Submitted:
01 July 2024
Posted:
02 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Design and Recruitment
2.2. Questionnaire
2.3. Anthropometry (Physical Measurements)
2.3. Procedures of 24-Hour Urine Collection
2.4. Statistical Analysis
3. Results
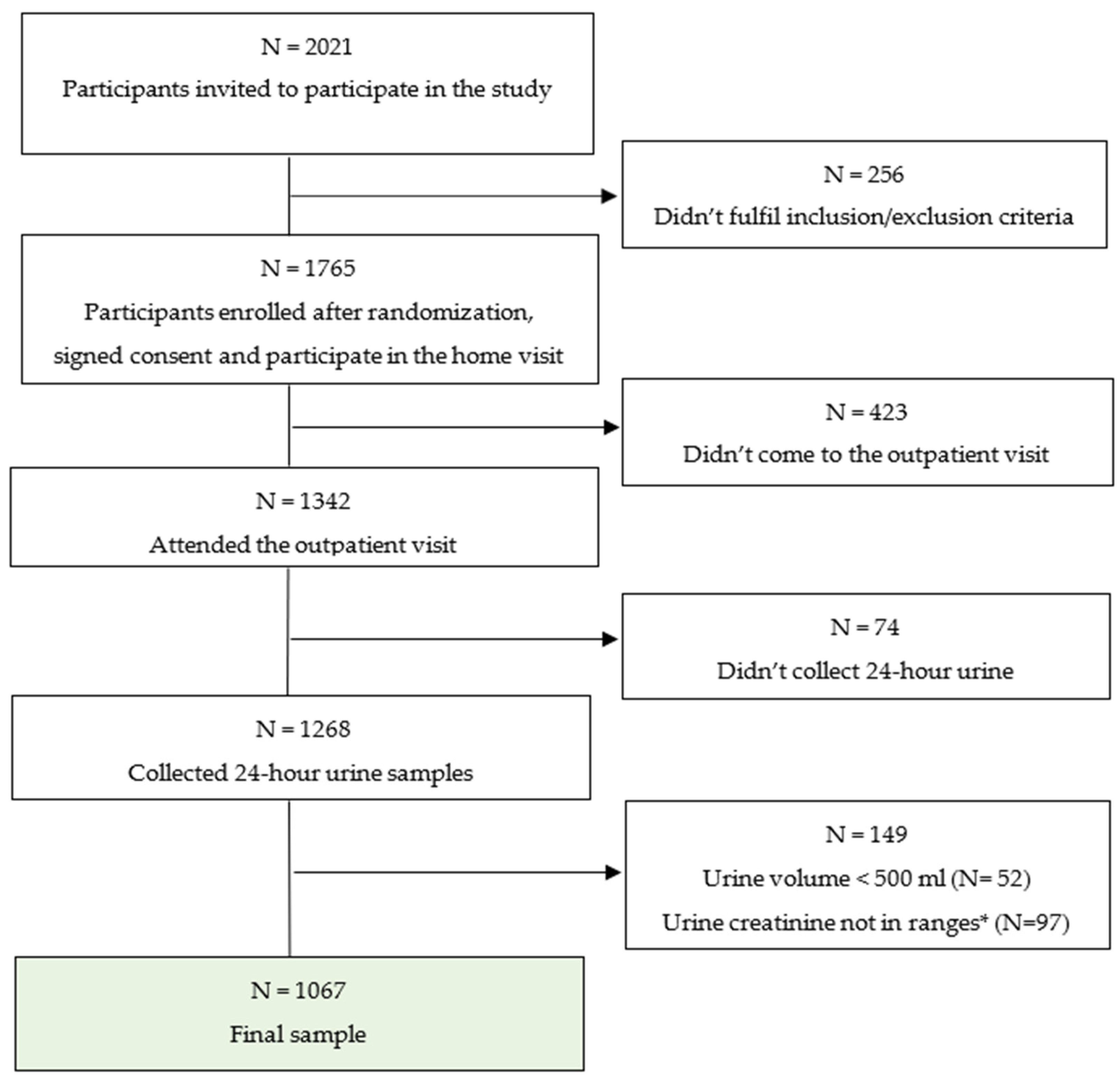
3.1. Completion of 24-Hour Urine Collection
3.2. Characteristics of the Participants Attended the Outpatient Visit and Asked to Participate in 24-Hour Urine Examination
3.3. Daily Urinary Excretions of Creatinine, Sodium, Potassium, Iodine, and Estimated Salt, Potassium and Iodine Intake in the Whole Group
3.4. Daily Salt, Potassium and Iodine Intake According to Residency and Geographic Regions
