1. Introduction
Language acquisition is a pivotal developmental milestone that emerges during early childhood, serving as the foundation for effective communication and interaction. When language development is disrupted, as in the case of Developmental Language Disorder (DLD), it can lead to significant challenges in social integration and academic achievement [
1]. DLD is a prevalent neurodevelopmental condition characterized by persistent difficulties in acquiring and using language. These difficulties are not attributable to sensory, neurological, or intellectual impairments [
2]. DLD typically manifests around the age of three and affects both language comprehension and production, leading to significant challenges in social integration and academic performance regardless of the language spoken [
3,
4]. In Spanish-speaking contexts, DLD is a significant concern within special education and is often detected early in educational settings where special resources are provided to support affected individuals [
5].
The neural bases of DLD involve several findings related to brain structure and function. Children with DLD exhibit differences in overall brain volume, with some studies reporting smaller or larger volumes compared to neurotypical children [
6,
7]. Alterations in both gray and white matter volume are also observed [
8], including increased white matter volumes in younger children, potentially indicating inefficient neural connectivity. Specific regions, such as the planum temporale, associated with language functions and typically larger in the left hemisphere in neurotypical individuals, do not show consistent asymmetry in children with DLD [
9]. The inferior frontal gyrus (IFG), crucial for language production, also exhibits abnormal activation and volume patterns [
10]; [
11]. The caudate nucleus, a subcortical structure involved in language processing and verbal memory, shows reduced gray matter volume. White matter tract integrity, particularly in the superior longitudinal fasciculus and arcuate fasciculus, is also compromised, affecting language processing and production. Functional MRI studies reveal atypical brain activation patterns, including hypoactivation in the left IFG and compensatory activation in the right IFG [
9]. Additionally, children with DLD exhibit reduced cerebral blood flow in left perisylvian regions, negatively impacting language processing [
12]. These findings indicate that DLD is associated with structural and functional alterations in key language-related brain regions and their connections, contributing to the linguistic difficulties observed in affected children.
Among the linguistic components impacted by DLD, verb acquisition and use are particularly affected [
13]. Verbs are crucial for constructing sentences and conveying actions, making their mastery essential for effective communication. Research has shown that a robust verb vocabulary enhances the ability to form complex sentences and is a stronger predictor of linguistic outcomes than noun knowledge, both in typically developing children and those with DLD [
14]. Errors in verb usage have been identified as clinical markers of DLD across various languages, including English, German, and Spanish [
15,
16,
17,
18].
One aspect of verb usage that has garnered recent research attention is telicity—the inherent endpoint of an action. Telic verbs denote actions with a clear endpoint (e.g., “close,” “break”), whereas atelic verbs describe actions without a defined conclusion (e.g., “run,” “paint”). Studies have shown that children with DLD struggle to associate telic verbs with completed actions, unlike their typically developing peers who do so from an early age [
17,
19,
20]. This difficulty suggests broader challenges in the semantic structuring of verbs for children with DLD [
21].
Understanding how children learn to process telic and atelic verbs can be further explained through the Event Structural Bootstrapping model [
21]. This model posits that children focus on event structure when learning verbs, initially acquiring those with clear final states (telic verbs) to facilitate semantic integration. In contrast, atelic verbs, with their ambiguous endpoints, present greater learning challenges and are acquired later. Studies comparing DLD and TD children in English and German support this model, showing that TD children process telic verbs more efficiently than atelic verbs, a difference not observed in DLD populations [
19,
21,
22]. Schulz [
17] suggests that this deficit might be universal, and Leonard [
23] hypothesizes that the difficulty in identifying the telicity of verbs may underlie future verb conjugation errors in individuals with DLD.
Given the critical role of telicity in linguistic development, it is important to investigate whether these differences are evident in Spanish-speaking children with DLD. In Spanish, verbs possess the classification and properties of being telic or atelic, making it plausible that telicity may also affect Spanish-speaking children with DLD. To date, only one study in Spanish has investigated the role of telicity. Grinstead et al. [
24] studied 38 Mexican children (19 with DLD and 19 typically developing) with a mean age of 6 years. They analyzed spontaneous speech samples to determine a preference in the use of aspect (telicity) versus verb tense. The researchers found that both DLD and control children showed a preference for past tense telic verbs and present tense atelic verbs. Although the total number of verbs used was lower in DLD subjects than in controls, the groups did not show significant differences in lexical aspect use. However, the authors suggest that this difference in the total number of verbs used might be influenced by the presence of an adult during the speech elicitation process. Adults may inadvertently guide the conversation or prompt the children in ways that encourage the use of certain types of verbs, thereby affecting the spontaneous speech samples collected from the children.
This study aims to address this gap by examining the effect of telicity on verb recognition in Spanish-speaking children with DLD compared to their TD peers using the Event-Related Potential (ERP) technique. ERPs are a non-invasive electrophysiological method that measures brain responses to specific sensory, cognitive, or motor events. As an online method, ERPs provide immediate access to relevant processes and allow for the evaluation of highly automated and unconscious mental and neural processes [
25]. By analyzing the N400 component—a negative-going wave that peaks around 400 milliseconds after stimulus onset and is associated with the processing of meaning—we can gain insights into the neural mechanisms underlying language processing.
Previous studies [
26] have shown that TD children exhibit a strong N400 effect in earlier time windows (300-500 ms and 500-800 ms), while children with DLD only show a reliable N400 effect in the later window (500-800 ms). Additionally, the topographical distribution of the N400 in children with DLD is less focalized compared to TD children, who show typical posterior effects. Courteau et al. [
27] found similar centroparietal N400 effects in preadolescent French children with DLD, although with a brief delay in onset compared to their TD peers. Although the findings are different, we expect that the differences in the N400 component between telic and atelic verbs will provide a deeper understanding of how children with DLD process these linguistic features compared to their TD peers.
Our hypothesis is that school-aged children with DLD will show a delayed N400 effect in response to incoherent sentences, with no difference between telic and atelic verbs. In contrast, typically developing school-aged children will exhibit a larger N400 component for incoherent sentences with atelic verbs compared to those with telic verbs, as atelic verbs are processed differently.
2. Materials and Methods
The research design is experimental using a mixed factorial approach. The design included one between-group factor (subjects with DLD/subjects with TD) and two within-group factors (telicity: telic/atelic and coherence: coherent/incoherent).
2.1. Participants
The study included 38 school-aged children, 19 with DLD (10 boys, 9 girls) and 19 TD (9 boys, 10 girls), who were finishing the second year of primary school. Initially, 38 subjects were recruited; however, one subject from each group was excluded during the analysis phase due to excessive artifacts in their EEG recordings, resulting in 18 participants per group (
Table 1). For the estimation of the minimum required sample size, the following parameters were considered: a) Effect size (f) = 0.25, b) Statistical power (1- β) = 0.95; c) Significance level (α) = 0.05; d) Number of measurements = 4. According to these variables, a minimum of 18 individuals per group is needed, as calculated by the G*Power program version 3.1.7. [
28]
The children were recruited from subsidized private schools in Concepción (Chile), and all were native Spanish speakers. Written consent was obtained from the parents and/or legal guardians, and the participants provided verbal assent prior to participating in the experiment. This study was approved by the Ethics, Bioethics, and Biosafety Committee (Protocol No. CEBB 731-2020) at the University of Concepción (Chile).
Participants were selected based on specific inclusion criteria, ensuring that all children were attending school, were right-handed, and had normal or corrected vision. The diagnosis of DLD was made using the Chilean IDTEL instrument [
29], which assesses language in children between the ages of 6 years and 9 years and 11 months and was developed and validated in Chile. The test uses oral response items to assess the 4 levels of language: phonological, morphosyntactic, semantic and pragmatic. It was administered by a single speech-language pathologist. Subjects in the DLD group had to score below the cut-off for the full test, and TD subjects had to score above the cut-off. Details of the sample are shown in
Table 1.
Participants were excluded if they had a known sensory or developmental disorder, such as autism, cognitive impairment, cerebral palsy, or Attention Deficit Disorder. This information was verified through interviews with parents or caregivers.
2.2. Stimuli and Procedure
2.2.1. Linguistic Material
Thirty-two telic verbs and thirty-two atelic verbs were used, which in counterbalance allowed for the creation of sixty-four sentences for each subject. These sentences followed the subject-verb-object structure and were divided into four categories: sixteen telic-coherent, sixteen telic-incoherent, sixteen atelic-coherent, and sixteen atelic-incoherent.
To ensure accurate classification of the verbs, a written survey was conducted with university students studying education. They rated the sentences according to their continuity or completion. Significant differences were found (t=12.86, p<.001) between telic sentences (e.g. “Mom turns on the oven”) and atelic sentences (e.g. “Mom cleans the oven”).
The subjects of the sentences included four family members: Mom, Dad, Emma, and Charlie. Direct objects and verbs were controlled for frequency (t = .359, p = .723) and length (t = .372, p = .713). The sentences were recorded to ensure that the duration of each segment was consistent across all stimuli.
The sentences were recorded in sections to control the duration of each segment. Microsoft Clipchamp [
30] was used for this purpose. The accompanying videos had an average length of 4 seconds and were created using the stop-motion technique with images that did not differ in color or size. The images were made in PowerPoint [
31] and then recorded into a video in the same application. Expert judgment was used to assess the coherence of the videos, ensuring they accurately reflected the two variables (coherent, incoherent). Examples of each condition are presented in
Table 2.
2.2.2. Task
The task consisted of viewing a video accompanied by an oral and written sentence that may or may not agree with the video.
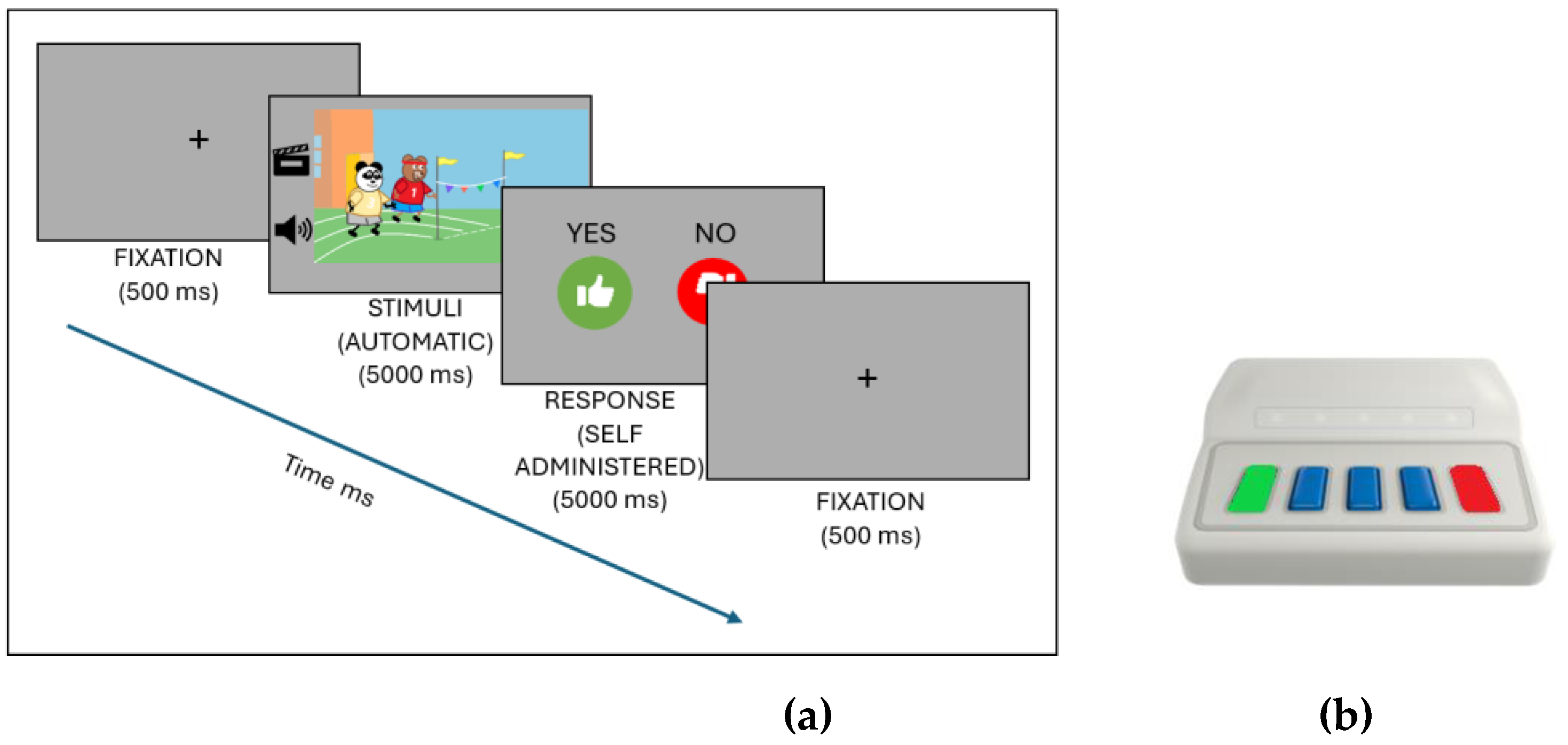
Participants received instructions through an interactive drawing that introduced them to the family and the task: “This is the Bear family: Dad, Mom, Emma and Charlie. They have a problem; they can’t remember if they did an action or not. To help them, you have to press the yes button (green) if you think they did the action and the no button (red) if they didn’t.” After receiving the instructions, participants completed four practice stimuli before beginning the experimental phase (
Figure 1a). The experimental phase was divided into two blocks, each containing 16 telic-coherent, 16 telic-incoherent, 16 atelic-coherent, and 16 atelic-incoherent stimuli presented in random order.
