Submitted:
01 July 2024
Posted:
02 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
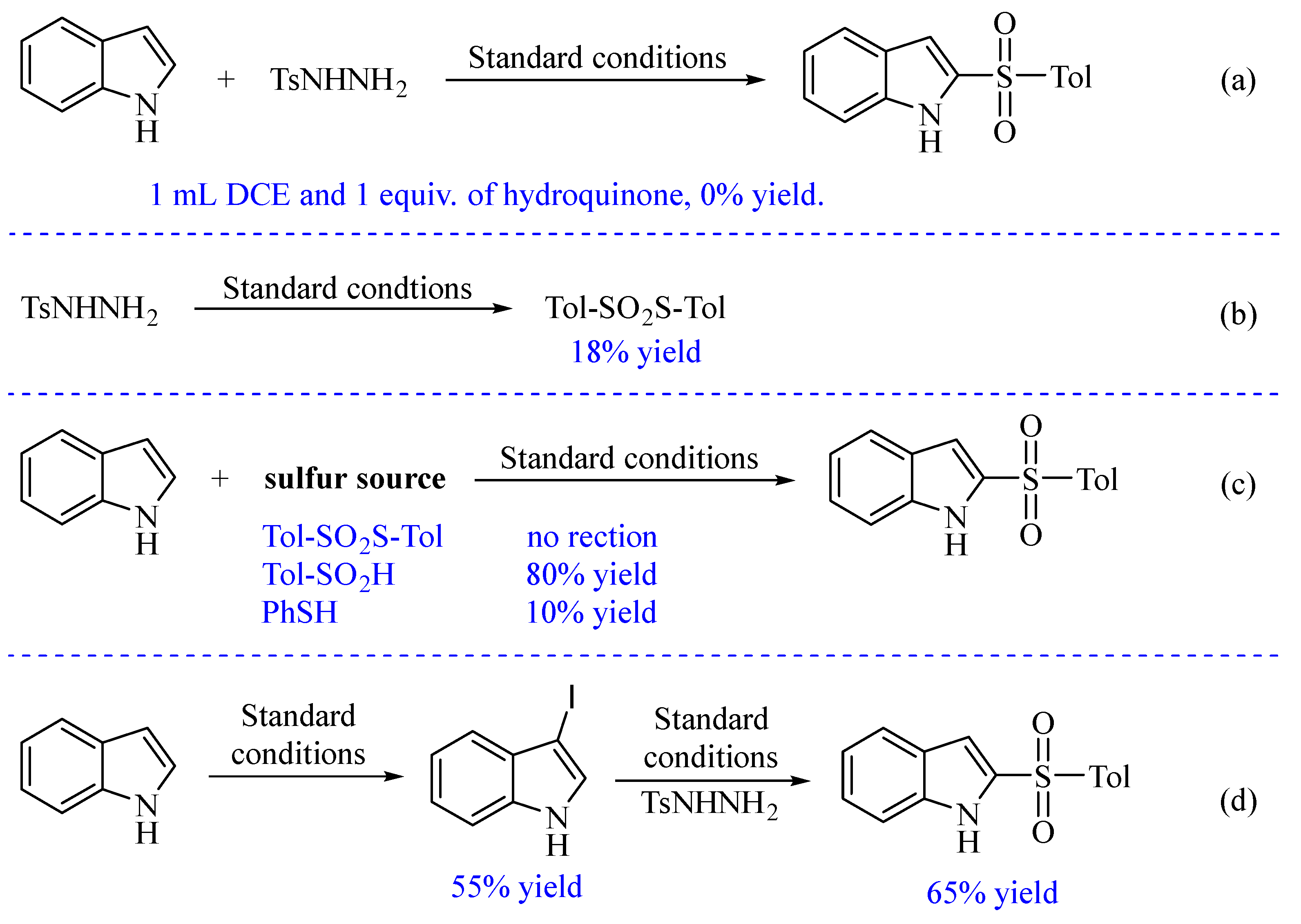
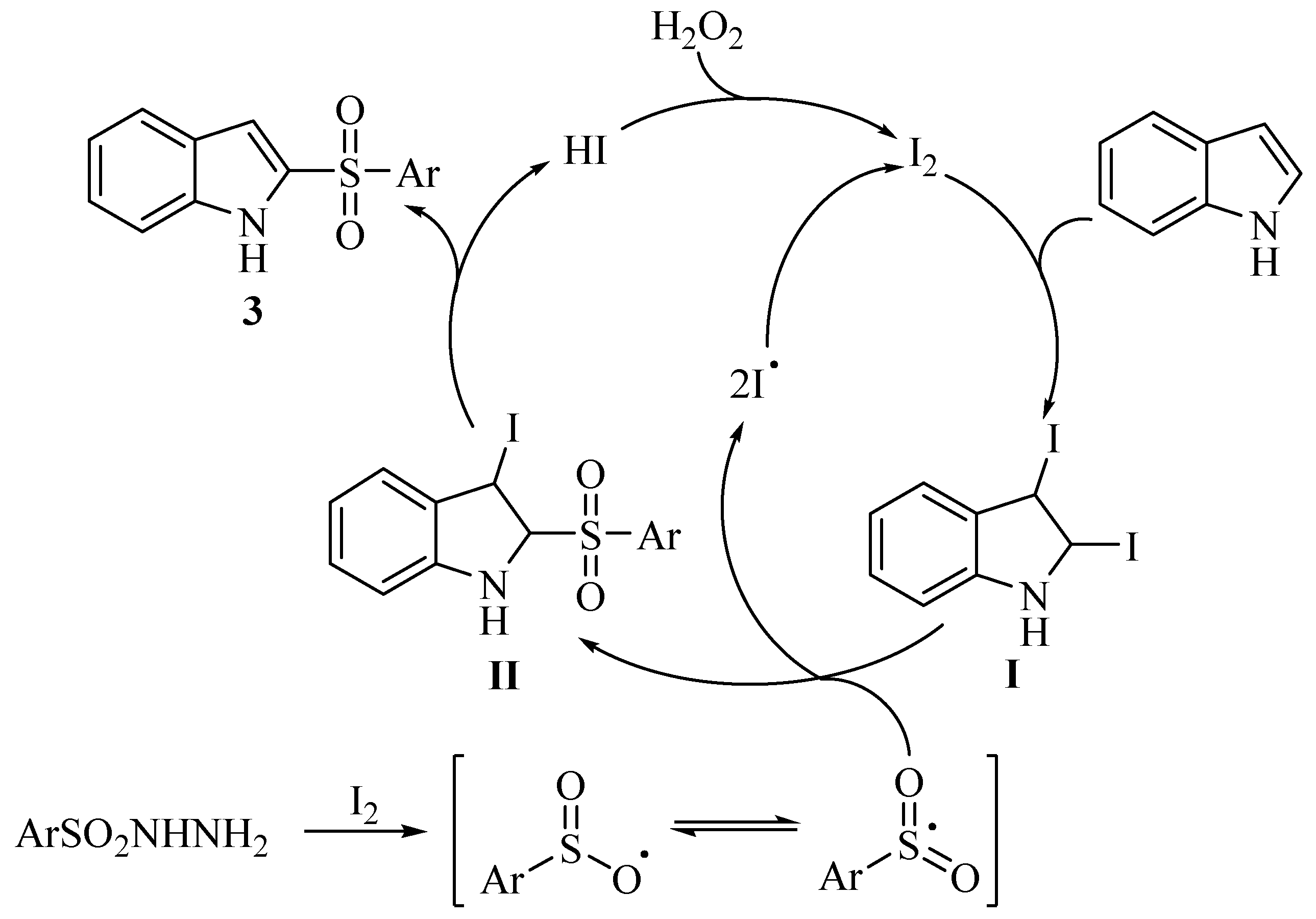
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ban, Y.; Murakami, Y.; Iwasawa, Y.; Tsuchiya, M.; Takano, N. Indole alkaloids in medicine. Med. Res. Rev., 1988, 8, 231–308. [Google Scholar] [CrossRef] [PubMed]
- Hibino, S.; Choshi, T. , Simple indole alkaloids and those with a nonrearranged monoterpenoid unit. Nat. Prod. Rep., 2002, 19, 148–180. [Google Scholar] [CrossRef] [PubMed]
- Takayama, H.; Tsutsumi, S.I.; Kitajima, M.; Santiarworn, D.; Liawruangrath, B.; Aimi, N. Gluco-indole Alkaloids from Nauclea cadamba in Thailand and Transformation of 3α-Dihydrocadambine into the Indolopyridine Alkaloid, 16-Carbomethoxynaufoline. Chem. Pharm. Bull., 2003, 51, 232–233. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.A.; Bordunov, V.; Borka, C.A.; Browner, M.F.; Kress, J.M.; Mirzadegan, T.; Ramesha, C.; Sanpablo, B.F.; Stabler, R.; Takahara, P.; Villasenor, A.; Walker, K.A.M.; Wang, J.H.; Welch, M.; Weller, P. , Rational design of 6-methylsulfonylindoles as selective cyclooxygenase-2 inhibitors. Bioorg. Med. Chem. Lett., 2004, 14, 4741–4745. [Google Scholar] [CrossRef]
- Holenz, J.; Pauwels, P.J.; Diaz, J.L.; Merce,̀ R.; Codony, X.; Buschmann, H. Medicinal chemistry strategies to 5-HT6 receptor ligands as potential cognitive enhancers and antiobesity agents. Drug Discov. Today, 2006, 11, 283–299. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Shankar, B.B.; Chen, L.; Rizvi, R.; Kelly, J.; Gilbert, E.; Huang, C.; Yang, D.Y.; Kozlowski, J.A.; Shih, N.Y.; Gonsiorek, W.; Hipkin, R.W.; Malikzay, A.; Lunn, C.A.; Lundell, D.J. Expansion of SAR studies on triaryl bis sulfone cannabinoid CB2 receptor ligands. Bioorg. Med. Chem. Lett., 2010, 20, 6785–6789. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.P.; Singh, O.M. Recent progress in biological activities of indole and indole alkaloids. Mini-rev. Med. Chem., 2018, 18, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Caddick, S.; Aboutayab, K.; West, R. An intramolecular radical cyclisation approach to fused [1,2-a]indoles. Synlett., 1993, 1993, 231–232. [Google Scholar] [CrossRef]
- Asai, T.; Takeuchi, T.; Diffenderfer, J.; Sibley, D.L. Identification of small-molecule inhibitors of nucleoside triphosphate hydrolase in toxoplasma gondii. Antimicrob. Agents Chemother., 2002, 46, 2393–2399. [Google Scholar] [CrossRef]
- Yang, F.L.; Tian, S.K. Iodine-catalyzed regioselective sulfenylation of indoles with sulfonyl hydrazides. Angew. Chem. Int. Ed., 2013, 52, 4929–4932. [Google Scholar] [CrossRef]
- Sang, P.; Chen, Z.K.; Zou, J.W.; Zhang, Y.H. K2CO3 promoted direct sulfenylation of indoles: a facile approach towards 3-sulfenylindoles. Green Chem., 2013, 15, 2096–2100. [Google Scholar] [CrossRef]
- Wang, P.; Tang, S.; Huang, P.F.; Lei, A.W. Electrocatalytic oxidant-free dehydrogenative C−H/S−H cross-coupling. Angew. Chem. Int. Ed., 2017, 56, 3009–3013. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.Q.; Bao, Y.S.; Dai, Z.H.; Zhou, Q.F.; Yang, F.L. Catalyst-free sulfenylation of indoles with sulfinic esters in ethanol. Green Chem., 2018, 20, 3727–3731. [Google Scholar] [CrossRef]
