Submitted:
03 July 2024
Posted:
03 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Effects of TrkB and BDNF Knockout on the Motor System
2.1. TrkB Knockout
2.2. BDNF Full Knockout
2.3. BDNF Conditional Knockout (Wnt1-Cre)
2.4. BDNF Conditional Knockout (Emx1-Cre)
2.5. BDNF Conditional Knockout (Tau-Cre)
3. Anterograde Transport and Release of BDNF from Corticostriatal Projection Neurons
4. The Role of BDNF in Corticostriatal Projection Neurons for Motor Learning
4.1. BDNF Is Necessary for LTP at Corticostriatal Synapses
4.2. Motor Activity Regulates BDNF Levels in the Brain
4.3. Implications of Cortical BDNF for Motor Learning
5. Alterations of BDNF in Parkinson’s Disease
5.1. Alterations of BDNF mRNA and Protein Expression
5.2. Altered Anterograde Transport of BDNF in Corticostriatal and Dopaminergic Projections
5.3. Release of BDNF in the Striatum
5.4. Potential Effects of Deep Brain Stimulation and Neuronal Activity Modulation
5.5. BDNF as a Therapeutic Agent for Parkinson’s Disease
6. Alterations of TrkB Signaling in Parkinson’s Disease
6.1. Alterations of Ntrk2 mRNA and TrkB Protein Expression
6.2. TrkB Depletion Induces Cell Loss in the Substantia Nigra
6.3. TrkB Interaction with α-Synuclein Is Linked to Pathology in Parkinson’s Disease
6.4. Changes in Subcellular Distribution of TrkB and Implications for Corticostriatal Synaptic Plasticity
7. BDNF/TrkB Signaling in Dystonia
7.1. Corticostriatal plasticity impairments in dystonia
7.2. Dopamine Signaling Is Involved in Dystonia Pathogenesis
7.3. BDNF/TrkB Signaling in Dystonia
7.4. Effects of Deep Brain Stimulation and Neuroplasticity Modulation in the Therapy of Dystonia
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barde, Y.A.; Edgar, D.; Thoenen, H. Purification of a new neurotrophic factor from mammalian brain. The EMBO journal 1982, 1, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Leibrock, J.; Lottspeich, F.; Hohn, A.; Hofer, M.; Hengerer, B.; Masiakowski, P.; Thoenen, H.; Barde, Y.-A. Molecular cloning and expression of brain-derived neurotrophic factor. Nature 1989, 341, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Levi-Montalcini, R.; Skaper, S.D.; Dal Toso, R.; Petrelli, L.; Leon, A. Nerve growth factor: from neurotrophin to neurokine. Trends Neurosci 1996, 19, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Thoenen, H. Neurotrophins and Neuronal Plasticity. Science 1995, 270, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Poo, M.-m. Neurotrophin regulation of neural circuit development and function. Nature Reviews Neuroscience 2013, 14, 7–23. [Google Scholar] [CrossRef]
- Barbacid, M. The Trk family of neurotrophin receptors. Journal of Neurobiology 1994, 25, 1386–1403. [Google Scholar] [CrossRef] [PubMed]
- Snider, W.D.; Johnson Jr., E. M. Neurotrophic molecules. Annals of Neurology 1989, 26, 489–506. [Google Scholar] [CrossRef] [PubMed]
- Chao, M.V. Neurotrophin receptors: A window into neuronal differentiation. Neuron 1992, 9, 583–593. [Google Scholar] [CrossRef]
- Chao, M.V. Neurotrophins and their receptors: A convergence point for many signalling pathways. Nature Reviews Neuroscience 2003, 4, 299–309. [Google Scholar] [CrossRef]
- Reichardt, L.F. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci 2006, 361, 1545–1564. [Google Scholar] [CrossRef]
- Sendtner, M.; Holtmann, B.; Kolbeck, R.; Thoenen, H.; Barde, Y.A. Brain-derived neurotrophic factor prevents the death of motoneurons in newborn rats after nerve section. Nature 1992, 360, 757–759. [Google Scholar] [CrossRef] [PubMed]
- Barde, Y.A. The nerve growth factor family. Prog Growth Factor Res 1990, 2, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Maisonpierre, P.C.; Belluscio, L.; Friedman, B.; Alderson, R.F.; Wiegand, S.J.; Furth, M.E.; Lindsay, R.M.; Yancopoulos, G.D. NT-3, BDNF, and NGF in the developing rat nervous system: Parallel as well as reciprocal patterns of expression. Neuron 1990, 5, 501–509. [Google Scholar] [CrossRef]
- Zheng, F.; Zhou, X.; Moon, C.; Wang, H. Regulation of brain-derived neurotrophic factor expression in neurons. Int J Physiol Pathophysiol Pharmacol 2012, 4, 188–200. [Google Scholar] [PubMed]
- Ernfors, P.; Wetmore, C.; Olson, L.; Persson, H. Identification of cells in rat brain and peripheral tissues expressing mRNA for members of the nerve growth factor family. Neuron 1990, 5, 511–526. [Google Scholar] [CrossRef] [PubMed]
- Rauskolb, S.; Zagrebelsky, M.; Dreznjak, A.; Deogracias, R.; Matsumoto, T.; Wiese, S.; Erne, B.; Sendtner, M.; Schaeren-Wiemers, N.; Korte, M.; et al. Global Deprivation of Brain-Derived Neurotrophic Factor in the CNS Reveals an Area-Specific Requirement for Dendritic Growth. The Journal of Neuroscience 2010, 30, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
- Katoh-Semba, R.; Takeuchi, I.K.; Semba, R.; Kato, K. Distribution of Brain-Derived Neurotrophic Factor in Rats and Its Changes with Development in the Brain. Journal of Neurochemistry 1997, 69, 34–42. [Google Scholar] [CrossRef] [PubMed]
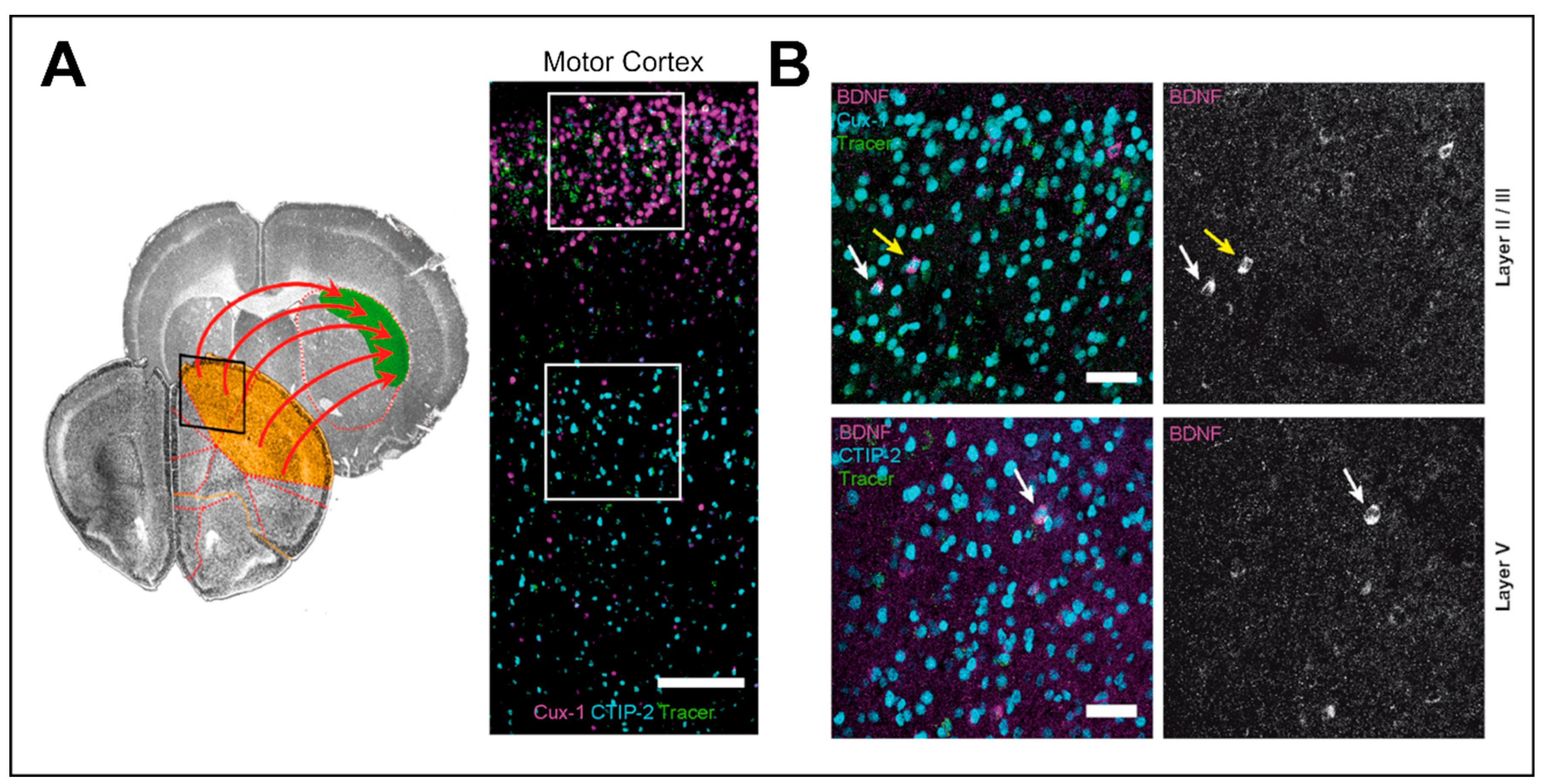
- Andreska, T.; Rauskolb, S.; Schukraft, N.; Lüningschrör, P.; Sasi, M.; Signoret-Genest, J.; Behringer, M.; Blum, R.; Sauer, M.; Tovote, P.; et al. Induction of BDNF Expression in Layer II/III and Layer V Neurons of the Motor Cortex Is Essential for Motor Learning. The Journal of Neuroscience 2020, 40, 6289–6308. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.-R.; Walther, D.; Drgon, T.; Polesskaya, O.; Lesnick, T.G.; Strain, K.J.; de Andrade, M.; Bower, J.H.; Maraganore, D.M.; Uhl, G.R. Human brain derived neurotrophic factor (BDNF) genes, splicing patterns, and assessments of associations with substance abuse and Parkinson’s Disease. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics 2005, 134B, 93–103. [Google Scholar] [CrossRef]
- Lu, B. BDNF and activity-dependent synaptic modulation. Learn Mem 2003, 10, 86–98. [Google Scholar] [CrossRef]
- Croll, S.D.; Ip, N.Y.; Lindsay, R.M.; Wiegand, S.J. Expression of BDNF and trkB as a function of age and cognitive performance. Brain Res 1998, 812, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Barde, Y.A. Neurotrophins: a family of proteins supporting the survival of neurons. Prog Clin Biol Res 1994, 390, 45–56. [Google Scholar] [PubMed]
- Shelton, D.L.; Reichardt, L.F. Studies on the expression of the beta nerve growth factor (NGF) gene in the central nervous system: level and regional distribution of NGF mRNA suggest that NGF functions as a trophic factor for several distinct populations of neurons. Proc Natl Acad Sci U S A 1986, 83, 2714–2718. [Google Scholar] [CrossRef] [PubMed]