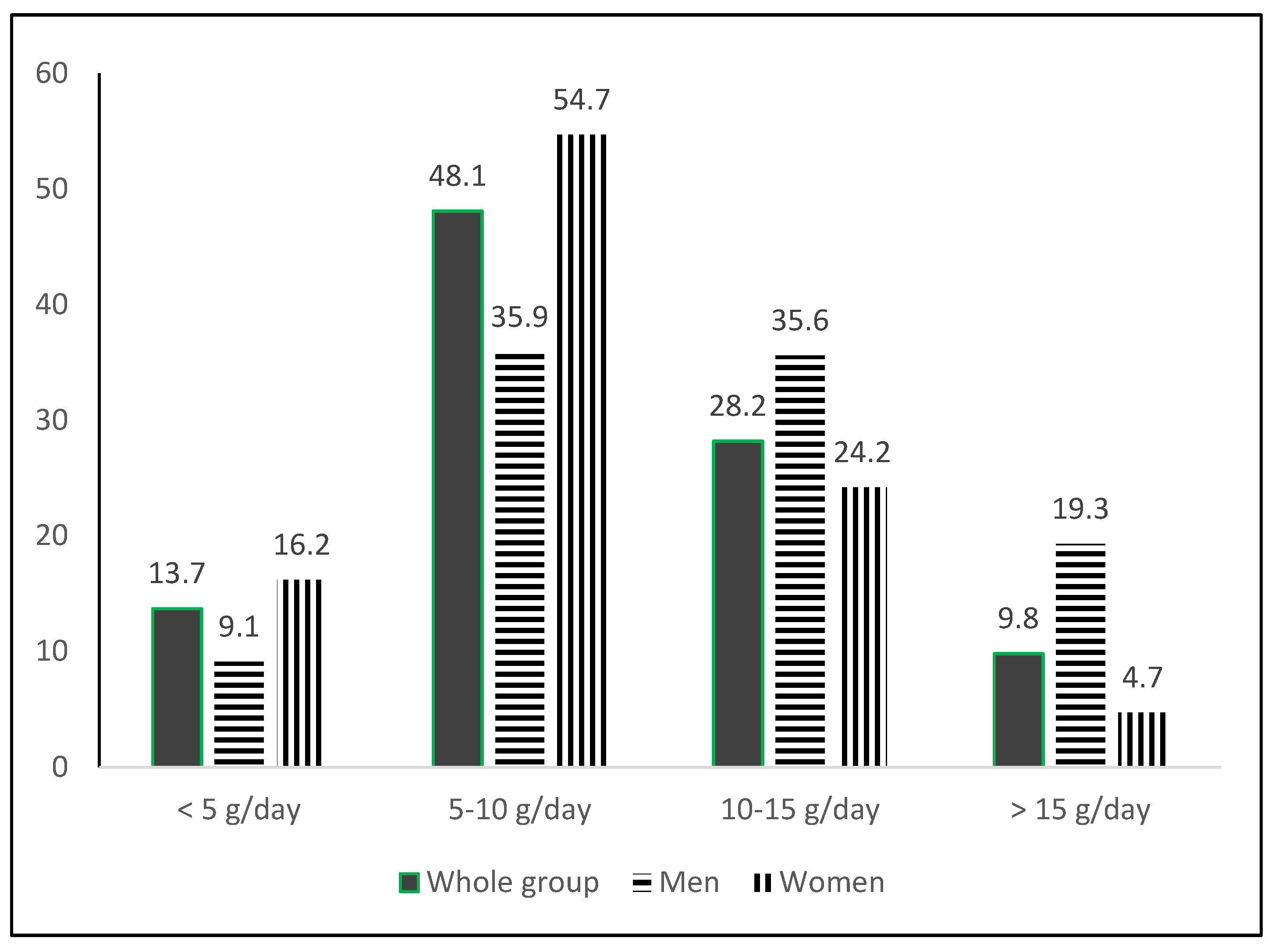

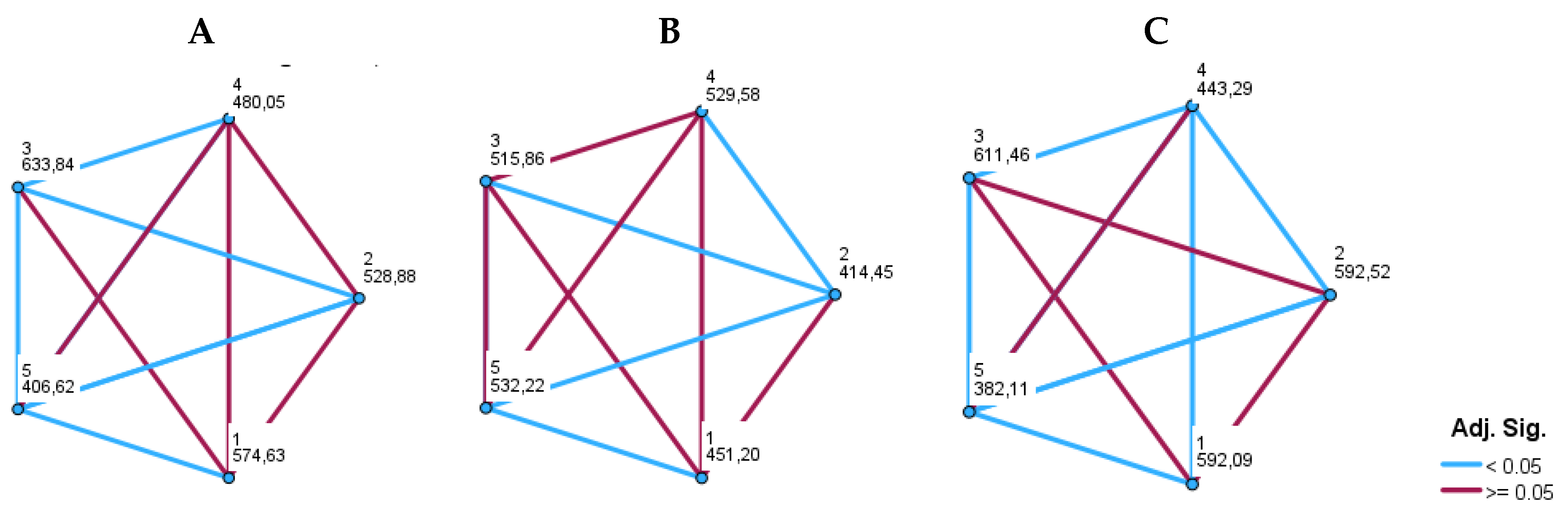
4. Discussion
1. Salt Consumption in General Adult Population
2. Potassium Consumption in Adult Population
3. Sodium-to-Potassium Ratio
4. Salt and Potassium Intake According to the Residency and Geographical Difference
5. Strenghts and Limitations
6. Conclusion
Supplementary Materials
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global report on hypertension: the race against a silent killer. World Health Organization: Geneva, Switzerland, 2023. Available online: https://www.who.int/publications/i/item/9789240081062 (accessed on 10 April 2024).
- GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018 Nov 10;392(10159):1736-1788. [CrossRef]
- Wang, K.; Jin, Y.; Wang, M.; Liu, J.; Bu, X.; Mu, J.; Lu, J. Global cardiovascular diseases burden attributable to high sodium intake from 1990 to 2019. J. Clin. Hypertens. 2023, 25 (9), 868–879. [CrossRef]
- Liu, W.; Zhou, L.; Yin, W.; Wang, J.; Zuo, X. Global, regional, and national burden of chronic kidney disease attributable to high sodium intake from 1990 to 2019. Front. Nutr. 2023, 10. [CrossRef]
- Wang, X.; Ma, H.; Kou, M.; Tang, R.; Xue, Q.; Li, X.; Harlan, T. S.; Heianza, Y.; Qi, L. Dietary sodium intake and risk of incident type 2 diabetes. Mayo Clinic Proceedings 2023, 98 (11), 1641–1652. [CrossRef]
- Filippini, T.; Malavolti, M.; Whelton, P. K.; Naska, A.; Orsini, N.; Vinceti, M. Blood pressure effects of sodium reduction. Circulation. 2021, 143 (16), 1542–1567. [CrossRef]
- McMahon, E.; Campbell, K. L.; Bauer, J.; Mudge, D. W.; Kelly, J. Altered dietary salt intake for people with chronic kidney disease. Cochrane Library 2021, 2021 (6). [CrossRef]
- Hodson, E. M.; Cooper, T. E. Altered dietary salt intake for preventing diabetic kidney disease and its progression. Cochrane Library 2023, 2023 (1). [CrossRef]
- World Health Organization. Noncommunicable Diseases, Rehabilitation and Disability (NCD). Global action plan for the prevention and control of noncommunicable diseases 2013-2020. Available online: https://www.who.int/publica-472tions/i/item/9789241506236 (accessed on 13 May 2024).
- Cobiac, L.; Vos, T.; Veerman, L. Cost-effectiveness of interventions to reduce dietary salt intake. Heart 2010, 96 (23), 1920–1925. [CrossRef]
- Webb, M.; Fahimi, S.; Singh, G.; Khatibzadeh, S.; Micha, R.; Powles, J.; Mozaffarian, D. Cost effectiveness of a government supported policy strategy to decrease sodium intake: global analysis across 183 nations. BMJ. 2017, i6699. [CrossRef]
- Trieu, K.; Neal, B.; Hawkes, C.; Dunford, E.; Campbell, N. R. C.; Rodríguez-Fernández, R.; Legetić, B.; McLaren, L.; Barberio, A.M.; Webster, J. Salt Reduction Initiatives around the World – A Systematic Review of Progress towards the Global Target. PloS 480 One 2015, 10 (7), e0130247. [CrossRef]
- Webster, J.; Dunford, E.; Hawkes, C.; Neal, B. Salt reduction initiatives around the world. J. Hypertens. 2011, 29 (6), 1043–1050. [CrossRef]
- Croatian Institute of Public Health. Epidemiological data on cardiovascular diseases. Available online: https://www.hzjz.hr/aktualnosti/epidemioloski-podaci-o-kardiovaskularnim-bolestima/ (accessed on 11 May 2024).
- Murray, C. J. L.; Aravkin, A. Y.; Zheng, P.; Abbafati, C.; Abbas, K.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abdelalim, A.; Abdollahi, M.; Abdollahpour, I. et al. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis 516 for the Global Burden of Disease Study 2019. Lancet 2020, 396 (10258), 1223–1249. [CrossRef]
- Global Burden of Disease 2017 Risk Factor Collaborators. Global, regional, and national comparative risk assessment of 84 407 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: 408 a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392 (10159), 1923–1994. [CrossRef]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in hypertension prevalence and progress in treatment and con-411 trol from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 2021, 412 398 (10304), 957–980. [CrossRef]
- Boateng, E. B.; Ampofo, A. G. A glimpse into the future: modelling global prevalence of hypertension. BMC Public Health 2023, 23 (1). [CrossRef]
- Less Salt—More Health. Croatian Action on Salt and Health (CRASH). Available online: https://pubmed.ncbi.nlm.nih.gov/19514255/ (accessed on 10 April 2024).
- Salt—Hidden Poison in Everyday Meal. Available online: https://pubmed.ncbi.nlm.nih.gov/19642535/ (accessed on 3 March 2024).
- Less salt—More Health: Possibilities of Prevention in Croatia. Available online: https://pubmed.ncbi.nlm.nih.gov/20649071/ (accessed on 5 April 2024).
- Jelaković, B.; Vrdoljak, A.; Pećin, I.; Buzjak, V.; Karanović, S.; Ivković, V.; Dapić, K.; Domislović, V.; Reiner, Z. Less Salt—More Health. Croatian Action on Salt and Health (46). J. Hypertens Res. 2016, 2, 61–68. Available online: http://hypertens.org/contents/pdfs/jhr-201606-020203.pdf (accessed on 15 May 2024).
- Jelaković, B.; Premužić, V.; Čvorišćec, D.; Erceg, I.; Fuček, M.; Jelaković, M.; Jovanović, A.; Kaić-Rak, A.; Laganović, M.; Lederer, P. et al. Salt Mapping in Croatia. Croatian Action on Salt and Health (CRASH). Kidney Blood Press Res. 2009, 32, 323.
- Sović, S.; Vitale, K.; Keranović, A.; Dražić; Džakula, A.; Jelaković, B. Prevalence, awareness, treatment and control of hypertension and salt intake in some rural area of Sisak—Moslavina county, Croatia. Period. Biol. 2011, 113, 321–326.
- World Health Organization. Reducing salt intake in populations. Available online: https://iris.who.int/bitstream/handle/10665/43653/9789241595377_eng.pdf?sequence=1&isAllowed=y (accessed on 13 March 2024).
- Jelaković, B.; Premužić, V.; Čvorišćec, D.; Erceg, I.; Fuček, M.; Jelaković, M.; Jovanović, A.; Kaić-Rak, A.; Laganović, M.; Lederer, 531 P. et al. Salt Mapping in Croatia. Croatian Action on Salt and Health (CRASH). Kidney and Blood Press Research, 2009; 32:323, 532 2009.
- Jelaković, B.; Marinović Glavić, M.; Batinić Sermek, M. Bilajac, L.; Bubaš, M.; Buzjak Služek, V.; Capak, K.; Drenjančević, I.; Gross Bošković, A.; Jelaković, A. et al. Croatian Action on Salt and Health (CRASH): On the Road to Success – Less Salt, More Health. Nutrients 2024, 16 (10), 1518. [CrossRef]
- Ordinance on cereals and cereal products (NN 101/2022) Available online: https://narodne-novine.nn.hr/clanci/sluzbeni/2022_09_101_1495.html (accessed on 5 May 2024).