The stimuli were presented electronically using E-Prime 3.0 software [
32] and for the response, the USB response device Chronos [
33] was used, which was adapted to use two outside buttons for yes and no (
Figure 1b).
2.2.3. EEG Recording
EEG data were recorded continuously at a sampling rate of 500 Hz using 32 cap-mounted active electrodes (actiCAP) from Brain Products GmbH, positioned according to the international 10/20 system. The electrodes covered the frontal, parietal, temporal, and occipital lobes, specifically at the following sites: FP1, FP2, F3, F4, F7, F8, Fz, FC1, FC2, FC5, FC6, FT9, FT10, C3, C4, Cz, CP1, CP2, CP5, CP6, P3, P4, Pz, T7, T8, TP9, TP10, P7, P8, O1, O2, and Oz. FCz was used as reference electrode and the ground electrode was located in the forehead.
Before each recording, preparation included impedance measurements to ensure that all electrode impedances were below 25 KΩ. Brain Products acquisition system consists of a battery powered amplifier and fiber optics communication to the acquisition PC, to minimize external electrical noise contamination.
Using BrainVision Analyzer software version 2.3, the recorded data was pre-processed according to standard practices. Data was filtered using an 8
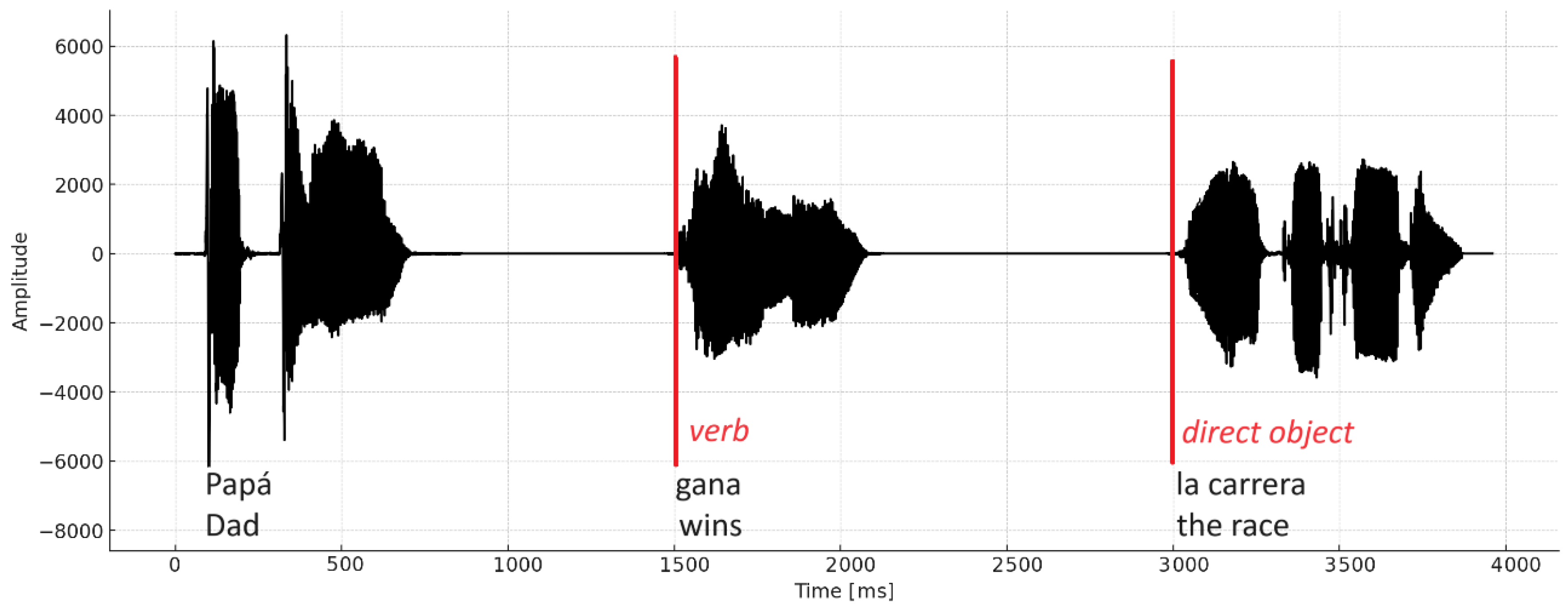
th order Butterworth bandpass filter, with cut-off frequencies of 0.1 Hz and 30 Hz. Artifacts were rejected by visual inspection from an expert. Both verb and direct object ERP were obtained. Stimulus phrases were designed so that the verb segment and the direct object segment start at specific times, as the example shown in
Figure 2.
Verb and direct object segments were standardized to 1200 ms (-200 ms to 1000 ms). Verb segment is obtained from 1300 to 2500 ms and direct object from 2800 to 4000 ms from the EEG recorded and synchronized to the start of the auditory and visual stimuli.
2.2.4. Analysis
Before the analysis, a visual inspection using a semiautomatic method was conducted to detect and reject artifacts. The thresholds used for the inspection were 150uV maximum deviation and a gradient of 50uV/ms. After visual inspection, ICA correction using FP2 (vertical) and F8 (horizontal) references for eye movement artifacts was applied.
