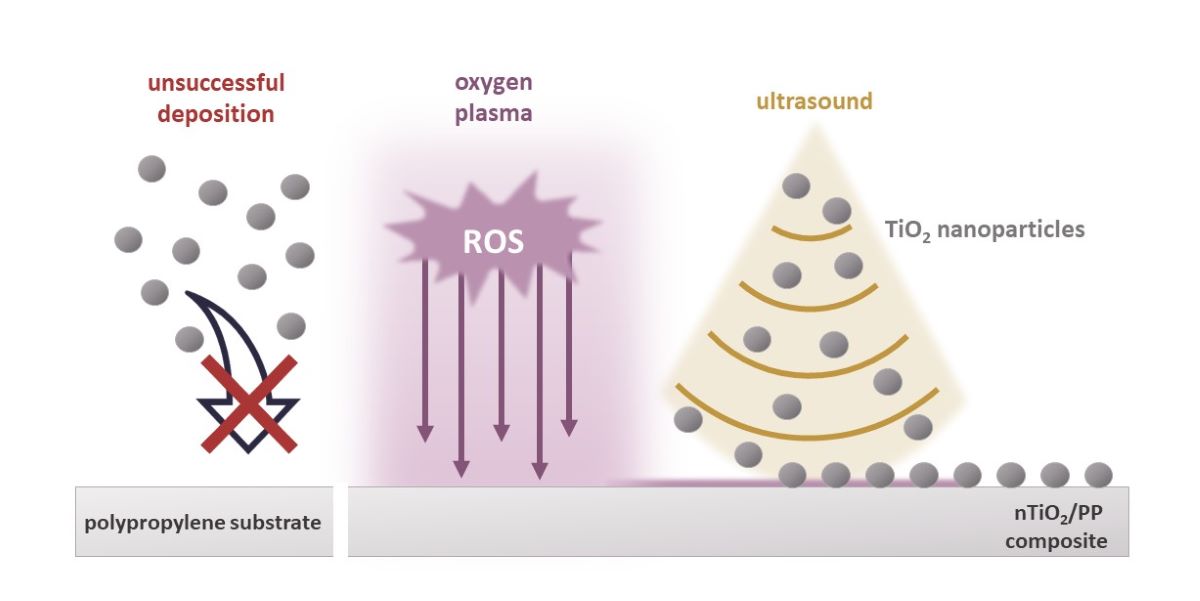
1. Introduction
Polypropylene (PP), due to its vast diversity of applications, is one of the most widespread polymeric materials in the world. It gained popularity almost instantly after its discovery, and the production of PP steadily increased [
1]. Diverse types of industries, including biomedical, automobile, aerospace, textiles, and packaging, to mention a few, employ polypropylene using their unique and adjustable characteristics. The most crucial advantages of these materials are chemical resistance, mechanical properties, and cost-effectiveness [
2]. Polypropylene properties could be additionally modified via chemical and physical methods, among which copolymerization and structural modification using additives and forming composites. The resultant materials could be processed in many ways, like injection or extrusion, and form final products. From the practical point of view, surface functionalization is particularly interesting, where the enhancement and/or addition of new surface properties is achieved without changing the bulk characteristics [
3,
4].
Nowadays, one of the modern trends in polymeric surface functionalization concerns antibacterial and self-cleaning surfaces, which can be achieved through the introduction of photocatalytic nanoparticles on the surface [
5]. When irradiated, the photocatalysts generate reactive oxygen species such as hydroxyl radical (HO
•) and superoxide radical (O
2•–). The primarily formed O
2•– species start a cascade of ROS formation, including H
2O
2, which, together with HO
•, can destroy the broad range of contaminants. They typically include inanimate matter, such as inorganic/organic substances, and living organisms, such as bacteria, fungi, and algae. Inorganic contaminants such as dust could be washed away due to superhydrophilic properties. In this case, the water contact angle should be below 10° so the water droplets can spread over and clean the surface [
6]. Organic impurities could be removed from the surface via total degradation to CO
2 and H
2O (mineralization) by photogenerated charges or reactive oxygen species. The main mechanism in destroying bacterial cell walls also involves damage to bacteria cell walls and operates for both Gram-negative and Gram-positive strains. However, it should be noted that the generated oxygen radicals are short-lived and are not found further than 1 μm from the photocatalyst surface [
7]. As the distance between the bacteria and the photocatalyst is essential for the self-disinfection process of all surfaces, the preparation of a system requires a high dispersion of photoactive nanoparticles over the polymer surface.
Titanium dioxide in the form of nanoparticles is one of the best known robust photocatalysts with superior properties, particularly photoactivity, nontoxicity and commercial availability. Thus, the deposition of titanium dioxide nanoparticles on the polymers could effectively trigger their photocatalytic cleaning [
8,
9]. The most commonly used approach is to add nanoparticles to monomers before forming them into a final product. There are several methods reported for successful preparation of PP-nTiO
2 composites like the direct blending of titania NPs with polymers, in situ formation of NPs within the polymeric matrix, copolymerization of surface-modified NPs, grafting or self-assembly of NPs and polymers [
10,
11]. It is worth mentioning that in such a case, a majority of nanoparticles are located in the bulk and, due to limited light penetration, are photocatalytic inactive. The bulk embedment could also result in undesired modifications of bulk properties of the commercial polymeric materials [
12].
Considering the above considerations, optimal functionalization should be limited to the surface decoration of polymeric materials with photocatalytic nanoparticles. The polypropylene surface is, however, chemically inert, which is a huge advantage for its stability in harsh chemical environments. However, it also makes functionalization difficult, especially via coating or attachment of nanoparticles [
13].
From a practical point of view, the best approach is to create surface chemical bonds between polypropylene and TiO
2 nanoparticles. The strategy involves the generation of abundant surface functional groups on the polypropylene to enhance nanoparticle anchoring [
14]. For this purpose, effective modification of the PP surface can be achieved by plasma treatment. Exposure of polymeric surfaces to ionized gas molecules, e.g. oxygen plasma, effectively changes their properties, like wettability and adhesion. Using low-temperature plasma the surface properties can be changed effectively by generating functional groups while preserving the bulk properties of the polymeric materials [
15]. Moreover, the surface modification could be precisely controlled by adjusting plasma parameters (power, oxygen partial pressure, treatment time) [
16,
17]. Such functionalized surfaces can then be decorated with photoactive nanoparticles with the use of a sonochemical process. The advantages of applying ultrasound for inorganic particle deposition over polymeric surface consist in employing ultrasound energy not only for the deposition process but also for softening the polymeric matrix, thus making the nanoparticle embedding effective. Consequently, the final product is expected to exhibit higher mechanical stability [
18].
In this paper, oxygen plasma treatment on polypropylene material has been investigated. New functional groups generated on the polymer surface after plasma treatment were monitored by the water contact angle, while their chemical nature was characterized by employing FTIR and XPS analysis. The process of depositing nanoparticles on the surface was performed using the sonochemical method, modifying the sonication time and concentration of the nTiO2 suspension. The influence of oxygen surface groups on the attachment and dispersion of TiO2 nanoparticles was documented by SEM images. The photocatalytic activity was tested in the methyl orange (as model contamination compound) degradation test using UV irradiation. The effect of plasma treatment on the properties of the obtained nTiO2/PP composites is discussed in terms of the nanoparticles dispersion and related photocatalytic activity.
2. Materials and Methods
2.1. Material Preparation
Titanium dioxide nanoparticles were purchased from Cinkarna Celje d. d. The particles were in a 20% colloidal solution, which was diluted to 1% or 0.1% before the experiments.
In experiments, 1 mm-thick polypropylene sheets (Merck) were used. The sheets were washed with isopropanol and dried in air to prepare the surface for further functionalization and measurement.
To functionalize the polymer's surface, oxygen plasma treatment (FEMTO system, Diener Electronics) was used with a controlled oxygen partial pressure of 0.2 mbar, plasma generator power of 100 W, and a defined sample exposure time (0, 1, 2, 5, or 10 min).
A sonochemical method was used to anchor nanoparticles to oxygen plasma-modified polypropylene. After plasma treatment, the polymer was immediately immersed in 5 ml of titanium dioxide solution (0.1%/0.2%/0.5%/1%). The samples were irradiated at a frequency of 20 kHz, amplitude of 30%, and time from 1 min to 5 min using a homogeniser (Ti-horn, QSonica Q500). A cold-water bath was used to avoid overheating.