- Hrvatska agencija za poljoprivredu i hranu, HAPIH (Croatian Agency for Agriculture and Food, CAAF), 2020. Scientific report 550 on the intake of salt through the consumption of bread and bakery products. Ad hoc. Task force of the Centre for Food Safety. 551 Available online: https://www.hapih.hr/wp-content/uploads/2021/01/Znanstveno-izvjesce-o-unosu-kuhinjske-soli-konzumaci-552jom-kruha-i-pekarskih-proizvoda_compressed.pdf (accessed on 15 April 2024).
- Gonçalves, C.; Abreu, S. Sodium and potassium intake and cardiovascular disease in Older people: a systematic review. Nutrients 2020, 12 (11), 3447. [CrossRef]
- Sodium and potassium intake: Effects on chronic disease Outcomes and risks [Internet]. PubMed. https://pubmed.ncbi.nlm.nih.gov/30125063/.
- Academies of Sciences, Engineering, and Medicine. Dietary Reference Intakes for Sodium and Potassium. Washington, DC; The National Academies Press; 2019. https://ods.od.nih.gov/factsheets/Potassium-HealthProfessional/#en11.
- World Health Organization. WHO issues new guidance on dietary salt and potassium. Available online: https://www.who.int/news/item/31-01-2013-who-issues-new-guidance-on-dietary-salt-and-potassium (accessed on 11 May 2024).
- European Food Safety Authority. Dietary reference values for potassium. European Food Safety Authority. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/4592 (accessed on 10 May).
- Reddin, C.; Ferguson, J.; Murphy, R.; Clarke, A.; Judge, C.; Griffith, V.; Alvarez, A.; Smyth, A.; Mente, A.; Yusuf, S. et al. Global mean potassium intake: a systematic review and Bayesian meta-analysis. European Journal of Nutrition 2023, 62 (5), 2027–2037. [CrossRef]
- Huang, L.; Li, Q.; Wu, J. H.; Tian, M.; Yin, X.; Yu, J.; Liu, Y.; Zhang, X.; Wu, Y.; Paige, E.; Trieu, K.; Marklund, M.; Rodgers, A.; Neal, B. The contribution of sodium reduction and potassium increase to the blood pressure lowering observed in the Salt Substitute and Stroke Study. Journal of Human Hypertension 2024, 38 (4), 298–306. [CrossRef]
- Yang, Y.; Wu, Q.; Lv, Q.; Li, J.; Li, L.; Wang, S. Dietary sodium, potassium intake, sodium-to-potassium ratio and risk of hypertension: a protocol for systematic review and dose–response meta-analysis of cohort studies. BMJ Open 2023, 13 (2), e065470. [CrossRef]
- Morrissey, E.; Giltinan, M.; Kehoe, L.; Nugent, A. P.; McNulty, B. A.; Flynn, A.; Walton, J. Sodium and Potassium Intakes and Their Ratio in Adults (18–90 y): Findings from the Irish National Adult Nutrition Survey. Nutrients 2020, 12 (4), 938. [CrossRef]
- Salt Ordinance, Narodne novine, number 70/2019. Available online: https://mingo.gov.hr/print.aspx?id=7930&url=print (accessed on 20 May 2024).
- Kusić, Z.; Lechpammer, S.; Labar, Ž.; Rončević, S.; Lukinac, Lj.; Notig-Hus, D.; Đaković, N.; Đokić, D.; Staničić, A.; Kaić-Rak, A. et al. Endemska gušavost i jodna profilaksa u Hrvatskoj / Znanstveni skup Nedostatak joda i gušavnost u Hrvatskoj - epidemiologija i jodna profilaksa : Gušavost u Hrvatskoj : prošireni zbornik / Kusić, Zvonko (ur.). Zagreb: Hrvatska akademija znanosti i umjetnosti ; : Klinička bolnica Sestre milosrdnice, 2000. str. 69-95.
- Kusić, Z.; Jukić, T.; Rogan SA, Juresa ,V.; Dabelić, N.; Stanicić, J.; Borić, M.; Lukinac, L.; Mihaljević, I.; Punda, A. et al. Current status of iodine intake in Croatia –the results of 2009 survey. Coll Antropol. 2012;36(1):123–128. https://pubmed.ncbi.nlm.nih.gov/22816208/.
- Kusić, Z.; Novosel, SA.; Dabelić, N.; Punda, M.; Roncević, S.; Labar Z, Lukinac, Lj.; Nothig-Hus, D.; Staničić, A.; Kaić-Rak, A. et al. Croatia has reached iodine sufficiency. J Endocrinol Invest. 2003; 26:738–742. https://link.springer.com/article/10.1007/BF03347356.
- Vidranski, V. (2019) Povezanost koncentracije joda u mokraći, antropoloških obilježja i fizičke aktivnosti djece u dobi od 6 do 12 godina . Doktorski rad. Osijek: Sveučilište J.J. Strossmayera, Medicinski fakultet Osijek. Available online: https://repozitorij.mefos.hr/islandora/object/mefos:1084 (accessed on 5 May 2024).
- Gharib H. Does iodine cause thyroid cancer? Acta Endocrinol (Buchar). 2018;14(4):525-526. [CrossRef]
- Vasiljev, V.; Subotić, A.; Marinović Glavić, M.; Jurga, D.; Bilajac, L.; Jelaković, B.; Rukavina, T. Overview of iodine intake. SEEMEDJ 2022, Vol 6, No 1.
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D. L.; Coca, A.; de Simone, G.; Dominiczak, A. et al. 2018 ESC/ESH Guidelines for the Management of Arterial Hypertension. Journal of Hypertension 2018, 36 (10), 1953–2041. [CrossRef]
- Vrdoljak, A.; Željković Vrkić, T.; Kos, J.; Vitale, K.; Premužić, V.; Laganović, M.; Jelaković, B. Blood pressure measurement – do not sweat the small stuff and it is all small stuff?!. Liječ Vjesn. 2014. https://hrcak.srce.hr/file/254652.