ERP analysis was conducted in R version 4.3 [
34]. The segments were baseline corrected in the interval between -200 to 0 ms. First, a point-by-point student t-test is conducted for each segment to detect intervals with significant differences between groups, telicity and coherence. The selected segments to be studied are: 250—500 ms and 500—700 ms in the Verb segment, and 250—500 ms and 600 – 1000 ms in the direct object segment.
For the 4 segments selected, and for each EEG signal channel, a general ANOVA between group (DLD and TD), telicity (telic or atelic verb), coherence (coherent or incoherent), hemisphere (left, right and center) and region (frontal, central, parietal, and temporal) was conducted, comparing segment means. The significance level was set at 5%. In the cases where significant differences were detected, a ROI exploration was conducted by combining neighboring electrodes.
3. Results
3.1. ERP Results
3.1.1. N400 Effects In Verb
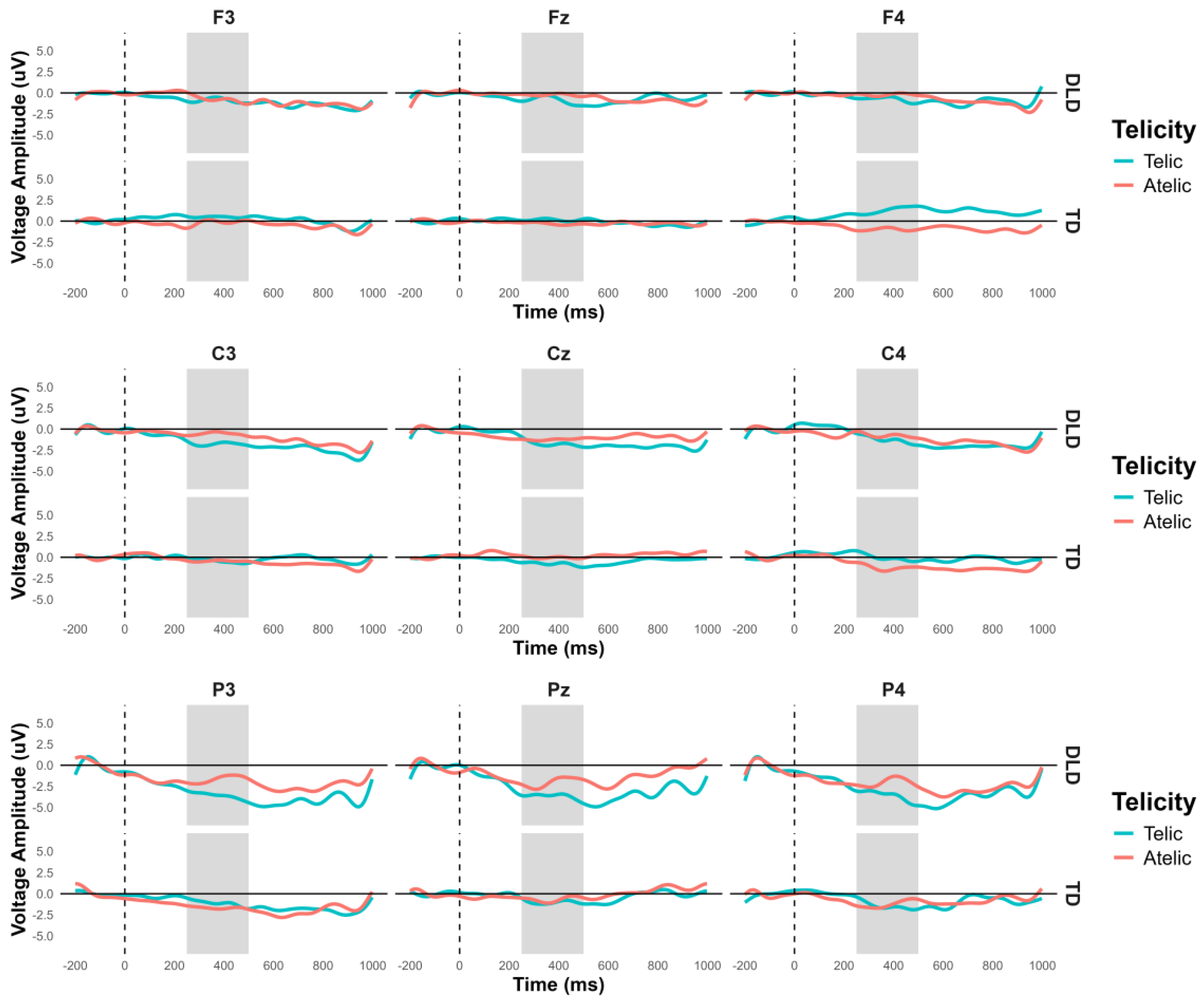
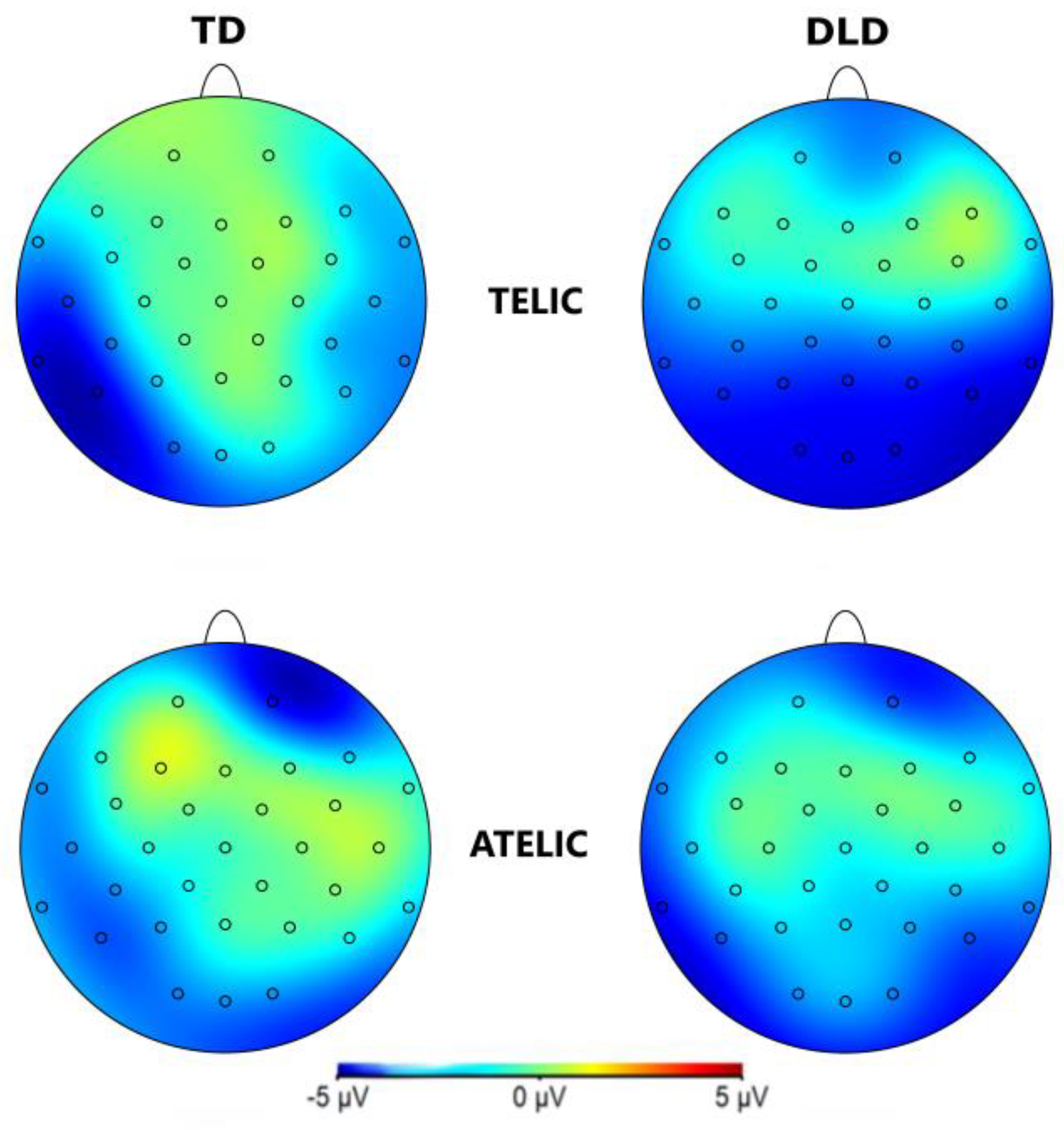
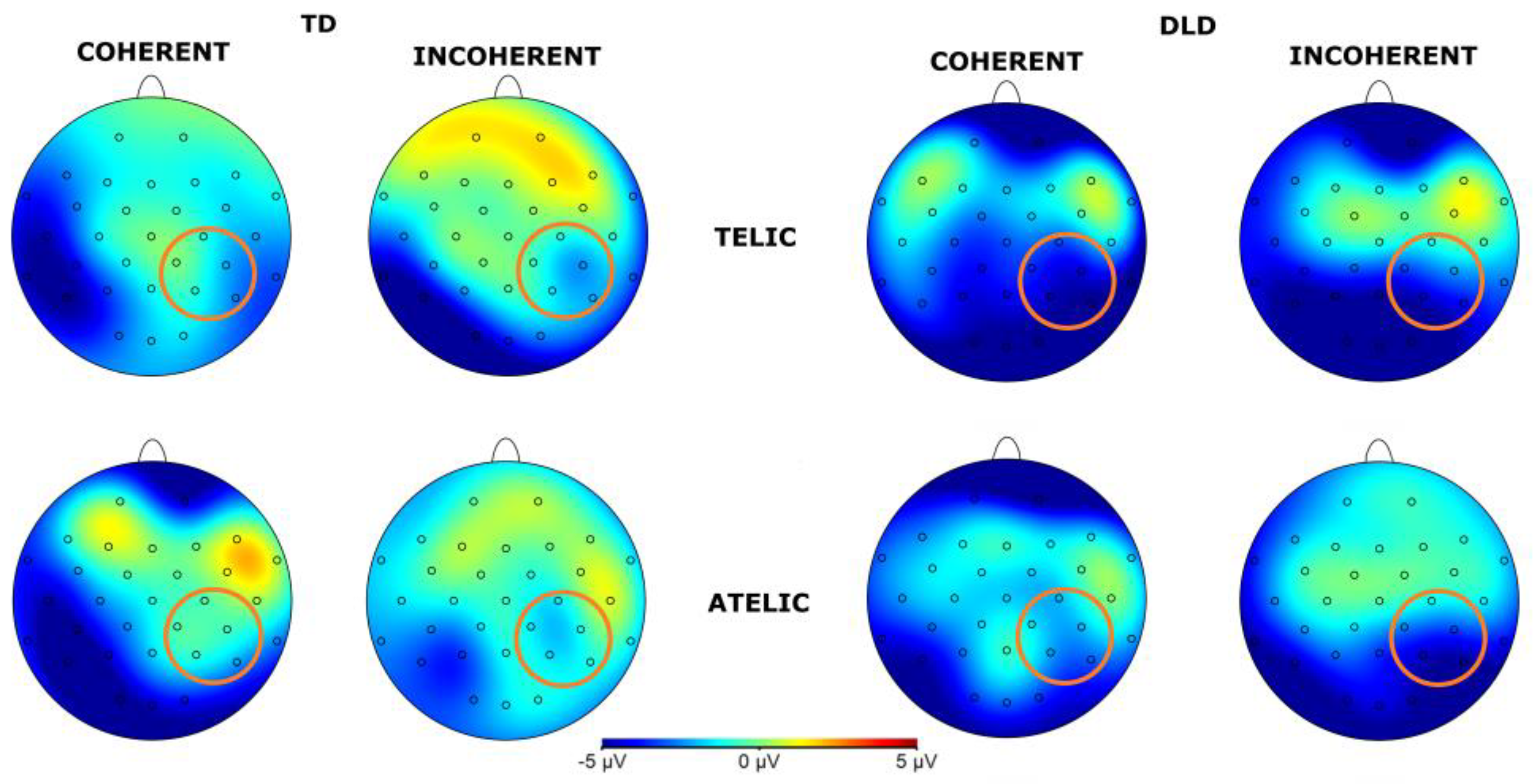
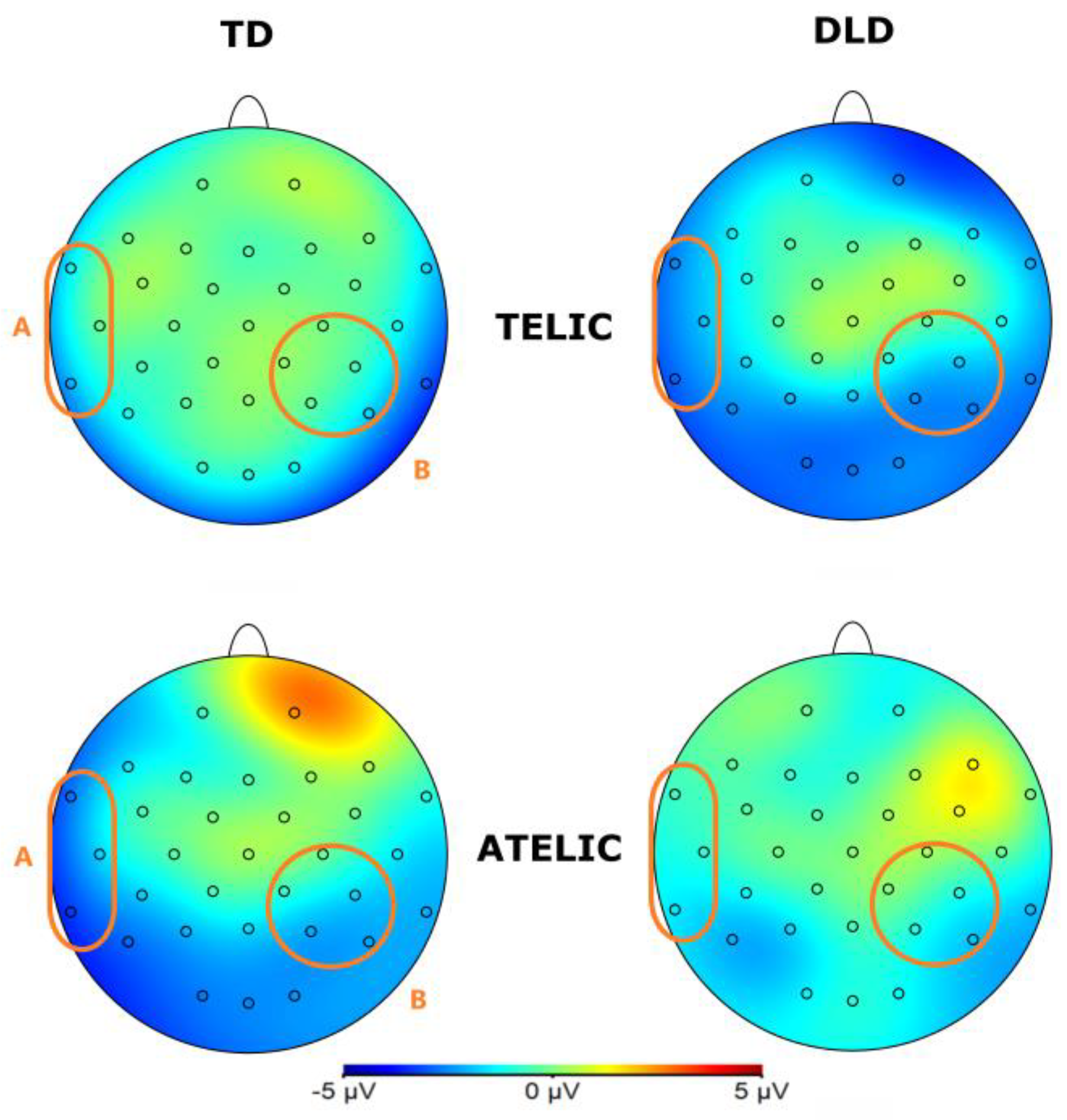
The general ANOVA in the time window of 250 to 500 ms revealed a significant interaction between group and telicity, F (1,354) = 4.705, p = .030, where DLD children show more negative amplitude in telic condition compared to children with TD (see
Figure 3 and
Figure 4). Specifically, comparisons between the DLD and TD groups showed significant differences in both telic and atelic conditions. For telic conditions, the difference between TD and DLD groups was M= 1.307, SE = .19, t (3596) = 6.881, p < .001. For atelic conditions, the difference was M = .733, SE = .19, t (3596) = 3.859, p < .001 for atelic conditions. Within the DLD group, there was a significant contrast between atelic and telic conditions, where the telic condition show more negative amplitude than the atelic, with a contrast estimate M = .712, SE = .19, t (3596) = 3.748, p < .001., No significant contrast was found within the TD group M = .712, SE = .19, t (3596) = .727, p = .467.
