] [CrossRef] [PubMed]
- Stoyanov, D.; Aryutova, K.; Kandilarova, S.; Paunova, R.; Arabadzhiev, Z.; Todeva-Radneva, A.; Kostianev, S.; Borgwardt, S. Diagnostic Task Specific Activations in Functional MRI and Aberrant Connectivity of Insula with Middle Frontal Gyrus Can Inform the Differential Diagnosis of Psychosis. Diagnostics 2021, 11, 95. [Google Scholar] [CrossRef] [PubMed]
- Seeley, W.W. The Salience Network: A Neural System for Perceiving and Responding to Homeostatic Demands. J. Neurosci. 2019, 39, 9878–9882. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Friston, K.; Mody, M.; Wang, H.; Lu, H.; Hu, D. A brain network model for depression: From symptom understanding to disease intervention. CNS Neurosci. Ther. 2018, 24, 1004–1019. [Google Scholar] [CrossRef] [PubMed]
- Duran, M.; Miller, C. Functional Connectivity of the Triple Network Model in Major Depressive Disorder: A Meta-Analysis. Biol. Psychiatry 2020, 87, S290. [Google Scholar] [CrossRef]
- Zheng, H.; Xu, L.; Xie, F.; Guo, X.; Zhang, J.; Yao, L.; Wu, X. The Altered Triple Networks Interaction in Depression under Resting State Based on Graph Theory. BioMed Res. Int. 2015, 2015, 386326. [Google Scholar] [CrossRef] [PubMed]
- Bluhm, R.L.; Miller, J.; Lanius, R.A.; Osuch, E.A.; Boksman, K.; Neufeld, R.; Theberge, J.; Schaefer, B.; Williamson, P. Spontaneous Low-Frequency Fluctuations in the BOLD Signal in Schizophrenic Patients: Anomalies in the Default Network. Schizophr. Bull. 2007, 33, 1004–1012. [Google Scholar] [CrossRef] [PubMed]
- Camchong, J.; MacDonald, A.W.; Bell, C.; Mueller, B.A.; Lim, K.O. Altered Functional and Anatomical Connectivity in Schizophrenia. Schizophr. Bull. 2011, 37, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zeng, L.-L.; Chen, Y.; Yin, H.; Tan, Q.; Hu, D. Evidence of a dissociation pattern in default mode subnetwork functional connectivity in schizophrenia. Sci. Rep. 2015, 5, 14655. [Google Scholar] [CrossRef] [PubMed]
- Mannell, M.V.; Franco, A.R.; Calhoun, V.D.; Cañive, J.M.; Thoma, R.J.; Mayer, A.R. Resting state and task-induced deactivation: A methodological comparison in patients with schizophrenia and healthy controls. Hum. Brain Mapp. 2009, 31, 424–437. [Google Scholar] [CrossRef] [PubMed]
- Salvador, R.; Sarró, S.; Gomar, J.J.; Ortiz-Gil, J.; Vila, F.; Capdevila, A.; Bullmore, E.; McKenna, P.J.; Pomarol-Clotet, E. Overall brain connectivity maps show cortico-subcortical abnormalities in schizophrenia. Hum. Brain Mapp. 2010, 31, 2003–2014. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Wang, Q.; Greenshaw, A.J.; Li, X.; Deng, W.; Ren, H.; Zhang, C.; Yu, H.; Wei, W.; Zhang, Y.; et al. Aberrant triple-network connectivity patterns discriminate biotypes of first-episode medication-naive schizophrenia in two large independent cohorts. Neuropsychopharmacology 2021, 46, 1502–1509. [Google Scholar] [CrossRef] [PubMed]
-
Alternative Perspectives on Psychiatric Validation; Oxford University Press (OUP): Oxford, Oxfordshire, United Kingdom, 2014.