- Korsching, S.; Thoenen, H. Developmental changes of nerve growth factor levels in sympathetic ganglia and their target organs. Developmental Biology 1988, 126, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Levi-Montalcini, R. The nerve growth factor 35 years later. Science 1987, 237, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- Sofroniew, M.V.; Howe, C.L.; Mobley, W.C. Nerve growth factor signaling, neuroprotection, and neural repair. Annu Rev Neurosci 2001, 24, 1217–1281. [Google Scholar] [CrossRef] [PubMed]
- Patel, T.D.; Kramer, I.; Kucera, J.; Niederkofler, V.; Jessell, T.M.; Arber, S.; Snider, W.D. Peripheral NT3 Signaling Is Required for ETS Protein Expression and Central Patterning of Proprioceptive Sensory Afferents. Neuron 2003, 38, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Maisonpierre, P.C.; Belluscio, L.; Squinto, S.; Ip, N.Y.; Furth, M.E.; Lindsay, R.M.; Yancopoulos, G.D. Neurotrophin-3: a neurotrophic factor related to NGF and BDNF. Science 1990, 247, 1446–1451. [Google Scholar] [CrossRef] [PubMed]
- Kucera, J.; Fan, G.; Jaenisch, R.; Linnarsson, S.; Ernfors, P. Dependence of developing group Ia afferents on neurotrophin-3. Journal of Comparative Neurology 1995, 363, 307–320. [Google Scholar] [CrossRef]
- Ernfors, P.; Persson, H. Developmentally Regulated Expression of HDNF/NT-3 mRNA in Rat Spinal Cord Motoneurons and Expression of BDNF mRNA in Embryonic Dorsal Root Ganglion. European Journal of Neuroscience 1991, 3, 953–961. [Google Scholar] [CrossRef]
- Meyer, M.; Matsuoka, I.; Wetmore, C.; Olson, L.; Thoenen, H. Enhanced synthesis of brain-derived neurotrophic factor in the lesioned peripheral nerve: different mechanisms are responsible for the regulation of BDNF and NGF mRNA. Journal of Cell Biology 1992, 119, 45–54. [Google Scholar] [CrossRef]
- Schäbitz, W.-R.; Schwab, S.; Spranger, M.; Hacke, W. Intraventricular Brain-Derived Neurotrophic Factor Reduces Infarct Size after Focal Cerebral Ischemia in Rats. Journal of Cerebral Blood Flow & Metabolism 1997, 17, 500–506. [Google Scholar] [CrossRef]
- Kokaia, Z.; Gidö, G.; Ringstedt, T.; Bengzon, J.; Kokaia, M.; Siesjö, B.K.; Persson, H.; Lindvall, O. Rapid increase of BDNF mRNA levels in cortical neurons following spreading depression: regulation by glutamatergic mechanisms independent of seizure activity. Molecular Brain Research 1993, 19, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Lindvall, O.; Ernfors, P.; Bengzon, J.; Kokaia, Z.; Smith, M.L.; Siesjö, B.K.; Persson, H. Differential regulation of mRNAs for nerve growth factor, brain-derived neurotrophic factor, and neurotrophin 3 in the adult rat brain following cerebral ischemia and hypoglycemic coma. Proceedings of the National Academy of Sciences 1992, 89, 648–652. [Google Scholar] [CrossRef]
- Tsukahara, T.; Yonekawa, Y.; Tanaka, K.; Ohara, O.; Watanabe, S.; Kimura, T.; Nishijima, T.; Taniguchi, T. The Role of Brain-derived Neurotrophic Factor in Transient Forebrain Ischemia in the Rat Brain. Neurosurgery 1994, 34, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Thoenen, H.; Zafra, F.; Hengerer, B.; Lindholm, D. The synthesis of nerve growth factor and brain-derived neurotrophic factor in hippocampal and cortical neurons is regulated by specific transmitter systems. Ann N Y Acad Sci 1991, 640, 86–90. [Google Scholar] [CrossRef]
- Zafra, F.; Hengerer, B.; Leibrock, J.; Thoenen, H.; Lindholm, D. Activity dependent regulation of BDNF and NGF mRNAs in the rat hippocampus is mediated by non-NMDA glutamate receptors. The EMBO Journal 1990, 9, 3545–3550. [Google Scholar] [CrossRef] [PubMed]
- Poo, M.-m. Neurotrophins as synaptic modulators. Nature Reviews Neuroscience 2001, 2, 24–32. [Google Scholar] [CrossRef]
- Neeper, S.A.; Gómez-Pinilla, F.; Choi, J.; Cotman, C.W. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Research 1996, 726, 49–56. [Google Scholar] [CrossRef]
- Vaynman, S.; Ying, Z.; Gomez-Pinilla, F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci 2004, 20, 2580–2590. [Google Scholar] [CrossRef]
- Bekinschtein, P.; Oomen, C.A.; Saksida, L.M.; Bussey, T.J. Effects of environmental enrichment and voluntary exercise on neurogenesis, learning and memory, and pattern separation: BDNF as a critical variable? Seminars in Cell & Developmental Biology 2011, 22, 536–542. [Google Scholar] [CrossRef]
- Takahashi, K.; Maejima, H.; Ikuta, G.; Mani, H.; Asaka, T. Exercise combined with low-level GABAA receptor inhibition up-regulates the expression of neurotrophins in the motor cortex. Neuroscience Letters 2017, 636, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Korte, M.; Kang, H.; Bonhoeffer, T.; Schuman, E. A role for BDNF in the late-phase of hippocampal long-term potentiation. Neuropharmacology 1998, 37, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Patterson, S.L.; Grover, L.M.; Schwartzkroin, P.A.; Bothwell, M. Neurotrophin expression in rat hippocampal slices: A stimulus paradigm inducing LTP in CA1 evokes increases in BDNF and NT-3 mRNAs. Neuron 1992, 9, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Minichiello, L. TrkB signalling pathways in LTP and learning. Nature Reviews Neuroscience 2009, 10, 850–860. [Google Scholar] [CrossRef]
- Figurov, A.; Pozzo-Miller, L.D.; Olafsson, P.; Wang, T.; Lu, B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature 1996, 381, 706–709. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, J.-i.; Horiike, Y.; Matsuzaki, M.; Miyazaki, T.; Ellis-Davies, G.C.R.; Kasai, H. Protein Synthesis and Neurotrophin-Dependent Structural Plasticity of Single Dendritic Spines. Science 2008, 319, 1683–1687. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, X.-b.; Frerking, M.; Zhou, Q. Spine Expansion and Stabilization Associated with Long-Term Potentiation. The Journal of Neuroscience 2008, 28, 5740–5751. [Google Scholar] [CrossRef] [PubMed]
- Kellner, Y.; Goedecke, N.; Dierkes, T.; Thieme, N.; Zagrebelsky, M.; Korte, M. The BDNF effects on dendritic spines of mature hippocampal neurons depend on neuronal activity. Frontiers in Synaptic Neuroscience 2014, 6. [Google Scholar] [CrossRef]
- Bruel-Jungerman, E.; Laroche, S.; Rampon, C. New neurons in the dentate gyrus are involved in the expression of enhanced long-term memory following environmental enrichment. Eur J Neurosci 2005, 21, 513–521. [Google Scholar] [CrossRef]
- Korte, M.; Carroll, P.; Wolf, E.; Brem, G.; Thoenen, H.; Bonhoeffer, T. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proceedings of the National Academy of Sciences 1995, 92, 8856–8860. [Google Scholar] [CrossRef] [PubMed]
