Submitted:
02 July 2024
Posted:
03 July 2024
You are already at the latest version
Abstract
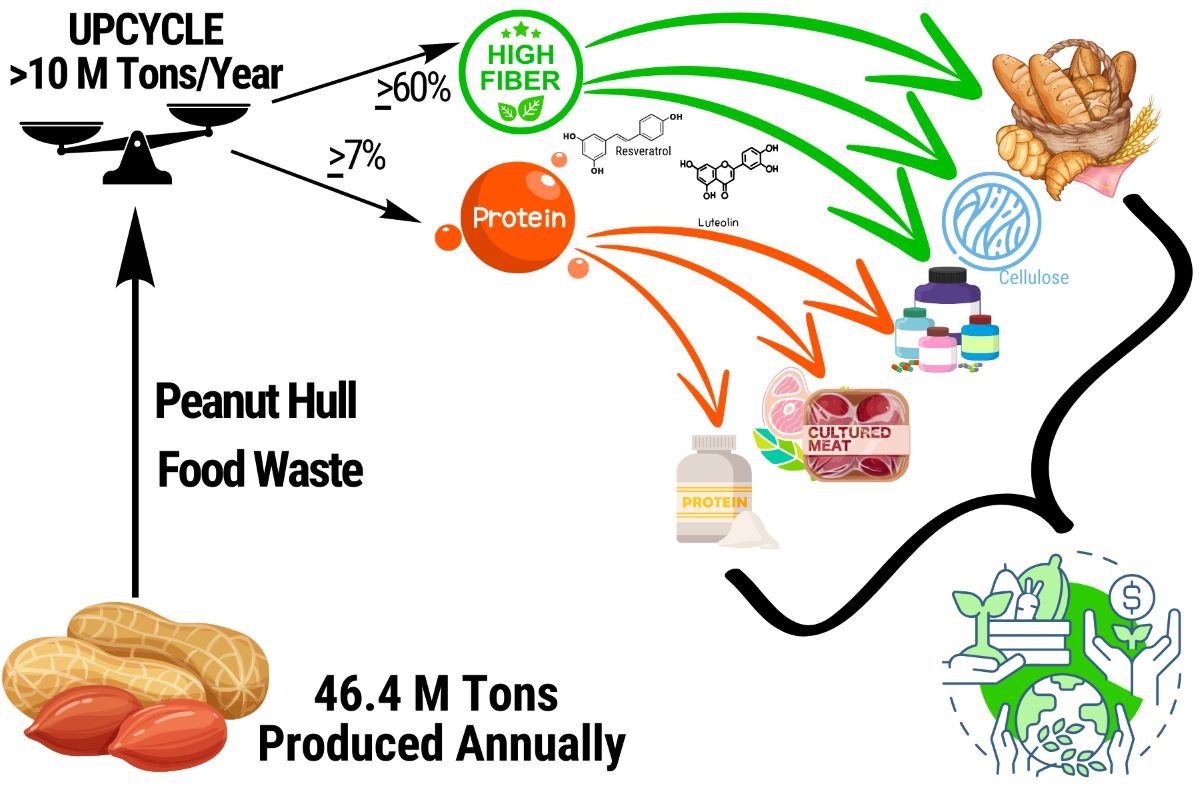
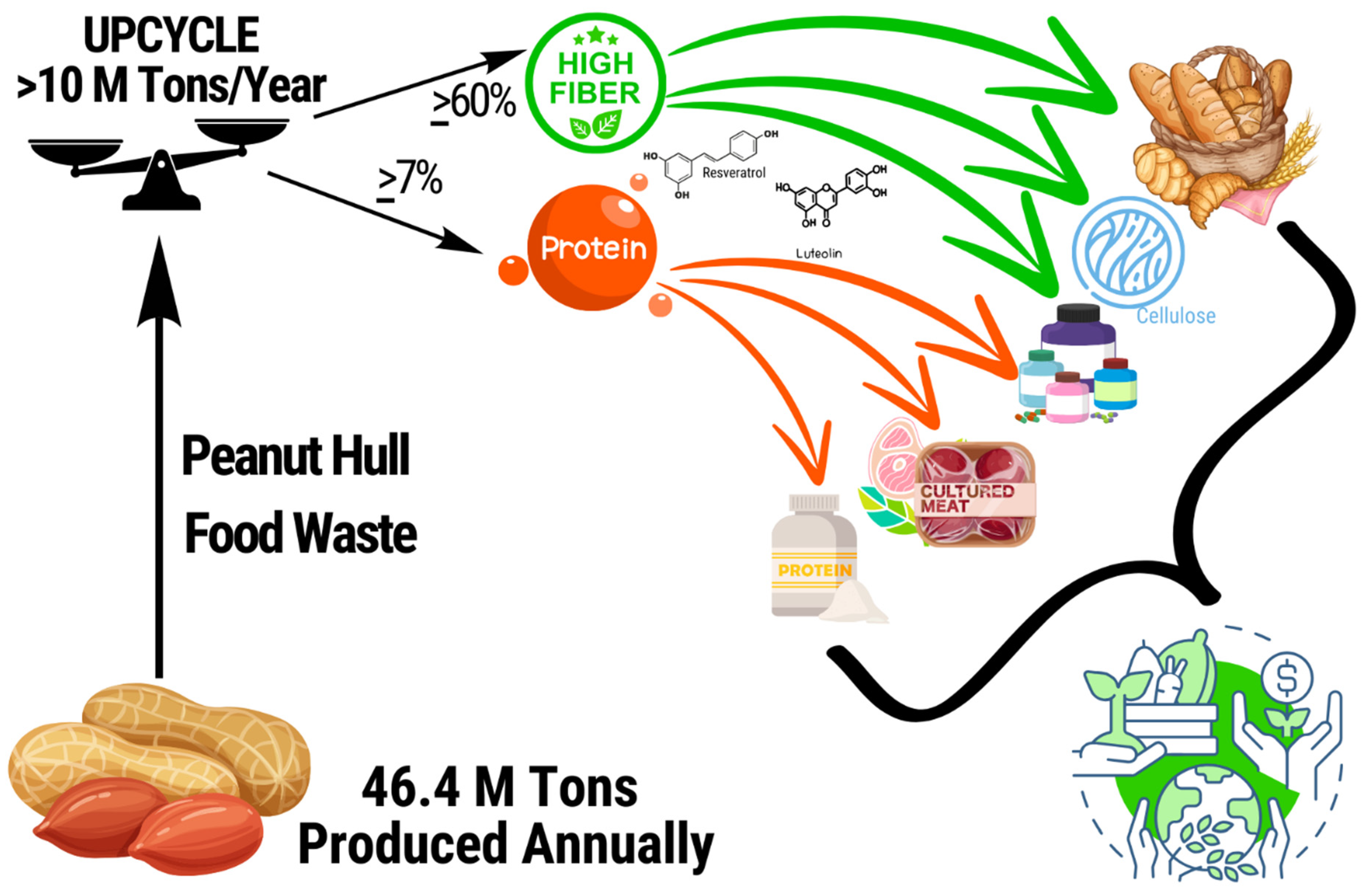
Keywords:
Background
Current Uses and Comparable Products
Processing and Manufacturing Requirements
Safety
Texture
Extraction
Other Uses
Conclusion
Acknowledgments
References
- Zhao, X., J. Chen, and F. Du, Potential use of peanut by-products in food processing: a review. J Food Sci Technol, 2012. 49(5): p. 521-9. [CrossRef] [PubMed]
- Perea-Moreno, M.-A., et al. Peanut Shell for Energy: Properties and Its Potential to Respect the Environment. Sustainability, 2018. 10. [CrossRef]
- Pączkowski, P., A. Puszka, and B. Gawdzik Effect of Eco-Friendly Peanut Shell Powder on the Chemical Resistance, Physical, Thermal, and Thermomechanical Properties of Unsaturated Polyester Resin Composites. Polymers, 2021. 13. [CrossRef]
- Chatterjee, A. and H. Singh, Development and Characterization of Peanut Shell Flour–Polypropylene Composite. Journal of The Institution of Engineers (India): Series D, 2019. 100(2): p. 147-153. [CrossRef]
- Collins, J.L. and J.F. Sanchez, EFFECTS OF PEANUT SHELL FLOUR ON, FIRMNESS, COLOR AND ACCEPTANCE OF PEANUT BUTTER. Journal of Food Science, 1979. 44(3): p. 944-945. [CrossRef]
- Collins, J.L., S.M. Kalantari, and A.R. Post, Peanut Hull Flour as Dietary Fiber in Wheat Bread. Journal of Food Science, 1982. 47(6): p. 1899-1902. [CrossRef]