Additionally, there was an effect between group and coherence, F(1,354) = 6.523, p = .011. However, we did not explore this effect further since at this point in the stimulus, children are not able to detect the coherence or incoherence yet.
3.1.2. Post N400 Effects In Verb
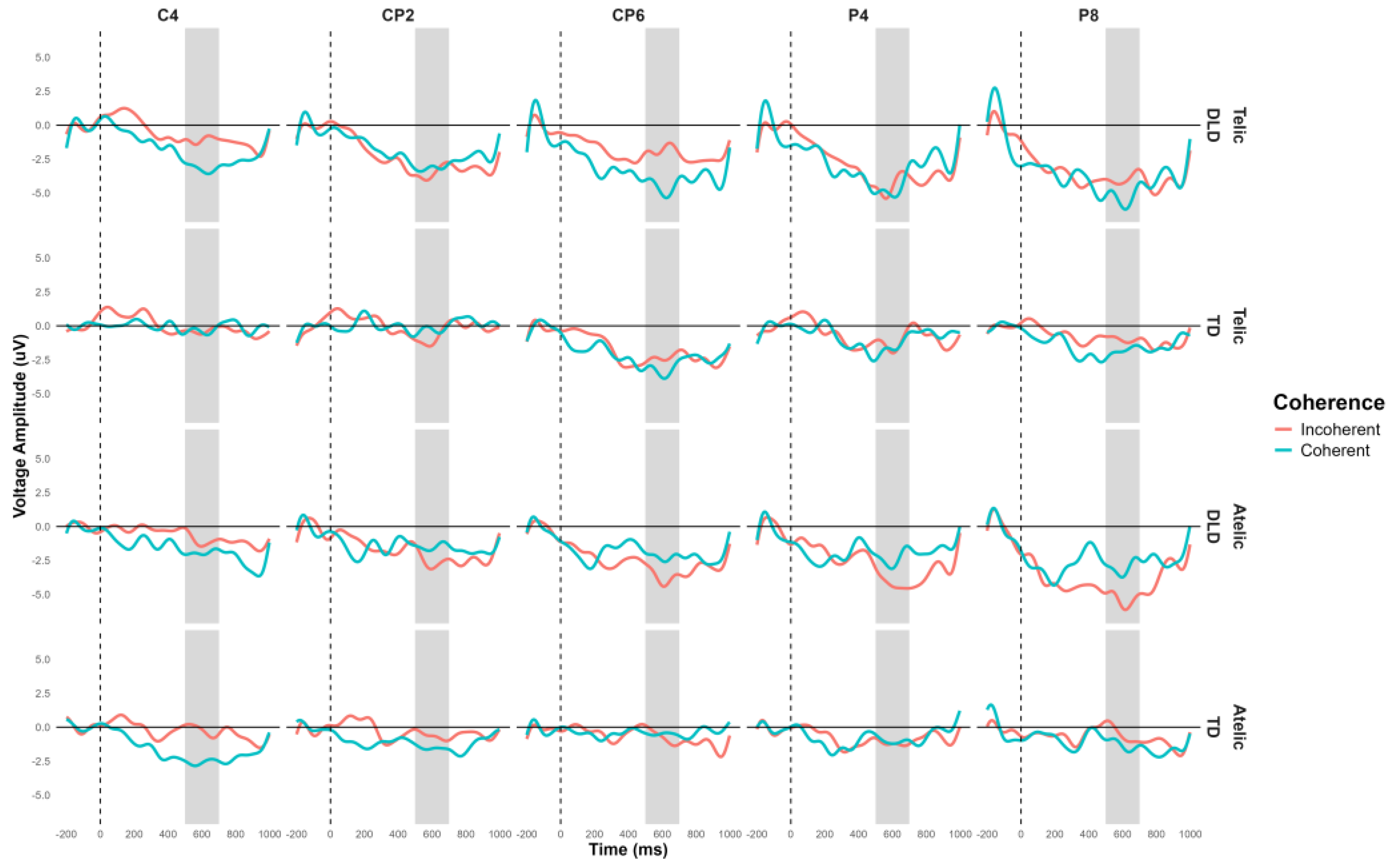
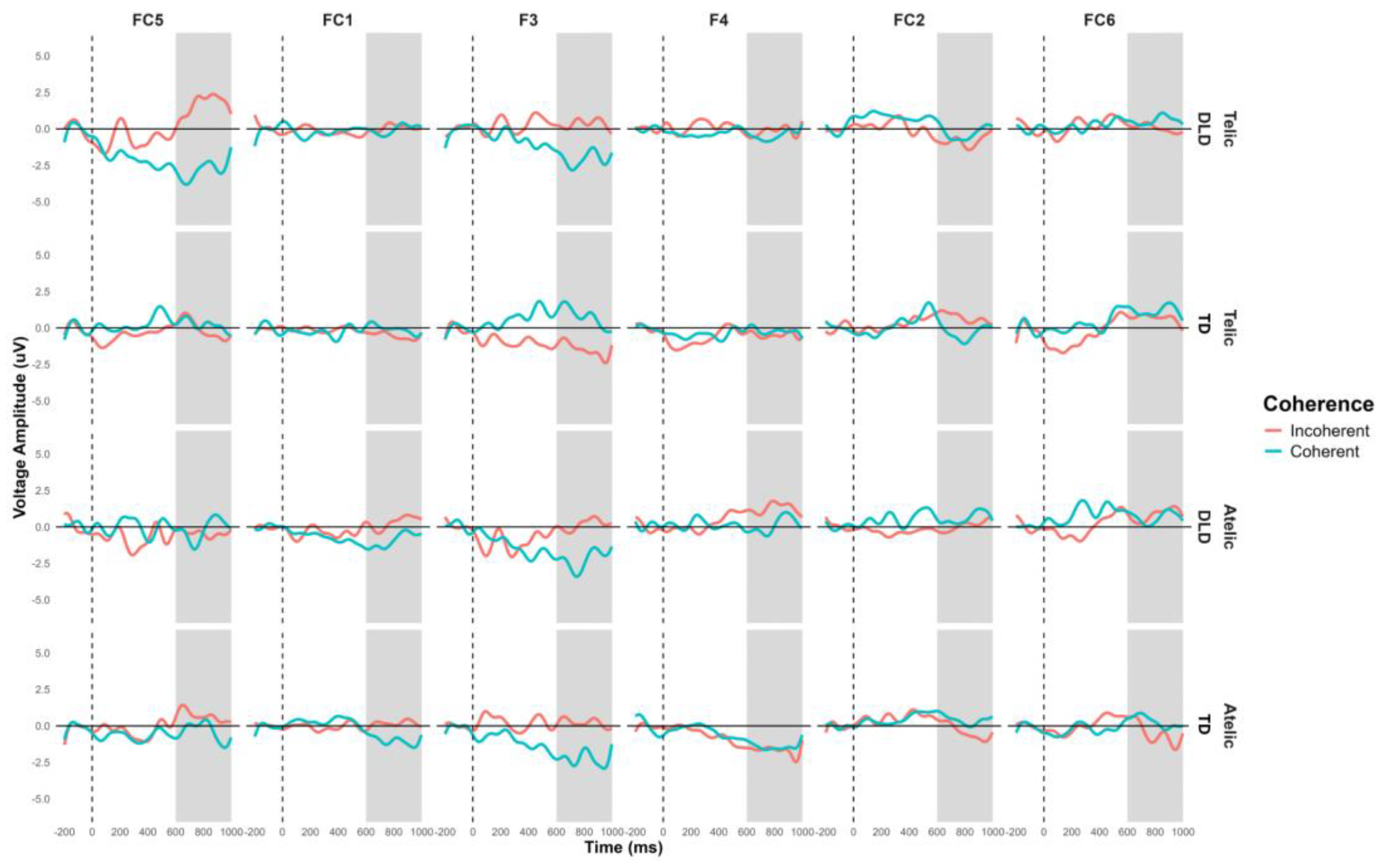
The general ANOVA in the time window of 500 to 700 indicated a significant triple interaction between group, telicity, and coherence, F (1,354) = 4.347, p = .0371. We focus in the right centroparietal ROI composed of electrodes C4, CP2, CP6, P4 and P8, F (1, 712) = 4.328, p = .038. This region was selected based on theoretical considerations, as previous research has indicated that the centroparietal areas are critically involved in the processing of semantic and syntactic information. The intergroup contrasts in this region revealed significant differences in several conditions. For telic coherent sentences DLD children show more negative amplitude than TD group, the mean difference was M = 2.746, SE = .711, t (712) = 3.865, p < .001. In telic incoherent sentences, although both groups process incoherence more positively, this effect shows greater amplitude in the DLD group, the difference was M = 1.726, SE = .711, t (712) = 2.429, p = .015. For atelic incoherent sentences, the DLD children show more negative amplitude than TD group, M = 2.710, SE = .711, t (712) = 3.813, p < .001 (see
Figure 5 and
Figure 6). However, the atelic coherent condition was not significant between the groups (M = .773, SE = .711, t (712) = 1.088, p = .277. In contrast, the intragroup comparisons did not reveal significant differences. The ERP waveforms show distinct patterns of neural activity between the groups across these conditions, highlighting the interaction effects observed.
