Submitted:
01 July 2024
Posted:
02 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
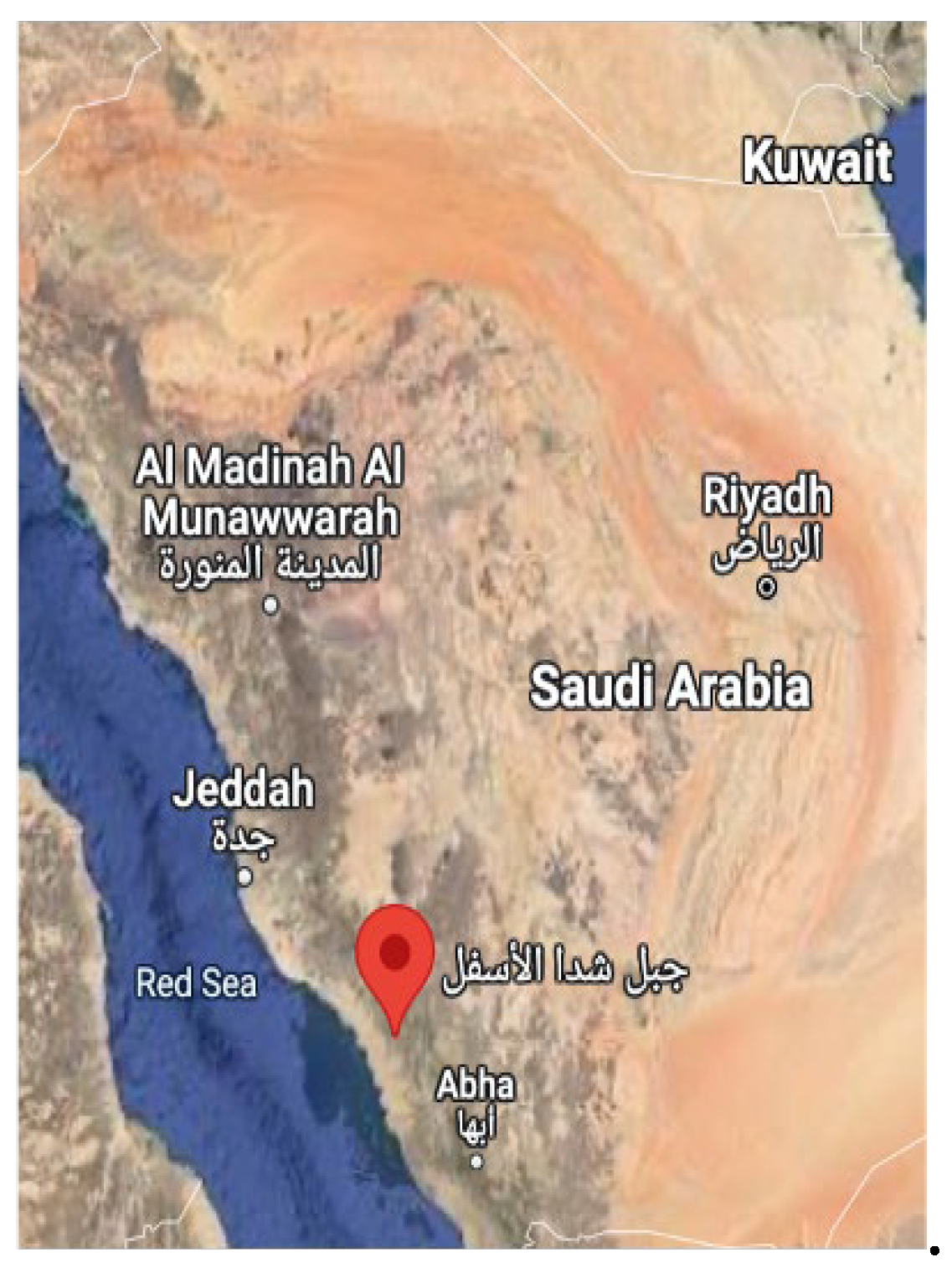
2.1. Plant Sampling and Study Area
2.2. Isolation of Endophytic Bacteria
2.3. Direct Mechanisms by Endophytic Bacteria
2.3.1. Solubilization of phosphate
2.3.2. Production of Indole-3-acetic acid (IAA)
2.3.3. Ammonia Production
2.4. Indirect Mechanisms by Endophytic Bacteria
2.4.1. Antifungal Activities
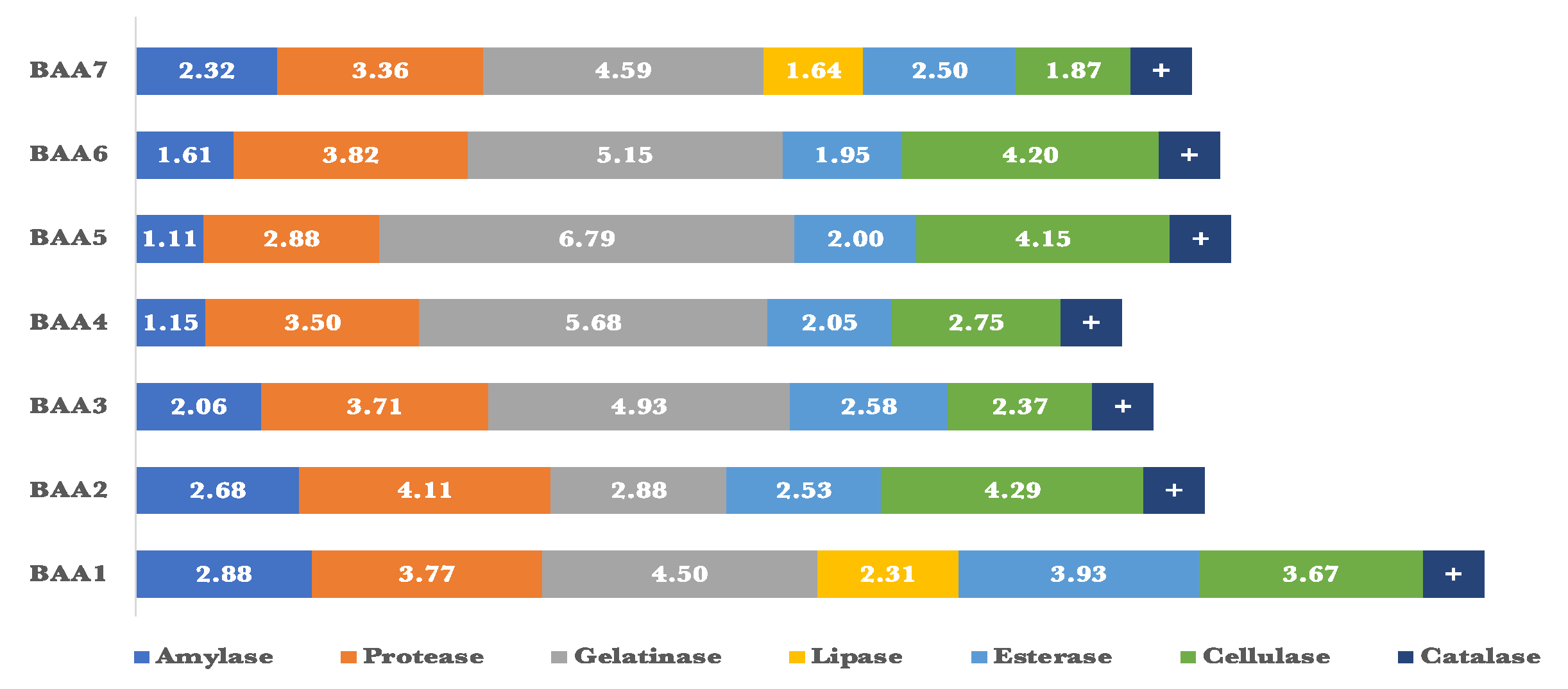
2.4.2. Production of Extracellular Enzymes
2.4.3. Production of Hydrogen Cyanide (HCN)
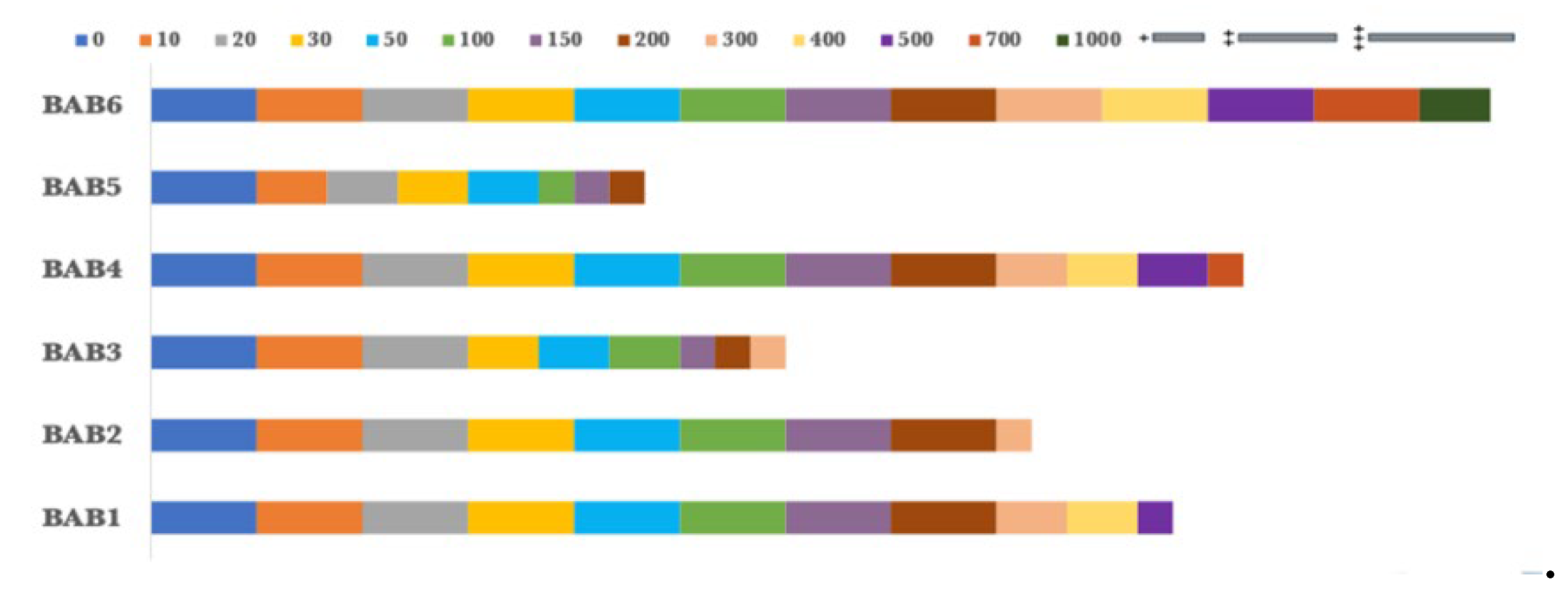