- Xi, Y.-B.; Guo, F.; Liu, W.-M.; Fu, Y.-F.; Li, J.-M.; Wang, H.-N.; Chen, F.-L.; Cui, L.-B.; Zhu, Y.-Q.; Li, C.; et al. Triple network hypothesis-related disrupted connections in schizophrenia: A spectral dynamic causal modeling analysis with functional magnetic resonance imaging. Schizophr. Res. 2021, 233, 89–96. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders; American Psychiatric Association: Arlington, VA, USA, 2013. [Google Scholar] [CrossRef]
- Boer, J.A.D.; Reinders, A.S.; Glas, G. Special Section: On Looking Inward. Theory Psychol. 2008, 18, 380–403. [Google Scholar] [CrossRef]
- van Tol, M.-J.; Demenescu, L.R.; van der Wee, N.J.; Kortekaas, R.; M. A., N.M.; Boer, J.D.; Renken, R.J.; van Buchem, M.A.; Zitman, F.G.; Aleman, A.; et al. Functional Magnetic Resonance Imaging Correlates of Emotional Word Encoding and Recognition in Depression and Anxiety Disorders. Biol. Psychiatry 2012, 71, 593–602. [Google Scholar] [CrossRef] [PubMed]
- The ICD-10 classification of mental and behavioural disorders : clinical descriptions and diagnostic guidelines. (n.d.). Retrieved June 28, 2024, from https://iris.who.int/handle/10665/37958.
- Stoyanov, D.; Stanghellini, G.; Broome, M. Conceptual Issues in Psychiatric Neuroimaging: An Update. Curr. Top. Med. Chem. 2013, 12, 2348–2356. [Google Scholar] [CrossRef]
- Stoyanov, D.; Kandilarova, S.; Borgwardt, S.; Stieglitz, R.-D.; Hugdahl, K.; Kostianev, S. Psychopathology Assessment Methods Revisited: On Translational Cross-Validation of Clinical Self-Evaluation Scale and fMRI. Front. Psychiatry 2018, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- von Zerssen, D. Clinical Self-Rating Scales (CSRS) of the Munich Psychiatric Information System (PSYCHIS München). Assessment of Depression, 1986, 270–303. [CrossRef]
- Stoyanov, D.; Kandilarova, S.; Arabadzhiev, Z.; Paunova, R.; Schmidt, A.; Borgwardt, S. Cross-Validation of Paranoid-Depressive Scale and Functional MRI: New Paradigm for Neuroscience Informed Clinical Psychopathology. Front. Psychiatry 2019, 10, 711. [Google Scholar] [CrossRef] [PubMed]
- Stoyanov, D.; Kandilarova, S.; Paunova, R.; Garcia, J.B.; Latypova, A.; Kherif, F. Cross-Validation of Functional MRI and Paranoid-Depressive Scale: Results From Multivariate Analysis. Front. Psychiatry 2019, 10, 869. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Kucyi, A.; Raya, J.; Nielsen, A.N.; Nomi, J.S.; Damoiseaux, J.S.; Greene, D.J.; Horovitz, S.G.; Uddin, L.Q.; Whitfield-Gabrieli, S. What have we really learned from functional connectivity in clinical populations? NeuroImage 2021, 242, 118466. [Google Scholar] [CrossRef] [PubMed]
- Stoyanov, D.; Paunova, R.; Dichev, J.; Kandilarova, S.; Khorev, V.; Kurkin, S. Functional magnetic resonance imaging study of group independent components underpinning item responses to paranoid-depressive scale. World J. Clin. Cases 2023, 11, 8458–8474. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-J.; Zeng, L.-L.; Shen, H.; Yuan, L.; Qin, J.; Zhang, P.; Hu, D. Functional network connectivity alterations in schizophrenia and depression. Psychiatry Res. Neuroimaging 2017, 263, 113–120. [Google Scholar] [CrossRef] [PubMed]
- A Ross, C.; Pearlson, G.D. Schizophrenia, the heteromodal association neocortex and development: potential for a neurogenetic approach. Trends Neurosci. 1996, 19, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Pearlson, G.D.; Marsh, L. Structural brain imaging in schizophrenia: a selective review. Biol. Psychiatry 1999, 46, 627–649. [Google Scholar] [CrossRef] [PubMed]