3.1.3. N400 Effects in Direct Object
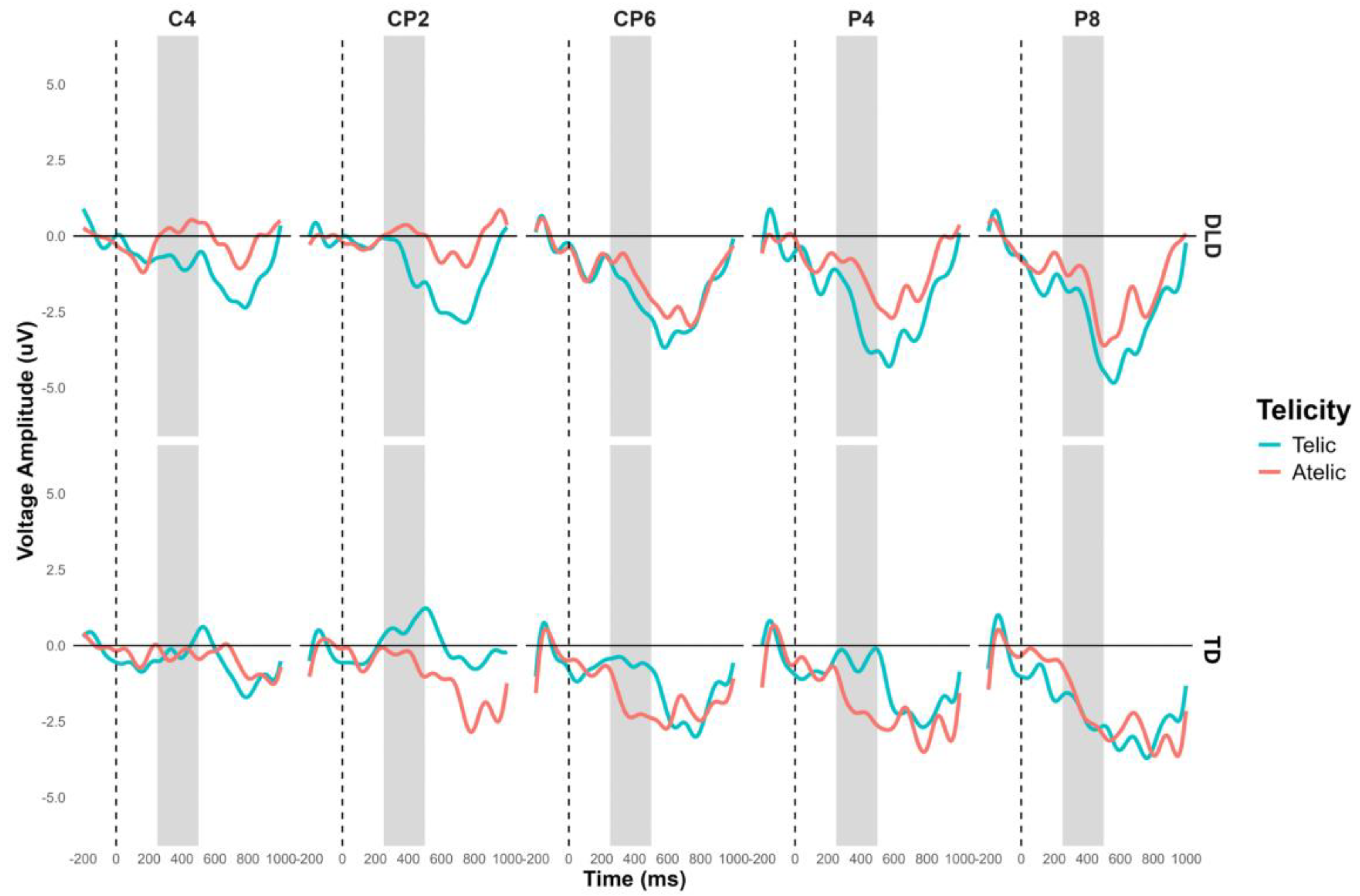
The general ANOVA in the time window of 250 to 500 ms showed a significant interaction between group and telicity, F (1,3728) = 13.504, p < .001. As mentioned previously, we focus on the right centroparietal ROI composed of electrodes C4, CP2, CP6, P4 and P8, F (1, 752) = 9.702, p < .001. The contrast in this region shows that in the telic condition, children with DLD show more negative amplitude than TD children, M = −1.184, SE = .409, t (756) = −2.899, p < .001. In the atelic condition, the difference was not significant, M = 0.614, SE = .409, t (756) = 1.503, p = .133. Intragroup contrasts for the DLD group showed more negative amplitude in telic condition than atelic condition, M = 1.010, SE = .409, t (756) = 2.472, p = 0.014. For the TD group, the difference between telic and atelic conditions is marginally significant, showing a more negative trend in the atelic condition compared to the telic condition, M = −0.789, SE = .409, t (756) = −1.931, p = 0.054 (see
Figure 7). The ERP waveforms highlight the significant differences observed, particularly in the telic condition between groups.
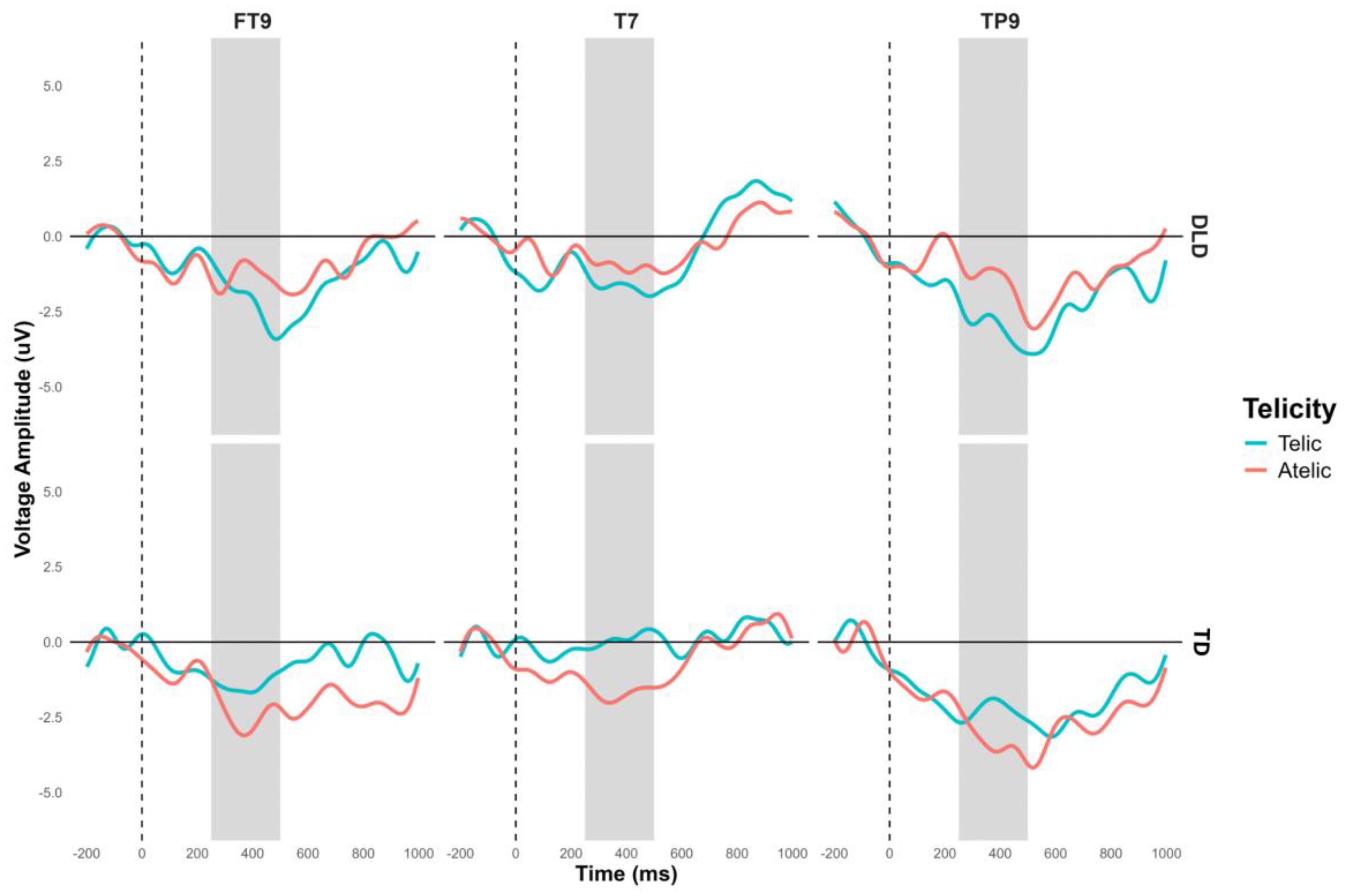
Additionally, a significant interaction between group and telicity was observed in the left temporal region composed of electrodes FT9, T7, and TP9, F (1, 448) = 5.804, p = .016. The contrast in this region approaches significance in several conditions. In the telic condition, both groups show in general a negativity that is slightly more pronounced in children with DLD, M = -1.09, SE = .677, t (452) = -1.604, p = .109. In the atelic condition, the difference was marginally significant, M = 1.23, SE = .677, t (452) = 1.814, p = .07. Intragroup contrasts for the TD group showed a marginally significant difference between telic and atelic conditions M = -1.30, SE = .677, t (452) = -1.913, p = .056. This interaction showed that children with TD showed different neural responses in this region compared to the DLD group (see
Figure 8 and
Figure 9).
3.1.4. P600 Effects in Direct Object
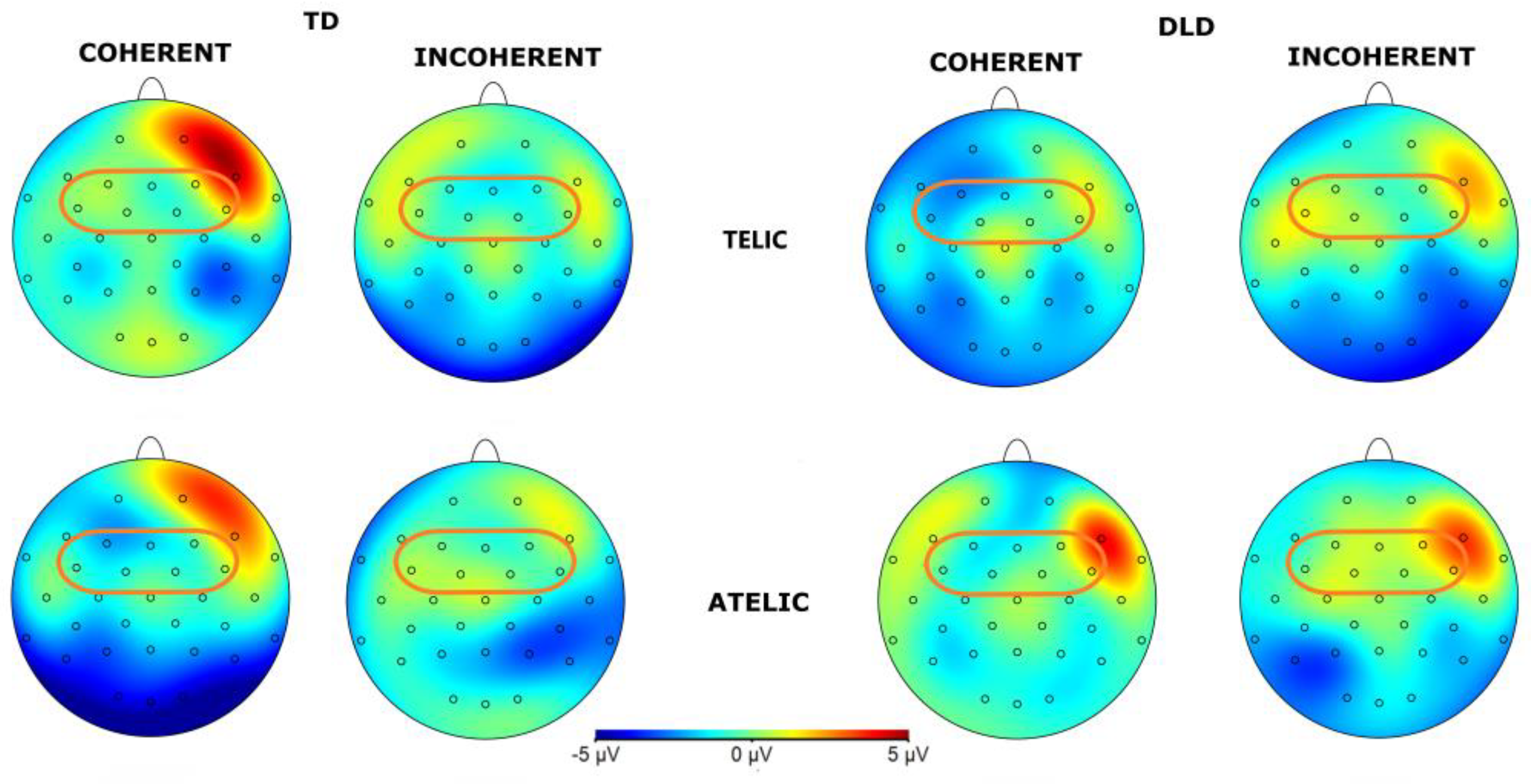
The general ANOVA in the time window of 600 to 1000 ms revealed a significant interaction between group, telicity, coherence, and hemisphere, F (1,3728) = 6.525, p = .0107. When we focus on the frontal ROI conformed by F3, FC1, FC5, F4, FC2 and FC6, F (1, 896) = 5.127, p = .024, the intergroup contrasts indicated significant differences in several conditions. For telic coherent sentences in the left hemisphere, children with DLD show a more negative amplitude than TD children, M = −1.969, SE = .814, t (896) = −2.419, p = .016. In telic incoherent sentences in the left hemisphere, the difference is marginally significant, M = 1.455, SE = 0.814, t (896) = 1.787, p = .074. For atelic incoherent sentences in the right hemisphere, the difference was also significant, M = 1.631, SE = .814, t (896) = 2.004, p = .045 showing a more negative trend in the TD group. Intragroup contrasts for the DLD group showed significant differences between telic coherent and telic incoherent conditions in the left hemisphere, M = −2.3834, SE = .814, t (896) = −2.928, p = .018 showing more negative amplitude in telic coherent condition. No significant differences were found in the TD group. The ERP waveforms illustrate these differences, particularly highlighting the interaction effects observed across hemispheres and conditions (see
Figure 10 and
Figure 11). Similar effects were found with a more frontal distribution in studies involving similar semantic tasks, specifically focusing on object-verb manipulation in the Spanish language [
35].
