Submitted:
03 July 2024
Posted:
04 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
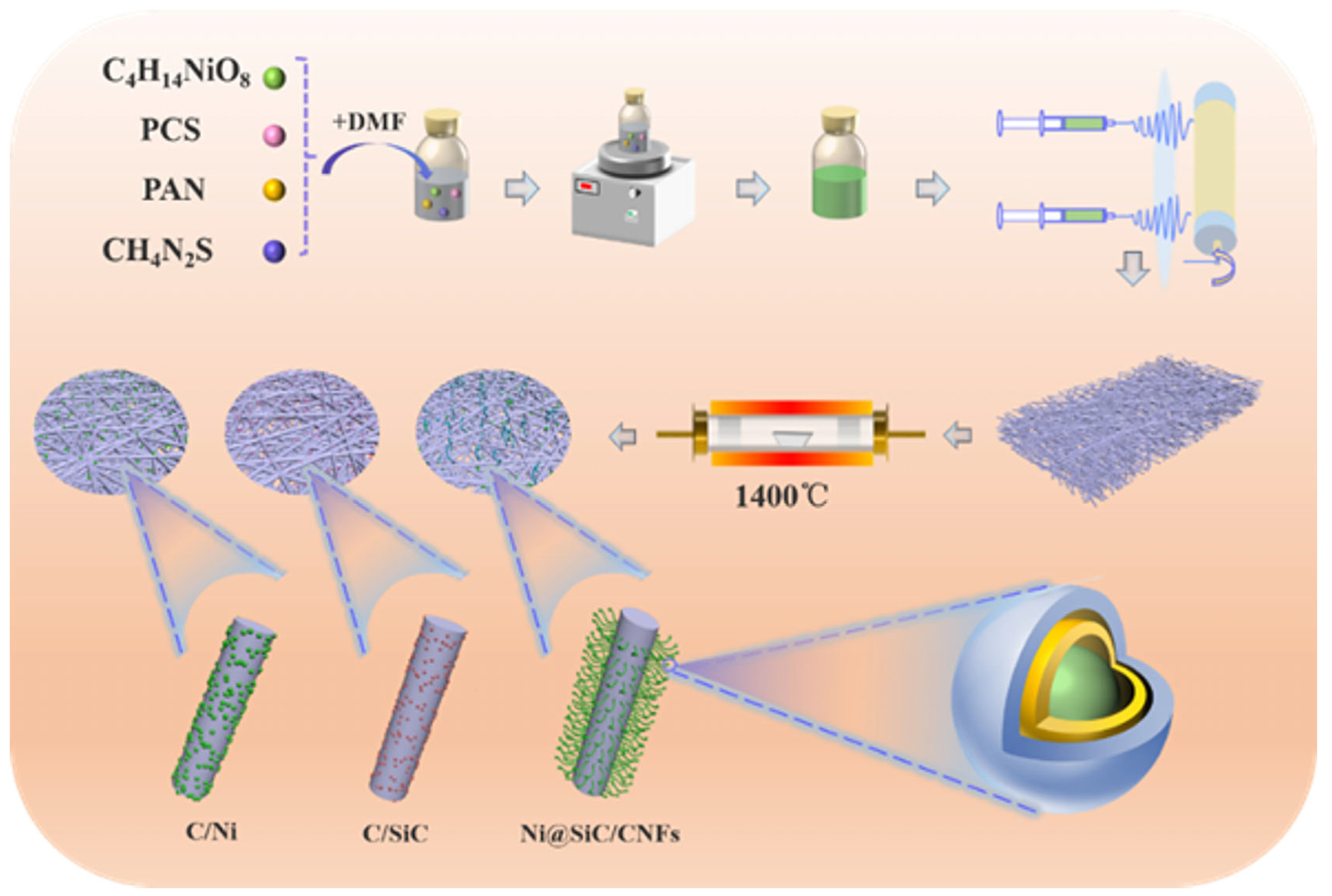
2.1. Synthesis of Ni@SiC/CNFs
2.2. Characterization
2.3. Degradation experiment using microwave
3. Results
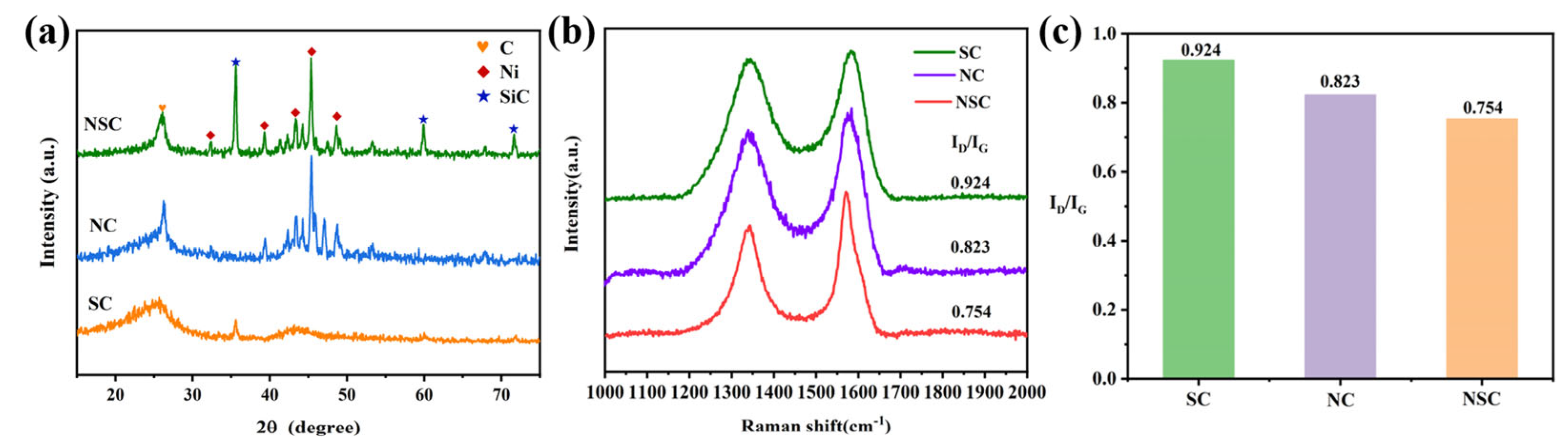
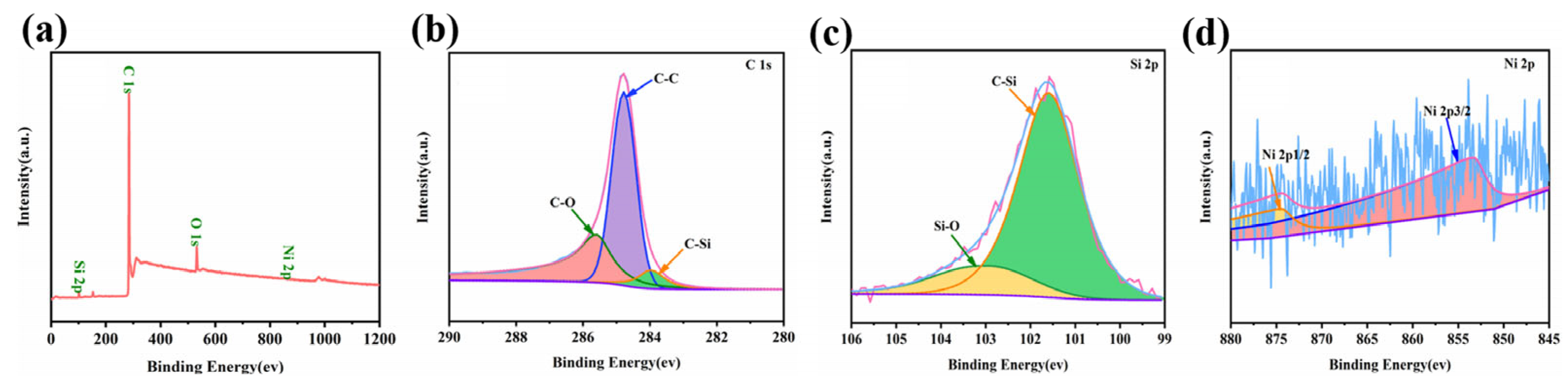
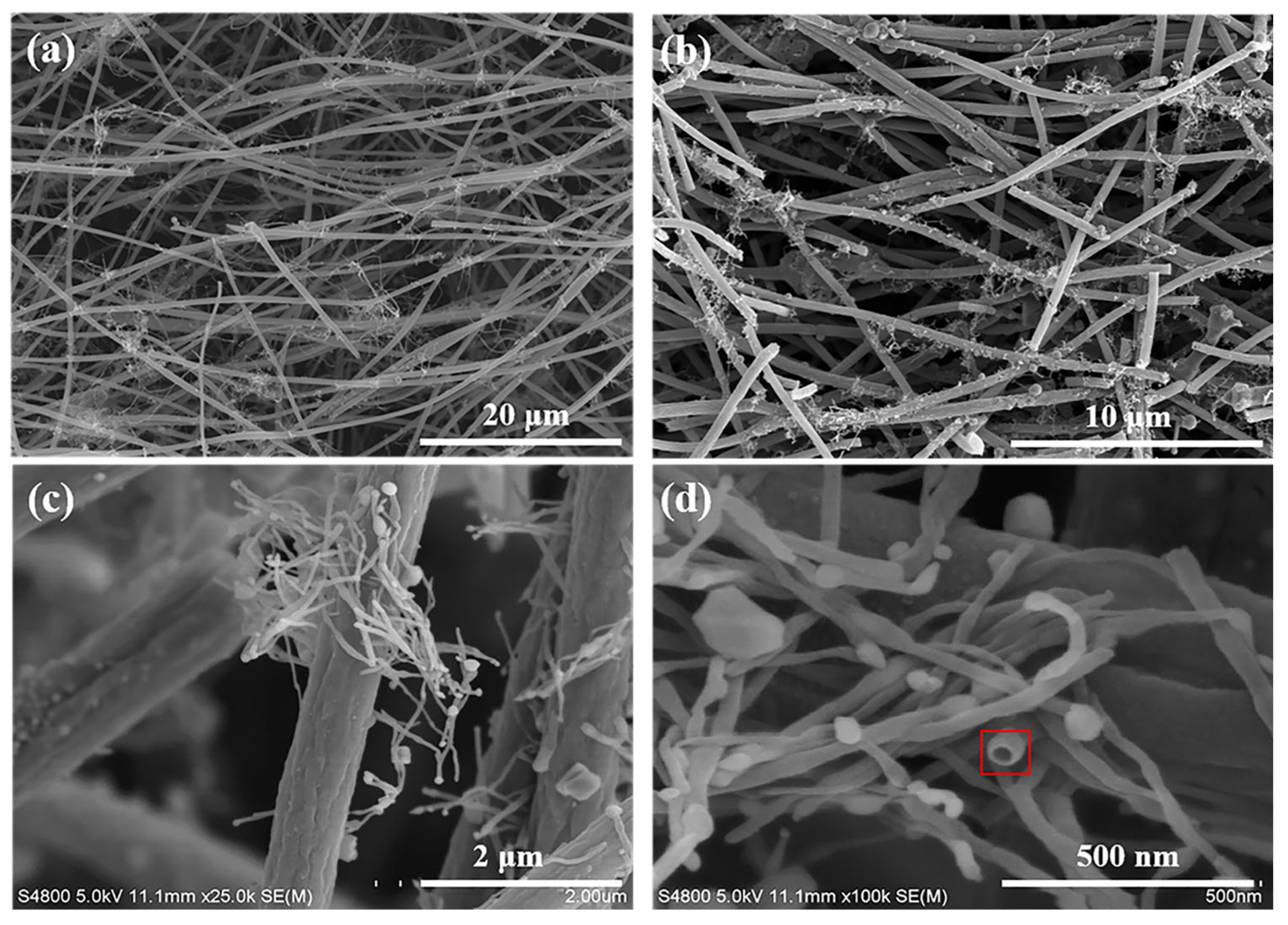
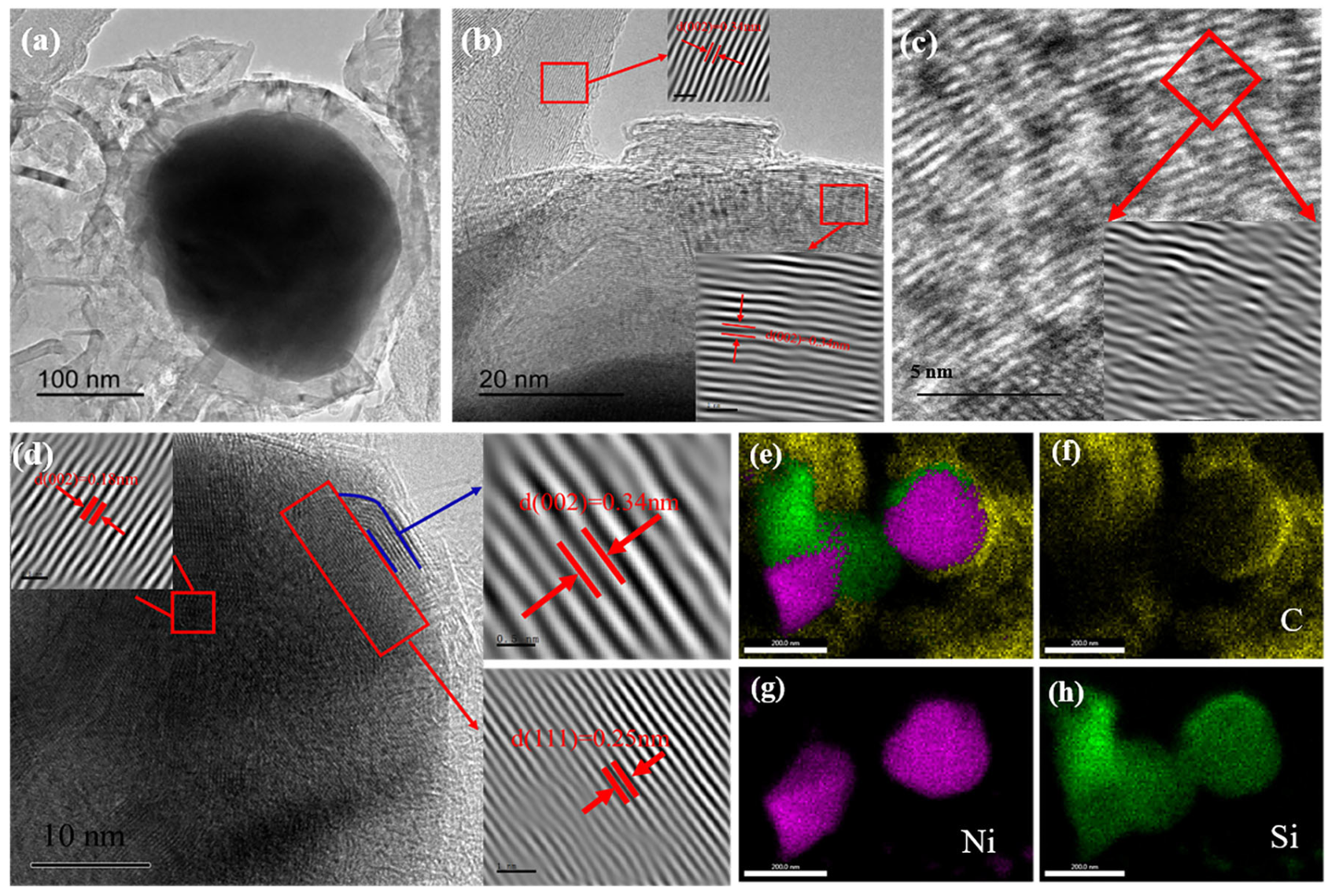
3.1. Microstructure and morphology
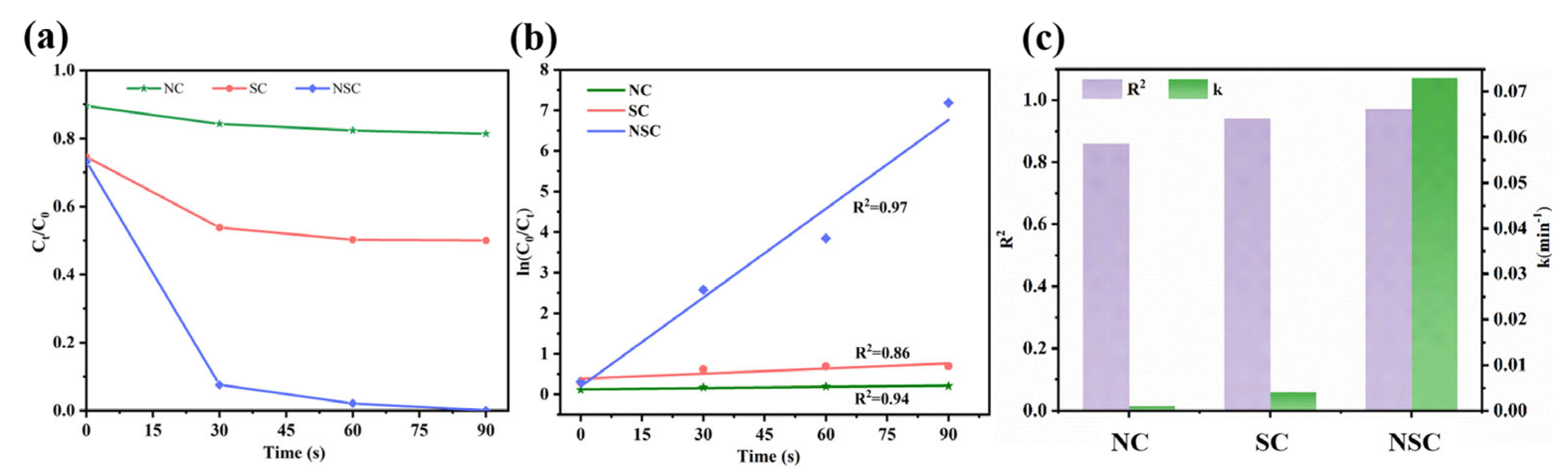
3.2. Microwave-induced catalytic degradation of MB
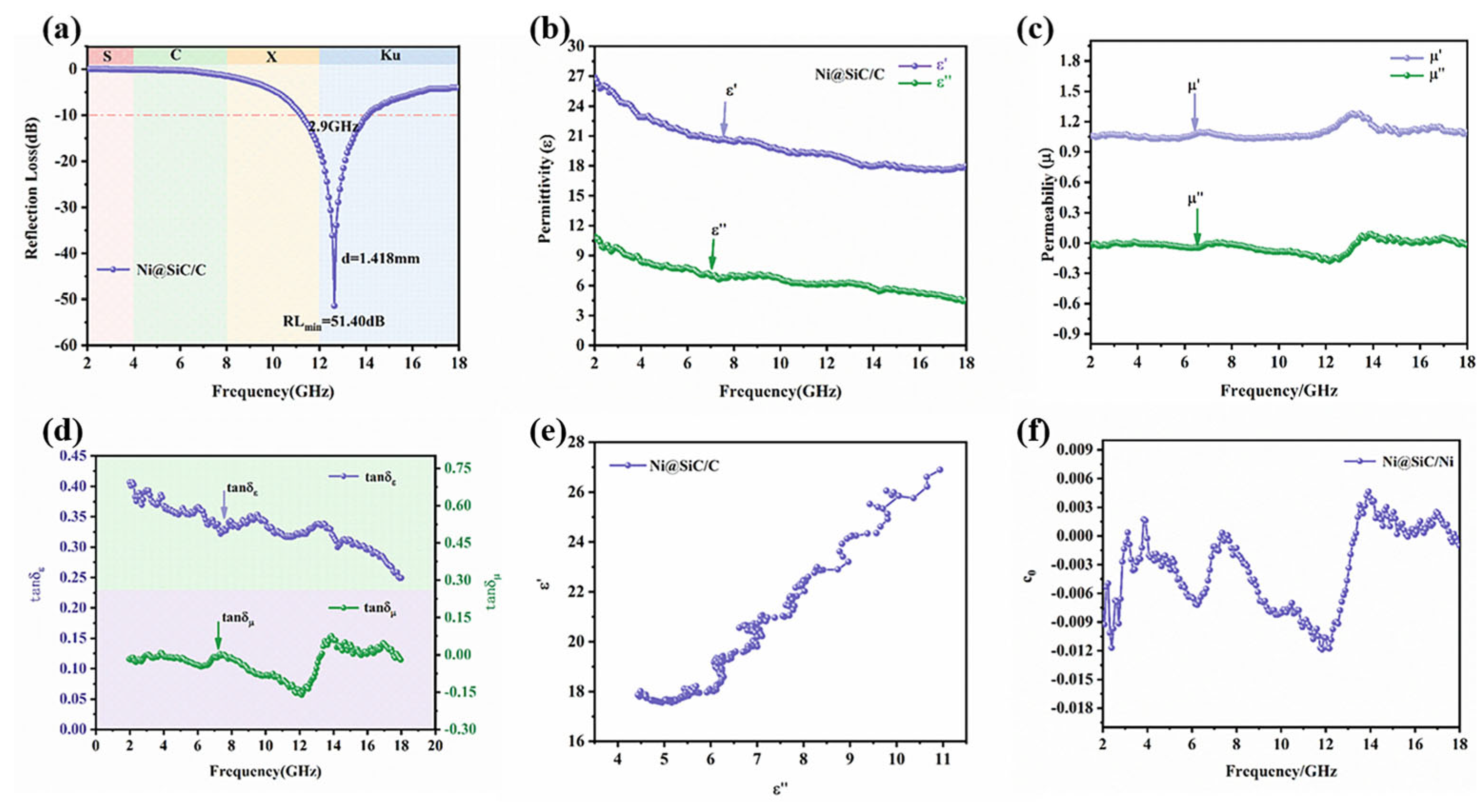
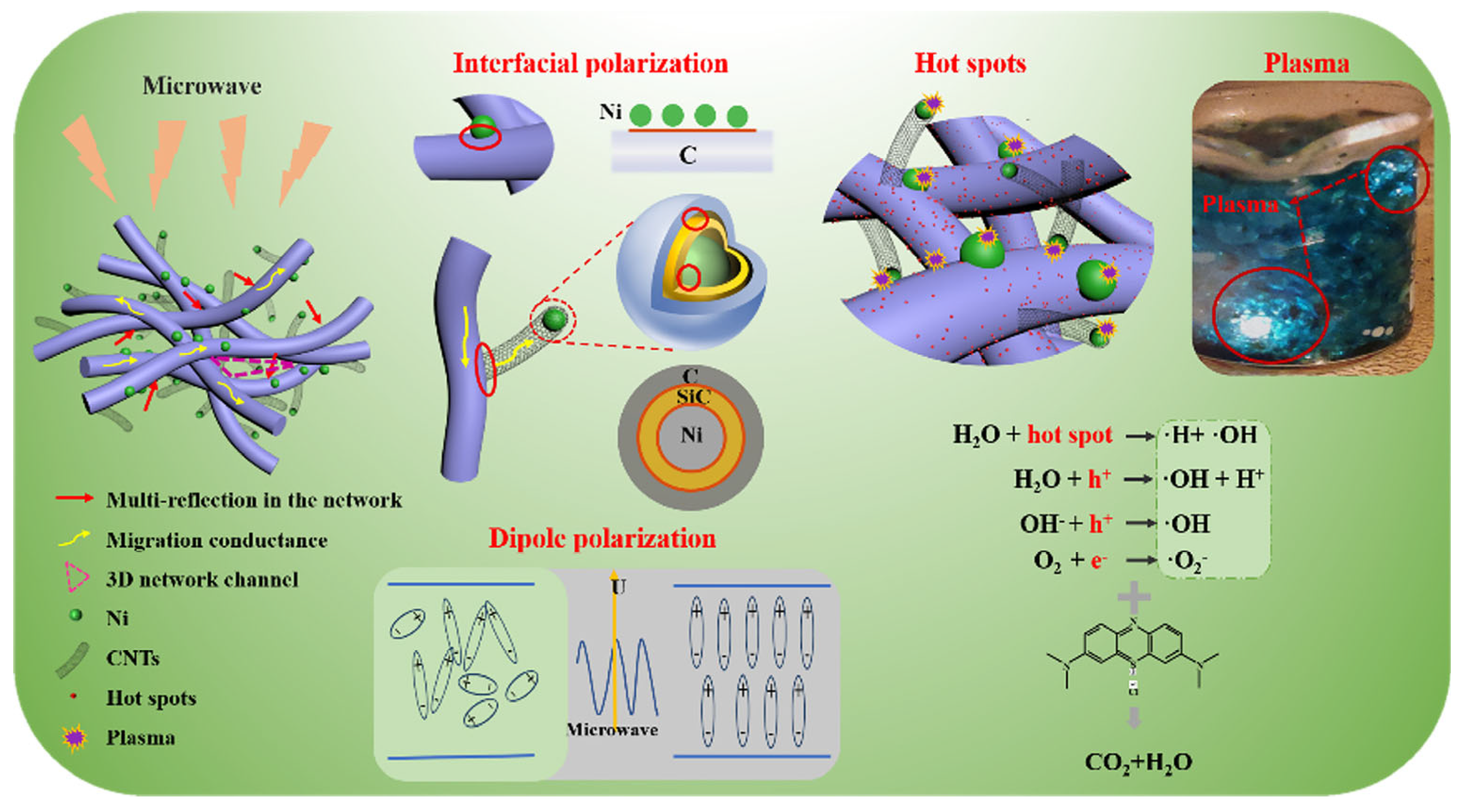
3.3. Electromagnetic wave absorption characteristics and catalytic degradation mechanism of NSC composite materials
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nasir, A.M.; Awang, N.; Jaafar, J.; Ismail, A.F.; Othman, M.H.D.; A. Rahman, M.; Aziz, F.; Mat Yajid, M.A. Recent progress on fabrication and