Submitted:
01 July 2024
Posted:
02 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Electron-Induced Charging Phenomena in Insulting Materials
3. Measuring the Surface Potential
4. Elastic Peak and Recoil Energy. Theory and Simulation
5. Elastic and Inelastic Scattering Cross-Sections
5.1. Elastic Scattering
5.2. Inelastic Scattering
6. Moving Atoms
7. Electron-Induced Hydrogen Desorption
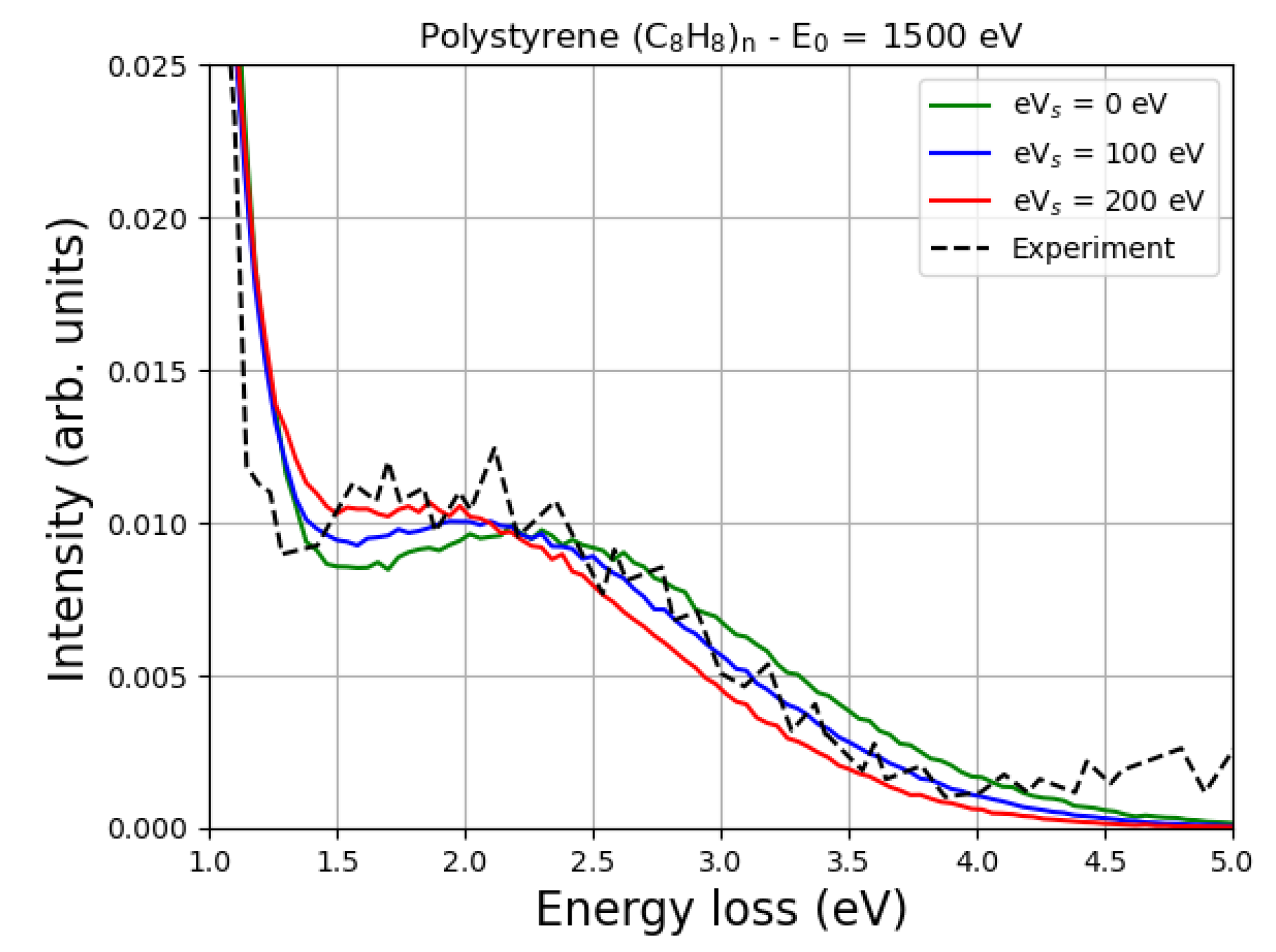
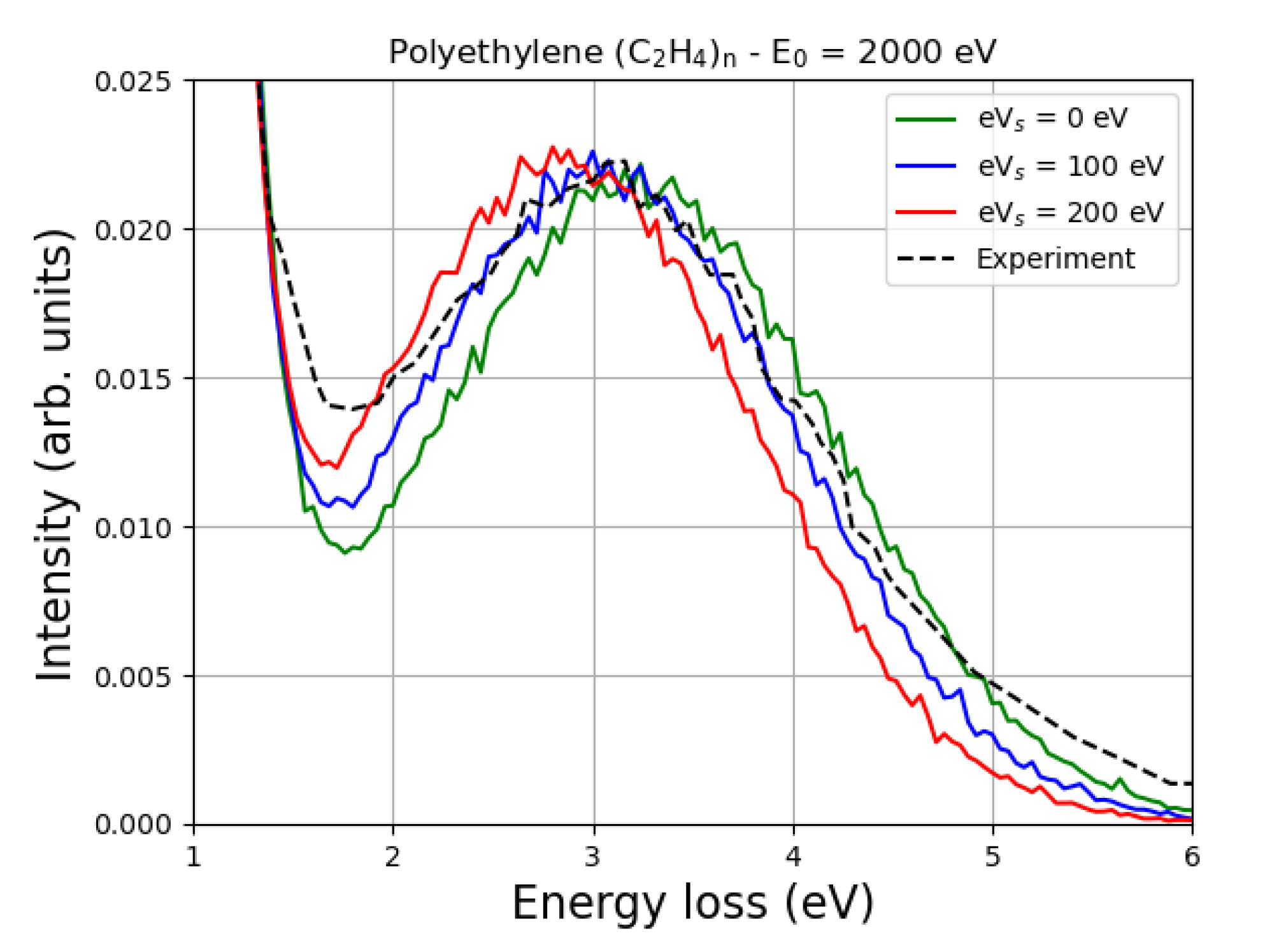
8. Simulating Elastic Peak Spectra
9. Conclusions
Acknowledgments
References
- Berger, P.; Raepsaet, C.; Khodja, H. Elastic Recoil Detection Analysis. In Neutron Scattering and Other Nuclear Techniques for Hydrogen in Materials; Springer International Publishing: Cham, 2016; pp. 277–314. [Google Scholar]
- Chatzidimitriou-Dreismann, C.A.; Vos, M.; Kleiner, C.; Abdul-Redah, T. Comparison of Electron and Neutron Compton Scattering from Entangled Protons in a Solid Polymer. Phys. Rev. Lett. 2003, 91, 057403 1-4. [Google Scholar] [CrossRef] [PubMed]
- Varga, D.; Tökési, K.; Berényi, Z.; Tóth, J.; Kövér, L.; Gergely, G.; Sulyok, A. Energy Shift and Broadening of the Spectra of Electrons Backscattered Elastically from Solid Surfaces. Surf. Interface Anal. 2001, 31, 1019–1026. [Google Scholar] [CrossRef]
- Kwei, C.M.; Li, Y.C.; Tung, C.J. Energy Spectra of Electrons Quasi-Elastically Backscattered from Solid Surfaces. J. Phys. D: Appl. Phys. 2004, 37, 1394–1399. [Google Scholar] [CrossRef]
- Vos, M. Observing Atom Motion by Electron-Atom Compton Scattering. Phys. Rev. A 2001, 65, 012703 1-5. [Google Scholar] [CrossRef]