2.2. Nanoparticle Tracking Analysis (NTA)
The hydrodynamic diameter of nanoparticles was investigated using the nanoparticle tracking analysis method with Nanosight LM10 (A.P. Instruments). The instrument's software calculates the particle diffusion coefficient and counts the particles' hydrodynamic diameter using the Stokes-Einstein equation. Every solution was investigated three times.
2.3. Attenuated Total Reflection – Fourier Transformation Infrared Spectroscopy (ATR-FTIR)
To examine changes with chemical groups on the surface after plasma treatment, the ATR-FTIR method was used with Nicolet Summit FTIR Spectrometers (ThermoFisher Scientific) equipment. Polypropylene samples were measured right after plasma treatment. Each studied sample was scanned 64 times in the range of 600–4000 cm–1.
2.4. X-ray Photoelectron Spectroscopy (XPS)
To identify the surface functional groups formed during plasma treatment, polypropylene samples were investigated by XPS. The measurements were conducted in an ultrahigh vacuum chamber (vacuum level >5×10–9 mbar) using an SES R4000 analyzer (Gammadata Scienta), with a monochromatic Al Kα X-ray source (1486.6 eV) at 250 W (pass energy: 100 eV) for the survey and narrow scans. The received XPS spectra were analysed using the Casa-XPS 2.3.15 software. The electron binding energy of the C 1s peak was calibrated at 285 eV.
2.5. SEM
The fabricated nanocomposites were investigated using an FEI Quanta 3D FEG field-emission scanning electron microscope (SEM) at low vacuum conditions (10 mbar of H2O pressure, evaporated from a distilled water reservoir) in the so-called Environmental SEM (ESEM) mode. This experimental setting was required due to the non-conductive nature of the polymeric substrate. To avoid disturbing the morphology of the nanocomposite, the samples were investigated in their native forms without any additional coatings. To verify the elemental composition of the nanocomposite, EDX measurements were also carried out for specific areas of interest.
2.6. TG/DTA
Thermogravimetric measurements with differential thermal analysis were performed with TGA 3+ equipment (Mettler Toledo). The measurements were carried out under the airflow in the temperature range of 30–700°C with a heating rate of 10°C/min. The measured mass loss of samples (~ 7 mg) was normalized to 100% for reliable comparison of the thermogravimetric profiles.
2.7. Photocatalytic Tests
The photocatalytic activity was tested using the methyl orange photodegradation reaction under UV-A light (365 nm) using a Sylvania blacklight bulb 20 W. The prepared nanocomposites – polypropylene (35 mm x 8 mm) with different nTiO2 coating – were placed in a quartz cuvette with standard 3.5 ml of methyl orange aqueous solution (25 mmol/dm3). Absorbance was measured periodically by UV-Vis spectrometer (Shimadzu, model 1900i) to determine changes in methyl orange concentration upon illumination. The characteristic absorbance maximum for methyl orange at 464 nm was used for kinetic measurements.
3. Results
3.1. Polypropylene Substrate
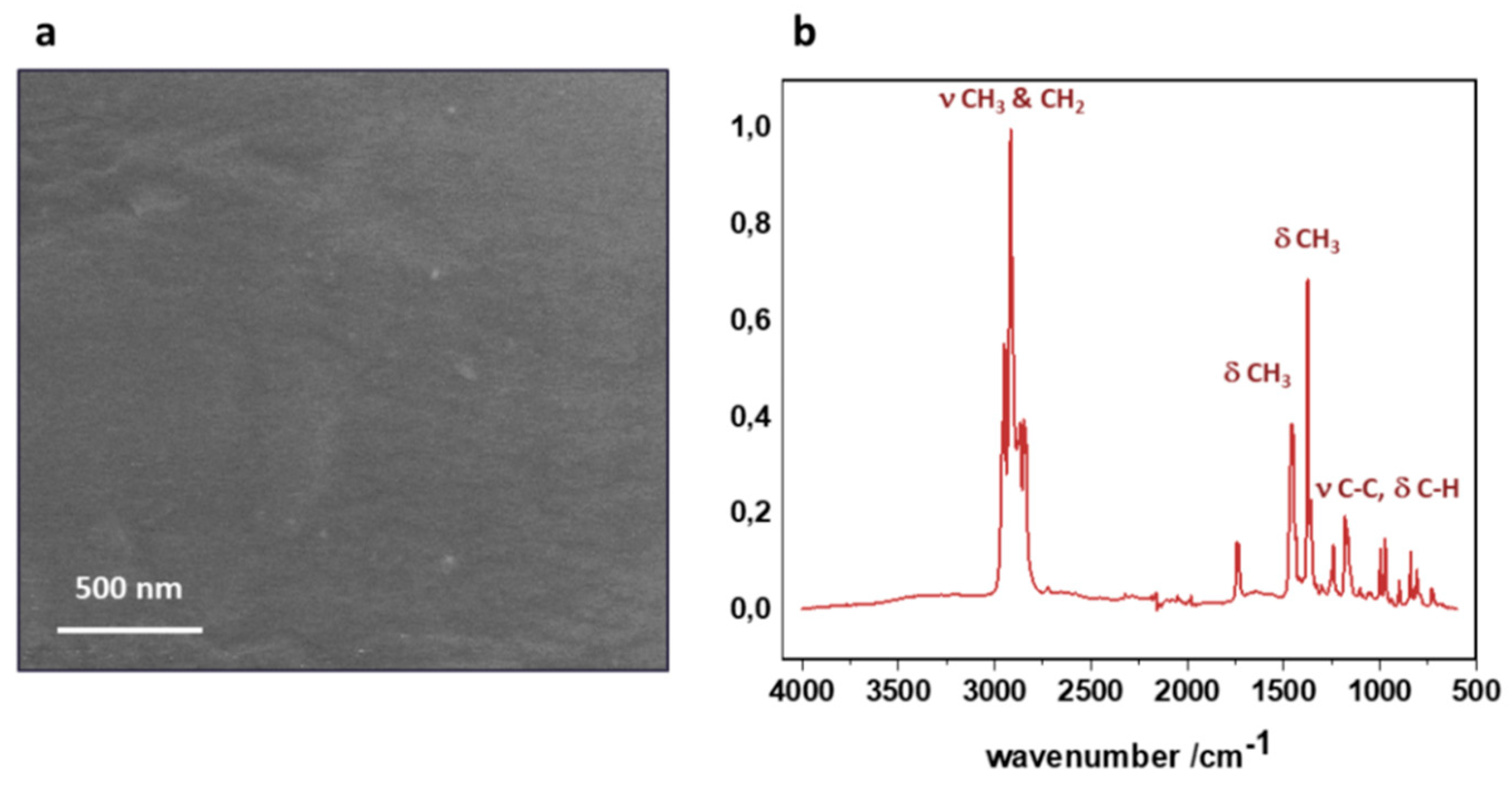
Before the preparation of the nTiO
2/PP nanocomposite, the main constituents were characterized in their pristine forms. The SEM and FTIR characterization of the polypropylene substrate is shown in
Figure 1. The SEM image demonstrates that the polypropylene exhibits a fairly smooth surface morphology on the micrometer scale, with scratches or irregularities caused by the production process not typically observed for polymers. A surface examination using ATR-FTIR (
Figure 1b) provides insight into the chemical structure of the polypropylene. All the characteristic bands present in the polypropylene fingerprint can be easily observed [
19], whereas no maxima characteristic for any contaminants or impurities were identified.