application of electrospun nanofibrous photocatalytic membranes for wastewater treatment: A review. Journal of Water Process Engineering 2021, 40. [Google Scholar] [CrossRef]
- Xia, H.; Li, C.; Yang, G.; Shi, Z.; Jin, C.; He, W.; Xu, J.; Li, G. A review of microwave-assisted advanced oxidation processes for wastewater treatment. Chemosphere 2022, 287. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, W.; Liang, Q.; Huang, J.; Shao, B.; Liu, Y.; Liu, Y.; He, Q.; Wu, T.; Gong, J.; et al. Microwave-assisted high-efficiency degradation of methyl orange by using CuFe2O4/CNT catalysts and insight into degradation mechanism. Environ Sci Pollut Res Int 2021, 28, 42683–42693. [Google Scholar] [CrossRef]
- Khoshnam, M.; Farahbakhsh, J.; Zargar, M.; Mohammad, A.W.; Benamor, A.; Ang, W.L.; Mahmoudi, E. alpha-Fe2O3/graphene oxide powder and thin film nanocomposites as peculiar photocatalysts for dye removal from wastewater. Sci Rep 2021, 11, 20378. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Su, P.; Weathersby, D.; Zhang, Q.; Zheng, J.; Fan, R.; Zhang, J.; Dai, Q. Synthesis of γ-Fe2O3-ZnO-biochar nanocomposites for Rhodamine B removal. Applied Surface Science 2020, 501. [Google Scholar] [CrossRef]
- Wong, S.; Ghafar, N.A.; Ngadi, N.; Razmi, F.A.; Inuwa, I.M.; Mat, R.; Amin, N.A.S. Effective removal of anionic textile dyes using adsorbent synthesized from coffee waste. Sci Rep 2020, 10, 2928. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Song, L.; Du, L.; Gao, P.; Liang, N.; Wu, S.; Minami, T.; Zang, L.; Yu, C.; Xu, X. Preparation of Polyaniline/Emulsion Microsphere Composite for Efficient Adsorption of Organic Dyes. Polymers (Basel) 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Long, F.; Chen, S.; Cao, Y.; Pan, X. Magnetic chitosan biopolymer as a versatile adsorbent for simultaneous and synergistic removal of different sorts of dyestuffs from simulated wastewater. Chemical Engineering Journal 2020, 385. [Google Scholar] [CrossRef]