3.2. Behavorial Results
The behavioral analyses were done with R [
34]. To ensure data accuracy, outliers were removed using a criterion of two standard deviations from the mean. This approach effectively eliminated data points significantly deviating from the norm [
36]. Approximately 8 % of the reaction time (RT) data points in the entire sample were identified as outliers.
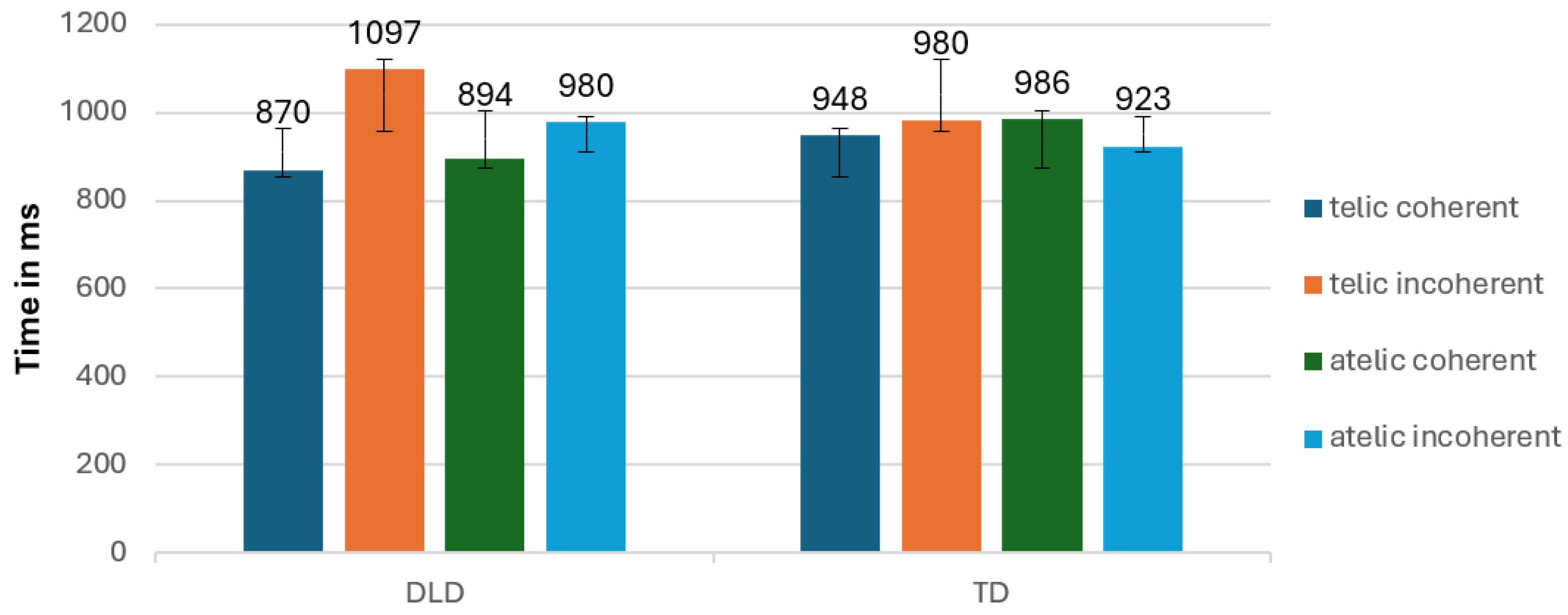
The results of the repeated measures ANOVA revealed significant interactions. A significant interaction was observed between group and coherence, F(1, 34) = 7.130, p < .001, suggesting that the difference in response to coherent and incoherent verbs varied between the DLD and TD groups (
Figure 12). The DLD group showed longer reaction times when the condition was incoherent and shorter reaction times when the condition was coherent, in comparison to the TD group. Following analysis of independent sample t-tests indicate no significant interaction.
In intragroup level the DLD group showed longer reaction times for incoherent conditions compared to coherent conditions, indicating greater difficulty or slower processing when the information presented was not coherent. In contrast, the TD group exhibited faster reaction times overall, with less pronounced differences between coherent and incoherent conditions, suggesting more efficient processing and better ability to handle both coherent and incoherent information.
Additionally, the interaction between telicity and coherence was significant, F(1, 34) = 7.348, p = .012, indicating that the combination of telicity and coherence affected participants differently.
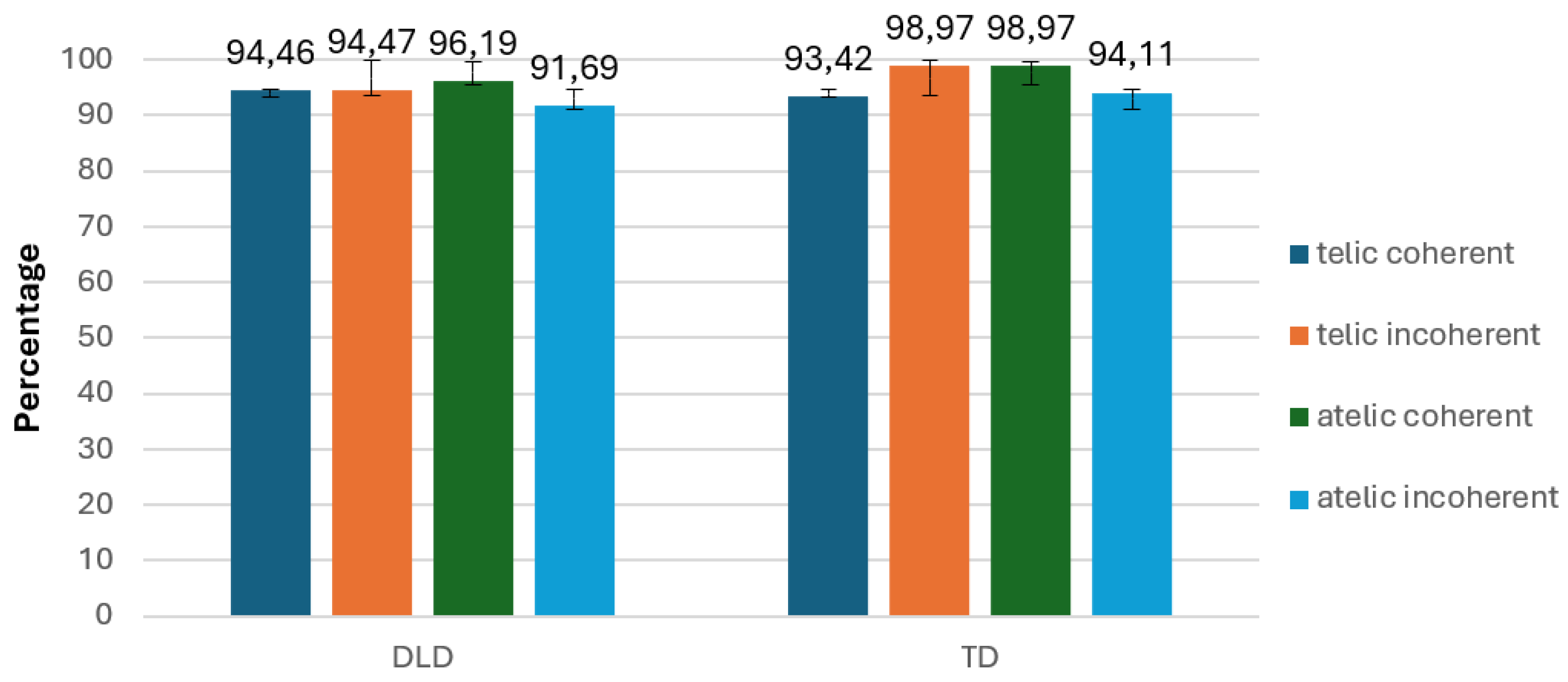
In terms of accuracy, we found a significant interaction between telicity and coherence, F(1, 34) = 14.576, p < .001, indicating that the combination of telicity and coherence affected accuracy differently (
Figure 13).
4. Discussion
The current research investigated the differences in neural and linguistic processing of telic and atelic verbs in Spanish-speaking children with DLD compared to the TD peers. An original experimental design was used, featuring sentences and images that express actions coherent with an endpoint or an ambiguous point of development, contrasted with actions incoherent with the telic and atelic semantics.
According to the proposed hypothesis, we expected to find difficulties in DLD children in distinguishing atelic verbs in contrast to telic verbs, through an attenuation of the N400 for incoherent atelic sentences. These assumptions are based on findings in other English- and German-speaking languages where atelic verbs are processed later and with greater difficulty than telic verbs [
19,
22].
Discussion of Verb Results
Based on the Event Structural Bootstrapping model, children with DLD deviate from normal language acquisition, seeking compensatory strategies such as overgeneralization in the semantic representation of verbs, which would lead to difficulties in distinguishing telic and atelic verbs [
21]. These assumptions are confirmed by the results found in the verb of the experimental sentences through the N400 component. The significant double interaction of telicity by group in the verb establishes that TD children show greater difficulty in integrating the linguistic meaning of atelic verbs, indicated by a greater amplitude of the N400 component, while the DLD group shows the opposite effect with greater difficulty in processing telic verbs compared to atelic verbs. This inverse effect might be due to the telicity in the present verb generating an effect of an unfinished action that could be affecting the DLD children [
37].
The contrast analysis indicates significant differences between telic and atelic verbs in both groups, clearly showing opposing intergroup trends. At the intragroup level, only the DLD group showed significant differences between telic and atelic verbs, with a higher cognitive cost for telic verbs. According to the literature, the past tense fits better and seems easier with telic verbs that imply a completed action than with the present tense as used in the current experiment [
37,
38,
39]. When the present tense is used with telic verbs, their degree of telicity decreases, creating an imperfective aspect of the action, while progressive and present tense contexts may be easier with atelic verbs because the action is ongoing. These temporal differences could generate a semantic effect in DLD children with difficulties in processing tense markers and the telicity of a verb [
39].
The telicity effect manifests early in both groups; however, in a late stage, the incoherent atelicity that the TD group had detected from 250 ms, the DLD group detected in a late window of 500-700 ms. This effect could be interpreted as a post-N400 component, aligning with the hypothesis that the processing time for telicity in DLD children starts later. On the other hand, in terms of telicity, the TD group detected the difficulty of present tense verbs afterwards as a more semantic processing of the verb, although no significant intragroup differences are observed in the experimental variables. It is noteworthy that the telic and atelic effects in DLD children are generally much more negative than in TD children, indicating the cognitive cost of this type of verb for this population.
Similar results were found in the lexical-semantic condition in the study by Courteau et al. [
40], who identified the typical N400 component associated with increased lexico-semantic processing difficulty at 500-700 ms (post-N400), indicating additional post-lexical integration processes. In the same vein in a recent investigation, Courteau et al. [
27] reported that lexico-semantic mismatches elicited broadly distributed N400-like negativities over centro-parietal electrodes in TD and DLD groups with the N400 duration extending beyond the classical 300-500 ms window typical for reading studies. However, both studies were conducted in adults and adolescents respectively; therefore, there could be differences in lexical-semantic processing in children.