3.2. Characteristics of Titanium Dioxide Nanoparticles
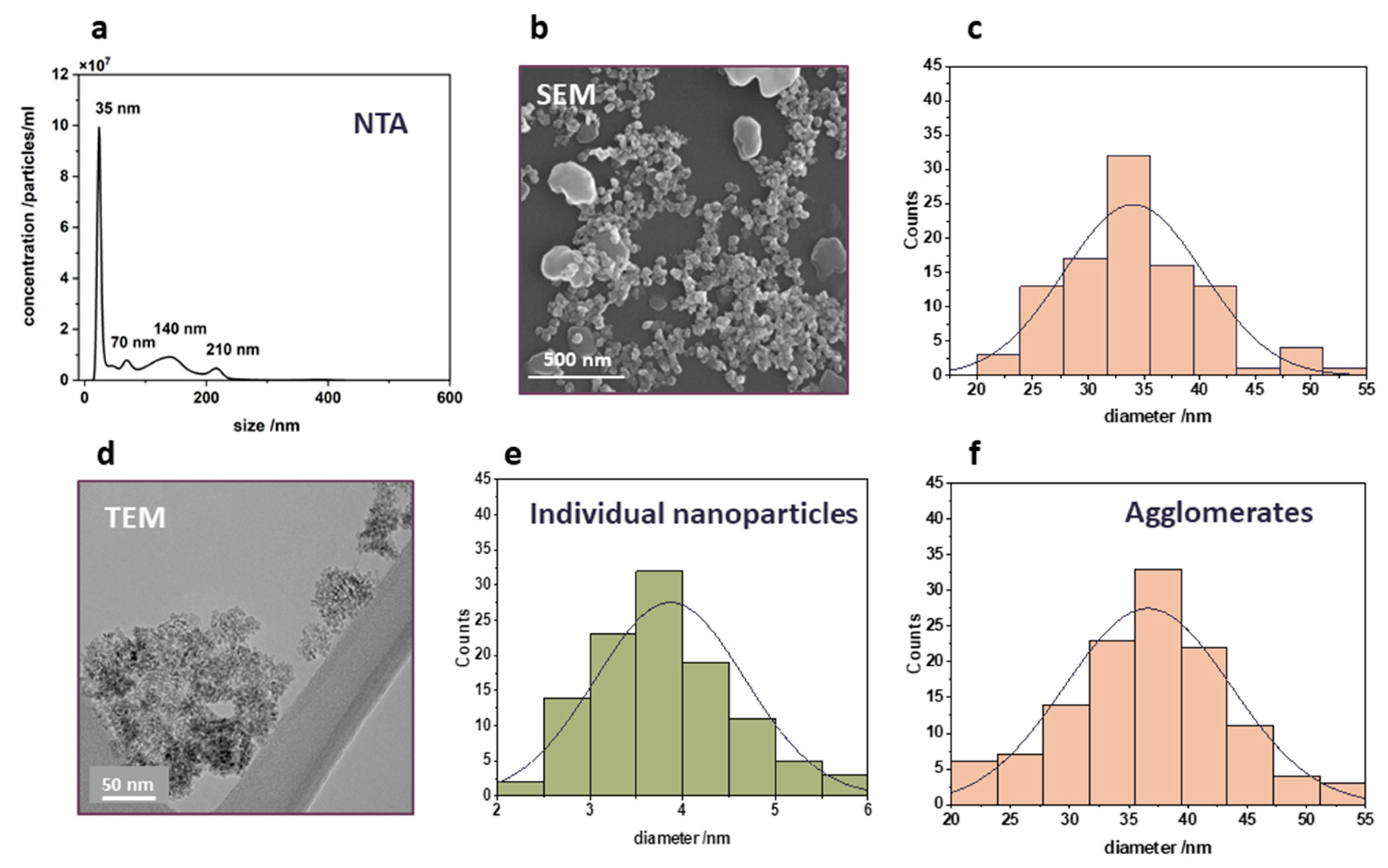
As the photocatalytic reactions occur on the surface of the photocatalyst, the size of the photocatalyst particles is a crucial factor for the efficacy of photocatalytic processes. Therefore, the second constituent of the prepared nanocomposites – photoactive titania nanoparticles – was characterized in terms of their size. The results are presented in
Figure 2 a-f. The most abundant hydrodynamic diameter of the used nTiO2 determined with the method of nanoparticle tracking analysis was found to be 35 nm (
Figure 2a). It is worth noting that other peaks at 70 nm, 140 nm, and 210 nm represent the multiplicity of the smallest particle diameter and can be attributed to agglomerates, which are formed due to a strong interaction between nanoparticles. The size distribution of nanoparticles was also examined by SEM, showing a particle size range between 20 and 55 nm (
Figure 2b). The majority of particles present are approximately 35 nm in size, as can be inferred from the particle size distribution presented in
Figure 2c. However, bigger particles of around 200 nm were also occasionally observed. The results of the EDX analysis confirmed that all the particles contain only titanium and oxygen. To analyze the nanoparticles in more detail, TEM analysis was conducted.
Figure 2d displays a typical TEM image of TiO₂ nanoparticles, illustrating that the nanoparticles observed in SEM are, in fact, agglomerates of much smaller crystallites. As can be inferred from
Figure 2e, the size distribution of the individual TiO₂ crystallites is in the range of 2–6 nm. Nevertheless, when the whole agglomerates are measured based on TEM images (
Figure 2f), the size matches the diameter of 35 nm, concluded from SEM.
3.3. Surface Functional Groups
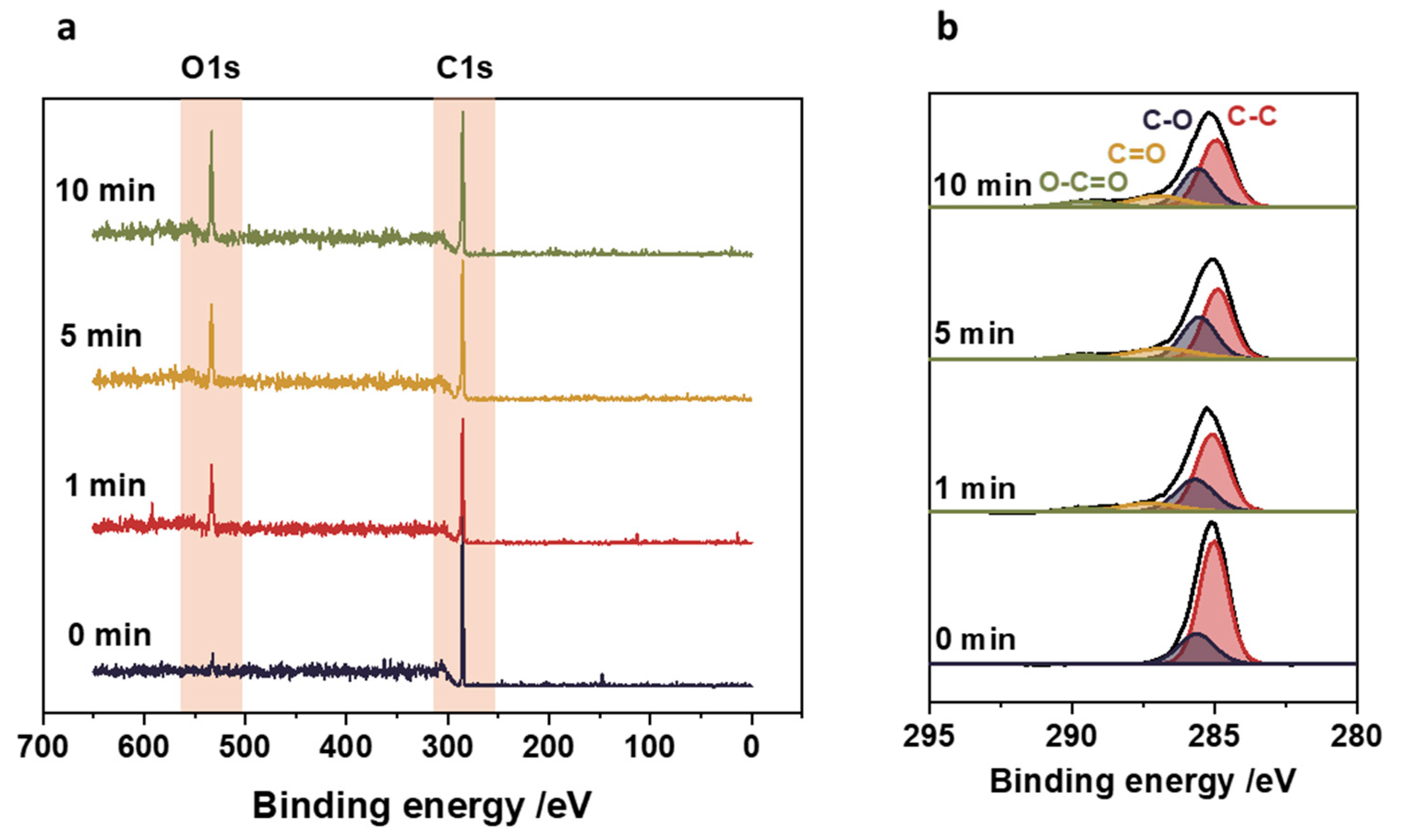
Oxygen plasma treatment was used to increase surface adhesion and facilitate the attachment of nanoparticles to polypropylene. The main changes introduced by low-temperature oxygen plasma on the polypropylene surface consist of generating surface functional groups. Modifying the polypropylene surface depends on the chemical nature and the surface concentration of the functional groups. Therefore, the surfaces before and after plasma treatment were characterized by XPS. The results are shown in
Figure 3. The XPS survey scans show only the main constituents of the polymeric substrate: oxygen O 1
s at 533 eV and carbon C 1
s at 285 eV. It can be observed that before plasma treatment, polypropylene contained surface oxygen at a level as low as 2.8%. Its concentration, however, dramatically changes upon plasma surface modification, as can be seen comparing the ratio of C 1
s to O 1
s. The longer the plasma treatment was applied, the higher the surface oxygen concentration was observed on polypropylene, with 21.3% reached after 10 minutes.