- Xue, C.; Mao, Y.; Wang, W.; Song, Z.; Zhao, X.; Sun, J.; Wang, Y. Current status of applying microwave-associated catalysis for the degradation of organics in aqueous phase - A review. J Environ Sci (China) 2019, 81, 119–135. [Google Scholar] [CrossRef]
- Kubra, K.T.; Salman, M.S.; Hasan, M.N. Enhanced toxic dye removal from wastewater using biodegradable polymeric natural adsorbent. Journal of Molecular Liquids 2021, 328. [Google Scholar] [CrossRef]
- Gągol, M.; Przyjazny, A.; Boczkaj, G. Wastewater treatment by means of advanced oxidation processes based on cavitation – A review. Chemical Engineering Journal 2018, 338, 599–627. [Google Scholar] [CrossRef]
- Tian, K.; Hu, L.; Li, L.; Zheng, Q.; Xin, Y.; Zhang, G. Recent advances in persulfate-based advanced oxidation processes for organic wastewater treatment. Chinese Chemical Letters 2022, 33, 4461–4477. [Google Scholar] [CrossRef]
- Liang, C.; Niu, H.-Y.; Guo, H.; Niu, C.-G.; Yang, Y.-Y.; Liu, H.-Y.; Tang, W.-W.; Feng, H.-P. Efficient photocatalytic nitrogen fixation to ammonia over bismuth monoxide quantum dots-modified defective ultrathin graphitic carbon nitride. Chemical Engineering Journal 2021, 406. [Google Scholar] [CrossRef]
- Peng, Y.; Li, Y.; Liu, L.; Hao, X.; Cai, K.; Xiong, J.; Hong, W.; Tao, J. New optimization approach for amphoteric/magnetic ramie biosorbent in dyestuff adsorption. Biochemical Engineering Journal 2022, 181. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, Z.; Zhang, X.; Dong, W.; Cao, Z.; He, L.; Wang, X. Rapid decomplexation of Ni-EDTA by microwave-assisted Fenton reaction. Chemical Engineering Journal 2020, 381. [Google Scholar] [CrossRef]