Kornilov et al. [
41] investigated lexical processing deficits in 23 Russian-speaking children with DLD compared to 16 TD peers using a picture-word matching paradigm According to their results, lexico-semantic mismatches elicited significantly attenuated N400 amplitudes in children with DLD compared to TD children in the semantically unrelated and initial phonological overlap conditions, indicating deficits in processing both phonological and lexico-semantic mismatches. The N400 component had a prominent parietal distribution, particularly over the midline parietal (Pz, PO3, PO4, POz, Oz) and right parietal (P4, P6, PO8) electrode clusters. On the other hand, in the study by Pijnacker et al. [
26] with preschoolers with DLD in comparison with TD, where the task involved listening to sentences containing either congruent or incongruent words. The N400 component, typically associated with semantic processing, was analyzed in two time windows: 300-500 ms and 500-800 ms. The TD group exhibited a robust N400 effect in both time windows, particularly over posterior electrode sites (e.g., Pz, P3, P4, CPz). In contrast, the DLD group only showed a significant N400 effect in the later 500-800 ms window, indicating a delayed semantic processing capability. These results are directly related to the findings found in the present study. However, both studies were conducted in languages other than Spanish, hence the relevance of this study in the field.
Discussion Direct Object Results
In the case of the direct object segment, in the early window of 250 to 500 ms, our analysis revealed a significant interaction between group and telicity. Specifically, the DLD group showed significantly different neural responses compared to the TD group when processing telic direct objects, with a higher cognitive cost associated with telic verbs. The observed N400 effect in the verb window continues to be present at this level, suggesting a persistent difficulty with telicity due to the present tense conjugation, which may affect the recognition of this component. In the TD group, atelic verbs begin to show a more negative response towards the end of the sentence, with the effect localized in the right parietal region, as expected from previous literature [
40]. This interaction highlights the distinct neural mechanisms between the groups, where children with DLD show less efficient processing of telic linguistic elements.
Additionally, the significant interaction found in the left temporal region can likely be attributed to the multimodal nature of the task, as the sentences were listened to by the participants. Significant interactions in this area are expected due to the auditory processing involved. This activation suggests that children with DLD may face challenges not only in semantic integration but also in auditory processing of the sentences, potentially complicating their ability to handle telic verbs in the present tense. This is consistent with findings by Malins et al. [
42], who investigated the neural mechanisms underlying auditory word recognition in children with DLD compared to TD children. Their study highlighted differences in the N400 response, indicating that children with DLD exhibit atypical lexical processing. Furthermore, Malins et al. [
42] emphasized the importance of the temporal region in early auditory processing and its role in subsequent phonological and lexical processing, noting that these differences can influence later stages of word recognition.
In the subsequent window of 600 to 1000 ms, our analysis revealed a significant interaction between group, telicity, coherence, and hemisphere. For telic coherent and incoherent sentences, children with DLD detect the effect of telicity later than their TD peers. These results are consistent with the hypothesis that children with DLD will show a delayed effect in response to incoherent sentences. In this case, the effects were detected in a later time window. On the other hand, the TD group seems to engage in a review of the coherence of atelic sentences, resulting in increased negativity. This suggests that at this stage, TD children are re-evaluating whether the sentence accurately describes an event that occurred, contributing to a higher cognitive cost. Children with DLD, however, do not appear to be performing this re-evaluation, indicating potential difficulties in their ability to reassess sentence coherence.
As discussed in the introduction, children with DLD appear to exhibit asymmetry at the developmental level, which could be reflected in these findings. Functional MRI studies reveal atypical brain activation patterns in children with DLD, including hypoactivation in the left Inferior Frontal Gyrus (IFG) and compensatory activation in the right IFG [
9].
This could explain why children with DLD elicited more negativity in the telic coherent condition compared to TD children in the left hemisphere, whereas the same population showed more negativity in the atelic incoherent condition compared to the TD population. This right hemisphere activation in the DLD group may indicate compensatory neural mechanisms due to their atypical neural development.
In sum, the delayed detection of telicity with a greater cognitive burden in a coherent context at this stage of analysis, at the end of the sentence, and the right hemisphere engagement in more complex experimental conditions explain the difficulties in distinguishing between telic and atelic verbs, a challenge that may be related to the overgeneralization strategies in the DLD children’s population. These findings underscore the unique challenges faced by children with DLD in processing complex linguistic information.
Discussion Behavioral Results
The behavioral task in this study involved a semantic judgment related to the action depicted in the sentences. In analyzing the behavioral results, it is important to consider that while reaction times (RT) offer a mediated access to underlying mental processes, ERP is a true online method that provides immediate access to these processes [
25]. This difference might mean that the effects of telicity were not as pronounced in the RT data as they were in the ERP data. This is reflected in the significant interaction between group and coherence in reaction times, which is the only significant interaction observed. The nature of the RT task could potentially contribute to the attenuation of the telicity effect in the reaction times.
Children with DLD showed a trend of more heterogeneous reaction times, suggesting a potential greater difficulty in recognizing the telicity of verbs compared to their TD peers. This variability in RTs may indicate that children with DLD struggle more with processing the endpoint of telic actions, which aligns with the ERP results showing delayed detection of telicity. The influence of verb tense on the recognition of telicity appears to be evident in both neural and behavioral responses.
Interestingly, the DLD group also exhibited faster reaction times in some conditions, which could be attributed to impulsivity. This faster response time might not necessarily indicate better processing but rather a tendency to respond quickly without thorough processing of the semantic content. Zapparrata et al. [
43], in their meta-analysis of time-based tasks in children with DLD, found that individuals with DLD often exhibit slower processing across various tasks. However, their analysis also highlighted that in some contexts, this slow processing can be masked by impulsive responses. Children with DLD might prioritize speed over accuracy, leading to faster reaction times but not reflecting efficient or accurate semantic processing.
5. Conclusions
This study investigated the neural and linguistic processing of telic and atelic verbs in Spanish-speaking children with Developmental Language Disorder (DLD) compared to their typically developing (TD) peers. Using ERP measures and behavioral tasks, we identified distinct patterns of verb processing in these groups.
Our findings highlight that children with DLD exhibit greater difficulty in processing telic verbs, likely due to challenges in integrating semantic and temporal information. This was evident in the N400 component, where DLD children showed a delayed and attenuated response compared to TD children.
Overall, this research provides valuable insights into the neural and behavioral correlates of verb processing in children with DLD, emphasizing the importance of these findings for understanding the acquisition and development of language in this population. By the age of 7, children are expected to have an understanding of telicity that typically develops around the age of 4 [
20]. This underscores the need for early and targeted interventions that include the semantics of the verb at an early stage to support language development in children with DLD. Current interventions by specialists often focus more on general aspects of semantics rather than specifically targeting the development of telicity [
44]. Promoting the development of telicity in children with DLD may be critical to their overall language acquisition and proficiency.
Author Contributions
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. The following statements should be used “Conceptualization, M.U.; methodology, M.U., S.S., E.P., software, M.T-S..; validation, H.M., E.P. and M.U..; formal analysis, S.S., M.T.S.; investigation, S.S..; resources, M.U..; data curation, E.P., M.U.., H.M.; writing—original draft preparation, S.S. and M.U..; writing—review and editing, E.P. and H.M..; visualization, M.T-S and S.S..; supervision, M.U., E.P..; project administration, M.U..; funding acquisition, M.U. All authors have read and agreed to the published version of the manuscript.
Funding
Funding from ANID/Fondecyt Regular 1210653 and PIA-CONICYT Basal Funds for Centers of Excellence Project BF0003 is gratefully acknowledged.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Universidad de Concepción (CEBB 731-2020).
Informed Consent Statement
Written informed consent was obtained from all parents/guardians of the children. Additionally, verbal and written assent was obtained from the children before their participation in the experiment.
Data Availability Statement
The data generated and analyzed in this study are available on reasonable request from the corresponding author. The data are not publicly available as they are human data from adults and children in neurotypical and clinical groups.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- E. Aguilar-Mediavilla, L. Buil-Legaz, A. Esteller-Cano and J. Pérez-Castelló, “Desde el trastorno Específico del lenguaje (TEL) hasta el trastorno del Desarrollo del Lenguaje (TDL): un cambio de concepción sobre los trastornos del lenguaje (TDL): un cambio de concepción sobre los trastornos del lenguaje.,” Lengua, Sociedad y Comunicación, vol. 17, 2019.
- D. V. Bishop, M. J. Snowling, P. A. Thomson, T. Greenhalgh and C.-2. Consortium., “Phase 2 of CATALISE: A multinational and multidisciplinary Delphi consensus study of problems with language development: Terminology,” Journal of Child Psychology and Psychiatry, vol. 58, no. 10, pp. 1068-1080, 2017. [CrossRef]
- C. F. Norbury, D. Gooch, C. Wray, G. Baird, T. Charman, E. Simonoff, G. Vamvakas and A. Pickles, “The impact of non-verbal ability on prevalence and clinical presentation of language disorder: Evidence from a population study.,” J. Child Psychol and Psyc., vol. 57, no. 11, p. 1247–1257, 2016.
- L. Andreu, A. Igualada, N. Ahufinger and M. Sanz-Torrent, “La situación del trastorno específico del lenguaje en los países hispanohablantes,” Rev. Invest. Logopedia, vol. 12, no. 1, 2021.
- MINEDUC, “Informe de Necesidades Educativas Especiales 2017,” Ministerio de Educación de Chile, Santiago, 2018.
- J. Lee, P. Nopoulos and J. Tomblin, “Abnormal subcortical components of the corticostriatal system in young adults with DLI: A combined structural MRI and DTI study.,” Neuropsychologia, vol. 51, pp. 2154-2161, 2013. [CrossRef]
- M. Herbert, D. Ziegler, N. Makris, A. Bakardjiev, J. Hodgson, K. Adrien, D. Kennedy, P. Filipek and V. J. Caviness, “Larger brain and white matter volumes in children with developmental language disorder.,” Dev. Sci. , vol. 6, p. F11–F22, 2003. [CrossRef]
- C. Soriano-Mas, J. Pujol, H. Ortiz, J. Deus, A. López-Sala and A. Sans, “ Age-related brain structural alterations in children with specific language impairment.,” Hum. Brain Mapp. , vol. 30, p. 1626–1636, 2009. [CrossRef]
- N. Abbott and T. Love, “Bridging the Divide: Brain and Behavior in Developmental Language Disorder.,” Brain Sci., vol. 13, no. 1606, 2023. [CrossRef]
- J. Lee, A. Dick and J. Tomblin, “Altered brain structures in the dorsal and ventral language pathways in individuals with and without developmental language disorder (DLD).,” Brain Imaging Behav. , vol. 14, p. 2569–2586, 2020. [CrossRef]
- K. Watkins, F. Vargha-Khadem, J. Ashburner, R. Passingham, A. Connelly, K. Friston, R. Frackowiak, M. Mishkin and D. Gadian, “MRI analysis of an inherited speech and language disorder: Structural brain abnormalities.,” Brain, vol. 125, p. 465–478, 2002. [CrossRef]
- H. Lou, L. Henriksen and P. Bruhn, “Focal cerebral dysfunction in developmental learning disabilities.,” Lancet, vol. 335, pp. 8-11, 1990.
- L. B. Leonard, P. Deevy, S. Horvath, S. L. Christ, J. Karpicke and J. B. Kueser, “Can Retrieval Practice Facilitate Verb Learning in Children With Developmental Language Disorder and Their Peers With Typical Language Development?.,” J. Speech Lang. Hear. R., vol. 66, no. 4, p. 1309–1333., 2023. [CrossRef]
- S. Horvath and S. Arunachalam, “Optimal Contexts for Verb Learning,” Perspectives of the ASHA special interest groups, vol. 4, no. 6, p. 1239–1249, 2019.