2.4.4. Abiotic Stress Tolerance
2.5. Molecular Identification of Endophytic Bacteria
3. Results
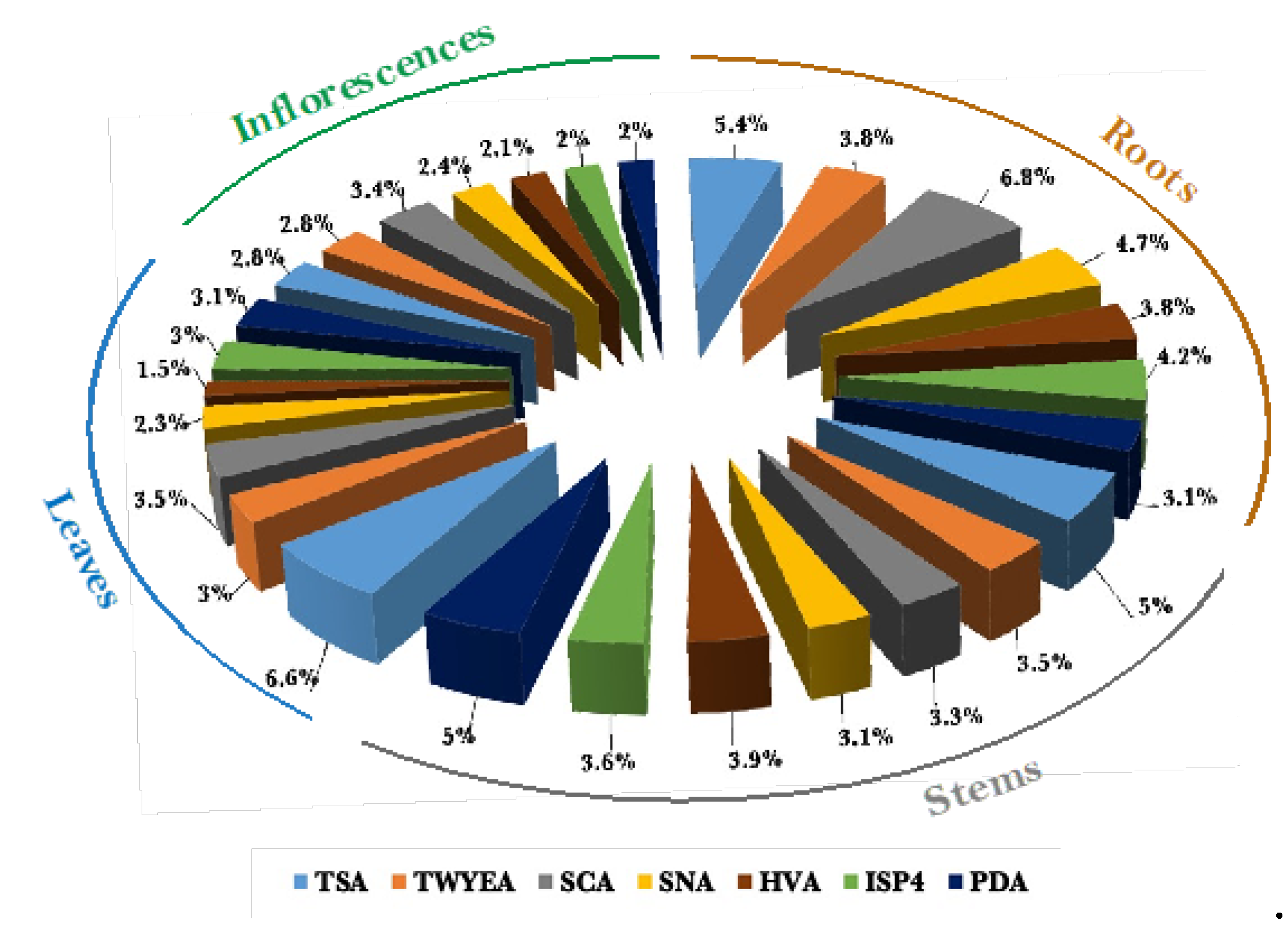
3.1. Isolation of Endophytic Bacteria
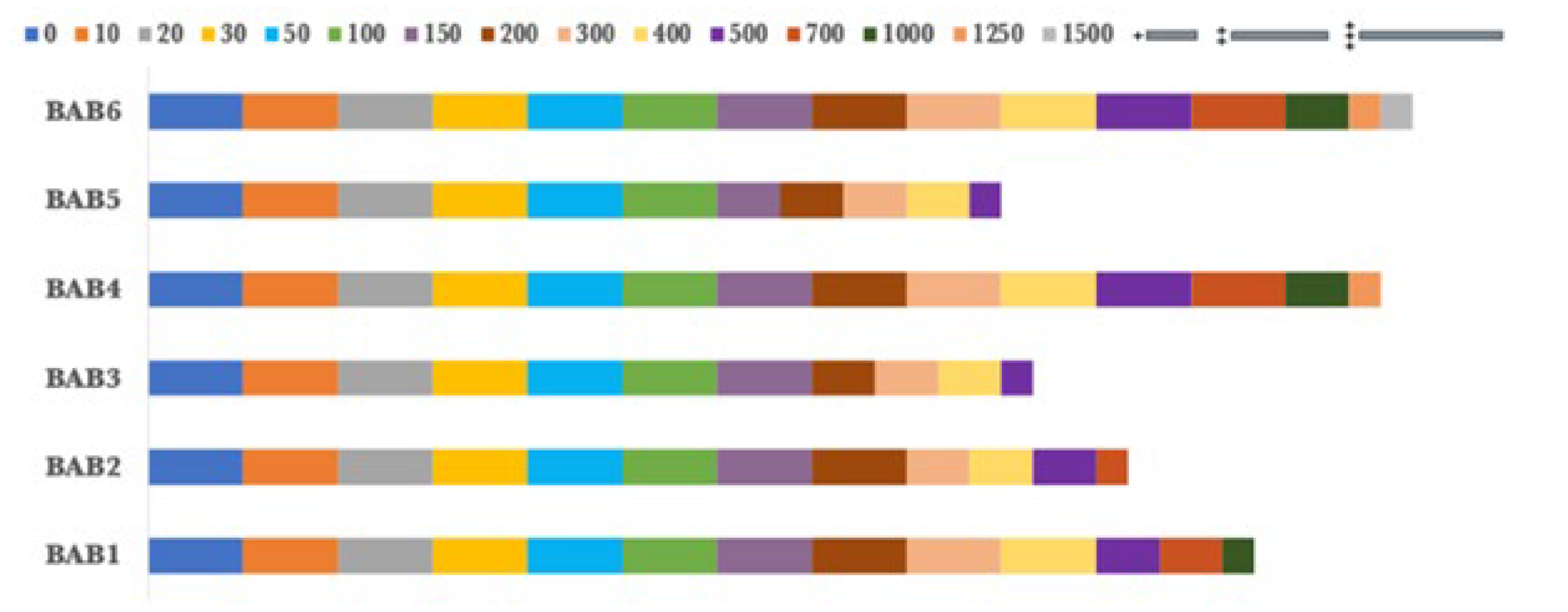
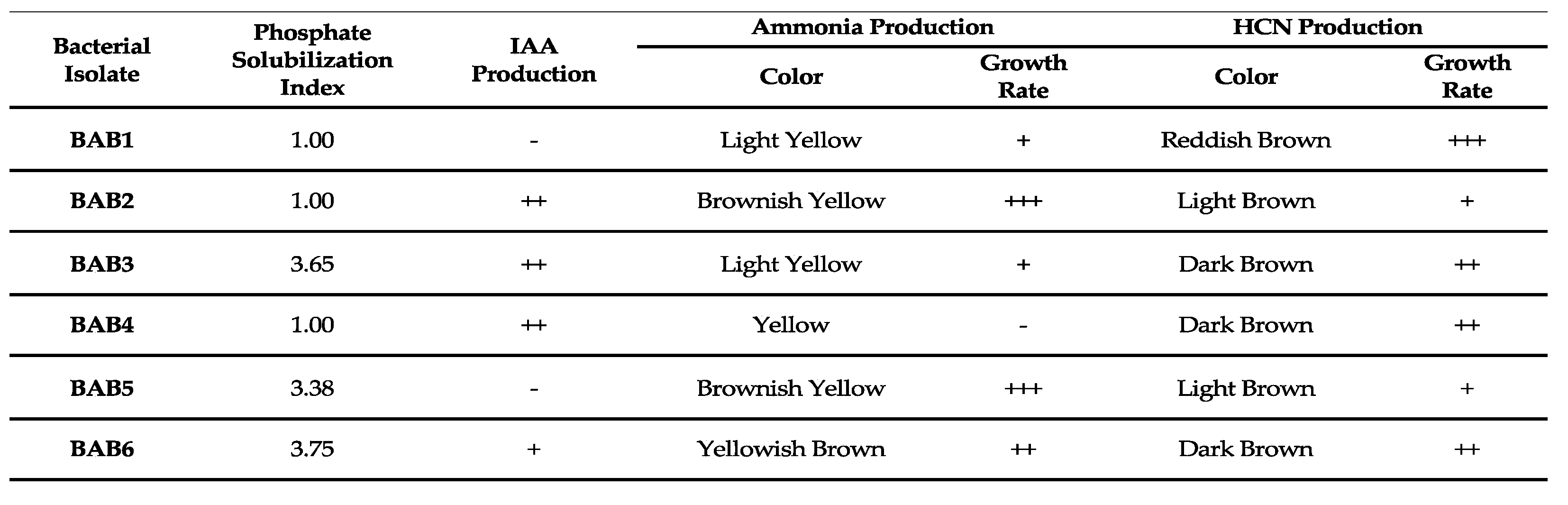
3.2. Direct PGP Activities of A. javanica Bacterial Endophytes
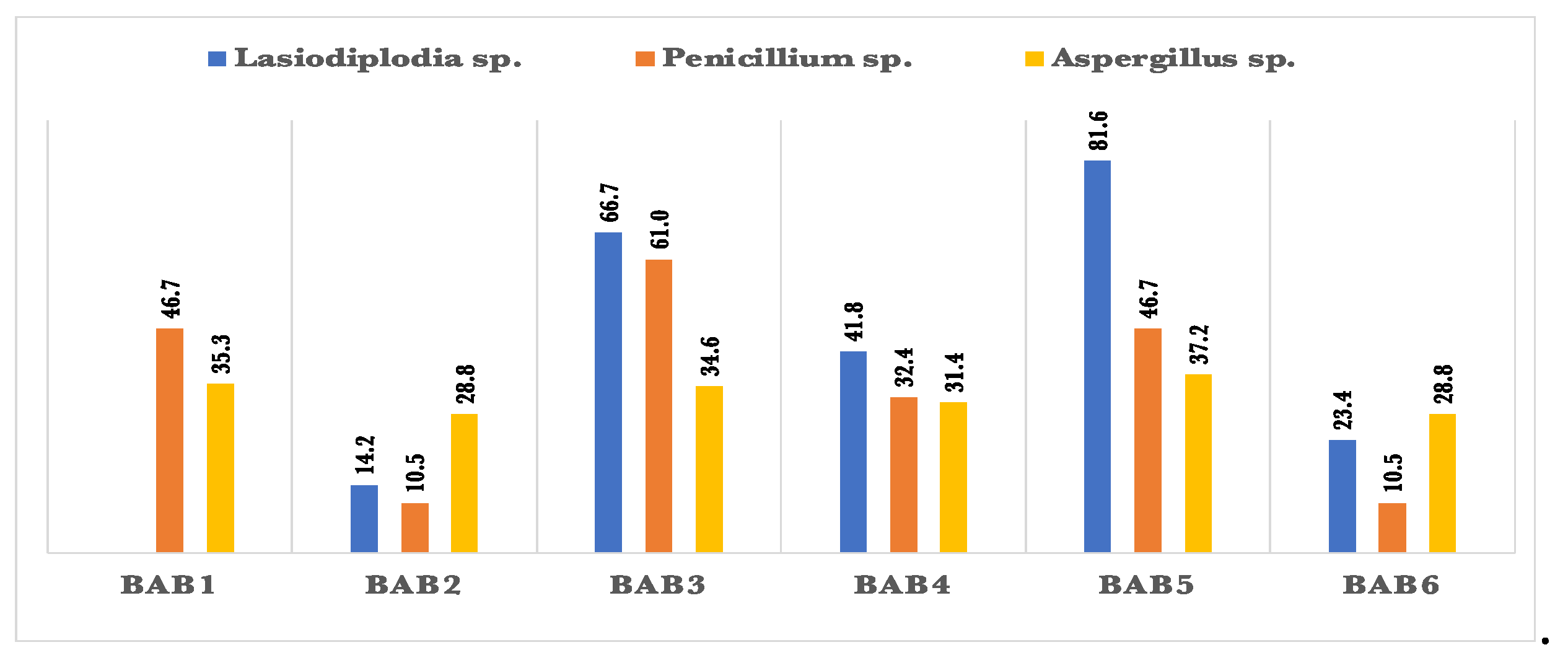
3.3. Indirect PGP Activities of A. javanica Bacterial Endophytes
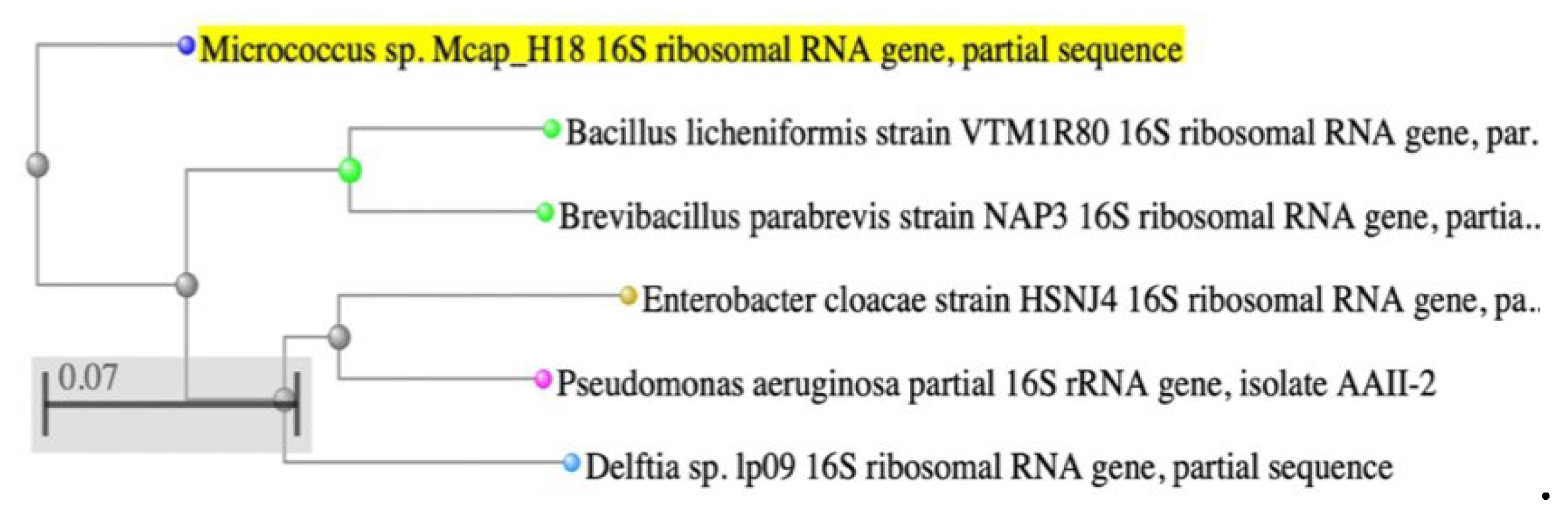
3.4. Molecular Identification of Selected Endophytic Bacteria
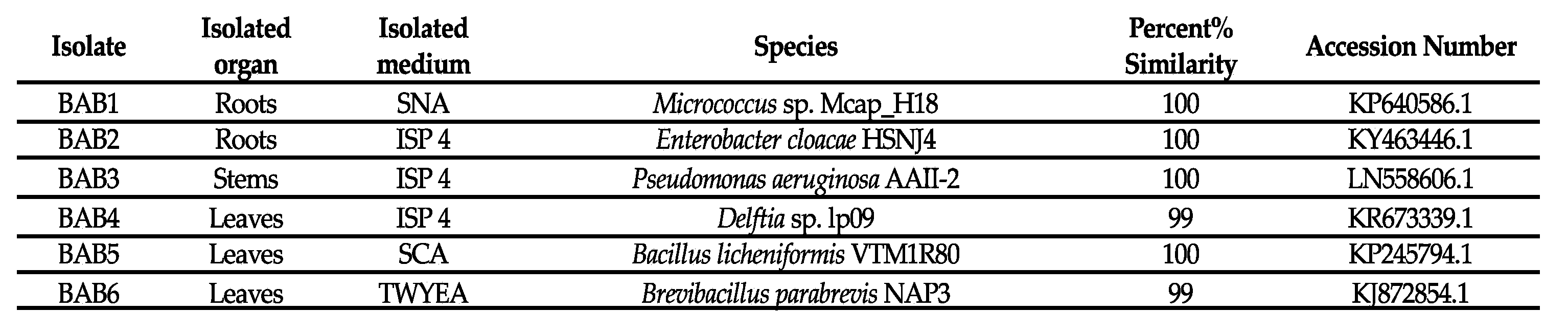 |