- Equbal, D.; Singh, R.; Saima; Lavekar, A.G., Sinha, A.K. Synergistic dual role of [hmim]Br-ArSO2Cl in cascade sulfenylation–halogenation of indole: mechanistic insight into regioselective C–S and C–S/C–X (X = Cl and Br) bond formation in one pot. J. Org. Chem.; 2019, 84, 2660–2675. [Google Scholar] [CrossRef] [PubMed]
- Nalbandian, C.J.; Miller, E.M.; Toenjes, S.T.; Gustafson, J.L. A conjugate Lewis base-Brønsted acid catalyst for the sulfenylation of nitrogen containing heterocycles under mild conditions. Chem. Commun., 2017, 53, 1494–1497. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Ghosh, M.; Hajra, A.; Majee, A. A simple and efficient approach for the sulfonylation of indoles catalyzed by CuI. J. Sulfur Chem., 2013, 34, 342–346. [Google Scholar] [CrossRef]
- Tocco, G.; Begala, M.; Esposito, F., Caboni, P.; Cannas, V.; Tramontano, E. ZnO-mediated regioselective C-arylsulfonylation of indoles: a facile solvent-free synthesis of 2- and 3-sulfonylindoles and preliminary evaluation of their activity against drug-resistant mutant HIV-1 reverse transcriptases (RTs). Tetrahedron Lett. 2013, 54, 6237–6241. [Google Scholar] [CrossRef]
- Xiao, F.H.; Chen, H.; Xie, H.; Chen, S.Q.; Yang, L.; Deng, G.J. Iodine-catalyzed regioselective 2-sulfonylation of indoles with sodium sulfinates. Org. Lett., 2014, 16, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Katrun, P.; Mueangkaew, C.; Pohmakotr, M.; Reutrakul, V.; Jaipetch, T.; Jaipetch, D.; Jaipetch, C. Regioselective C2 sulfonylation of indoles mediated by molecular iodine. J. Org. Chem.; 2014, 79, 1778–1785. [Google Scholar] [CrossRef]
- Pagire, S.K.; Hossain, A.; Reiser, O. Temperature controlled selective C–S or C–C bond formation: Photocatalytic sulfonylation versus arylation of unactivated heterocycles utilizing aryl sulfonyl chlorides. Org. Lett., 2018, 20, 648–651. [Google Scholar] [CrossRef]