- Patterson, S.L.; Pittenger, C.; Morozov, A.; Martin, K.C.; Scanlin, H.; Drake, C.; Kandel, E.R. Some Forms of cAMP-Mediated Long-Lasting Potentiation Are Associated with Release of BDNF and Nuclear Translocation of Phospho-MAP Kinase. Neuron 2001, 32, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Kernie, S.G.; Liebl, D.J.; Parada, L.F. BDNF regulates eating behavior and locomotor activity in mice. Embo j 2000, 19, 1290–1300. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.; Yeo, G.S.; Cox, J.J.; Morton, J.; Adlam, A.L.; Keogh, J.M.; Yanovski, J.A.; El Gharbawy, A.; Han, J.C.; Tung, Y.C.; et al. Hyperphagia, severe obesity, impaired cognitive function, and hyperactivity associated with functional loss of one copy of the brain-derived neurotrophic factor (BDNF) gene. Diabetes 2006, 55, 3366–3371. [Google Scholar] [CrossRef]
- Rios, M.; Fan, G.; Fekete, C.; Kelly, J.; Bates, B.; Kuehn, R.; Lechan, R.M.; Jaenisch, R. Conditional Deletion Of Brain-Derived Neurotrophic Factor in the Postnatal Brain Leads to Obesity and Hyperactivity. Molecular Endocrinology 2001, 15, 1748–1757. [Google Scholar] [CrossRef] [PubMed]
- Yeo, G.S.H.; Connie Hung, C.-C.; Rochford, J.; Keogh, J.; Gray, J.; Sivaramakrishnan, S.; O’Rahilly, S.; Farooqi, I.S. A de novo mutation affecting human TrkB associated with severe obesity and developmental delay. Nature Neuroscience 2004, 7, 1187–1189. [Google Scholar] [CrossRef] [PubMed]
- Petzold, A.; Psotta, L.; Brigadski, T.; Endres, T.; Lessmann, V. Chronic BDNF deficiency leads to an age-dependent impairment in spatial learning. Neurobiology of Learning and Memory 2015, 120, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Bekinschtein, P.; Cammarota, M.; Katche, C.; Slipczuk, L.; Rossato, J.I.; Goldin, A.; Izquierdo, I.; Medina, J.H. BDNF is essential to promote persistence of long-term memory storage. Proc Natl Acad Sci U S A 2008, 105, 2711–2716. [Google Scholar] [CrossRef] [PubMed]
- Snider, W.D. Functions of the neurotrophins during nervous system development: What the knockouts are teaching us. Cell 1994, 77, 627–638. [Google Scholar] [CrossRef]
- Conover, J.C.; Yancopoulos, G.D. Neurotrophin Regulation of the Developing Nervous System: Analyses of Knockout Mice. Reviews in the Neurosciences 1997, 8, 13–28. [Google Scholar] [CrossRef]
- Klein, R.; Parada, L.F.; Coulier, F.; Barbacid, M. trkB, a novel tyrosine protein kinase receptor expressed during mouse neural development. The EMBO Journal 1989, 8, 3701–3709. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Conway, D.; Parada, L.F.; Barbacid, M. The trkB tyrosine protein kinase gene codes for a second neurogenic receptor that lacks the catalytic kinase domain. Cell 1990, 61, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Smeyne, R.J.; Wurst, W.; Long, L.K.; Auerbach, B.A.; Joyner, A.L.; Barbacid, M. Targeted disruption of the trkBneurotrophin receptor gene results in nervous system lesions and neonatal death. Cell 1993, 75, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Ernfors, P.; Lee, K.-F.; Jaenisch, R. Mice lacking brain-derived neurotrophic factor develop with sensory deficits. Nature 1994, 368, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.R.; Fariñas, I.; Backus, C.; Reichardt, L.F. Targeted disruption of the BDNF gene perturbs brain and sensory neuron development but not motor neuron development. Cell 1994, 76, 989–999. [Google Scholar] [CrossRef] [PubMed]
- Patterson, S.L.; Abel, T.; Deuel, T.A.S.; Martin, K.C.; Rose, J.C.; Kandel, E.R. Recombinant BDNF Rescues Deficits in Basal Synaptic Transmission and Hippocampal LTP in BDNF Knockout Mice. Neuron 1996, 16, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Prensa, L.; Giménez-Amaya, J.M.; Parent, A. Morphological features of neurons containing calcium-binding proteins in the human striatum. Journal of Comparative Neurology 1998, 390, 552–563. [Google Scholar] [CrossRef]
- McMahon, A.P.; Gavin, B.J.; Parr, B.; Bradley, A.; McMahon, J.A. The WNT Family of Cell Signalling Molecules in Postimplantation Development of the Mouse. In Ciba Foundation Symposium 165 - Postimplantation Development in the Mouse; 2007; pp. 199-218.
- Danielian, P.S.; Muccino, D.; Rowitch, D.H.; Michael, S.K.; McMahon, A.P. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Current Biology 1998, 8, 1323–1326. [Google Scholar] [CrossRef] [PubMed]
- Baquet, Z.C.; Bickford, P.C.; Jones, K.R. Brain-Derived Neurotrophic Factor Is Required for the Establishment of the Proper Number of Dopaminergic Neurons in the Substantia Nigra Pars Compacta. The Journal of Neuroscience 2005, 25, 6251–6259. [Google Scholar] [CrossRef]
- Gorski, J.A.; Talley, T.; Qiu, M.; Puelles, L.; Rubenstein, J.L.R.; Jones, K.R. Cortical Excitatory Neurons and Glia, But Not GABAergic Neurons, Are Produced in the Emx1-Expressing Lineage. The Journal of Neuroscience 2002, 22, 6309–6314. [Google Scholar] [CrossRef]
- Gorski, J.A.; Zeiler, S.R.; Tamowski, S.; Jones, K.R. Brain-Derived Neurotrophic Factor Is Required for the Maintenance of Cortical Dendrites. The Journal of Neuroscience 2003, 23, 6856–6865. [Google Scholar] [CrossRef] [PubMed]
- Baquet, Z.C.; Gorski, J.A.; Jones, K.R. Early Striatal Dendrite Deficits followed by Neuron Loss with Advanced Age in the Absence of Anterograde Cortical Brain-Derived Neurotrophic Factor. The Journal of Neuroscience 2004, 24, 4250–4258. [Google Scholar] [CrossRef] [PubMed]
- Gorski, J.A.; Balogh, S.A.; Wehner, J.M.; Jones, K.R. Learning deficits in forebrain-restricted brain-derived neurotrophic factor mutant mice. Neuroscience 2003, 121, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Tucker, K.L.; Meyer, M.; Barde, Y.-A. Neurotrophins are required for nerve growth during development. Nature Neuroscience 2001, 4, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Gerfen, C.R. Segregation of D1 and D2 dopamine receptors in the striatal direct and indirect pathways: An historical perspective. Front Synaptic Neurosci 2022, 14, 1002960. [Google Scholar] [CrossRef]
- Gerfen, C.R.; Surmeier, D.J. Modulation of striatal projection systems by dopamine. Annu Rev Neurosci 2011, 34, 441–466. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.Z.; Creed, M.C. Updating the striatal-pallidal wiring diagram. Nat Neurosci 2024, 27, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Calabresi, P.; Pisani, A.; Mercuri, N.B.; Bernardi, G. The corticostriatal projection: from synaptic plasticity to dysfunctions of the basal ganglia. Trends in Neurosciences 1996, 19, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Besusso, D.; Geibel, M.; Kramer, D.; Schneider, T.; Pendolino, V.; Picconi, B.; Calabresi, P.; Bannerman, D.M.; Minichiello, L. BDNF–TrkB signaling in striatopallidal neurons controls inhibition of locomotor behavior. Nature Communications 2013, 4, 2031. [Google Scholar] [CrossRef]
- Bastioli, G.; Arnold, J.C.; Mancini, M.; Mar, A.C.; Gamallo-Lana, B.; Saadipour, K.; Chao, M.V.; Rice, M.E. Voluntary Exercise Boosts Striatal Dopamine Release: Evidence for the Necessary and Sufficient Role of BDNF. The Journal of Neuroscience 2022, 42, 4725–4736. [Google Scholar] [CrossRef]