- Salem, M.A., et al., Potential Valorization of Edible Nuts By-Products: Exploring the Immune-Modulatory and Antioxidants Effects of Selected Nut Shells Extracts in Relation to Their Metabolic Profiles. Antioxidants (Basel), 2022. 11(3). [CrossRef]
- Adhikari, B., et al., Antioxidant activities, polyphenol, flavonoid, and amino acid contents in peanut shell. Journal of the Saudi Society of Agricultural Sciences, 2019. 18(4): p. 437-442. [CrossRef]
- Wang, Q., et al., Chapter 5 - Peanut By-Products Utilization Technology, in Peanuts: Processing Technology and Product Development, Q. Wang, Editor. 2016, Academic Press. p. 211-325.
- Win, M.M., et al., Phenolic Compounds and Antioxidant Activity of Peanut Skin, Hull, Raw Kernel, and Roasted Kernel Flour. Pakistani Journal of Botany, 2011. 43: p. 1635-1642.
- Gao, A.X., et al., The extract of peanut shell enhances neurite outgrowth of neuronal cells: Recycling of agricultural waste for development of nutraceutical products. Journal of Functional Foods, 2022. 91: p. 105023. [CrossRef]
- Lin, Y., et al., Luteolin, a flavonoid with potential for cancer prevention and therapy. Curr Cancer Drug Targets, 2008. 8(7): p. 634-46. [CrossRef]
- Prasher, P., et al., Luteolin: a flavonoid with a multifaceted anticancer potential. Cancer Cell International, 2022. 22(1): p. 386. [CrossRef]
- Wang, L., et al., Characterization of the structural, physicochemical, and functional properties of soluble dietary fibers obtained from the peanut shell using different extraction methods. Frontiers in Nutrition, 2023. 9. [CrossRef]