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alzahrani, K., Jastaniah, S., Amasha, R., et al. Diversity and Colonization of Endophytic Actinomycetes in some Medicinal Plants: Review. Journal of Contemporary Medical Sciences 2022, 8(3). [CrossRef]
- Goulart, M., Cueva-Yesquén, L., Hidalgo Martinez, K., et al. Comparison of specific endophytic bacterial communities in different developmental stages of Passiflora incarnata using culture-dependent and culture-independent analysis. Microbiology Open 2019, 8, e896. [CrossRef]
- Suman, A., Yadav, A. and Verma, P. Endophytic Microbes in Crops: Diversity and Beneficial Impact for Sustainable Agriculture. In: Singh D., Singh H., Prabha R. (eds) Microbial Inoculants in Sustainable Agricultural Productivity. Springer, New Delhi, 2016.
- Singh, M., Kumar, A., Singh, R., et al. Endophytic bacteria: A new source of bioactive compounds. 3 Biotech 2017, 7(5), 315. [CrossRef]
- Buatong, J., Phongpaichit, S., Rukachaisirikul, V., et al. Antimicrobial activity of crude extracts from mangrove fungal endophytes. World J Microbiol Biotechnol. 2011, 27(12), 3005–8. [CrossRef]
- Santoyo, G., Moreno-Hagelsieb, G., del Orozco-Mosqueda, M., et al. Plant growth-promoting bacterial endophytes. Microbiol. Res. 2016, 183, 92–99. [CrossRef]