- E Schlaepfer, T.; Harris, G.J.; Tien, A.Y.; Peng, L.W.; Lee, S.; Federman, E.B.; A Chase, G.; E Barta, P.; Pearlson, G.D. Decreased regional cortical gray matter volume in schizophrenia. Am. J. Psychiatry 1994, 151, 842–848. [Google Scholar] [CrossRef]
- Stoyanov, D.; Kandilarova, S.; Aryutova, K.; Paunova, R.; Mantarkov, M.; Mitrev, I.; Todeva-Radneva, A.; Specht, K. Effective Connectivity Between the Orbitofrontal Cortex and the Precuneus Differentiates Major Psychiatric Disorders: Results from a Transdiagnostic Spectral DCM Study. CNS Neurol. Disord. - Drug Targets 2023, 22, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Gold, J.M.; Waltz, J.A.; Prentice, K.J.; Morris, S.E.; Heerey, E.A. Reward Processing in Schizophrenia: A Deficit in the Representation of Value. Schizophr. Bull. 2008, 34, 835–847. [Google Scholar] [CrossRef] [PubMed]
- Orliac, F.; Naveau, M.; Joliot, M.; Delcroix, N.; Razafimandimby, A.; Brazo, P.; Dollfus, S.; Delamillieure, P. Links among resting-state default-mode network, salience network, and symptomatology in schizophrenia. Schizophr. Res. 2013, 148, 74–80. [Google Scholar] [CrossRef]
- Mingoia, G.; Wagner, G.; Langbein, K.; Maitra, R.; Smesny, S.; Dietzek, M.; Burmeister, H.P.; Reichenbach, J.R.; Schlösser, R.G.; Gaser, C.; et al. Default mode network activity in schizophrenia studied at resting state using probabilistic ICA. Schizophr. Res. 2012, 138, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Whitfield-Gabrieli, S.; Thermenos, H.W.; Milanovic, S.; Tsuang, M.T.; Faraone, S.V.; McCarley, R.W.; Shenton, M.E.; Green, A.I.; Nieto-Castanon, A.; LaViolette, P.; et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc. Natl. Acad. Sci. 2009, 106, 1279–1284. [Google Scholar] [CrossRef] [PubMed]
- Rotarska-Jagiela, A.; van de Ven, V.; Oertel-Knöchel, V.; Uhlhaas, P.J.; Vogeley, K.; Linden, D.E. Resting-state functional network correlates of psychotic symptoms in schizophrenia. Schizophr. Res. 2010, 117, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Kandilarova, S.; Stoyanov, D.S.; Paunova, R.; Todeva-Radneva, A.; Aryutova, K.; Maes, M. Effective Connectivity between Major Nodes of the Limbic System, Salience and Frontoparietal Networks Differentiates Schizophrenia and Mood Disorders from Healthy Controls. J. Pers. Med. 2021, 11, 1110. [Google Scholar] [CrossRef] [PubMed]
- Ohta, M.; Nakataki, M.; Takeda, T.; Numata, S.; Tominaga, T.; Kameoka, N.; Kubo, H.; Kinoshita, M.; Matsuura, K.; Otomo, M.; et al. Structural equation modeling approach between salience network dysfunction, depressed mood, and subjective quality of life in schizophrenia: an ICA resting-state fMRI study. Neuropsychiatr. Dis. Treat. 2018, ume 14, 1585–1597. [Google Scholar] [CrossRef]
- Forlim, C.G.; Klock, L.; Bächle, J.; Stoll, L.; Giemsa, P.; Fuchs, M.; Schoofs, N.; Montag, C.; Gallinat, J.; Kühn, S. Reduced Resting-State Connectivity in the Precuneus is correlated with Apathy in Patients with Schizophrenia. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Zhuo, C.-J.; Zhu, J.-J.; Wang, C.-L.; Wang, L.-N.; Li, J.; Qin, W. Increased Local Spontaneous Neural Activity in the Left Precuneus Specific to Auditory Verbal Hallucinations of Schizophrenia. Chin. Med J. 2016, 129, 809–813. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.-L.; Zong, X.-F.; Mann, J.J.; Zheng, J.-J.; Liao, Y.-H.; Li, Z.-C.; He, Y.; Chen, X.-G.; Tang, J.-S. A Review of the Functional and Anatomical Default Mode Network in Schizophrenia. Neurosci. Bull. 2016, 33, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Pu, W.; Wang, J.; Liu, H.; Wu, G.; Liu, C.; Mwansisya, T.E.; Tao, H.; Chen, X.; Huang, X.; et al. Inefficient DMN Suppression in Schizophrenia Patients with Impaired Cognitive Function but not Patients with Preserved Cognitive Function. Sci. Rep. 2016, 6, 21657. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Zhou, Y.; Zhou, J.; Liang, S.; Li, X.; Xu, C.; Xie, G.; Liang, J. Abnormalities of the Amygdala in schizophrenia: a real world study. BMC Psychiatry 2023, 23, 1–6. [Google Scholar] [CrossRef]