- Feng, M.L.; Xi, L.Y.; Chen, S.Y.; Yu, X.Q. Electrooxidative metal-free dehydrogenative α-sulfonylation of 1H-indole with sodium sulfinates. Eur. J. Org. Chem., 2017, 2017, 2746–2750. [Google Scholar] [CrossRef]
- Zheng, N.; Shi, W.Y; Ding, Y.N; Liu, X.Y.; Liang, Y.M. Chemo-and regioselective C−H sulfidation of indoles for the synthesis of tolylthioindoles under metal-free conditions. Adv. Synth. Catal., 2022, 364, 4310–4315. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Z.; Chen, L.J.; Liu, Y.; Liu, P.; Dai, B. The fast and efficient KI/H2O2 mediated 2-sulfonylation of indoles and N-methylpyrrole in water. RSC Adv., 2018, 8, 41651–41656. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, R.; Barman, P. A sulfonylation reaction: Direct synthesis of 2-sulfonylindoles from sulfonyl hydrazides and indoles. Synlett., 2017, 28, 684–690. [Google Scholar]
- Singh, R.; Raghuvanshi, D.S.; Singh, K.N. Regioselective hydrothiolation of alkynes by sulfonyl hydrazides using organic ionic Base–Brønsted Acid. Org. Lett., 2013, 15, 4202–4205. [Google Scholar] [CrossRef] [PubMed]
- Li, X.W.; Xu, Y.L.; Wu, W.Q.; Jiang, C.; Qi, C.R.; Jiang, H.F. Copper-catalyzed aerobic oxidative N–S bond functionalization for C–S bond formation: Regio- and stereoselective synthesis of sulfones and thioethers. Chem.–Eur. J., 2014, 20, 7911–7915. [Google Scholar] [CrossRef]
- Guo, S.R.; He, W.M.; Xiang, J.N.; Yuan, Y.Q. Palladium-catalyzed thiolation of alkanes and ethers with arylsulfonyl hydrazides. Chem. Commun., 2014, 50, 8578–8581. [Google Scholar] [CrossRef]
- Xu, K.; Khakyzadeh, V.; Bury, T.; Breit, B. Direct transformation of terminal alkynes to branched allylic sulfones. J. Am. Chem. Soc., 2014, 136, 16124–16127. [Google Scholar] [CrossRef] [PubMed]