- Ivkovic, S.; Ehrlich, M.E. Expression of the Striatal DARPP-32/ARPP-21 Phenotype in GABAergic Neurons Requires Neurotrophins In Vivo and In Vitro. The Journal of Neuroscience 1999, 19, 5409–5419. [Google Scholar] [CrossRef] [PubMed]
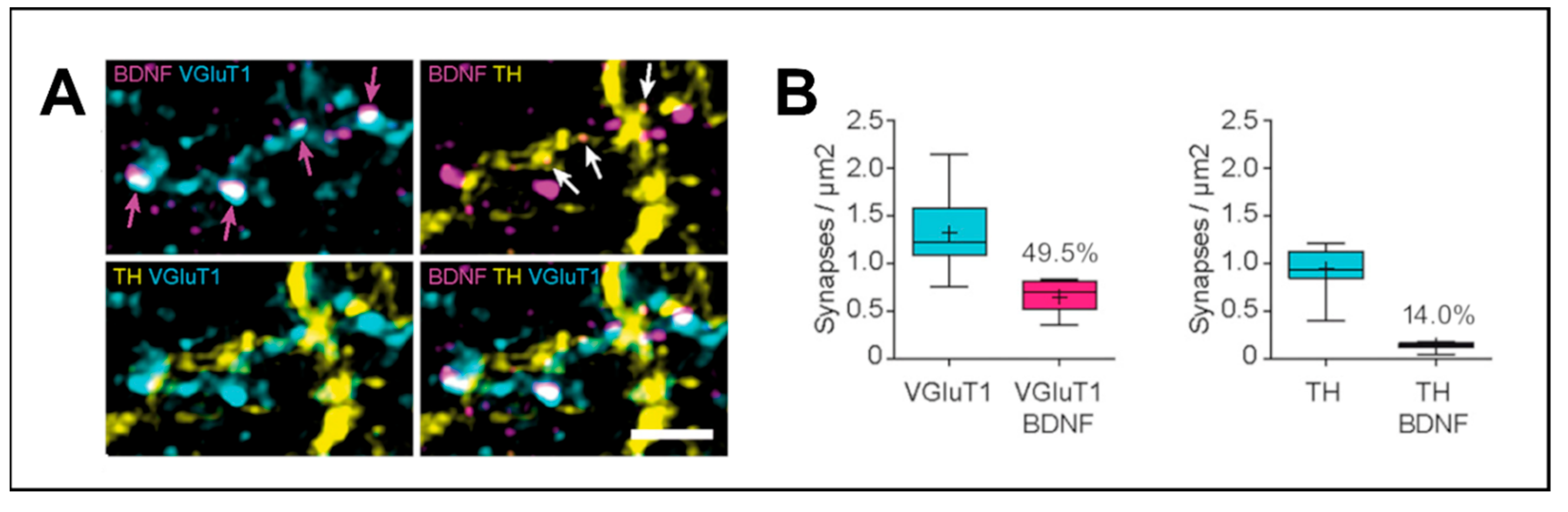
- Park, H.; Popescu, A.; Poo, M.-m. Essential Role of Presynaptic NMDA Receptors in Activity-Dependent BDNF Secretion and Corticostriatal LTP. Neuron 2014, 84, 1009–1022. [Google Scholar] [CrossRef]
- Calabresi, P.; Picconi, B.; Tozzi, A.; Di Filippo, M. Dopamine-mediated regulation of corticostriatal synaptic plasticity. Trends in Neurosciences 2007, 30, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Baydyuk, M.; Xu, B. BDNF signaling and survival of striatal neurons. Frontiers in Cellular Neuroscience 2014, 8. [Google Scholar] [CrossRef] [PubMed]
- Altar, C.A.; Cai, N.; Bliven, T.; Juhasz, M.; Conner, J.M.; Acheson, A.L.; Lindsay, R.M.; Wiegand, S.J. Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature 1997, 389, 856–860. [Google Scholar] [CrossRef] [PubMed]
- Conner, J.M.; Lauterborn, J.C.; Yan, Q.; Gall, C.M.; Varon, S. Distribution of Brain-Derived Neurotrophic Factor (BDNF) Protein and mRNA in the Normal Adult Rat CNS: Evidence for Anterograde Axonal Transport. The Journal of Neuroscience 1997, 17, 2295–2313. [Google Scholar] [CrossRef] [PubMed]
- Dieni, S.; Matsumoto, T.; Dekkers, M.; Rauskolb, S.; Ionescu, M.S.; Deogracias, R.; Gundelfinger, E.D.; Kojima, M.; Nestel, S.; Frotscher, M.; et al. BDNF and its pro-peptide are stored in presynaptic dense core vesicles in brain neurons. Journal of Cell Biology 2012, 196, 775–788. [Google Scholar] [CrossRef]
- Li, Y.; Yui, D.; Luikart, B.W.; McKay, R.M.; Li, Y.; Rubenstein, J.L.; Parada, L.F. Conditional ablation of brain-derived neurotrophic factor-TrkB signaling impairs striatal neuron development. Proceedings of the National Academy of Sciences 2012, 109, 15491–15496. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Gall, C.M.; Lynch, G. Presynaptic BDNF Promotes Postsynaptic Long-Term Potentiation in the Dorsal Striatum. The Journal of Neuroscience 2010, 30, 14440–14445. [Google Scholar] [CrossRef]
- West, M.O.; Carelli, R.M.; Pomerantz, M.; Cohen, S.M.; Gardner, J.P.; Chapin, J.K.; Woodward, D.J. A region in the dorsolateral striatum of the rat exhibiting single-unit correlations with specific locomotor limb movements. Journal of Neurophysiology 1990, 64, 1233–1246. [Google Scholar] [CrossRef]
- Grillner, S. Action: The Role of Motor Cortex Challenged. Current Biology 2015, 25, R508–R511. [Google Scholar] [CrossRef] [PubMed]
- Kawai, R.; Markman, T.; Poddar, R.; Ko, R.; Fantana, Antoniu L. ; Dhawale, Ashesh K.; Kampff, Adam R.; Ölveczky, Bence P. Motor Cortex Is Required for Learning but Not for Executing a Motor Skill. Neuron 2015, 86, 800–812. [Google Scholar] [CrossRef] [PubMed]
- Sheng, M.-j.; Lu, D.; Shen, Z.-m.; Poo, M.-m. Emergence of stable striatal D1R and D2R neuronal ensembles with distinct firing sequence during motor learning. Proceedings of the National Academy of Sciences 2019, 116, 11038–11047. [Google Scholar] [CrossRef] [PubMed]
- Hofer, M.; Pagliusi, S.R.; Hohn, A.; Leibrock, J.; Barde, Y.A. Regional distribution of brain-derived neurotrophic factor mRNA in the adult mouse brain. The EMBO Journal 1990, 9, 2459–2464. [Google Scholar] [CrossRef]
- Boatell, L.L.; Lindefors, N.; Ballarin, M.; Ernfors, P.; Mahy, N.; Persson, H. Activation of basal forebrain cholinergic neurons differentially regulates brain-derived neurotrophic factor mRNA expression in different projection areas. Neuroscience Letters 1992, 136, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Falkenberg, T.; Mohammed, A.K.; Henriksson, B.; Persson, H.; Winblad, B.; Lindefors, N. Increased expression of brain-derived neurotrophic factor mRNA in rat hippocampus is associated with improved spatial memory and enriched environment. Neuroscience Letters 1992, 138, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Timmusk, T.; Lendahl, U.; Funakoshi, H.; Arenas, E.; Persson, H.; Metsis, M. Identification of brain-derived neurotrophic factor promoter regions mediating tissue-specific, axotomy-, and neuronal activity-induced expression in transgenic mice. Journal of Cell Biology 1995, 128, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Metsis, M.; Timmusk, T.; Arenas, E.; Persson, H. Differential usage of multiple brain-derived neurotrophic factor promoters in the rat brain following neuronal activation. Proceedings of the National Academy of Sciences 1993, 90, 8802–8806. [Google Scholar] [CrossRef]
- Tao, X.; Finkbeiner, S.; Arnold, D.B.; Shaywitz, A.J.; Greenberg, M.E. Ca2+ Influx Regulates BDNF Transcription by a CREB Family Transcription Factor-Dependent Mechanism. Neuron 1998, 20, 709–726. [Google Scholar] [CrossRef]
- Neeper, S.A.; Góauctemez-Pinilla, F.; Choi, J.; Cotman, C. Exercise and brain neurotrophins. Nature 1995, 373, 109–109. [Google Scholar] [CrossRef]
- Cotman, C.W.; Berchtold, N.C. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends in Neurosciences 2002, 25, 295–301. [Google Scholar] [CrossRef]
- Pruunsild, P.; Kazantseva, A.; Aid, T.; Palm, K.; Timmusk, T. Dissecting the human BDNF locus: Bidirectional transcription, complex splicing, and multiple promoters. Genomics 2007, 90, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Oliff, H.S.; Berchtold, N.C.; Isackson, P.; Cotman, C.W. Exercise-induced regulation of brain-derived neurotrophic factor (BDNF) transcripts in the rat hippocampus. Molecular Brain Research 1998, 61, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Adlard, P.A.; Perreau, V.M.; Engesser-Cesar, C.; Cotman, C.W. The timecourse of induction of brain-derived neurotrophic factor mRNA and protein in the rat hippocampus following voluntary exercise. Neuroscience Letters 2004, 363, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Adlard, P.A.; Perreau, V.M.; Cotman, C.W. The exercise-induced expression of BDNF within the hippocampus varies across life-span. Neurobiology of Aging 2005, 26, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Berchtold, N.C.; Chinn, G.; Chou, M.; Kesslak, J.P.; Cotman, C.W. Exercise primes a molecular memory for brain-derived neurotrophic factor protein induction in the rat hippocampus. Neuroscience 2005, 133, 853–861. [Google Scholar] [CrossRef]
- Chen, K.; Zheng, Y.; Wei, J.-a.; Ouyang, H.; Huang, X.; Zhang, F.; Lai, C.S.W.; Ren, C.; So, K.-F.; Zhang, L. Exercise training improves motor skill learning via selective activation of mTOR. Science Advances 2019, 5, eaaw1888. [Google Scholar] [CrossRef]
- Zhai, S.; Shen, W.; Graves, S.M.; Surmeier, D.J. Dopaminergic modulation of striatal function and Parkinson’s disease. Journal of Neural Transmission 2019, 126, 411–422. [Google Scholar] [CrossRef]