- Ntalouka, F. and A. Tsirivakou, Luteolin: A promising natural agent in management of pain in chronic conditions. Front Pain Res (Lausanne), 2023. 4: p. 1114428. [CrossRef]
- Liu, W., L. Wang, and J. Zhang, Peanut Shell Extract and Luteolin Regulate Lipid Metabolism and Induce Browning in 3T3-L1 Adipocytes. Foods, 2022. 11(17). [CrossRef]
- Prakash, A., P. Nithyanand, and V. Vadivel, In vitro antibacterial activity of nut by-products against foodborne pathogens and their application in fresh-cut fruit model. J Food Sci Technol, 2018. 55(10): p. 4304-4310. [CrossRef]
- Sanders, T.H., J.L. McMeans, and J.I. Davidson, Aflatoxin content of peanut hulls. Journal of the American Oil Chemists’ Society, 1984. 61(12): p. 1839-1841. [CrossRef]
- McRae, M.P., Dietary fiber intake and type 2 diabetes mellitus: an umbrella review of meta-analyses. Journal of Chiropractic Medicine, 2018. 17(1): p. 44-53. [CrossRef] [PubMed]
- Raczyk, M., B. Kruszewski, and D. Michałowska, Effect of Coconut and Chestnut Flour Supplementations on Texture, Nutritional and Sensory Properties of Baked Wheat Based Bread. Molecules, 2021. 26(15). [CrossRef]
- Konak, Ü.İ., et al., Quality characteristics of functional snack foods prepared from hazelnut shell and teff flour. Journal of Food Measurement and Characterization, 2023. 17(6): p. 5721-5729. [CrossRef]
- Kahlaoui, M., et al., Almond Hull as a Functional Ingredient of Bread: Effects on Physico-Chemical, Nutritional, and Consumer Acceptability Properties. Foods, 2022. 11(6). [CrossRef]
- GMR., Global Hazelnut Shell Powders Market. 2022, Growth Market Reports.