- A. Castilla-Earls, A. Auza, A. T. Pérez-Leroux, K. Fulcher-Rood and C. Barr, “Morphological Errors in Monolingual Spanish-Speaking Children With and Without Developmental Language Disorders,” Language, speech, and hearing services in schools, vol. 51, no. 2, p. 270–281, 2020. [CrossRef]
- W. C. Krok and L. B. Leonard, “Past Tense Production in Children With and Without Specific Language Impairment Across Germanic Languages: A Meta-Analysis,” J. Speech Lang. Hear R., vol. 58, no. 4, p. 1326–1340, 2015. [CrossRef]
- P. Schulz, “Telicity in typical and impaired acquisition,” in Semantics in Language Acquisition., K. a. A. S. Syrett, Ed., John Benjamins Publishing Company., 2018.
- G. Morgan, M. Restrepo and A. Auza, “Comparison of Spanish morphology in monolingual and Spanish–English bilingual children with and without language impairment,” Bilingualism: Language and Cognition, vol. 16, no. 3, pp. 578-596, 2013. [CrossRef]
- P. Schulz, K. Wymann and Z. Penner, “The early acquisition of verb meaning in German by normally developing and language impaired children,” Brain and language,, vol. 77, no. 3, pp. 407-418, 2001. [CrossRef]
- P. Schulz and A. Wittek, “Opening doors and Sweeping floors: what children with specific language impairment know about telic and atelic verbs,” in BUCLD 27: Proceedings of the 27th annual Boston University Conference on Language Development, A. B. a. F. C. B. Beachley, Ed., Somerville, MA., Cascadilla Press, 2003, pp. 727-738.
- Z. Penner, P. Schulz and K. Wymann, “Learning the meaning of verbs: what distinguishes language-impaired from normally developing children,” Linguistics, vol. 41, no. 2, pp. 289-319, 2003. [CrossRef]
- L. B. Leonard and P. Deevy, “Tense and aspect in sentence interpretation by children with specific language impairment.,” Journal of Child Language, vol. 37, p. 395–418., 2010. [CrossRef]
- L. B. Leonard, “Time-related grammatical use by children with SLI across languages: Beyond tense.,” International journal of speech-language pathology, vol. 17, no. 6, p. 545–555., 2015. [CrossRef]
- J. Grinstead, P. Lintz, J. De la Mora, M. Cantú-Sánchez and B. Flores, “Prototypical tense-aspect alignment and the tense deficit in the spontaneous speech of Spanish-speaking children with SLI,” Probus, vol. 28, pp. 145-163, 2016. [CrossRef]
- B. Mertins, “The use of experimental methods in linguistic research: Advantages, problems and possible pitfalls. Slavic Languages in Psycholinguistics,,” pp. 15-33, 2016.
- J. Pijnacker, N. Davids, M. van Weerdenburg, L. Verhoeven, H. Knoors and P. Alphen, “Semantic Processing of Sentences in Preschoolers With Specific Language Impairment: Evidence From the N400 Effect.,” J. Speach Lang. Hear. R., vol. 60, no. 1, 2017. [CrossRef]
- É. Courteau, P. Royle and K. Steinhauer, “Number agreement processing in adolescents with and without developmental language disorder (DLD): evidence from event-related brain potentials. 1,” Scientific reports, vol. 13, no. 1, p. 22836, 2023.
- F. Faul, E. Erdfelder, A. Buchner and A. G. Lang, “Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses.,” Behavior Research Methods, vol. 41, pp. 1149-1160, 2009. [CrossRef]
- D. Pérez, P. Cáceres, S. Cáceres, C. Calderón and B. Góngora, Instrumento de diagnóstico para los trastornos específicos del lenguaje en edad escolar, Valparaiso, Chile: Ediciones Universidad de Valparaíso, 2014.
- M. Corporation., Microsoft Clipchamp, 2023.
- M. Corporation, Microsoft PowerPoint, Versión 2405., 2023.
- I. Psychology Software Tools, [E-Prime 3.0], 2016.
- I. Psychology Software Tools, [Chronos], 2016.
- Core Team, “A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna.,” pp. https://www.R-project.org., 2021.
- B. Leone-Fernandez, N. Molinaro, M. Carreiras and H. Barber, “Objects, events and ‘‘to be’’ verbs in Spanish – An ERP study of the syntax–semantics,” Brain & Language, vol. 120, pp. 127-134, 2012.
- A. Field, Discovering Statistics Using IBM SPSS Statistics, Los Angeles, CA, USA: Sage Publications, 2013.
- A. Owen Van Horne, M. Fey and M. Curran, “Do the Hard Things First: A Randomized Controlled Trial Testing the Effects of Exemplar Selection on Generalization Following Therapy for Grammatical Morphology,” J Speech Lang Hear Res., vol. 60, no. 9, pp. 2569-2588, 2017. [CrossRef]
- A. Owen Van Horne and M. Green Fager, “Quantifying the relative contributions of lexical and phonological factors to regular past tense accuracy,” Int J Speech Lang Pathol., vol. 17, no. 6, pp. 605-616, 2015.
- N. Stuart and H. van der Lely, “Role of aspect in understanding tense: an investigation with adolescents with SLI,” Int J Lang Commun Disord., vol. 50, no. 2, pp. 187-201, 2015.
- É. Courteau, L. Martignetti, P. Royle and K. Steinhauer, “ERPs for Number Mismatches in Grammatical Sentences,” Front. Psychol., vol. 10, no. 1152, 2019.
- S. Kornilov, J. Magnuson, N. Rakhlin, N. Landi and E. Grigorenko, “Lexical processing deficits in children with developmental language disorder: An event-related potentials study,” Dev Psychopathol, vol. 27, no. 2, pp. 459-476, 2015. [CrossRef]
- J. Malins, A. Desroches, E. Robertson, R. Newman, L. Archibald and M. Joanisse, “ERPs reveal the temporal dynamics of auditory word recognition in specific language impairment,” Dev Cogn Neurosci, vol. 5, pp. 134-148, 2013. [CrossRef]
- N. Zapparrata, P. Brooks and T. Ober, “Developmental Language Disorder Is Associated With Slower Processing Across Domains: A Meta-Analysis of Time-Based Tasks,” J. Speech Lang. Hear. Res., vol. 66, no. 1, pp. 325-246, 2023. [CrossRef]
- H. Lowe, L. Henry, L. Müller and J. V. , “Vocabulary intervention for adolescents with language disorder: a systematic review,” Int J Lang Commun Disord., vol. 53, no. 2, pp. 199-217, 2018. [CrossRef]
Figure 1.
(a) Presentation stimuli. (b) Chronos device.
Figure 1.
(a) Presentation stimuli. (b) Chronos device.
Figure 2.
Waveform of the audio file (mono) corresponding to the sentence. The red lines indicate the beginning of the areas of analysis: verb and direct object.
Figure 2.
Waveform of the audio file (mono) corresponding to the sentence. The red lines indicate the beginning of the areas of analysis: verb and direct object.
Figure 3.
N400 Grand-average ERPs for channels F3, Fz, F4, C3, Cz, C4, P3, Pz and P4 to represent the ANOVA effects in verb. Telic conditions are in light blue color and atelic condition in red.
Figure 3.
N400 Grand-average ERPs for channels F3, Fz, F4, C3, Cz, C4, P3, Pz and P4 to represent the ANOVA effects in verb. Telic conditions are in light blue color and atelic condition in red.
Figure 4.
Topographical map of N400 component for verb in the 250-500 ms time window for the Telic and Atelic (Telicity) conditions between groups (TD and DLD).
Figure 4.
Topographical map of N400 component for verb in the 250-500 ms time window for the Telic and Atelic (Telicity) conditions between groups (TD and DLD).
Figure 5.
Post N400 Grand-average ERPs for channels C4, CP2, CP6, P4 and P8 that form the right centroparietal ROI in verb. Coherent conditions are in light blue color and incoherent condition in red.
Figure 5.
Post N400 Grand-average ERPs for channels C4, CP2, CP6, P4 and P8 that form the right centroparietal ROI in verb. Coherent conditions are in light blue color and incoherent condition in red.
Figure 6.
Topographical map of Post N400 component for verb in the 500-700 ms time window for Telicity (Telic and Atelic) and Coherence (Coherent and Incoherent) conditions between groups (TD and DLD) on ROI involving channels C4, CP2, CP6, P4 and P8.
Figure 6.
Topographical map of Post N400 component for verb in the 500-700 ms time window for Telicity (Telic and Atelic) and Coherence (Coherent and Incoherent) conditions between groups (TD and DLD) on ROI involving channels C4, CP2, CP6, P4 and P8.
Figure 7.
N400 Grand-average ERPs for channels C4, CP2, CP6, P4 and P8 that form the right centroparietal ROI for object segment. Telic conditions are in light blue color and atelic condition in red.
Figure 7.
N400 Grand-average ERPs for channels C4, CP2, CP6, P4 and P8 that form the right centroparietal ROI for object segment. Telic conditions are in light blue color and atelic condition in red.
Figure 8.
N400 Grand-average ERPs for channels FT9, T7 and TP9, that form the left temporal ROI, for object segment. Telic conditions are in light blue color and atelic condition in red.
Figure 8.
N400 Grand-average ERPs for channels FT9, T7 and TP9, that form the left temporal ROI, for object segment. Telic conditions are in light blue color and atelic condition in red.
Figure 9.
Topographical map of N400 component for object in the 250-500 ms time window for the Telic and Atelic (Telicity) conditions between groups (TD and DLD). A: ROI involving channels FT9, T7 and TP10; B: ROI involving channels C4, CP2, CP6, P4 and P8.
Figure 9.
Topographical map of N400 component for object in the 250-500 ms time window for the Telic and Atelic (Telicity) conditions between groups (TD and DLD). A: ROI involving channels FT9, T7 and TP10; B: ROI involving channels C4, CP2, CP6, P4 and P8.
Figure 10.
Grand-average ERPs for channels F3, FC1, FC5, F4, FC2 and FC6, that form the frontal ROI for object segment. Coherent conditions are in light blue color and incoherent condition in red.
Figure 10.
Grand-average ERPs for channels F3, FC1, FC5, F4, FC2 and FC6, that form the frontal ROI for object segment. Coherent conditions are in light blue color and incoherent condition in red.
Figure 11.
Topographical map of P600 component for object in the 600-1000 ms time window for Telicity (Telic and Atelic) and Coherence (Coherent and Incoherent) conditions between groups (TD and DLD) on ROI involving channels F3, FC1, FC5, F4, FC2 and FC6.
Figure 11.
Topographical map of P600 component for object in the 600-1000 ms time window for Telicity (Telic and Atelic) and Coherence (Coherent and Incoherent) conditions between groups (TD and DLD) on ROI involving channels F3, FC1, FC5, F4, FC2 and FC6.
Figure 12.
Mean reaction times (RTs;± SE) according to conditions and Group.
Figure 12.
Mean reaction times (RTs;± SE) according to conditions and Group.
Figure 13.
Accuracy (%;± SE) according to conditions and Group.
Figure 13.
Accuracy (%;± SE) according to conditions and Group.
Table 1.
Participant characteristics.
Table 1.
Participant characteristics.
| Variable |
DLD (n=18) |
TD (n=18) |
Comparison |
| M |
SD |
Min-Max |
M |
SD |
Min-Max |
| Age (months) |
91.83 |
3.70 |
83-95 |
91.78 |
4.50 |
83-97 |
t = -.04 p = 0.98 |
| IDTEL Score |
56.39 |
6.71 |
38-66 |
104.56 |
13.70 |
83-124 |
t = -13.39 p = <0.001 |
Table 2.
Items examples.
| Variable |
Telic-coherent |
Telic-incoherent |
Atelic-coherent |
Atelic-incoherent |
| Sentence |
Papá gana la carrera/ Dad wins the race |
Papá gana la carrera/ Dad wins the race |
Papá corre la carrera/ Dad runs the race |
Papá corre la carrera/ Dad runs the race |
| Video |
Dad crosses the finish line |
Another runner wins, dad is right behind him |
Dad comes running |
Dad is sitting watching the race |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).