- López-Arredondo, D., Leyva-González, M., Alatorre-Cobos, F., et alBiotechnology of nutrient uptake and assimilation in plants. Int. J. Dev. Biol. 2013, 57, 595–610.
- Noemi, C. and Everlon, C. (2022) Endophytic fungi: a tool for plant growth promotion and sustainable agriculture, Mycology 2022, 13(1), 39-55.
- Glick, B. Plant growth-promoting bacteria: mechanisms and applications. Scientifica 2012, 1–15. [CrossRef]
- Gupta, P., Samant, K., and Sahu, A. Isolation of cellulose-degrading bacteria and determination of their cellulolytic potential. International journal of microbiology 2012, 578925. [CrossRef]
- Nagei, T., Alshaeri, S., Alsulaimany, F., et al. The Role of Aerva Javanica (Burm.F.) Juss. Growing in Jazan, Saudi Arabia as Antimicrobial and Coagulating Factor. European Journal of Applied Sciences, 2021, 9(5), 456-471. [CrossRef]
- Thomas, J., El-Sheikh, M. and Alatar, A. Endemics and endangered species in the biodiversity hotspot of the Shada Mountains, Saudi Arabia. Journal of Arid Land 2017, 9(1), 109–121. [CrossRef]
- El-Tayeh, N., Galal, H., Soliman, M., et al. Association of Morphological, Ecological, and Genetic Diversity of Aerva javanica Populations Growing in the Eastern Desert of Egypt. Agronomy 2020, 10, 402.