- Howells, D.W.; Porritt, M.J.; Wong, J.Y.F.; Batchelor, P.E.; Kalnins, R.; Hughes, A.J.; Donnan, G.A. Reduced BDNF mRNA Expression in the Parkinson’s Disease Substantia Nigra. Experimental Neurology 2000, 166, 127–135. [Google Scholar] [CrossRef]
- Seroogy, K.B.; Lundgren, K.H.; Tran, T.M.D.; Guthrie, K.M.; Isackson, P.J.; Gall, C.M. Dopaminergic neurons in rat ventral midbrain express brain-derived neurotrophic factor and neurotrophin-3 mRNAs. Journal of Comparative Neurology 1994, 342, 321–334. [Google Scholar] [CrossRef]
- Venero, J.L.; Beck, K.D.; Hefti, F. 6-Hydroxydopamine lesions reduce BDNF mRNA levels in adult rat brain substantia nigra. NeuroReport 1994, 5, 429–432. [Google Scholar] [CrossRef] [PubMed]
- Parain, K.; Murer, M.G.; Yan, Q.; Faucheux, B.; Agid, Y.; Hirsch, E.; Raisman-Vozari, R. Reduced expression of brain-derived neurotrophic factor protein in Parkinson’s disease substantia nigra. NeuroReport 1999, 10, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Mogi, M.; Togari, A.; Kondo, T.; Mizuno, Y.; Komure, O.; Kuno, S.; Ichinose, H.; Nagatsu, T. Brain-derived growth factor and nerve growth factor concentrations are decreased in the substantia nigra in Parkinson’s disease. Neuroscience Letters 1999, 270, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Collier, T.J.; Dung Ling, Z.; Carvey, P.M.; Fletcher-Turner, A.; Yurek, D.M.; Sladek, J.R.; Kordower, J.H. Striatal trophic factor activity in aging monkeys with unilateral MPTP-induced parkinsonism. Experimental Neurology 2005, 191, S60–S67. [Google Scholar] [CrossRef]
- Saha, A.R.; Hill, J.; Utton, M.A.; Asuni, A.A.; Ackerley, S.; Grierson, A.J.; Miller, C.C.; Davies, A.M.; Buchman, V.L.; Anderton, B.H.; et al. Parkinson’s disease α-synuclein mutations exhibit defective axonal transport in cultured neurons. Journal of Cell Science 2004, 117, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.Y.; Koprich, J.B.; Siddiqi, H.; Isacson, O. Dynamic Changes in Presynaptic and Axonal Transport Proteins Combined with Striatal Neuroinflammation Precede Dopaminergic Neuronal Loss in a Rat Model of AAV α-Synucleinopathy. The Journal of Neuroscience 2009, 29, 3365–3373. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Morfini, G.A.; Langhamer, L.B.; He, Y.; Brady, S.T.; Kordower, J.H. Alterations in axonal transport motor proteins in sporadic and experimental Parkinson’s disease. Brain 2012, 135, 2058–2073. [Google Scholar] [CrossRef] [PubMed]
- Volpicelli-Daley, L.A. Effects of α-synuclein on axonal transport. Neurobiology of Disease 2017, 105, 321–327. [Google Scholar] [CrossRef]
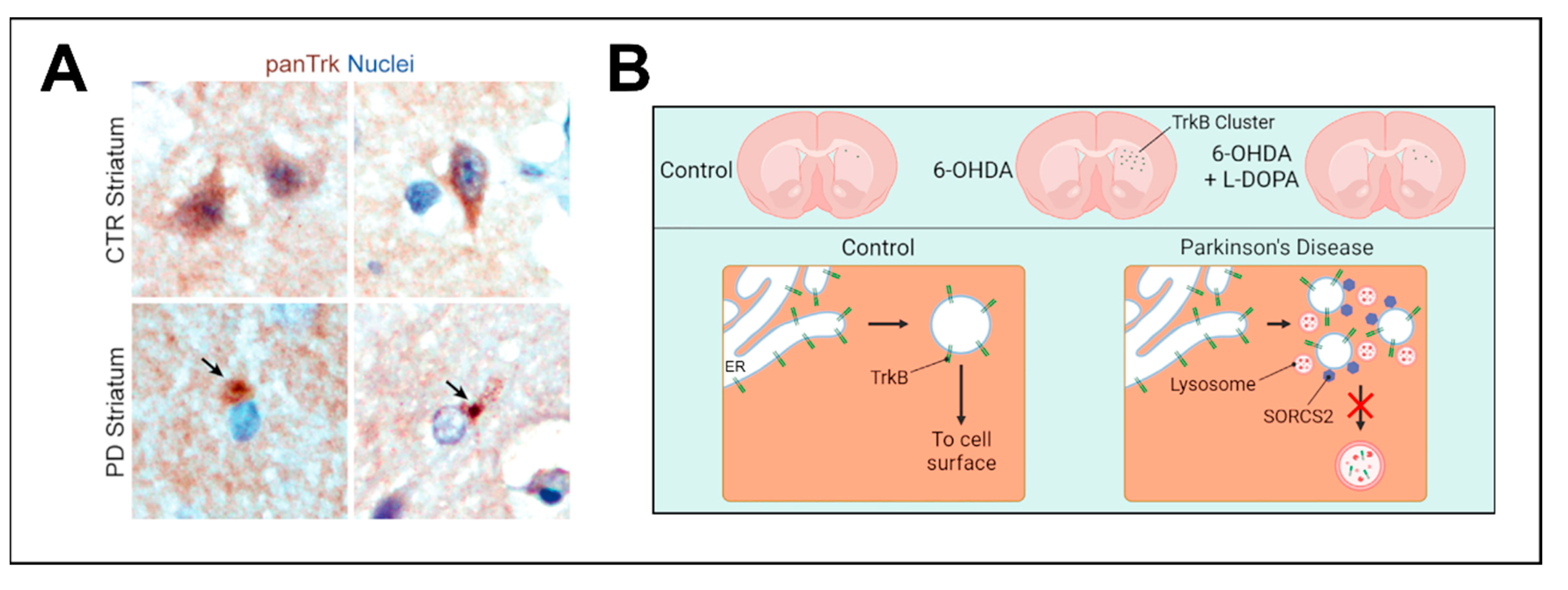
- Fang, F.; Yang, W.; Florio, J.B.; Rockenstein, E.; Spencer, B.; Orain, X.M.; Dong, S.X.; Li, H.; Chen, X.; Sung, K.; et al. Synuclein impairs trafficking and signaling of BDNF in a mouse model of Parkinson’s disease. Scientific Reports 2017, 7, 3868. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.M.; Patterson, J.R.; Kochmanski, J.; Kemp, C.J.; Stoll, A.C.; Onyekpe, C.U.; Cole-Strauss, A.; Steece-Collier, K.; Howe, J.W.; Luk, K.C.; et al. Striatal Afferent BDNF Is Disrupted by Synucleinopathy and Partially Restored by STN DBS. The Journal of Neuroscience 2021, 41, 2039–2052. [Google Scholar] [CrossRef]
- Brigadski, T.; Leßmann, V. The physiology of regulated BDNF release. Cell and Tissue Research 2020, 382, 15–45. [Google Scholar] [CrossRef]
- Balkowiec, A.; Katz, D.M. Activity-Dependent Release of Endogenous Brain-Derived Neurotrophic Factor from Primary Sensory Neurons Detected by ELISA In Situ. The Journal of Neuroscience 2000, 20, 7417–7423. [Google Scholar] [CrossRef]
- Balkowiec, A.; Katz, D.M. Cellular Mechanisms Regulating Activity-Dependent Release of Native Brain-Derived Neurotrophic Factor from Hippocampal Neurons. The Journal of Neuroscience 2002, 22, 10399–10407. [Google Scholar] [CrossRef] [PubMed]
- Larson, J.; Wong, D.; Lynch, G. Patterned stimulation at the theta frequency is optimal for the induction of hippocampal long-term potentiation. Brain Research 1986, 368, 347–350. [Google Scholar] [CrossRef]
- Staubli, U.; Lynch, G. Stable hippocampal long-term potentiation elicited by ‘theta’ pattern stimulation. Brain Research 1987, 435, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Larson, J.; Munkácsy, E. Theta-burst LTP. Brain Research 2015, 1621, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Volkmann, J. Deep Brain Stimulation for the Treatment of Parkinson’s Disease. Journal of Clinical Neurophysiology 2004, 21, 6–17. [Google Scholar] [CrossRef]
- Su, D.; Chen, H.; Hu, W.; Liu, Y.; Wang, Z.; Wang, X.; Liu, G.; Ma, H.; Zhou, J.; Feng, T. Frequency-dependent effects of subthalamic deep brain stimulation on motor symptoms in Parkinson’s disease: a meta-analysis of controlled trials. Scientific Reports 2018, 8, 14456. [Google Scholar] [CrossRef]
- McIntyre, C.C.; Savasta, M.; Kerkerian-Le Goff, L.; Vitek, J.L. Uncovering the mechanism(s) of action of deep brain stimulation: activation, inhibition, or both. Clinical Neurophysiology 2004, 115, 1239–1248. [Google Scholar] [CrossRef]
- Anderson, M.E.; Postupna, N.; Ruffo, M. Effects of High-Frequency Stimulation in the Internal Globus Pallidus on the Activity of Thalamic Neurons in the Awake Monkey. Journal of Neurophysiology 2003, 89, 1150–1160. [Google Scholar] [CrossRef]
- Hashimoto, T.; Elder, C.M.; Okun, M.S.; Patrick, S.K.; Vitek, J.L. Stimulation of the Subthalamic Nucleus Changes the Firing Pattern of Pallidal Neurons. The Journal of Neuroscience 2003, 23, 1916–1923. [Google Scholar] [CrossRef] [PubMed]
- Spieles-Engemann, A.L.; Steece-Collier, K.; Behbehani, M.M.; Collier, T.J.; Wohlgenant, S.L.; Kemp, C.J.; Cole-Strauss, A.; Levine, N.D.; Gombash, S.E.; Thompson, V.B.; et al. Subthalamic Nucleus Stimulation Increases Brain Derived Neurotrophic Factor in the Nigrostriatal System and Primary Motor Cortex. Journal of Parkinson’s Disease 2011, 1, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Fischer, D.L.; Sortwell, C.E. BDNF provides many routes toward STN DBS-mediated disease modification. Movement Disorders 2019, 34, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Hyman, C.; Hofer, M.; Barde, Y.-A.; Juhasz, M.; Yancopoulos, G.D.; Squinto, S.P.; Lindsay, R.M. BDNF is a neurotrophic factor for dopaminergic neurons of the substantia nigra. Nature 1991, 350, 230–232. [Google Scholar] [CrossRef] [PubMed]
- Beck, K.D.; Knüsel, B.; Hefti, F. The nature of the trophic action of brain-derived neurotrophic factor, des(1-3)-insulin-like growth FACTOR-1, and basic fibroblast growth factor on mesencephalic dopaminergic neurons developing in culture. Neuroscience 1993, 52, 855–866. [Google Scholar] [CrossRef]