- Vos, M.; Went, M.R. Elastic electron scattering at high momentum transfer: A possible new analytic tool. J. Electron Spectrosc. Relat. Phenomena 2006, 155, 35–39. [Google Scholar] [CrossRef]
- Vos, M.; Marmitt, G.G.; Grande, P.L. A Comparison of ERBS Spectra of Compounds with Monte Carlo Simulations. Surf. Interface Anal. 2016, 48, 415–421. [Google Scholar] [CrossRef]
- Yubero, F.; Rico, V.J.; Espinós, J.P.; Cotrino, J.; González-Elipe, A.R. Quantification of the H Content in Diamond-Like Carbon and Polymeric Thin Films by Reflection Electron Energy Loss Spectroscopy. Appl. Phys. Lett. 2005, 87, 084101 1-3. [Google Scholar] [CrossRef]
- Yubero, F.; Pauly, N.; Dubus, A.; Tougaard, S. Test of Validity of the V-type Approach for Electron Trajectories in Reflection Electron Energy Loss Spectroscopy. Phys. Rev. B 2008, 77, 245405 1-11. [Google Scholar] [CrossRef]
- Alvarez, R.; Yubero, F. Interpretation of Electron Rutherford Backscattering Spectrometry for Hydrogen Quantification. Surf. Interface Anal. 2014, 46, 812–816. [Google Scholar] [CrossRef]
- Filippi, M.; Calliari, L. On the Use of Elastic Peak Electron Spectroscopy (EPES) to Measure the H Content of Hydrogenated Amorphous Carbon Films. Surf. Interface Anal. 2008, 40, 1469–1474. [Google Scholar] [CrossRef]
- Dapor, M. Electron-induced Hydrogen Desorption from Selected Polymers (Polyacetylene, Polyethylene, Polystyrene, and Polymethyl-methacrylate). Phys. Scr. 2024, 99, 0659b4. [Google Scholar] [CrossRef]
- Orosz, G.T.; Gergely, G.; Menyhard, M.; Tóth, J.; Varga, D.; Lesiak, B.; Jablonski, A. Hydrogen and Surface Excitation in Electron Spectra of Polyethylene. Surf. Sci. 2004, 566–568, 544–548. [Google Scholar] [CrossRef]