- Suleiman, M. Ethnobotanical, Phytochemical, and Biological Study of Tamarix aphylla and Aerva javanica Medicinal Plants Growing in the Asir Region, Saudi Arabia. Tropical Conservation Science 2019, 12.
- Srinivas, P. and Reddy S. Screening for antibacterial principle and activity of Aerva javanica (Burm.f) Juss. ex Schult. Asian Pacific Journal of Tropical Biomedicine 2012, S838-S845. [CrossRef]
- Singh, R. and Jha, P. Plant Growth Promoting Potential of ACC Deaminase Rhizospheric Bacteria Isolated from Aerva javanica: A Plant Adapted to Saline Environments. Int.J.Curr.Microbiol.App.Sci 2015, 4(7), 142-152.
- Mookherjee, A., Mitra, M., Kutty, N., et al. Characterization of endo-metabolome exhibiting antimicrobial and antioxidant activities from endophytic fungus Cercospora sp. PM018. South African journal of botany 2020, 134. [CrossRef]
- Qin, S., Li, J., Chen, H., et al. Isolation, diversity, and antimicrobial activity of rare actinobacteria from medicinal plants of tropical rain forests in Xishuangbanna, China. Appl. Environ. Microbiol. 2009, 75, 6176-6186. [CrossRef]
- Musa, Z., Ma, J., Egamberdieva, D., et al. Diversity and Antimicrobial Potential of Cultivable Endophytic Actinobacteria Associated with the Medicinal Plant Thymus roseus. Frontiers in Microbiology. 2020, 11. [CrossRef]
- Phongsopitanuna, W., Sripreechasakc, P., Rueangsawang, K., et al. Diversity and antimicrobial activity of culturable endophytic actinobacteria associated with Acanthaceae plants. Science Asia 2020, 46, 288-296.
- Islam, M., Madhaiyan, M., Deka Boruah, H., et al. Characterization of plant growth-promoting traits of free-living diazotrophic bacteria and their inoculation effects on growth and nitrogen uptake of crop plants. J Microbiol Biotechnol. 2009, 19(10), 1213–1222.
- Ndeddy Aka, R. and Babalola, O. Effect of bacterial inoculation of strains of Pseudomonas aeruginosa, Alcaligenes feacalis and Bacillus subtilis on germination, growth and heavy metal (Cd, Cr, and Ni) uptake of Brassica juncea. Int J Phytorem. 2016, 18, 200–209.
- Pitiwittayakul N, Wongsorn D, Tanasupawat S. Characterisation of Plant Growth-Promoting Endophytic Bacteria from Sugarcane and Their Antagonistic Activity against Fusarium moniliforme. Trop Life Sci Res. 2021, 32(3), 97-118. [CrossRef]
- Mohamed, A., Abd El-Megeed, F., Hassanein, N., et al. Native Rhizospheric and Endophytic Fungi as Sustainable Sources of Plant Growth Promoting Traits to Improve Wheat Growth under Low Nitrogen Input. J. Fungi 2022, 8, 94. [CrossRef]
- Minotto, E., Milagre, L., Oliveira, M., et al. Enzyme Characterization of Endophytic Actinobacteria Isolated from Tomato Plants. Journal of Advanced Scientific Research 2014, 5(02), 16-23.
- Pranay, K., Padmadeo, S., Jha, V., et al. Screening and identification of amylase producing strains of Bacillus. J. Appl. Biol. Biotech 2019, 7, 57–62.
- Medina, P. and Baresi, L. (2007). Rapid identification of gelatin and casein hydrolysis using TCA. J Microbiol Meth. 2007, 69, 391-3. [CrossRef]
- Mahdi, I., Fahsi, N., Hafidi, M., et al. Plant Growth Enhancement using Rhizospheric Halotolerant Phosphate Solubilizing Bacterium Bacillus licheniformis QA1 and Enterobacter asburiae QF11 Isolated from Chenopodium quinoa Willd. Microorganisms 2020, 8, 948. [CrossRef]
- Bakker, A. and Schippers, B. Microbial cyanide production in the rhizosphere in relation to potato yield reduction and Pseudomonas spp. mediated plant growth-stimulation. Soil Biol Biochem. 1987, 19, 451–457. [CrossRef]
- Kumar, A., Singh, R., Yadav, A., et al. Isolation and characterization of bacterial endophytes of Curcuma longa L. 3 Biotech 2016, 6, 60. [CrossRef]
- Perelomov, L., Sizova, O., Rahman, M., et al. Metal-Tolerant Bacteria of Wastewater Treatment Plant in a Large City. Sustainability 2022, 14, 11335. [CrossRef]
- Nxumalo, C., Ngidi, L., Shandu, J., et al. Isolation of endophytic bacteria from the leaves of Anredera cordifolia CIX1 for metabolites and their biological activities. BMC Complement Med Ther. 2020, 20, 300. [CrossRef]
- Weiland, J. Rapid procedure for the extraction of DNA from fungal spores and mycelia. Fungal Genet. Rep. 1997, 44, 60–63. [CrossRef]
- Altschul, S., Gish, W., Miller, W., et al. Basic local alignment search tool. J Mol Biol. 1990, 215,3, 403–410. [CrossRef]
- Chaturvedi, H., Singh, V. and Gupta, G. Potential of bacterial endophytes as plant growth promoting factors. Journal of Plant Pathology Microbiology 2016, 7, 376. [CrossRef]
- Hassan, S. Plant growth-promoting activities for bacterial and fungal endophytes isolated from medicinal plant of Teucrium polium L. J. Adv. Res. 2017, 8, 687–695. [CrossRef]
- Ben Slama, H., Triki, M., Chenari Bouket, A., et al. Screening of the High-Rhizosphere Competent Limoniastrum monopetalum’ Culturable Endophyte Microbiota Allows the Recovery of Multifaceted and Versatile Biocontrol Agents. Microorganisms 2019, 7(8), 249. [CrossRef]
- Pinto, M., Inocente, L., Oliveira, P., et al. Plant Growth-Promoting (PGP) Traits of Endophytic Bacteria from In Vitro Cultivated Tectona grandis L.f. Forests 2022, 13(10), 1539. [CrossRef]
- Bamisile, B., Dash, C., Akutse, K., et al. Fungal endophytes: beyond herbivore management. Front Microbiol. 2018, 9(11).