- Knüsel, B.; Winslow, J.W.; Rosenthal, A.; Burton, L.E.; Seid, D.P.; Nikolics, K.; Hefti, F. Promotion of central cholinergic and dopaminergic neuron differentiation by brain-derived neurotrophic factor but not neurotrophin 3. Proceedings of the National Academy of Sciences 1991, 88, 961–965. [Google Scholar] [CrossRef]
- SPINA, M.B.; HYMAN, C.; SQUINTO, S.; LINDSAY, R.M. Brain-Derived Neurotrophic Factor Protects Dopaminergic Cells from 6-Hydroxydopamine Toxicity. Annals of the New York Academy of Sciences 1992, 648, 348–350. [Google Scholar] [CrossRef]
- Tsukahara, T.; Takeda, M.; Shimohama, S.; Ohara, O.; Hashimoto, N. Effects of Brain-derived Neurotrophic Factor on 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinsonism in Monkeys. Neurosurgery 1995, 37, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Chan, N.G.; Bannon, M.J.; Orozco-Barrios, C.E.; Escobedo, L.; Zamudio, S.; De la Cruz, F.; Gongora-Alfaro, J.L.; Armendáriz-Borunda, J.; Reyes-Corona, D.; Espadas-Alvarez, A.J.; et al. Neurotensin-polyplex-mediated brain-derived neurotrophic factor gene delivery into nigral dopamine neurons prevents nigrostriatal degeneration in a rat model of early Parkinson’s disease. Journal of Biomedical Science 2015, 22, 59. [Google Scholar] [CrossRef]
- Nie, S.; Xu, Y.; Chen, G.; Ma, K.; Han, C.; Guo, Z.; Zhang, Z.; Ye, K.; Cao, X. Small molecule TrkB agonist deoxygedunin protects nigrostriatal dopaminergic neurons from 6-OHDA and MPTP induced neurotoxicity in rodents. Neuropharmacology 2015, 99, 448–458. [Google Scholar] [CrossRef]
- Kang, S.S.; Wu, Z.; Liu, X.; Edgington-Mitchell, L.; Ye, K. Treating Parkinson’s Disease via Activation of BDNF/TrkB Signaling Pathways and Inhibition of Delta-Secretase. Neurotherapeutics 2022, 19, 1283–1297. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Kong, L.; Wang, X.; Lu, X.-g.; Gao, Q.; Geller, A.I. Comparison of the capability of GDNF, BDNF, or both, to protect nigrostriatal neurons in a rat model of Parkinson’s disease. Brain Research 2005, 1052, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Sauer, H.; Campbell, K.; Wiegand, S.J.; Lindsay, R.M.; Björklund, A. Brain-derived neurotrophic factor enhances striatal neuropeptide expression in both the intact and the dopamine-depleted rat striatum. NeuroReport 1994, 5, 609–612. [Google Scholar] [CrossRef] [PubMed]
- Palasz, E.; Wysocka, A.; Gasiorowska, A.; Chalimoniuk, M.; Niewiadomski, W.; Niewiadomska, G. BDNF as a Promising Therapeutic Agent in Parkinson’s Disease. International Journal of Molecular Sciences 2020, 21, 1170. [Google Scholar] [CrossRef] [PubMed]
- Nagahara, A.H.; Tuszynski, M.H. Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nature Reviews Drug Discovery 2011, 10, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Bjorklund, T.; Kordower, J.H. Gene therapy for Parkinson’s disease. Movement Disorders 2010, 25, S161–S173. [Google Scholar] [CrossRef] [PubMed]
- Mak, M.K.Y.; Wong-Yu, I.S.K. Chapter One - Exercise for Parkinson’s disease. In International Review of Neurobiology, Yau, S.-Y., So, K.-F., Eds.; Academic Press: 2019; Volume 147, pp. 1-44.
- Ernst, M.; Folkerts, A.K.; Gollan, R.; Lieker, E.; Caro-Valenzuela, J.; Adams, A.; Cryns, N.; Monsef, I.; Dresen, A.; Roheger, M.; et al. Physical exercise for people with Parkinson’s disease: a systematic review and network meta-analysis. Cochrane Database of Systematic Reviews 2024. [Google Scholar] [CrossRef]
- Kaagman, D.G.M.; van Wegen, E.E.H.; Cignetti, N.; Rothermel, E.; Vanbellingen, T.; Hirsch, M.A. Effects and Mechanisms of Exercise on Brain-Derived Neurotrophic Factor (BDNF) Levels and Clinical Outcomes in People with Parkinson’s Disease: A Systematic Review and Meta-Analysis. Brain Sciences 2024, 14, 194. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, M.A.; van Wegen, E.E.H.; Newman, M.A.; Heyn, P.C. Exercise-induced increase in brain-derived neurotrophic factor in human Parkinson’s disease: a systematic review and meta-analysis. Translational Neurodegeneration 2018, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Paterno, A.; Polsinelli, G.; Federico, B. Changes of brain-derived neurotrophic factor (BDNF) levels after different exercise protocols: a systematic review of clinical studies in Parkinson’s disease. Frontiers in Physiology 2024, 15. [Google Scholar] [CrossRef]
- Benisty, S.; Boissiere, F.; Faucheux, B.; Agid, Y.; Hirsch, E.C. Tyrosine kinase B messenger RNA expression in normal human brain and in the substantia nigra of parkinsonian patients: an in situ hybridization study. Neuroscience 1998, 86, 813–826. [Google Scholar] [CrossRef] [PubMed]
- Riquelme, E.; Abarca, J.; Campusano, J.M.; Bustos, G. An NR2B-Dependent Decrease in the Expression of trkB Receptors Precedes the Disappearance of Dopaminergic Cells in Substantia Nigra in a Rat Model of Presymptomatic Parkinson′s Disease. Parkinson’s Disease 2012, 2012, 129605. [Google Scholar] [CrossRef]
- Venero, J.L.; Vizuete, M.L.; Revuelta, M.; Vargas, C.; Cano, J.; Machado, A. Upregulation of BDNF mRNA and trkB mRNA in the Nigrostriatal System and in the Lesion Site Following Unilateral Transection of the Medial Forebrain Bundle. Experimental Neurology 2000, 161, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Numan, S.; Seroogy, K.B. Increased Expression of trkB mRNA in Rat Caudate-Putamen Following 6–OHDA Lesions of the Nigrostriatal Pathway. European Journal of Neuroscience 1997, 9, 489–495. [Google Scholar] [CrossRef]
- Pelosi, A.; Nakamura, Y.; Girault, J.-A.; Hervé, D. BDNF/TrkB pathway activation in D1 receptor-expressing striatal projection neurons plays a protective role against L-DOPA-induced dyskinesia. Neurobiology of Disease 2023, 185, 106238. [Google Scholar] [CrossRef]
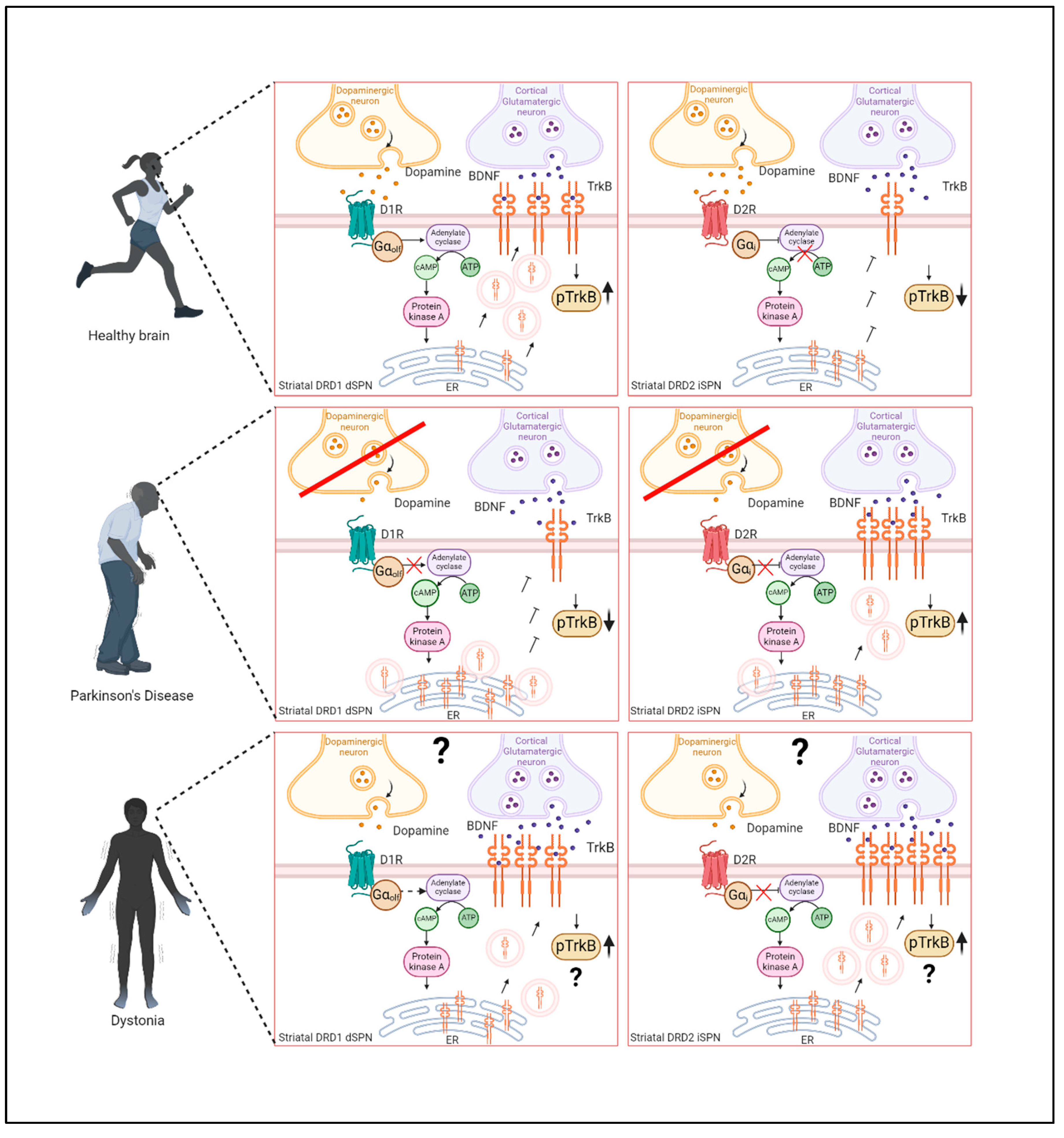
- Andreska, T.; Lüningschrör, P.; Wolf, D.; McFleder, R.L.; Ayon-Olivas, M.; Rattka, M.; Drechsler, C.; Perschin, V.; Blum, R.; Aufmkolk, S.; et al. DRD1 signaling modulates TrkB turnover and BDNF sensitivity in direct pathway striatal medium spiny neurons. Cell Reports 2023, 42. [Google Scholar] [CrossRef] [PubMed]
- Dragunow, M.; Butterworth, N.; Waldvogel, H.; Faull, R.L.M.; Nicholson, L.F.B. Prolonged expression of Fos-related antigens, Jun B and TrkB in dopamine-denervated striatal neurons. Molecular Brain Research 1995, 30, 393–396. [Google Scholar] [CrossRef] [PubMed]
- von Bohlen und Halbach, O.; Minichiello, L.; Unsicker, K. Haploinsufficiency for trkB and trkC receptors induces cell loss and accumulation of α-synuclein in the substantia nigra. The FASEB Journal 2005, 19, 1740–1742. [Google Scholar] [CrossRef] [PubMed]
- Baydyuk, M.; Nguyen, M.T.; Xu, B. Chronic deprivation of TrkB signaling leads to selective late-onset nigrostriatal dopaminergic degeneration. Experimental Neurology 2011, 228, 118–125. [Google Scholar] [CrossRef]