- Houston, B.E., Method and system for producing peanut hull flour, in Google. 2007, Individual: USA.
- CPG Sec 570.350 Peanuts, Shelled and Unshelled - Adulteration with Filth and Reject Nuts, FDA, Editor. 2005, Center for Food Safety and Applied Nutrition, Office of Regulatory Affairs.
- Galvez, F.C.F., et al., Manual Sorting To Eliminate Aflatoxin from Peanuts. Journal of Food Protection, 2003. 66(10): p. 1879-1884. [CrossRef] [PubMed]
- Darko, C., et al., Effects of packaging and pre-storage treatments on aflatoxin production in peanut storage under controlled conditions. J Food Sci Technol, 2018. 55(4): p. 1366-1375. [CrossRef]
- Milled Peanuts-- Inspection Instructions, USDA, Editor. 2023, USDA.
- APSA, Industry Handbook for the Safe Shelling of Peanuts. APSA Committe on Regulatory Compliance, 2020.
- Anco, D.J. and S. Rustgi, Mangement of Aflatoxins in Peanut. Land-Grant Press, 2020.
- Dorner, J.W., Management and prevention of mycotoxins in peanuts. Food Additives & Contaminants: Part A, 2008. 25(2): p. 203-208. [CrossRef]
- Lameiras, M.M. Field to Jar. College of Agricultural and Environmental Sciences; Available from: https://discover.caes.uga.edu/peanut-protectors/index.html.
- EPA, Peanut Processing, EPA, Editor.
- Rossbach, J.D., et al., Factors influencing the Salmonella internalization into seedpods and whole plants of Arachis hypogaea (L.). Food Microbiology, 2017. 66: p. 184-189. [CrossRef]
- Uppala, S.S., Factors Affecting Pre-Harvest Aflatoxin Contamination of Peanut (Arachis Hypogaea L.). Auburn University, 2011. Dissertation.
- Waliyar, F., et al., Mycotoxins: detection methods, management, public health and agricultural trade Pre-and post-harvest management of aflatoxin contamination in peanuts., ed. R.B.a.A.V. J.F. Leslie. 2008: Wallingford: CAB International.
- Shen, M.-H. and R.K. Singh, Detoxifying aflatoxin contaminated peanuts by high concentration of H2O2 at moderate temperature and catalase inactivation. Food Control, 2022. 142: p. 109218. [CrossRef]
- Shabeer, S., et al. Aflatoxin Contamination, Its Impact and Management Strategies: An Updated Review. Toxins, 2022. 14. [CrossRef]
- Sipos, P., et al., Physical and Chemical Methods for Reduction in Aflatoxin Content of Feed and Food. Toxins (Basel), 2021. 13(3). [CrossRef]
- Dhanshetty, M., C.T. Elliott, and K. Banerjee, Decontamination of aflatoxin B1 in peanuts using various cooking methods. J Food Sci Technol, 2021. 58(7): p. 2547-2554. [CrossRef] [PubMed]
- FDA, Field Science - Laboratory Manual, FDA, Editor. 2023.
- McGlynn, W., Guidelines for the Use of Chlorine Bleach as a Sanitizer in Food Processing Operations, in Food and Agricultural Products Research and Technology Center, O.S. University, Editor.
- Lieberman, J.A., et al., The global burden of illness of peanut allergy: A comprehensive literature review. Allergy, 2021. 76(5): p. 1367-1384. [CrossRef]
- Cuadrado, C., Á. Sanchiz, and R. Linacero Nut Allergenicity: Effect of Food Processing. Allergies, 2021. 1, 150-162. [CrossRef]
- Chen, Y.-X., et al., Effects of wheat tempering with slightly acidic electrolyzed water on the microbial, biological, and chemical characteristics of different flour streams. LWT, 2020. 118: p. 108790. [CrossRef]
- Kumar, A., et al., Effect of ultraviolet irradiation on wheat (Triticum aestivum) flour: Study on protein modification and changes in quality attributes. Journal of Cereal Science, 2020. 96: p. 103094. [CrossRef]
- Rashwan, A.K. and e. al., Plant-based proteins: advanced extraction technologies, interactions, physicochemical and functional properties, food and related applications, and health benefits. Critical Reviews in Food Science and Nutrition.