- Belbahri, L., Chenari Bouket, A., Rekik, I., et al. Comparative genomics of Bacillus amyloliquefaciens strains reveals a core genome with traits for habitat adaptation and a secondary metabolites rich accessory genome. Front. Microbiol 2017, 8(1438).
- Ji, S., Gururani, M. and Chun, S. Isolation and characterization of plant growth promoting endophytic diazotrophic bacteria from Korean rice cultivars. Microbiol. Res. 2014, 169, 83–98.
- Defago, G., Berling, C., Burger, U., et al. Suppression of black root rot of tobacco and other root diseases by strains of Pseudomonas fluorescens: Potential applications and mechanisms. Biol. Control Soil Borne Plant Pathog. 1990, 34, 93–108.
- Rijavec, T. and Lapanje, A. Hydrogen cyanide in the rhizosphere: not suppressing plant pathogens, but rather regulating a vailability of phosphate. Front. Microbiol. 2016, 7, 1785. [CrossRef]
- Agbodjato, N., Noumavo, P., Baba Moussa, F., et al. Characterization of potential plant growth promoting rhizobacteria isolated from Maize (Zea mays L.) in central and Northern Benin (West Africa). Appl. Environ. Soil Sci. 2015, 01-09.
- Kloepper, J., Tuzun, S., Liu, L., et al. Plant growth promoting rhizobacteria as inducers of systemic disease resistance. Pest Management: Biologically Based Technologies. American Chemical Society Books, Washington, DC. 1993, 156–165.
- Mendes, R., Kruijt, M., De Bruijn, I., et al. (2011). Deciphering the rhizosphere microbiome for disease suppressive bacteria. Science 2011, 332, 1097–1100. [CrossRef]
- Choi, Y., Hodgkiss, I. and Hyde, K. Enzyme production by endophytes of Brucea javanica. J Agric Tech 2005, 1.
- Singh, R., Singh, T. and Pandey, A. Chapter 1, Microbial Enzymes-An Overview. In Biomass, Biofuels, Biochemicals. Advances in Enzyme Technology, Elsevier, 2019, 1-40.
- Zandalinas, S., Mittler, R., Balfagón, D., et al. Plant adaptations to the combination of drought and high temperatures. Physiol. Plant 2018, 162 (1), 2–12.
- Singh, D., Kaur, S. and Kumar, A. In vitro drought tolerance in selected elite clones of Eucalyptus tereticornis Sm. Acta Physiol. Plant 2020, 42, 1–9.
- Koza, N., Adedayo, A. Babalola, O., et al. Microorganisms in Plant Growth and Development: Roles in Abiotic Stress Tolerance and Secondary Metabolites Secretion. Microorganisms 2022, 10, 1528. [CrossRef]
- Balliu, A., Zheng, Y., Sallaku, G., et al. Environmental and Cultivation Factors Affect the Morphology, Architecture and Performance of Root Systems in Soilless Grown Plants. Horticulturae 2021, 7(8), 243.
- Ljubej, V., Karalija, E., Salopek-Sondi, B., et al. Effects of Short-Term Exposure to Low Temperatures on Proline, Pigments, and Phytochemicals Level in Kale (Brassica oleracea var. acephala). Horticulturae 2021, 7, 341. [CrossRef]
- Msimbira, L. and Smith, D. The Roles of Plant Growth Promoting Microbes in Enhancing Plant Tolerance to Acidity and Alkalinity Stresses. Front. Sustain. Food Syst. 2020, 4, 106. [CrossRef]
- Burt, R. Soil Survey Staff: Soil Survey Field and Laboratory Methods Manual- Soil Survey Investigations Report. Washington, DC: US Department of Agriculture 2014, 51, 227–234.
- Läuchli, A. and Grattan, S. “Soil pH extremes,” in Plant Stress Physiology; ed. S. Shabala; Wallingford: Centre for Agriculture and Bioscience International, 2012, 194.


 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