The XPS results also permit to characterize the chemical nature of the generated functional groups. The narrow scan XPS spectra, including analysis of C 1
s peak, are shown in
Figure 3b. As expected, a broadening of the C 1
s peak was observed after plasma treatment, which is related to its oxidation resulting from the generation of oxygen groups. After 1 minute of oxygen plasma, the new component can be distinguished at 287 eV, corresponding to hydroxyl (-OH) and carbonyl groups (-C=O). After 5 minutes of plasma treatment, a new peak at 288 eV can be distinguished and assigned to carboxyl bonds (O–C=O) [
19]. Following the application of a plasma treatment for 10 minutes, the peak with the broadest distribution is observed, corresponding to the carbon-oxygen bonds. This clearly indicates the increase in concentration of functional groups on the surface of the polypropylene with time of exposure to oxygen plasma.
The surface changes induced by plasma were also confirmed by FTIR measurements (
Figure S1). After plasma treatment, two new bands appeared in the polypropylene spectra. A broad band at 3000-3500 cm
–1 corresponds to the vibration of –OH bonds. A second band at 1730 cm
–1 can be assigned to –C=O vibrations. Both of these maxima indicate surface oxidation. Additionally, the intensity of the observed bands increased with the time of exposure to plasma, which aligns with the XPS results described above.
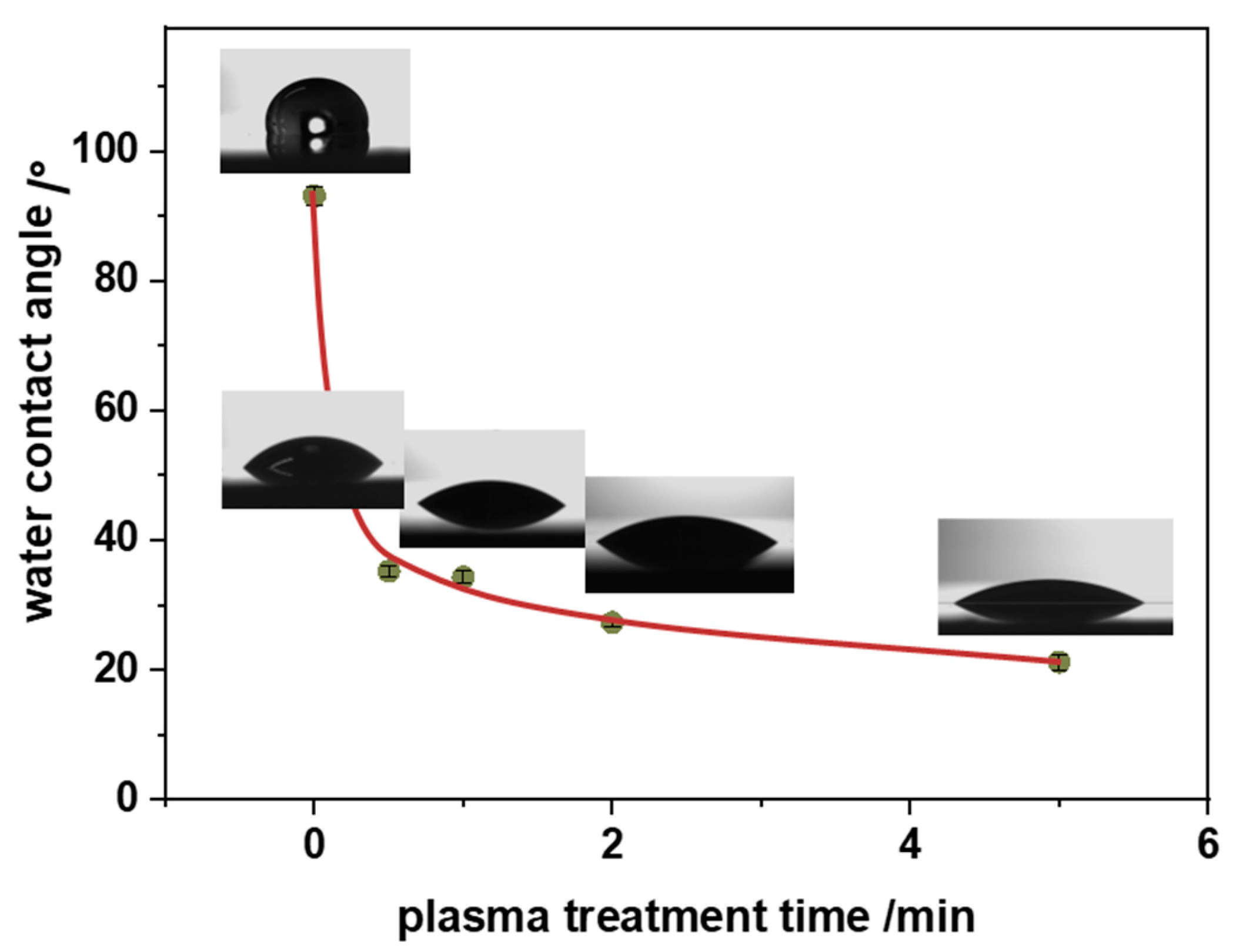
Introducing surface functional groups on the hydrophobic surface of polypropylene has very important practical implications. Since oxygen-containing functional groups are polar in nature, they stimulate stronger interactions with water molecules. Consequently, the surface becomes more hydrophilic as can be quantified experimentally by water contact angle values. They are presented as a function of plasma treatment time in
Figure 4. It can be noticed that after just 1 minute of plasma the water contact angle drops down from 95° for pristine PP to 30°. After that, a gentler water contact angle decrease is observed, reaching a stable level of about 20° after 5 minutes of plasma. The induced wettability of PP has a further impact on the deposition of nTiO
2, as discussed below.