- Biswas, A., et al., Conversion of agricultural by-products to methyl cellulose. Industrial Crops and Products, 2013. 46: p. 297-300. [CrossRef]
- Statista, Market size of plant-based protein ingredients. 2023.
- Yen, F.-C., et al., Cultured meat platform developed through the structuring of edible microcarrier-derived microtissues with oleogel-based fat substitute. NATURE COMMUNICATIONS, 2023. 14(1). [CrossRef]
| Processing for Flour | |||
| Chemical or Bioactive Reagent | Damage control | Moisture Reduction | |
| Field Methods to Reduce Pathogen Activity | Lime and calcium soil amendment; A.flavus or other resistant strain competition | ||
| Harvest Methods to Reduce Pathogen Activity | Manual Inspection | Rapid Shelling | |
| Systematic Sampling | Pathogen testing to initiate effective quarantining and discarding, as well as soil amelioration. | Moisture testing to ensure low water activity inhospitable to microbial growth. | |
| Wet Sanitization | Hydrogen peroxide, hypochlorite, electrolyzed water, and others may kill and in some cases remove mycotoxins. | ||
| Texture Maturing and Conditioning | UV irradiation to mature flavor and enhance milling texture; sodium bicarbonate and sodium bisulfate to reduce mixtures and produce finer flour. | ||
| Rapid Drying | Baking, roasting, fan, and sun drying. UV irradiation for rapid drying, producing finer and more consistent milling textures while controlling toxigenic species; Ozone gas may also be used, but is best applied after milling to support finer textures. | ||
| Milling and Equipment Sanitization | Burr grinders should be sanitized with food-safe dilutions of hypochlorite, or equivalent, in between each processing lot and at the end of working periods to reduce equipment-related pathogen transfer. | ||
| Bagging and Storage | Fully dried, milled flour should be tested for water activity, and stored as flour in rooms without the threat of flood or high humidity. | ||
| Processing for Nutrient Extraction | |||
| Chemical or Bioactive Reagent | Damage control | Moisture Reduction | |
| Field Methods to Reduce Pathogen Activity | Lime and calcium soil amendment; A.flavus or other resistant strain competition | ||
| Harvest Methods to Reduce Pathogen Activity | Manual Inspection | Rapid Shelling | |
| Systematic Sampling | Pathogen testing to initiate effective quarantining and discarding, as well as soil amelioration. | Moisture testing to ensure low water activity inhospitable to microbial growth. | |
| Texture Maturing and Conditioning | UV irradiation to mature flavor and enhance milling texture; sodium bicarbonate and sodium bisulfate to reduce mixtures and produce finer flour. | UV irradiation also assists drying, producing finer and more consistent milling textures while controlling toxigenic species; Ozone gas may also be used but is best applied after milling to support finer textures which may improve protein yield. | |
| Milling and Equipment Sanitization | Burr grinders should be sanitized with food-safe dilutions of hypochlorite, or equivalent, in between each processing lot and at the end of working periods to reduce equipment-related pathogen transfer. | ||
| Preparation for Extraction (by method) | Dissolved in water or other solvents, specific to each method. Additional use of food-safe acids and enzymes may also be applied to extract one or more nutritional compounds. | Dry methods may include ultrasound- and microwave-assisted extraction, or high-pressure extractions without direct water contact, enabling less need for drying final substrates. | |
| Nutrient Retention and Single-step production of multiple ingredients | Different heat, enzymes and multiple pass methods may be used to obtain the maximum recycled yield from materials with single processing. | ||
| Bagging and Storage | Fully dried, powdered extracts should be tested for water activity, and stored in sealed, air-tight containers impervious to moisture infiltration. | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