- World Health Organization. Protocol for population level sodium determination in 24-hour urine samples. Available online: https://www3.paho.org/hq/dmdocuments/2013/24h-urine-Protocol-eng.pdf (accessed on 29 May 2024).
- World Health Organization World Health Organization Guideline: Potassium Intake for Adults and Children. 2012.Available online: https://www.who.int/publications/i/item/9789241504829 (accessed on 1 June 2024).
- Turck, D.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.-I.; Kearney, J.; Knutsen, H. K.; Maciuk, A.; Mangelsdorf, I.; McArdle, H. J.; Pelaez, C. et al. Dietary reference values for sodium. EFSA Journal 2019, 17 (9). [CrossRef]
- World Health Organization. United Nations Children’s Fund & International Council for the Control of Iodine Deficiency Disorders. Assessment of iodine deficiency disorders and monitoring their elimination. 3rd ed. Geneva, Switzerland: WHO, 2007). Available online: https://iris.who.int/bitstream/handle/10665/43781/9789241595827_eng.pdf (accessed on 2 June 2024).
- WMA - the World Medical Association-WMA Declaration of Helsinki – Ethical principles for medical research involving human subjects. WMA - the World Medical Association-WMA Declaration of Helsinki – Ethical Principles for Medical Research Involving Human Subjects. https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects.
- Croatian Bureau of Statistics. Population estimate of Republic of Croatia, 2021. Available online: https://podaci.dzs.hr/2022/hr/29032 (accessed on 3 June 2024).
- Kwong, E. J. L.; Whiting, S.; Bunge, A. C.; Leven, Y.; Breda, J.; Rakovac, I.; Cappuccio, F. P.; Wickramasinghe, K. Population-level salt intake in the WHO European Region in 2022: a systematic review. Public Health Nutrition 2022, 26 (S1), s6–s19. [CrossRef]
- Santos, J. A.; Tekle, D.; Rosewarne, E.; Flexner, N.; Cobb, L.; Al-Jawaldeh, A.; Kim, W. J.; Breda, J.; Whiting, S.; Campbell, N.; Neal, B.; Webster, J.; Trieu, K. A Systematic Review of Salt Reduction Initiatives around the world: A midterm evaluation of progress towards the 2025 Global Non-Communicable Diseases Salt Reduction Target. Advances in Nutrition 2021, 12 (5), 1768–1780. [CrossRef]
- McLaren, L.; Sumar, N.; Barberio, A. M.; Trieu, K.; Lorenzetti, D. L.; Tarasuk, V.; Webster, J.; Campbell, N. R. Population-level interventions in government jurisdictions for dietary sodium reduction. Cochrane Library 2016, 2017 (3). [CrossRef]
- Laatikainen, T.; Pietinen, P.; Valsta, L.; Sundvall, J.; Reinivuo, H.; Tuomilehto, J. Sodium in the Finnish diet: 20-year trends in urinary sodium excretion among the adult population. European Journal of Clinical Nutrition 2006, 60 (8), 965–970. [CrossRef]
- Donfrancesco, C.; Lo Noce, C.; Russo, O.; Minutoli, D.; Di Lonardo, A.; Profumo, E.; Buttari, B.; Iacone, R.; Vespasiano, F.; Vannucchi, S. et al. Trend of salt intake measured by 24-h urine collection in the Italian adult population between the 2008 and 2018 CUORE project surveys. NMCD. Nutrition Metabolism and Cardiovascular Diseases 2021, 31 (3), 802–813. [CrossRef]
- World Health Organization Regional Office for Europe. Meeting of the WHO action network on salt reduction in the population in the European region (ESAN): meeting report 2–10 May 2017, Dublin, Ireland. Copenhagen (Denmark): World Health Organization Regional Office for Europe; 2017. Available online: https://www.who.int/publications/i/item/WHO-EURO-2017-3289-43048-60245 (accessed on 18 May 2024).
- International Food Policy Research Institute. Global nutrition report 2016: from promise to impact: ending malnutrition by 2030. Washington (DC): International Food Policy Research Institute; 2016. Available online: https://wedocs.unep.org/20.500.11822/9677 (accessed on 15 May 2024).
- Nowson, C.; Lim, K.; Grimes, C.; O’Halloran, S.; Land, M.; Webster, J.; Shaw, J.; Chalmers, J.; Smith, W.; Flood, V. et al. Dietary salt intake and discretionary salt use in two general population samples in Australia: 2011 and 2014. Nutrients 2015, 7 (12), 10501–10512. [CrossRef]
- Pillay, A.; Trieu, K.; Santos, J.; Sukhu, A.; Schultz, J.; Wate, J.; Bell, C.; Moodie, M.; Snowdon, W.; Ma, G.; Rogers, K.; Webster, J. Assessment of a salt reduction intervention on adult population salt intake in Fiji. Nutrients 2017, 9 (12), 1350. [CrossRef]
- World Health Organization Regional Office for Europe. Progress in reducing salt consumption in Turkey [Internet]. World Health Organization Regional Office for Europe; 2013. Available online: http://www.euro.who.int/en/countries/turkey/news/news/2013/04/progress-in-reducing-salt-consumption-in-turkey (accessed on 1 June 2024).
- Public Health England. National diet and nutrition survey: assessment of salt intake from urinary sodium in adults (aged 19 to 64 years) in England, 2018 to 2019. London (UK): Public Health England; 2020. Available online: https://assets.publishing.service.gov.uk/media/5e7cd9cdd3bf7f133ed1b6a1/Report_England_Sodium_Survey_2018-to-2019__3_.pdf (accessed on 1 June 2024).