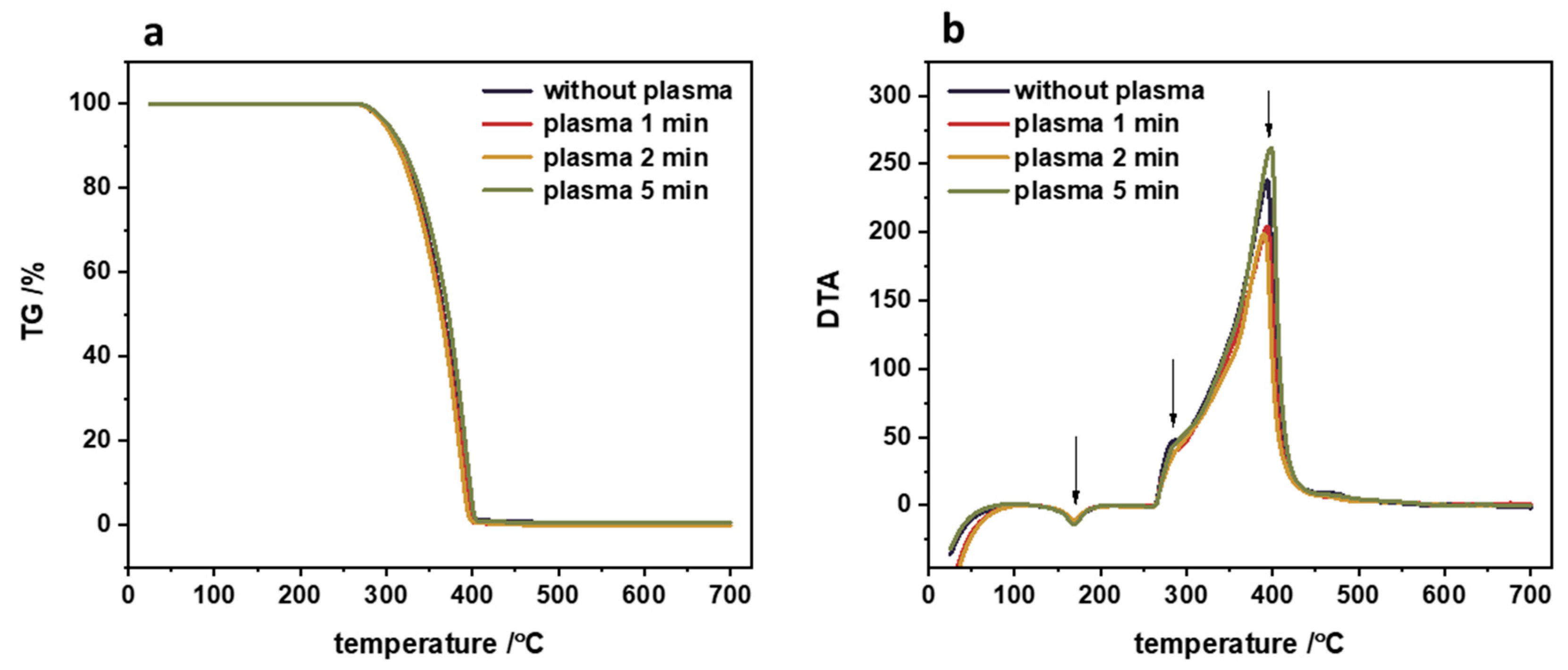
During the plasma treatment, several processes occur on the polymer surface that can be distinguished, such as cleaning, functionalization and etching. The optimal strategy of polymer functionalization via plasma treatment is to add functional groups to the surface without disturbing the optimized bulk properties of the polymer. The effect of plasma treatment on the structural properties of the polypropylene can be easily checked by TG/DTA measurements. The results of such thermogravimetric analysis are presented in
Figure 5. The TG profiles clearly illustrated that the polymeric material is stable up to 270°C independently of the plasma treatment time. This result strongly supports the working hypothesis that bulk polymeric properties are preserved. Additionally, there is no change in melting temperature after plasma treatment for 1 to 5 minutes (the typical melting temperature of polypropylene is approximately 170°C [
20]). Even the rapid thermal degradation of untreated and plasma-treated polypropylene, observed in the temperature range of 270-400°C, is the same within the experimental error.
Summarizing the effect of oxygen plasma on the polypropylene substrate, it can be concluded that while the XPS and FTIR spectroscopic results, as well as water contact angle measurement, confirm the generation of the oxygen functional groups on the polymeric surface, its bulk properties remain unchanged. Such surface-modified polypropylene was then used as the substrate for nTiO2 deposition to prepare a functional nanocomposite, which is described in the next section.
3.4. Nanocomposite
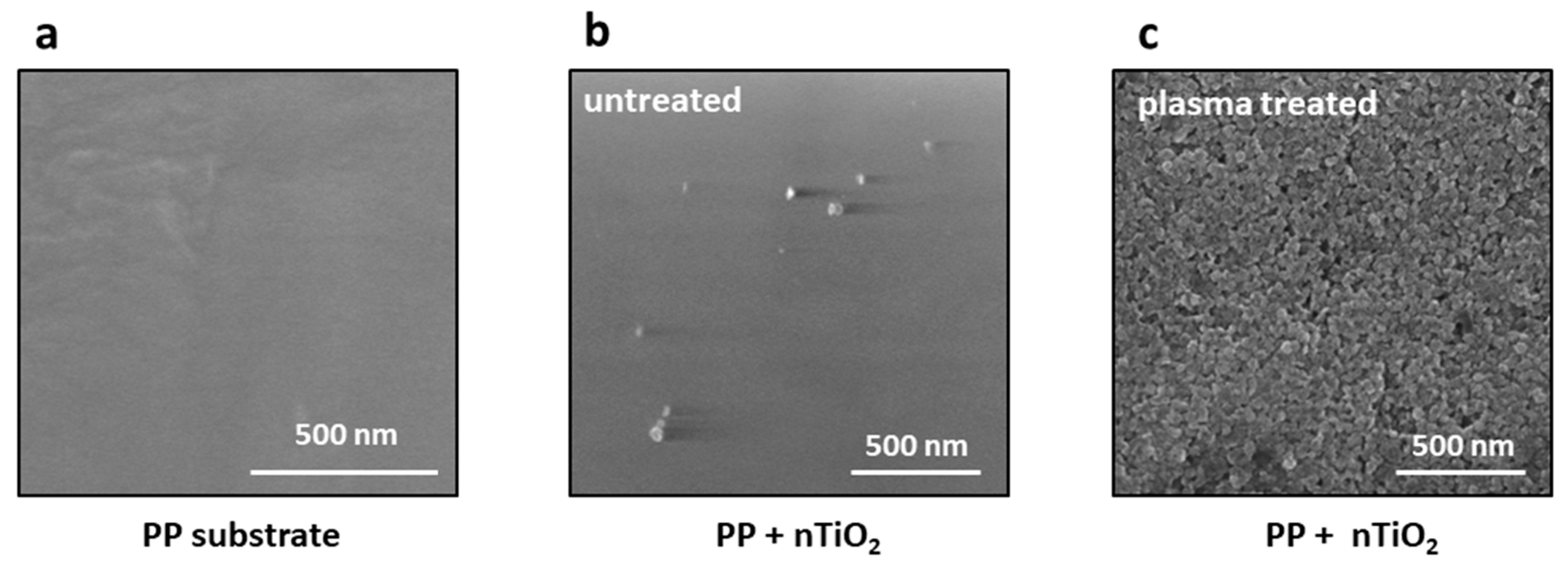
The deposition of titania nanoparticles on untreated or plasma-modified polypropylene substrates was performed via the sonochemical method.
Figure 6 presents SEM images comparing the effectiveness of nTiO
2 deposition on these surfaces together with the pristine PP substrate. The images represent the surfaces of a) polypropylene, b) polypropylene untreated by plasma after the deposition of nanoparticles from a 1% titanium dioxide suspension, and c) plasma-treated polypropylene after the deposition of nanoparticles from a 1% titanium dioxide suspension. As can be inferred from the images in
Figure 6b,c, the effect of oxygen plasms is tremendous. To assure a rational comparison during the deposition process on untreated and plasma-treated PP surfaces, the identical sonication parameters, i.e. TiO₂ concentration (1%), deposition time, power and amplitude, were employed. Single titania nanoparticles were deposited on the untreated polypropylene substrate by oxygen plasma, whereas the plasma-treated surface exhibited a completely covered surface with TiO
2 nanoparticles. It is evident that the efficiency of nanoparticles deposition on polymer substrates is influenced by the plasma treatment process and the introduction of functional groups on their surfaces.