- Garcia-Costa, A.L.; Zazo, J.A.; Casas, J.A. Microwave-assisted catalytic wet peroxide oxidation: Energy optimization. Separation and Purification Technology 2019, 215, 62–69. [Google Scholar] [CrossRef]
- Qi, Y.; Mei, Y.; Li, J.; Yao, T.; Yang, Y.; Jia, W.; Tong, X.; Wu, J.; Xin, B. Highly efficient microwave-assisted Fenton degradation of metacycline using pine-needle-like CuCo2O4 nanocatalyst. Chemical Engineering Journal 2019, 373, 1158–1167. [Google Scholar] [CrossRef]
- Hu, L.; Wang, P.; Liu, G.; Zheng, Q.; Zhang, G. Catalytic degradation of p-nitrophenol by magnetically recoverable Fe3O4 as a persulfate activator under microwave irradiation. Chemosphere 2020, 240, 124977. [Google Scholar] [CrossRef]
- Garcia-Costa, A.L.; Zazo, J.A.; Rodriguez, J.J.; Casas, J.A. Microwave-assisted catalytic wet peroxide oxidation. Comparison of Fe catalysts supported on activated carbon and γ-alumina. Applied Catalysis B: Environmental 2017, 218, 637–642. [Google Scholar] [CrossRef]