- Kim, W.-S.; Shen, G.; Liu, C.; Kang, N.-I.; Lee, K.-H.; Sui, J.; Chung, Y.-C. Altered amygdala-based functional connectivity in individuals with attenuated psychosis syndrome and first-episode schizophrenia. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Zhang, H.; Wei, X.; Tao, H.; Mwansisya, T.E.; Pu, W.; He, Z.; Hu, A.; Xu, L.; Liu, Z.; Shan, B.; et al. Opposite Effective Connectivity in the Posterior Cingulate and Medial Prefrontal Cortex between First-Episode Schizophrenic Patients with Suicide Risk and Healthy Controls. PLOS ONE 2013, 8, e63477. [Google Scholar] [CrossRef] [PubMed]
- Mellem, M.S.; Liu, Y.; Gonzalez, H.; Kollada, M.; Martin, W.J.; Ahammad, P. Machine Learning Models Identify Multimodal Measurements Highly Predictive of Transdiagnostic Symptom Severity for Mood, Anhedonia, and Anxiety. Biol. Psychiatry: Cogn. Neurosci. Neuroimaging 2020, 5, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Chen, C.; Rong, B.; Wan, Q.; Chen, J.; Liu, Z.; Zhou, Y.; Wang, G.; Wang, H. Resting-state functional connectivity of salience network in schizophrenia and depression. Sci. Rep. 2022, 12, 1–8. [Google Scholar] [CrossRef]
- Davey, C.G.; Harrison, B.J.; Yücel, M.; Allen, N.B. Regionally specific alterations in functional connectivity of the anterior cingulate cortex in major depressive disorder. Psychol. Med. 2012, 42, 2071–2081. [Google Scholar] [CrossRef]
- Guha, A.; Yee, C.M.; Heller, W.; Miller, G.A. Alterations in the default mode-salience network circuit provide a potential mechanism supporting negativity bias in depression. Psychophysiology 2021, 58, e13918. [Google Scholar] [CrossRef] [PubMed]
- Sambataro, F.; Thomann, P.A.; Nolte, H.M.; Hasenkamp, J.; Hirjak, D.; Kubera, K.M.; Hofer, S.; Seidl, U.; Depping, M.S.; Stieltjes, B.; et al. Transdiagnostic modulation of brain networks by electroconvulsive therapy in schizophrenia and major depression. Eur. Neuropsychopharmacol. 2019, 29, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Farrer, C.; Franck, N.; Frith, C.D.; Decety, J.; Georgieff, N.; D'Amato, T.; Jeannerod, M. Neural correlates of action attribution in schizophrenia. Psychiatry Res. Neuroimaging 2004, 131, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Seghier, M.L. The Angular Gyrus: multiple functions and multiple subdivisions. Neuroscientist 2013, 19, 43–61. [Google Scholar] [CrossRef]
- Nelson, S.M.; Dosenbach, N.U.F.; Cohen, A.L.; Wheeler, M.E.; Schlaggar, B.L.; Petersen, S.E. Role of the anterior insula in task-level control and focal attention. Anat. Embryol. 2010, 214, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Warrier, C.; Wong, P.; Penhune, V.; Zatorre, R.; Parrish, T.; Abrams, D.; Kraus, N. Relating Structure to Function: Heschl's Gyrus and Acoustic Processing. J. Neurosci. 2009, 29, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Wang, X.; Wang, Q.; Yan, R.; Chattun, M.R.; Yao, Z.; Lu, Q. Altered fractional amplitude of low-frequency fluctuations in the superior temporal gyrus: a resting-state fMRI study in anxious depression. BMC Psychiatry 2023, 23, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Peng, W.; Sweeney, J.A.; Jia, Z.; Gong, Q. Brain structure alterations in depression: Psychoradiological evidence. CNS Neurosci. Ther. 2018, 24, 994–1003. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).