- D’Elia, L.; Obreja, G.; Ciobanu, A.; Breda, J.; Jewell, J.; Cappuccio, F. P. Sodium, potassium and iodine intake, in a national adult population sample of the Republic of Moldova. Nutrients 2019, 11 (12), 2896. [CrossRef]
- Zakauskiene, U.; Macioniene, E.; Zabuliene, L.; Sukackiene, D.; Linkeviciute-Dumce, A.; Banys, V.; Bratcikoviene, N.; Karosiene, D.; Slekiene, V. et al. Sodium, potassium and iodine intake in an adult population of Lithuania. Nutrients 2022, 14 (18), 3817. [CrossRef]
- Sarkadi-Nagy, E.; Horváth, A.; Varga, A.; Zámbó, L.; Török, A.; Guba, G.; Szilfai, N.; Zentai, A.; Bakacs, M. Dietary Sodium and Potassium Intake in Hungarian Elderly: Results from the Cross-Sectional Biomarker2019 Survey. International Journal of Environmental Research and Public Health/International Journal of Environmental Research and Public Health 2021, 18 (16), 8806. [CrossRef]
- Vasara, E.; Marakis, G.; Breda, J.; Skepastianos, P.; Hassapidou, M.; Kafatos, A.; Rodopaios, N.; Koulouri, A.; Cappuccio, F. Sodium and potassium intake in healthy adults in Thessaloniki Greater Metropolitan Area—The salt intake in Northern Greece (SING) study. Nutrients 2017, 9 (4), 417. [CrossRef]
- Palaniveloo, L.; Ambak, R.; Othman, F.; Zaki, N. A. M.; Baharudin, A.; Aziz, N. S. A.; Salleh, R. Low potassium intake and its association with blood pressure among adults in Malaysia: findings from the MyCoSS (Malaysian Community Salt Survey). Journal of Health, Population and Nutrition 2021, 40 (S1). [CrossRef]
- Reddin, C.; Ferguson, J.; Murphy, R.; Clarke, A.; Judge, C.; Griffith, V.; Alvarez, A.; Smyth, A.; Mente, A.; Yusuf, S. et al. Global mean potassium intake: a systematic review and Bayesian meta-analysis. European Journal of Nutrition 2023, 62 (5), 2027–2037. [CrossRef]
- D’Elia, L.; Brajović, M.; Klisic, A.; Breda, J.; Jewell, J.; Cadjenović, V.; Cappuccio, F. P. Sodium and potassium intake, knowledge attitudes and behaviour towards salt consumption amongst adults in Podgorica, Montenegro. Nutrients 2019, 11 (1), 160. [CrossRef]
- Stamler, J.; Rose, G.; Stamler, R.; Elliott, P.; Dyer, A.; Marmot, M. INTERSALT study findings. Public health and medical care implications. Hypertension 1989, 14 (5), 570–577. [CrossRef]
- Meyer, H. E.; Johansson, L.; Eggen, A. E.; Johansen, H.; Holvik, K. Sodium and potassium intake assessed by spot and 24-H urine in the Population-Based Tromsø Study 2015–2016. Nutrients 2019, 11 (7), 1619. [CrossRef]
- Cogswell, M. E.; Loria, C. M.; Terry, A. L.; Zhao, L.; Wang, C.-Y.; Chen, T.-C.; Wright, J. D.; Pfeiffer, C. M.; Merritt, R.; Moy, C. S. et al. Estimated 24-Hour urinary sodium and potassium excretion in US adults. JAMA 2018, 319 (12), 1209. [CrossRef]
- Vasara, E.; Marakis, G.; Breda, J.; Skepastianos, P.; Hassapidou, M.; Kafatos, A.; Rodopaios, N.; Koulouri, A.; Cappuccio, F. Sodium and potassium intake in healthy adults in Thessaloniki Greater Metropolitan Area—The salt intake in Northern Greece (SING) study. Nutrients 2017, 9 (4), 417. [CrossRef]
- McLean, R.; Edmonds, J.; Williams, S.; Mann, J.; Skeaff, S. Balancing Sodium and Potassium: Estimates of Intake in a New Zealand Adult Population Sample. Nutrients 2015, 7, 8930–8938. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4663567/.
- Donfrancesco, C.; Ippolito, R.; Lo Noce, C.; Palmieri, L.; Iacone, R.; Russo, O.; Vanuzzo, D.; Galletti, F.; Galeone, D.; Giampaoli, S. et al. Excess dietary sodium and inadequate potassium intake in Italy: Results of the MINISAL study. NMCD. Nutrition Metabolism and Cardiovascular Diseases 2013, 23 (9), 850–856. [CrossRef]
- Christoforou, A.; Ng, A.; Bernstein, J.; L’Abbe, M. Estimating usual sodium intake and Sodium-to-Potassium molar ratios from urine excretion among Canadian adults: An analysis of the Canadian Health Measures Survey. Current Developments in Nutrition 2021, 5, 1020. [CrossRef]