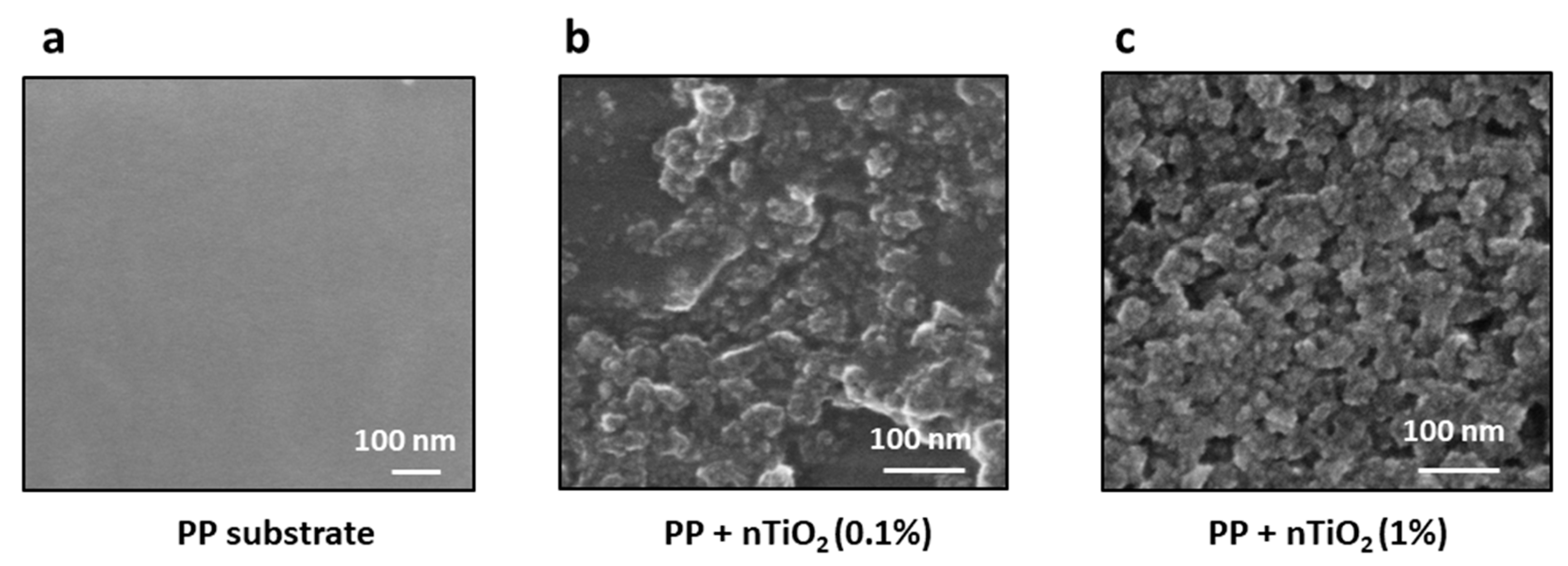
In order to ascertain the optimal degree of surface coverage and, consequently, the most developed photocatalytically active surface, the effect of nanoparticle suspension concentrations on the application process was also screened.
Figure 7 illustrates scanning electron microscopy (SEM) images of a polypropylene substrate following the application of TiO₂ from 0.1% and 1% suspensions (
Figure 7b, 7c, respectively).
Figure 7a presents a polypropylene substrate as a reference; the surface of this polymer is not decorated with nanoparticles. As expected, less surface coverage was evident when employing a reduced initial concentration. It is possible to discern individual nanoparticles attached to the surface, as well as clusters of nanoparticles or unoccupied regions. The optimal concentration of TiO
2 in the initial suspension and the most effective sonication time will yield the most favorable results. The obtained results showed that by optimizing both plasma treatment and sonochemical deposition parameters, the surface concentration and the accessibility of the photocatalyst surface can be controlled.
3.5. Photocatalytic Tests
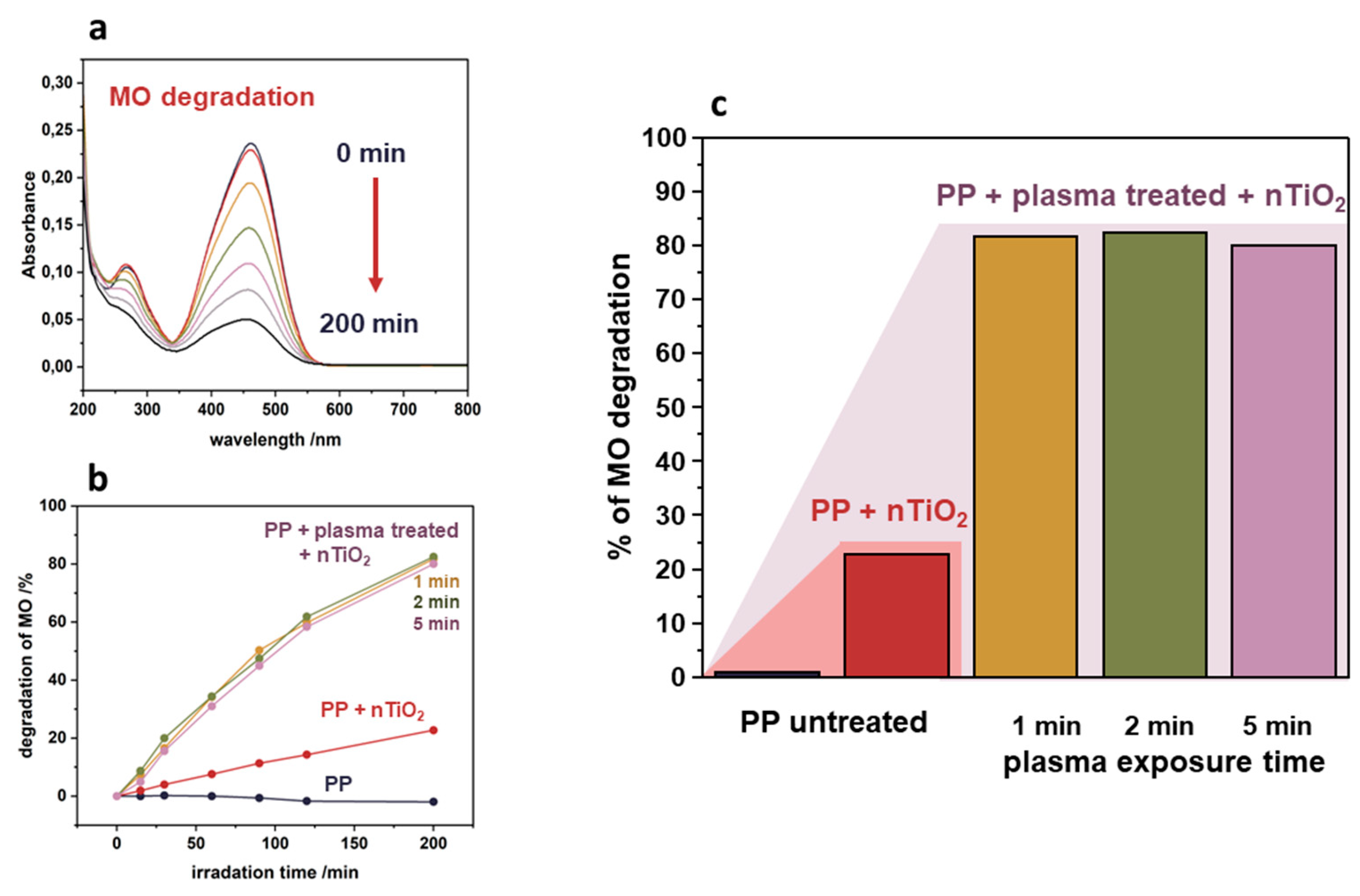
The photocatalytic activity was investigated in the degradation of methyl orange (as a model pollutant). The degradation of methyl orange in the presence of TiO
2/PP nanocomposite upon UVA light exposure was observed by monitoring the decrease of absorbance at the characteristic band of 464 nm in a time range of 0-200 min (
Figure 8a). Based on these results the degradation kinetics curves were determined and are presented in
Figure 8b. It can be observed that the polymer (PP) without nanoparticles (reference sample) does not exhibit photocatalytic activity. However, the deposition of TiO₂ nanoparticles onto its surface strongly promotes its activity. A huge difference between plasma-untreated (degradation of ~20% of methyl orange after 200 minutes) and plasma-treated PP (~80% degradation for 200 min) can also be noted. This results from the much more efficient attachment of nanoparticles onto the functionalized PP substrate (see
Figure 6c). The effect of plasma treatment is schematically presented in
Figure 8c. It can also be noted that a 1-minute plasma treatment is enough to make a significant impact on the activity, and a further increase of the plasma treatment time does not result in any discernible changes.