- Kang, S.S.; Zhang, Z.; Liu, X.; Manfredsson, F.P.; Benskey, M.J.; Cao, X.; Xu, J.; Sun, Y.E.; Ye, K. TrkB neurotrophic activities are blocked by α-synuclein, triggering dopaminergic cell death in Parkinson’s disease. Proceedings of the National Academy of Sciences 2017, 114, 10773–10778. [Google Scholar] [CrossRef]
- Meyer-Franke, A.; Wilkinson, G.A.; Kruttgen, A.; Hu, M.; Munro, E.; Hanson, M.G.; Reichardt, L.F.; Barres, B.A. Depolarization and cAMP Elevation Rapidly Recruit TrkB to the Plasma Membrane of CNS Neurons. Neuron 1998, 21, 681–693. [Google Scholar] [CrossRef]
- Du, J.; Feng, L.; Yang, F.; Lu, B. Activity- and Ca2+-Dependent Modulation of Surface Expression of Brain-Derived Neurotrophic Factor Receptors in Hippocampal Neurons. Journal of Cell Biology 2000, 150, 1423–1434. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Pang, P.T.; Feng, L.; Lu, B. Cyclic AMP controls BDNF-induced TrkB phosphorylation and dendritic spine formation in mature hippocampal neurons. Nature Neuroscience 2005, 8, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Glatt, C.E.; Snyder, S.H. Cloning and expression of an adenylyl cyclase localized to the corpus striatum. Nature 1993, 361, 536–538. [Google Scholar] [CrossRef] [PubMed]
- Corvol, J.C.; Studler, J.M.; Schonn, J.S.; Girault, J.A.; Hervé, D. Gαolf is necessary for coupling D1 and A2a receptors to adenylyl cyclase in the striatum. Journal of Neurochemistry 2001, 76, 1585–1588. [Google Scholar] [CrossRef] [PubMed]
- Albert, P.R.; Neve, K.A.; Bunzow, J.R.; Civelli, O. Coupling of a cloned rat dopamine-D2 receptor to inhibition of adenylyl cyclase and prolactin secretion. Journal of Biological Chemistry 1990, 265, 2098–2104. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Zhou, D.; Chase, K.; Gusella, J.F.; Aronin, N.; DiFiglia, M. Immunohistochemical localization of the D1 dopamine receptor in rat brain reveals its axonal transport, pre- and postsynaptic localization, and prevalence in the basal ganglia, limbic system, and thalamic reticular nucleus. Proceedings of the National Academy of Sciences 1992, 89, 11988–11992. [Google Scholar] [CrossRef]
- Kreitzer, A.C.; Malenka, R.C. Striatal Plasticity and Basal Ganglia Circuit Function. Neuron 2008, 60, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Ince, E.; Ciliax, B.J.; Levey, A.I. Differential expression of D1 and D2 dopamine and m4 muscarinic acetylcholine receptor proteins in identified striatonigral neurons. Synapse 1997, 27, 357–366. [Google Scholar] [CrossRef]
- Pollack, A.E.; Harrison, M.B.; Wooten, G.F.; Fink, J.S. Differential localization of A2a adenosine receptor mRNA with D1 and D2 dopamine receptor mRNA in striatal output pathways following a selective lesion of striatonigral neurons. Brain Research 1993, 631, 161–166. [Google Scholar] [CrossRef]
- Iwakura, Y.; Nawa, H.; Sora, I.; Chao, M.V. Dopamine D1 Receptor-induced Signaling through TrkB Receptors in Striatal Neurons*. Journal of Biological Chemistry 2008, 283, 15799–15806. [Google Scholar] [CrossRef] [PubMed]
- Ayon-Olivas, M.; Wolf, D.; Andreska, T.; Granado, N.; Lüningschrör, P.; Ip, C.W.; Moratalla, R.; Sendtner, M. Dopaminergic Input Regulates the Sensitivity of Indirect Pathway Striatal Spiny Neurons to Brain-Derived Neurotrophic Factor. Biology 2023, 12, 1360. [Google Scholar] [CrossRef] [PubMed]
- Lüningschrör, P.; Andreska, T.; Veh, A.; Wolf, D.; Giridhar, N.J.; Moradi, M.; Denzel, A.; Sendtner, M. Calnexin controls TrkB cell surface transport and ER-phagy in mouse cerebral cortex development. Developmental Cell 2023, 58, 1733–1747. [Google Scholar] [CrossRef] [PubMed]
- Zhai, S.; Tanimura, A.; Graves, S.M.; Shen, W.; Surmeier, D.J. Striatal synapses, circuits, and Parkinson’s disease. Current Opinion in Neurobiology 2018, 48, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Zhai, S.; Surmeier, D.J. Striatal synaptic adaptations in Parkinson’s disease. Neurobiology of Disease 2022, 167, 105686. [Google Scholar] [CrossRef]
- Calabresi, P.; Maj, R.; Pisani, A.; Mercuri, N.; Bernardi, G. Long-term synaptic depression in the striatum: physiological and pharmacological characterization. The Journal of Neuroscience 1992, 12, 4224–4233. [Google Scholar] [CrossRef] [PubMed]
- Calabresi, P.; Saiardi, A.; Pisani, A.; Baik, J.-H.; Centonze, D.; Mercuri, N.B.; Bernardi, G.; Borrelli, E. Abnormal Synaptic Plasticity in the Striatum of Mice Lacking Dopamine D2 Receptors. The Journal of Neuroscience 1997, 17, 4536–4544. [Google Scholar] [CrossRef] [PubMed]
- Centonze, D.; Gubellini, P.; Picconi, B.; Calabresi, P.; Giacomini, P.; Bernardi, G. Unilateral Dopamine Denervation Blocks Corticostriatal LTP. Journal of Neurophysiology 1999, 82, 3575–3579. [Google Scholar] [CrossRef]
- Pawlak, V.; Kerr, J.N.D. Dopamine Receptor Activation Is Required for Corticostriatal Spike-Timing-Dependent Plasticity. The Journal of Neuroscience 2008, 28, 2435–2446. [Google Scholar] [CrossRef]
- Yagishita, S.; Hayashi-Takagi, A.; Ellis-Davies, G.C.R.; Urakubo, H.; Ishii, S.; Kasai, H. A critical time window for dopamine actions on the structural plasticity of dendritic spines. Science 2014, 345, 1616–1620. [Google Scholar] [CrossRef]
- Defazio, G. The epidemiology of primary dystonia: current evidence and perspectives. European Journal of Neurology 2010, 17, 9–14. [Google Scholar] [CrossRef]
- di Biase, L.; Di Santo, A.; Caminiti, M.L.; Pecoraro, P.M.; Di Lazzaro, V. Classification of Dystonia. Life 2022, 12, 206. [Google Scholar] [CrossRef] [PubMed]
- Balint, B.; Mencacci, N.E.; Valente, E.M.; Pisani, A.; Rothwell, J.; Jankovic, J.; Vidailhet, M.; Bhatia, K.P. Dystonia. Nature Reviews Disease Primers 2018, 4, 25. [Google Scholar] [CrossRef]
- Albanese, A.; Bhatia, K.; Bressman, S.B.; DeLong, M.R.; Fahn, S.; Fung, V.S.C.; Hallett, M.; Jankovic, J.; Jinnah, H.A.; Klein, C.; et al. Phenomenology and classification of dystonia: A consensus update. Movement Disorders 2013, 28, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Peterson, D.A.; Sejnowski, T.J.; Poizner, H. Convergent evidence for abnormal striatal synaptic plasticity in dystonia. Neurobiology of Disease 2010, 37, 558–573. [Google Scholar] [CrossRef]
- Downs, A.M.; Roman, K.M.; Campbell, S.A.; Pisani, A.; Hess, E.J.; Bonsi, P. The neurobiological basis for novel experimental therapeutics in dystonia. Neurobiology of Disease 2019, 130, 104526. [Google Scholar] [CrossRef]
- Ribot, B.; Aupy, J.; Vidailhet, M.; Mazère, J.; Pisani, A.; Bezard, E.; Guehl, D.; Burbaud, P. Dystonia and dopamine: From phenomenology to pathophysiology. Progress in Neurobiology 2019, 182, 101678. [Google Scholar] [CrossRef] [PubMed]
- Quartarone, A.; Siebner, H.R.; Rothwell, J.C. Task-specific hand dystonia: can too much plasticity be bad for you? Trends Neurosci 2006, 29, 192–199. [Google Scholar] [CrossRef]