- Buzina, R.; Keys, A.; Mohaček, I.; Hahn, A.; Brozek, J.; Blackburn, H. C3. Rural men in Dalmatia and Slavonia, Yugoslavia. Acta Medica Scandinavica 1966, 180 (s460), 147–168. [CrossRef]
- Cappuccio, F. P.; Ji, C.; Donfrancesco, C.; Palmieri, L.; Ippolito, R.; Vanuzzo, D.; Giampaoli, S.; Strazzullo, P. Geographic and socioeconomic variation of sodium and potassium intake in Italy: results from the MINISAL-GIRCSI programme. BMJ Open 2015, 5 (9), e007467. [CrossRef]
- Shoaibi, A.; Ghandour, R.; Khatib, R.; Mason, H.; O’Flaherty, M.; Capewell, S.; Husseini, A. Salt reduction as a population-based intervention for the prevention of coronary heart diseases: an economic assessment. Lancet 2013, 382, S33. [CrossRef]
| Whole group | Men | Women | P | |
| N | 1067 | 374 | 693 | |
| Age (years) | ||||
| Mean (SD) | 57.2 (13.9) | 57.2 (13.8) | 56.4 | 0.352 |
| S.E. | 0.37 | 0.75 | 0.55 | |
| Median (IQ range) |
59.0 (48.0-67.0) |
59.0 (48-68) |
59.0 (48-66) |
|
| Systolic BP (mmHg) | ||||
| Mean (SD) | 133.7 (19.0) | 137.9 (18.3) | 131.4 (19.2) | < 0.001 |
| S.E. | 0.5 | 0.99 | 0.77 | |
| Median (IQ range) |
132 (120.0-145.0) |
137.0 (124.5-149.0) |
130.0 (117.0-133.8) |
|
| Diastolic BP (mmHg) | ||||
| Mean (SD) | 82.5 (10.2) | 83.9 (10.2) | 81.5 (10.0) | < 0.001 |
| S.E. | 0.2 | 0.55 | 0.4 | |
| Median (IQ range) |
82.0 (76.0-89.0) |
84.0 (77.5-90.5) |
81.0 (75.1-87.5) |
|
| Heart rate (bpm) | ||||
| Mean (SD) | 75.7 (12.2) | 75.1 (13.0) | 76.4 (12.0) | 0.084 |
| S.E. | 0.3 | 0.71 | 0.48 | |
| Median (IQ range) |
75.0 (67.0-83.0) |
73.0 (65-0-83.3) |
75.6 (68.0-83.0) |
|
| Height (cm) | ||||
| Mean (SD) | 169.1 (16.7) | 178.4 (7.7) | 165.3 (6.8) | < 0.001 |
| S.E. | 0.4 | 0.42 | 0.27 | |
| Median (IQ range) |
168 (162-175) |
178.0 (174.0-183.0) |
165.0 (161.0-170.0) |
|
| Weight (kg) | ||||
| Mean (SD) | 81.4 (16.7) | 92.4 (16.1) | 76.6 (14.5) | < 0.001 |
| S.E. | 0.4 | 0.88 | 0.58 | |
| Median (IQ range) |
80.0 (69.0-91.0) |
90.0 (82.0-100.7) |
75.5 (66.0-85.8) |
|
| Body mass index (kg/m2) | ||||
| Mean (SD) | 28.3 (5.0) | 28.0 (4,6) | 28.0 (5.3) | < 0.001 |
| S.E. | 0.1 | 0.25 | 0.21 | |
| Median (IQ range) |
27.9 (24.8-31.2) |
28.4 (26.0-31,4) |
27.4 (24.0-31.1) |
| 24- hour urine | Whole group | Men | Women | p |
| N | 1067 | 374 | 693 | |
| Volume (ml) | ||||
| Mean (SD) | 1589.9 (628.5) | 1670 (613.2) | 1546 (632.9) | <0.001 |
| S.E. | 19.24 | 31.7 | 24.0 | |
| Median (IQ range) |
1500 (1100-1950) |
1590 (1215-2000) |
1500 (1100-1900) |
|
| Creatinine (g/l) | ||||
| Mean (SD) | 0.8 (0.4) | 1.0 (0.5) | 0.8 (0.4) | <0.001 |
| S.E. | 0.01 | 0.02 | 0.01 | |
| Median (IQ range) |
0.7 (0.5-0.1) |
0.9 (0.6-1.3) |
0.7 (0.5-0,9) |
|
| Sodium (dU) | ||||
| Mean (SD) | 151.8 (68.8) | 179.1 (74.9) | 137.1 (60.4) | <0.001 |
| S.E. | 2.14 | 3.8 | 2.29 | |
| Median (IQ range) |
140.5 (101.5-191.3) |
170.9 (119.7-229.7) |
130.6 (96.7-171.4) |
|
| Potassium (dU) | ||||
| Mean (SD) | 58.5 (22.3) | 64.5 (24.8) | 55.3 (20.1) | <0.001 |
| S.E. | 0.68 | 1.2 | 0.76 | |
| Median (IQ range) |
56.0 (43.0-71.0) |
62.0 (47.0-78.0) |
53 (41-66.5) |
|
| NaCl intake (g/day) |
||||
| Mean (SD) | 9.3 (4.2) | 10.9 (4.5) | 8.4 (3.7) | <0.001 |
| S.E. | 0.12 | 0.23 | 0.14 | |
| Median (IQ range) |
8.6 (6.2-11.7) |
10.5 (7.3-14.0) |
8.0 (5.8-10.5) |
|
| Potassium intake (g/day) |
||||
| Mean (SD) | 2.9 (1.1) | 3.3 (1.2) | 2.8 (1.0) | <0.001 |
| S.E. | 0.03 | 0.06 | 0.03 | |
| Median (IQ range) |
2.8 (2.1-3.5) |
3.1 (2.3-3.9) |
2.6 (2.0-3.3) |
|
| Na-to-K ratio |
||||
| Mean (SD) | 2.8 (1.4) | 3.0 (1.6) | 2.6 (1.2) | <0.001 |
| S.E. | 0.04 | 0.08 | 0.04 | |
| Median (IQ range) |
2.6 (1.8-3.5) |
2.8 (1.9-3.8) |
2.5 (1.8-3.3) |
|
| Urinary iodine excretion (µg/l) | N=577 | N= 208 | N=369 | |
| Mean (SD) | 156.2 (142.19) | 172.6 (144.9) | 147.3 (139.9) | <0.001 |
| S.E. | 7.0 | 10.2 | 7.2 | |
| Median (IQ range) |
131.8 (95.4-174) |
145.9 (108.58-191.6) |
121.7 (91.4-167.1) |
| Iodine status |
Iodine concentration (µg/l)) |
Whole group N= 577 |
Men N=208 |
Women N=369 |
|||
| Insufficient Severe Moderate Mild |