- Werner, W. Electron Beams Near Surfaces: The Concept of Partial Intensities for Surface Analysis and Perspective on the Low Energy Regime. Front. Mater. 2023, 10, 1202456 1–23. [CrossRef]
- Cazaux, J. Some Considerations on the Electric Field Induced in Insulators by Electron Bombardment. J. Appl. Phys. 1986, 59, 1418–1430. [Google Scholar] [CrossRef]
- Cazaux, J. Charging in Scanning Electron Microscopy “from Inside and Outside”. Scanning 2004, 26, 181–203. [Google Scholar] [CrossRef] [PubMed]
- Cazaux, J. A New Model of Dependence of Secondary Electron Emission Yield on Primary Electron Energy for Application to Polymers. J. Phys. D: Appl. Phys. 2005, 38, 2433–2441. [Google Scholar] [CrossRef]
- Joy, D.C. A Model for Calculating Secondary and Backscattered Electron Yields. J. Microsc. 1987, 147, 51–64. [Google Scholar] [CrossRef]
- Joy, D.C. Control of Charging in Low-Voltage SEM. Scanning 1989, 11, 1–4. [Google Scholar] [CrossRef]
- Joy, D.C.; Joy, C.S. Low Voltage Scanning Electron Microscopy. Micron 1996, 27, 247–263. [Google Scholar] [CrossRef]
- Ciappa, M.; Koschik, A.; Dapor, M.; Fichtner, W. Modeling secondary electron images for linewidth measurement by critical dimension scanning electron microscopy. Microelectron. Reliab. 2010, 50, 1407–1412. [Google Scholar] [CrossRef]
- Koschik, A.; Ciappa, M.; Holzer, S.; Dapor, M.; Fichtner, W. A Novel Monte Carlo Simulation Code for Linewidth Measurement in Critical Dimension Scanning Electron Microscopy. Proc. of SPIE 2010, 7729, 1–12. [Google Scholar]
- Miotello, A. Mobility and Surface Recombination Process of Primary Electrons in Dielectric System during Auger Electron Spectroscopy. Phys. Lett. 1984, 103A, 279–282. [Google Scholar] [CrossRef]