- Quartarone, A.; Sant’Angelo, A.; Battaglia, F.; Bagnato, S.; Rizzo, V.; Morgante, F.; Rothwell, J.C.; Siebner, H.R.; Girlanda, P. Enhanced long-term potentiation-like plasticity of the trigeminal blink reflex circuit in blepharospasm. J Neurosci 2006, 26, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Cenci, M.A.; Konradi, C. Maladaptive striatal plasticity in L-DOPA-induced dyskinesia. Prog Brain Res 2010, 183, 209–233. [Google Scholar] [CrossRef]
- Guillin, O.; Diaz, J.; Carroll, P.; Griffon, N.; Schwartz, J.-C.; Sokoloff, P. BDNF controls dopamine D3 receptor expression and triggers behavioural sensitization. Nature 2001, 411, 86–89. [Google Scholar] [CrossRef]
- Edwards, M.J.; Huang, Y.Z.; Mir, P.; Rothwell, J.C.; Bhatia, K.P. Abnormalities in motor cortical plasticity differentiate manifesting and nonmanifesting DYT1 carriers. Mov Disord 2006, 21, 2181–2186. [Google Scholar] [CrossRef] [PubMed]
- Martella, G.; Tassone, A.; Sciamanna, G.; Platania, P.; Cuomo, D.; Viscomi, M.T.; Bonsi, P.; Cacci, E.; Biagioni, S.; Usiello, A.; et al. Impairment of bidirectional synaptic plasticity in the striatum of a mouse model of DYT1 dystonia: role of endogenous acetylcholine. Brain 2009, 132, 2336–2349. [Google Scholar] [CrossRef] [PubMed]
- Grundmann, K.; Glöckle, N.; Martella, G.; Sciamanna, G.; Hauser, T.-K.; Yu, L.; Castaneda, S.; Pichler, B.; Fehrenbacher, B.; Schaller, M.; et al. Generation of a novel rodent model for DYT1 dystonia. Neurobiology of Disease 2012, 47, 61–74. [Google Scholar] [CrossRef]
- Maltese, M.; Stanic, J.; Tassone, A.; Sciamanna, G.; Ponterio, G.; Vanni, V.; Martella, G.; Imbriani, P.; Bonsi, P.; Mercuri, N.B.; et al. Early structural and functional plasticity alterations in a susceptibility period of DYT1 dystonia mouse striatum. eLife 2018, 7, e33331. [Google Scholar] [CrossRef] [PubMed]
- Sanna, A.; Follesa, P.; Puligheddu, M.; Cannas, A.; Serra, M.; Pisu, M.G.; Dagostino, S.; Solla, P.; Tacconi, P.; Marrosu, F. Cerebellar continuous theta burst stimulation reduces levodopa-induced dyskinesias and decreases serum BDNF levels. Neurosci Lett 2020, 716, 134653. [Google Scholar] [CrossRef] [PubMed]
- Wichmann, T. Commentary: Dopaminergic dysfunction in DYT1 dystonia. Experimental Neurology 2008, 212, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Vidailhet, M.; Dupel, C.; Lehéricy, S.; Remy, P.; Dormont, D.; Serdaru, M.; Jedynak, P.; Veber, H.; Samson, Y.; Marsault, C.; et al. Dopaminergic dysfunction in midbrain dystonia: anatomoclinical study using 3-dimensional magnetic resonance imaging and fluorodopa F 18 positron emission tomography. Arch Neurol 1999, 56, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Breakefield, X.O.; Blood, A.J.; Li, Y.; Hallett, M.; Hanson, P.I.; Standaert, D.G. The pathophysiological basis of dystonias. Nature Reviews Neuroscience 2008, 9, 222–234. [Google Scholar] [CrossRef]
- Furukawa, Y.; Hornykiewicz, O.; Fahn, S.; Kish, S.J. Striatal dopamine in early-onset primary torsion dystonia with the DYT1 mutation. Neurology 2000, 54, 1193–1195. [Google Scholar] [CrossRef]
- Augood, S.J.; Hollingsworth, Z.; Albers, D.S.; Yang, L.; Leung, J.-C.; Muller, B.; Klein, C.; Breakefield, X.O.; Standaert, D.G. Dopamine transmission in DYT1 dystonia: A biochemical and autoradiographical study. Neurology 2002, 59, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Shashidharan, P.; Sandu, D.; Potla, U.; Armata, I.A.; Walker, R.H.; McNaught, K.S.; Weisz, D.; Sreenath, T.; Brin, M.F.; Olanow, C.W. Transgenic mouse model of early-onset DYT1 dystonia. Hum Mol Genet 2005, 14, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Scarduzio, M.; Hess, E.J.; Standaert, D.G.; Eskow Jaunarajs, K.L. Striatal Synaptic Dysfunction in Dystonia and Levodopa-Induced Dyskinesia. Neurobiology of Disease 2022, 166, 105650. [Google Scholar] [CrossRef] [PubMed]
- Nygaard, T.G.; Marsden, C.D.; Fahn, S. Dopa-responsive dystonia. Neurology 1991, 41, 174–174. [Google Scholar] [CrossRef] [PubMed]
- Trender-Gerhard, I.; Sweeney, M.G.; Schwingenschuh, P.; Mir, P.; Edwards, M.J.; Gerhard, A.; Polke, J.M.; Hanna, M.G.; Davis, M.B.; Wood, N.W.; et al. Autosomal-dominant GTPCH1-deficient DRD: clinical characteristics and long-term outcome of 34 patients. J Neurol Neurosurg Psychiatry 2009, 80, 839–845. [Google Scholar] [CrossRef]
- Yu-Taeger, L.; Ott, T.; Bonsi, P.; Tomczak, C.; Wassouf, Z.; Martella, G.; Sciamanna, G.; Imbriani, P.; Ponterio, G.; Tassone, A.; et al. Impaired dopamine- and adenosine-mediated signaling and plasticity in a novel rodent model for DYT25 dystonia. Neurobiology of Disease 2020, 134, 104634. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Flajolet, M.; Greengard, P.; Surmeier, D.J. Dichotomous dopaminergic control of striatal synaptic plasticity. Science 2008, 321, 848–851. [Google Scholar] [CrossRef] [PubMed]
- Avchalumov, Y.; Sander, S.E.; Richter, F.; Porath, K.; Hamann, M.; Bode, C.; Kirschstein, T.; Köhling, R.; Richter, A. Role of striatal NMDA receptor subunits in a model of paroxysmal dystonia. Experimental Neurology 2014, 261, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Köhling, R.; Koch, U.-R.; Hamann, M.; Richter, A. Increased excitability in cortico-striatal synaptic pathway in a model of paroxysmal dystonia. Neurobiology of Disease 2004, 16, 236–245. [Google Scholar] [CrossRef]
- Ahn, J.H.; Kim, A.R.; Kim, N.K.D.; Park, W.Y.; Kim, J.S.; Kim, M.; Park, J.; Lee, J.I.; Cho, J.W.; Cho, K.R.; et al. The Effect of Globus Pallidus Interna Deep Brain Stimulation on a Dystonia Patient with the GNAL Mutation Compared to Patients with DYT1 and DYT6. J Mov Disord 2019, 12, 120–124. [Google Scholar] [CrossRef]
- Tisch, S.; Kumar, K.R. Pallidal Deep Brain Stimulation for Monogenic Dystonia: The Effect of Gene on Outcome. Frontiers in Neurology 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Volkmann, J.; Mueller, J.; Deuschl, G.; Kühn, A.A.; Krauss, J.K.; Poewe, W.; Timmermann, L.; Falk, D.; Kupsch, A.; Kivi, A.; et al. Pallidal neurostimulation in patients with medication-refractory cervical dystonia: a randomised, sham-controlled trial. The Lancet Neurology 2014, 13, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Jacksch, C.; Zeuner, K.E.; Helmers, A.-K.; Witt, K.; Deuschl, G.; Paschen, S. Long-term efficacy with deep brain stimulation of the globus pallidus internus in cervical dystonia: a retrospective monocentric study. Neurological Research and Practice 2022, 4, 48. [Google Scholar] [CrossRef] [PubMed]
- Eisinger, R.S.; Cernera, S.; Gittis, A.; Gunduz, A.; Okun, M.S. A review of basal ganglia circuits and physiology: Application to deep brain stimulation. Parkinsonism & Related Disorders 2019, 59, 9–20. [Google Scholar] [CrossRef]
- Hu, W.; Stead, M. Deep brain stimulation for dystonia. Translational Neurodegeneration 2014, 3, 2. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