N | % | N | % | N | % | |
| < 20 | 2 | 0.3 | 1 | 0.5 | 1 | 0.3 | |
| 20-49 | 16 | 2.8 | 8 | 3.7 | 8 | 2.2 | |
| 50-99 | 111 | 19.2 | 31 | 14.5 | 80 | 21.7 | |
| Adequate consumption | 100-199 | 298 | 51.7 | 100 | 46.7 | 198 | 53.7 |
| Above requirement | 200-299 | 111 | 19.2 | 54 | 25.2 | 57 | 15.4 |
| Excessive consumption | >300 | 39 | 6.8 | 14 | 6.5 | 25 | 6.8 |
| 24- hour urine | Urban | Rural | p | Mediterranean | Continental | P |
| N | 608 | 376 | 434 | 473 | ||
| NaCl intake (g/day) |
||||||
| Mean (SD) | 8.8 (3.9) | 9.9 (4.4) | < 0.001 | 8.0 (3.6) | 10.3 (4.4) | <0.001 |
| S.E. | 0.16 | 0.23 | 0.17 | 0.23 | ||
| Median (IQ range) |
8.3 (5.8-11.2) |
9.4 (6.5-12.8) |
7.4 (5.3-10.2) |
9.9 (7.2 -13.3) |
||
| Potassium intake (g/day) |
||||||
| Mean (SD) | 3.0 (1.1) | 2.8 (1.0) | 0.006 | 3.0 (1.2) | 2.8 (1.0) | <0.001 |
| S.E. | 0.04 | 0.09 | 0.05 | 0.04 | ||
| Median (IQ range) |
2.9 (2.1-3.7) |
2.7 (2.0-3.4) |
2.9 (2.2-3.8) |
2.6 (2.0-3.3) |
||
| Na-to-K ratio | ||||||
| Mean (SD) | 2.6 (1.4) | 3.0 (1.4) | < 0.001 | 2.4 (1.4) | 3.0 (1.3) | <0.001 |
| S.E. | 0.05 | 0.07 | 0.06 | 0.06 | ||
| Median (IQ range) |
2.3 (1.6-3.1) |
2.9 (2.1-3.7) |
2.0 (1.5-2.9) |
3.0 (2.3-3.8) |
||
| Urinary iodine excretion (µg/l) |
N= 183 | N= 334 | N= 219 | N= 251 | ||
| Mean (SD) | 168.9 (133.1) | 146.4 (130.0) | 0.007 | 145.8 (116.5) | 161.5 (124.3) | 0.012 |
| S.E. | 9.84 | 7.1 | 7.9 | 7.8 | ||
| Median (IQ range) |
127.7 (103.2-193.2) |
119.8 (92.6-109.2) |
124.9 (90.3-171.2) |
141.2 (103.9-183.0) |
| Continental |
Coast | ||||
| Central part |
North-East part |
North-West Part |
North part |
South part |
|
| N | 143 | 234 | 154 | 102 | 434 |
| 24-hour urine volume (ml) | |||||
| Mean (SD) |
1631.3 (667.3) | 1621.6 (652.5) |
1515.7 (579.4) |
1764.6 (840.2) |
1544.3 (564.1) |
| S.E. | 58.9 | 44.0 | 48.9 | 101.8 | 27.2 |
| Median (IQ range) |
1500.0 (1200-1972.5) |
1560.0 (1152-5-1987.9) |
1400.0 (1100.0-1800.0) |
1500.0 (1180.0-2100.0) |
1500.0 (1100.0-1900.0) |
| Urine creatinine (g/l) | |||||
| Mean (SD) |
0.7 (0.3) |
0.91 (0.414) |
0.6 (0.3) |
0.6 (0.3) |
0.9 (0.4) |
| S.E. | 0.02 | 0.02 | 0.02 | 0.03 | 0.02 |
| Median (IQ range) |
0.71 (0.52-0.93) |
0.81 (0.61-1.11) |
0.6 0.42-0.87) |
0.5 (0.42-0.79) |
0.9 (0.6-1.2) |
| 24- hour sodium (dU) | |||||
| Mean (SD) |
169.0 (69.8) |
160.2 (75.3) |
182.3 (66.7) |
147.0 (63.4) |
130.5 (59.6) |
| S.E. | 6.1 | 5.1 | 5.6 | 7.6 | 2.8 |
| Median (IQ range) |
158.2 (119.7-216.3) |
146.9 (109.0-204.0) |
179.5 (133.3-220.8) |
137.8 (98.8-185.0) |
120.1 (87.0-163.2) |
| 24-hour potassium (dU) | |||||
| Mean (SD) |
55.6 (20.1) |
52.2 (20.0) |
59.4 (18.8) |
61.2 (21.4) |
62.4 (25.0) |
| S.E. | 1.7 | 1.3 | 1.5 | 2.5 | 1.2 |
| Median (IQ range) |
52.0 (41.0-66.8) |
49.0 (38.0-66.0) |
58.0 (46.0-71.7) |
59.5 (47.0-74.5) |
60.0 (45-0-76.0) |
| NaCl intake (g/day) | |||||
| Mean (SD) |
10.3 (4.2) |
9.8 (4.6) |
11.1 (4.0) |
9.0 (3.8) |
8.0 (3.6) |
| S.E. | 0.3 | 0.3 | 0.3 | 0.4 | 0.1 |
| Median (IQ range) |
9.7 (7.3-13.2) |
9.0 (6.7-12.6) |
11.0 (8.1-13.5) |
8.4 (6.0-11.3) |
7.3 (5.3-9.9) |
| Potassium intake (g/day) | |||||
| Mean (SD) |
2.8 (1.0) |
2.6 (1.0) |
3.0 (0.9) |
3.1 (1.0) |
3.1 (1.2) |
| S.E. | 0.09 | 0.06 | 0.08 | 0.13 | 0.06 |
| Median (IQ range) |
2.6 (2.1-3.4) |
2.4 (1.9-3.3) |
2.9 (2.3-3.6) |
3.0 (2.3-3.7) |
3.0 (2.3-3.8) |
| Na-to-K ratio | |||||
| Mean (SD) |
3.1 (1.2) |
3.2 (1.5) |
3.1 (1.1) |
2.4 (0.8) |
2.3 (1.4) |
| S.E. | 0.1 | 0.1 | 0.09 | 0.1 | 0.07 |
| Median (IQ range) |
2.9 (2.2-3.9) |
2.9 (2.2-3.9) |
3.1 (2.4-3.7) |
2.3 (1.7-3.0) |
2.0 (1.4-2.9) |
| Urinary iodine excretion (µg/l) |
114 | 60 | 78 | 43 | 223 |
| Mean (SD) | 169.1 (164.4) | 157.3 (83.5) | 153.4 (64.2) | 110.9 (147.3) | 146.1 (136.7) |
| S.E. | 15.5 | 10.7 | 7.3 | 22.4 | 9.2 |
| Median (IQ range) |
140.5 (105.8-181.2) |
138.6 (99.9-196.1) |
146.6 (111.3-179.5) |
126.7 (79.0-169.3) |
122.3 (90.4-171.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