- Rong, G.W.; Mao, J.C.; Yan, H.; Zheng, Y.; Zhang, G.Q. Iron/Copper co-catalyzed synthesis of vinyl sulfones from sulfonyl hydrazides and alkyne derivatives. J. Org. Chem., 2015, 80, 4697–4703. [Google Scholar] [CrossRef]
- Singh, R.; Allam, B.K.; Singh, N.; Kumari, K.; Singh, S.K.; Singh, K.N. Nickel-catalyzed C–S bond formation: synthesis of aryl sulfides from arylsulfonyl hydrazides and boronic acids. Adv. Synth. Catal., 2015, 357, 1181–1186. [Google Scholar] [CrossRef]
- Margraf, N.; Manolikakes, G. One-pot synthesis of aryl sulfones from organometallic reagents and iodonium salts. J. Org. Chem., 2015, 80, 2582–2600. [Google Scholar] [CrossRef] [PubMed]
| Entry | Oxidation | Iodophor (mL) | T (℃) | t (min) | Yield (%)b |
|---|---|---|---|---|---|
| 1 | H2O2 (1 equiv.) | 2 (0.04 mmol I2) | 25 | 120 | 28 |
| 2 | H2O2 (1 equiv.) | 2 (0.04 mmol I2) | 25 | 10 | 30 |
| 3 | H2O2 (1 mL) | 2 (0.04 mmol I2) | 25 | 10 | 42 |
| 4 | H2O2 (1 mL) | 2 (0.04 mmol I2) | 50 | 10 | 45 |
| 5 | H2O2 (1 mL) | 2 (0.04 mmol I2) | 60 | 10 | 70 |
| 6 | H2O2 (1 mL) | 2 (0.04 mmol I2) | 80 | 10 | 50 |
| 7 | H2O2 (1 mL) | 2 (0.04 mmol I2) | 90 | 10 | 40 |
| 8 | H2O2 (1 mL) | 2 (0.04 mmol I2) | 100 | 10 | 36 |
| 9 | H2O2 (1 mL) | 1 (0.02 mmol I2) | 25 | 10 | 35 |
| 10 | TBHP (1 mL) | 2 (0.04 mmol I2) | 60 | 10 | 55 |
| 11 | H2O2 (0.5 mL) | 2 (0.04 mmol I2) | 60 | 10 | 58 |
| 12 | H2O2 (0.5 mL) | 2 (0.04 mmol I2) | 25 | 10 | 38 |
| 13 | H2O2 (0.5 mL) | 2 (0.04 mmol I2) | 90 | 10 | 32 |
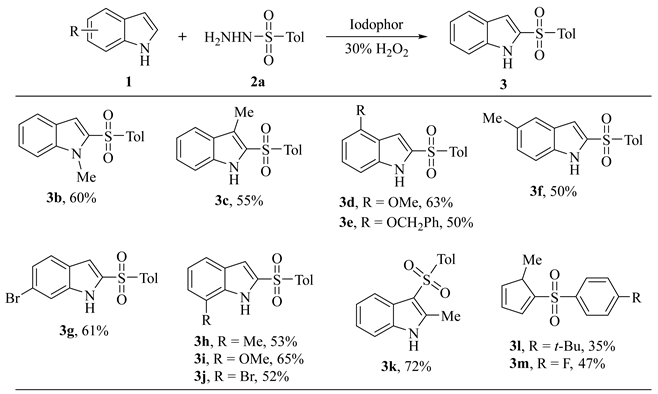 |
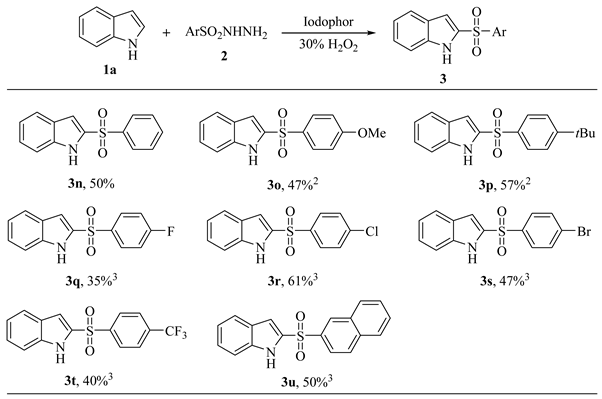 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