- Miotello, A.; Dapor, M. Slow Electrons Impinging on Dielectric Solids. II. Implantation Profiles, Electron Mobility, and Recombination Processes. Phys. Rev. B 1997-II, 56, 2241–2247.
- Frank, L.; Müllerová, I.; Delong, A. Microscopy with slow electrons. Czech. J. Phys. 1994, 44, 195–238. [Google Scholar] [CrossRef]
- Paoli, M.; Holt, R. Anisotropy in the Atomic Momentum Distribution of Pyrolytic Graphite. J. Phys. C: Solid State Phys. 1988, 21, 3633–3639. [Google Scholar] [CrossRef]
- Shimizu, R.; Ding, Z.J. Monte Carlo Modelling of Electron-Solid Interactions. Rep. Prog. Phys. 1992, 55, 487–531. [Google Scholar] [CrossRef]
- Dapor, M. Transport of Energetic Electrons in Solids; Vol. 290, Springer Tracts in Modern Physics, Springer Nature Switzerland AG, 2023.
- Mayol, R.; Salvat, F. Total and Transport Cross Sections for Elastic Scattering of Electrons by Atoms. At. Data Nucl. Data Tables 1997, 65, 55–154. [Google Scholar] [CrossRef]
- Dapor, M. Electron-Atom Collisions. Quantum-Relativistic Theory and Exercises; De Gruyter, Berlin, Boston, 2022.
- Ritchie, R.H. Plasma Losses of Fast Electrons in Thin Films. Phys. Rev. 1957, 106, 874–881. [Google Scholar] [CrossRef]
- Tanuma, S.; Powell, C.J.; Penn, D.R. Calculation of Electron Inelastic Mean Free Paths. Surf. Interface Anal. 1993, 21, 165–176. [Google Scholar] [CrossRef]
- Mayers, J.; Burke, T.M.; Newport, R.J. Neutron Compton Scattering from Amorphous Hydrogenated Carbon. J. Phys.: Condens. Matter 1994, 6, 641–658. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