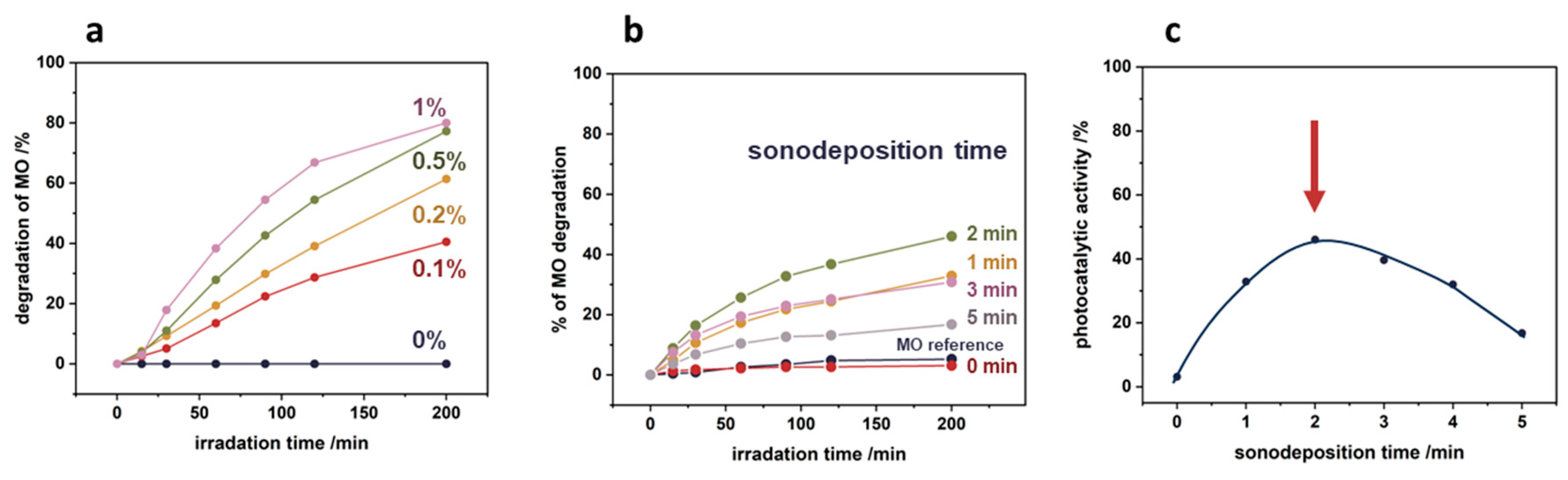
Figure 9a illustrates the effect of the initial concentration of the nTiO
2 suspension on the degradation of methyl orange (MO) with the irradiation time. As the concentration of the TiO
2 suspension used increases, the level of MO degradation increases in the whole-time range of irradiation. After 200 min of irradiation, for 0.1% TiO
2 and 0.2% TiO
2, approximately 40% and 60% degradation of the model pollutant was achieved, whereas for higher nTiO
2 concentrations of 0.5% and 1%, the MO degradation reached 80%. As can be observed, the degradation of the model pollutant did not increase linearly with the content of TiO
2. This may be attributed to the addition of subsequent layers of nanoparticles, which lead to limitations in the accessibility of TiO
2 surface for photocatalytic processes.
To achieve optimal photocatalytic effect, optimization of the sonochemical deposition stage was essential. The degradation results of methyl orange upon light exposure, as a function of sonication time using a 0.1% TiO
2 suspension, are depicted in
Figure 9b, while the relationship between photocatalytic activity and sonodeposition time is illustrated in
Figure 9c. It becomes evident that insufficient and excessive sonication times can reduce the effective anchoring of nanoparticles onto the polypropylene surface. Experimental data indicate that a sonication time of precisely two minutes is required to maximize the photocatalytic efficiency of the nTiO₂/PP nanocomposite system.
Furthermore, the analysis of plasma treatment durations revealed that beyond one minute, there were no significant improvements in the photocatalytic properties of the nTiO₂/PP. This suggests that while initial plasma exposure is crucial for generating surface functional groups necessary for nanoparticle adhesion, extended treatment times do not proportionally enhance photocatalytic activity. Therefore, a balanced approach is necessary: ensuring adequate plasma treatment to functionalize the surface, coupled with optimized sonochemical deposition parameters, is essential for achieving high photocatalytic performance. The findings illustrate the importance of both surface functionalization and precise control of sonodeposition conditions in the development of effective photocatalytic nanocomposites. Finally, it is worth noting that the proposed approach combining oxygen plasma treatment and ultrasound shows significant potential and can be used for any polymer-nanoparticle system to develop functional composites for diverse specific applications.
4. Conclusions
The low-temperature oxygen plasma treatment effectively introduced polar surface functional groups (mainly -OH and -C=O) on polypropylene, transforming its surface from hydrophobic to hydrophilic. This increase in wettability is evidenced by a significant reduction in water contact angle from 95° to 20°. Moreover, the generated surface oxygen groups were found to be crucial as they enhance the interaction between the polymer and nanoparticles and thus significantly improve the anchoring efficiency of TiO2 nanoparticles. Optimized parameters for plasma treatment (100 W power, 0.2 mbar O2 pressure, 1 minute duration) ensured effective surface modification. The sonochemical method was found to be facile and effective for depositing TiO2 nanoparticles on the plasma-treated PP surface. Optimal nanoparticle dispersion and surface coverage were achieved with a 1% TiO2 suspension and a sonication time of 2 min at 20 kHz, resulting in well-distributed photocatalytic TiO2 nanoparticles on the PP substrate. The developed nTiO2/PP nanocomposite demonstrated significant photocatalytic activity, degrading 80% of methyl orange under UVA irradiation within 200 minutes. Despite the superior surface modifications, the bulk properties of PP remained unchanged, as confirmed by thermogravimetric analysis (TG/DTA). The polymer maintained its stability up to 270°C and did not exhibit any changes in melting temperature after plasma treatment. The proposed two-step method (plasma treatment followed by sonochemical deposition) can be considered universal, versatile and scalable, making it applicable for functionalizing various polymeric materials with photoactive nanoparticles. From a broader perspective, this approach shows high potential for designing and fabricating self-cleaning and antibacterial polymeric surfaces for diverse applications.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Figure S1. Introducing functional groups after plasma treatment on ATR-FTIR spectra.
Author Contributions
Conceptualization, K.Z., J.M, A.K., W.M.; methodology, K.Z., K.S.; validation, J.M., F.K., W.M., A.K.; formal analysis, J.M., F.K, W.M, A.K.; investigation, K.Z; K.S.; data curation, K.Z., K.S.; writing—original draft preparation, K.Z.; writing—review and editing, J.M. F.K. W.M. A.K.; visualization, K.Z. A.K.; supervision, J.M, A.K.; project administration, J.M, A.K.; funding acquisition, J.M, A.K.; All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Ministry of Education and Science, Implementation Doctorate within “Funkcjonalne powłoki nanokompozytowe” project (DWD/6/257/2022). The study was carried out using research infrastructure purchased with the funds of the European Union in the framework of the Smart Growth Operational Programme, Measure 4.2; Grant No. POIR.04.02.00-00-D001/20, “ATOMIN 2.0 - ATOMic scale science for the INnovative economy”.
Data Availability Statement
Acknowledgments
The authors acknowledge D. Majda and M. Drozdek for performing the TG/DTA and XPS measurements.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Statista Research Department, Global Production of Plastics since 1950, 2024, available at: https://www.statista.com/statistics/282732/global-production-of-plastics-since-1950/.
- Hisham A. Maddah, Polypropylene as a Promising Plastic: A Review, American Journal of Polymer Science, 2016, 6(1), 1-11. [CrossRef]
- Hossain, M.T., Shahid, M.A., Mahmud, N. et al. Research and application of polypropylene: a review. Discover Nano, 2024, 19, 2. [CrossRef]
- Harish, V.; Ansari, M.M.; Tewari, D.; Gaur, M.; Yadav, A.B.; García-Betancourt, M.-L.; Abdel-Haleem, F.M.; Bechelany, M.; Barhoum, A. Nanoparticle and Nanostructure Synthesis and Controlled Growth Methods. Nanomaterials 2022, 12, 3226. [CrossRef]
- Ubaldi, F.; Valeriani, F.; Volpini, V.; Lofrano, G.; Romano Spica, V. Antimicrobial Activity of Photocatalytic Coatings on Surfaces: A Systematic Review and Meta Analysis. Coatings, 2024, 14, 92. [CrossRef]
- Wei Y., Wu Q., Meng H., Zhang Y., Cao C., Recent advances in photocatalytic self-cleaning performances of TiO2-based building materials, RSC Adv., 2023, 13, 20584-20597.
- Howard A. Foster Photocatalytic disinfection using titanium dioxide: spectrum and mechanism of antimicrobial activity; Appl Microbiol Biotechnol, 2011, 90, 1847–1868. [CrossRef]
- Qing Guo, Chuanyao Zhou, Zhibo Ma, Xueming Yang, Fundamentals of TiO2 Photocatalysis: Concepts, Mechanisms, and Challenges, Adv. Mater. 2019, 1901997. [CrossRef]
- Iesalnieks, M.; Eglıtis, R.; Juhna, T.; Šmits, K.; Šutka, A. Photocatalytic Activity of TiO2 Coatings Obtained at Room Temperature on a Polymethyl Methacrylate Substrate. Int. J. Mol. Sci. 2022, 23, 12936. [CrossRef]
- Sun L., Guan J., Xu Q., Yang X., Wang J., Hu X., Synthesis and Applications of Molecularly Imprinted Polymers Modified TiO2 Nanomaterials: A Review, Polymers 2018, 10, 1248. [CrossRef]
- Zhang, H., Han, J., & Yang, B. Structural Fabrication and Functional Modulation of Nanoparticle-Polymer Composites. Advanced Functional Materials, 2010, 20(10), 1533–1550. [CrossRef]
- Cazan C., Enesca A., Andronic L., Synergic Effect of TiO2 Filler on the Mechanical Properties of Polymer Nanocomposites, Polymers, 2021, 13, 2017 . [CrossRef]
- Lai J., Sunderland B., Xue J., Yan S., Zhao W., Folkard M., Michael B. D., Wang Y., Study on hydrophilicity of polymer surfaces improved by plasma treatment, Applied Surface Science, 2006, 252 (10), 3375-3379.
- Nemani, S. K., Annavarapu, R. K., Mohammadian, B., Raiyan, A., Heil, J., Haque, M. A., Sojoudi, H. Surface Modification of Polymers: Methods and Application, Advanced Materials Interfaces, 2018, 1801247. [CrossRef]
- Sadowski R., Wach A., Buchalska M., Kuśtrowski P., Macyk W., Photosensitized TiO2 films on polymers – Titania-polymer interactions and visible light induced photoactivity, Applied Surface Science 2019, 475, 710–719 . [CrossRef]
- Ricard A., The production of active plasma species for surface treatments, J. Phys. D: Appl. Phys. 1997, 30, 2261. [CrossRef]
- Fleischer, M.; Kielar Tuceková, Z.; Galmiz, O.; Baková, E.; Plšek, T.; Kolárová, T.; Kovácik, D.; Kelar, J. Plasma Treatment of Large-Area Polymer Substrates for the Enhanced Adhesion of UV–Digital Printing. Nanomaterials 2024, 14, 426. [CrossRef]
- Gedanken A.; Using sonochemistry for the fabrication of nanomaterials, Ultrasonics Sonochemistry, 2004, 11 (2), 47-55. [CrossRef]
- Morent R., De Geyter N., Leys C., Gengembre L., Payen E.; Comparison between XPS- and FTIR-analysis of plasma-treated polypropylene film surfaces, Surface and Interface Analysis, 2008, 40, 597-600. [CrossRef]
- Maddah H. A.; Polypropylene as a Promising Plastic: A Review, American Journal of Polymer Science, 2016, 6(1), 1-11. [CrossRef]
Figure 1.
Pristine polypropylene substrate: (a) SEM image and (b) ATR-FTIR spectrum indicating the main functional groups in the polypropylene structure.
Figure 1.
Pristine polypropylene substrate: (a) SEM image and (b) ATR-FTIR spectrum indicating the main functional groups in the polypropylene structure.
Figure 2.
Titanium dioxide nanoparticles: (a) nanoparticle tracking analysis showing the hydrodynamic diameter, (b) SEM image of TiO2, (c) particle size distribution based on SEM images, (d) TEM image revealing that observed SEM nanoparticles are agglomerates of smaller TiO2 crystallites, (e) size distribution of individual TiO2 crystallites, (f) size distribution of agglomerates from TEM images.
Figure 2.
Titanium dioxide nanoparticles: (a) nanoparticle tracking analysis showing the hydrodynamic diameter, (b) SEM image of TiO2, (c) particle size distribution based on SEM images, (d) TEM image revealing that observed SEM nanoparticles are agglomerates of smaller TiO2 crystallites, (e) size distribution of individual TiO2 crystallites, (f) size distribution of agglomerates from TEM images.
Figure 3.
XPS results for polypropylene surface before and after oxygen plasma treatment. (a) XPS survey scans showing the increase in surface oxygen to carbon ratio with the plasma treatment time, (b) narrow XPS scan of the C 1s peak indicating the presence of hydroxyl (-OH) and carbonyl (C=O) groups, and the appearance of carboxyl bonds (O–C=O) upon plasma treatment.
Figure 3.
XPS results for polypropylene surface before and after oxygen plasma treatment. (a) XPS survey scans showing the increase in surface oxygen to carbon ratio with the plasma treatment time, (b) narrow XPS scan of the C 1s peak indicating the presence of hydroxyl (-OH) and carbonyl (C=O) groups, and the appearance of carboxyl bonds (O–C=O) upon plasma treatment.
Figure 4.
Changes in the water contact angle of the polypropylene surface as a function of oxygen plasma treatment time.
Figure 4.
Changes in the water contact angle of the polypropylene surface as a function of oxygen plasma treatment time.
Figure 5.
Thermogravimetric analysis of pristine polypropylene and PP after plasma treatment: (a) loss of mass profile and the corresponding (b) differential thermal analysis.
Figure 5.
Thermogravimetric analysis of pristine polypropylene and PP after plasma treatment: (a) loss of mass profile and the corresponding (b) differential thermal analysis.
Figure 6.
SEM images of the polypropylene substrate showing the effectiveness of TiO2 nanoparticles deposition on untreated and plasma-treated substrates: (a) Pristine polypropylene surface, (b) TiO2 nanoparticles deposited on the untreated surface, and (c) TiO2 nanoparticles deposited on the plasma-treated surface.
Figure 6.
SEM images of the polypropylene substrate showing the effectiveness of TiO2 nanoparticles deposition on untreated and plasma-treated substrates: (a) Pristine polypropylene surface, (b) TiO2 nanoparticles deposited on the untreated surface, and (c) TiO2 nanoparticles deposited on the plasma-treated surface.
Figure 7.
SEM images of polypropylene substrate after deposition of TiO2 from suspension with varying nanoparticle concentrations: (a) reference polypropylene substrate before nanoparticles deposition, (b) polypropylene surface after deposition from TiO2 0.1% suspension, and (c) polypropylene surface after deposition from 1% TiO2 suspension.
Figure 7.
SEM images of polypropylene substrate after deposition of TiO2 from suspension with varying nanoparticle concentrations: (a) reference polypropylene substrate before nanoparticles deposition, (b) polypropylene surface after deposition from TiO2 0.1% suspension, and (c) polypropylene surface after deposition from 1% TiO2 suspension.
Figure 8.
Photocatalytic activity of nTiO2/PP nanocomposite in methyl orange degradation under UVA irradiation: (a) absorbance decrease at 464 nm upon irradiation, (b) kinetic curves illustrating enhanced activity for plasma-treated PP composite, and (c) schematic representation of the plasma treatment effect on photocatalytic performance of the developed nTiO2/PP composite.
Figure 8.
Photocatalytic activity of nTiO2/PP nanocomposite in methyl orange degradation under UVA irradiation: (a) absorbance decrease at 464 nm upon irradiation, (b) kinetic curves illustrating enhanced activity for plasma-treated PP composite, and (c) schematic representation of the plasma treatment effect on photocatalytic performance of the developed nTiO2/PP composite.
Figure 9.
Optimization of sonochemical deposition parameters for enhanced photocatalytic activity: (a) effect of initial nTiO2 suspension concentration, (b) effect of sonication time on methyl orange degradation using 0.1% TiO2 suspension, (c) photocatalytic activity as a function of sonodeposition time, with 2 minutes identified as the optimal for maximum efficiency.
Figure 9.
Optimization of sonochemical deposition parameters for enhanced photocatalytic activity: (a) effect of initial nTiO2 suspension concentration, (b) effect of sonication time on methyl orange degradation using 0.1% TiO2 suspension, (c) photocatalytic activity as a function of sonodeposition time, with 2 minutes identified as the optimal for maximum efficiency.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).