- Lei, Y.; Lin, X.; Liao, H. New insights on microwave induced rapid degradation of methyl orange based on the joint reaction with acceleration effect between electron hopping and Fe2+-H2O2 reaction of NiFeMnO4 nanocomposites. Separation and Purification Technology 2018, 192, 220–229. [Google Scholar] [CrossRef]
- Shen, M.; Fu, L.; Tang, J.; Liu, M.; Song, Y.; Tian, F.; Zhao, Z.; Zhang, Z.; Dionysiou, D.D. Microwave hydrothermal-assisted preparation of novel spinel-NiFe2O4/natural mineral composites as microwave catalysts for degradation of aquatic organic pollutants. J Hazard Mater 2018, 350, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.; Dai, J.; Tian, Y.; Duan, Y.; Li, Y. Efficient degradation of Fipronil in water by microwave-induced argon plasma: Mechanism and degradation pathways. Sci Total Environ 2020, 725, 138487. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Xue, L.; Zhou, Y.; Zhang, Y.; Huang, K. A microwave atmospheric plasma strategy for fast and efficient degradation of aqueous p-nitrophenol. J Hazard Mater 2021, 409, 124473. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Jiang, H.; Stapelberg, M.; Zhou, J.; Liu, M.; Li, Q.J.; Cao, Y.; Gao, R.; Cai, M.; Qiao, J.; et al. Self-Perpetuating Carbon Foam Microwave Plasma Conversion of Hydrocarbon Wastes into Useful Fuels and Chemicals. Environ Sci Technol 2021, 55, 6239–6247. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Liang, C.; Wu, M.; Chen, X.; Liu, D.; Ma, J. High-efficient microwave plasma discharging initiated conversion of waste plastics into hydrogen and carbon nanotubes. Energy Conversion and Management 2022, 268. [Google Scholar] [CrossRef]
- Pang, Y.; Lei, H. Degradation of p-nitrophenol through microwave-assisted heterogeneous activation of peroxymonosulfate by manganese ferrite. Chemical Engineering Journal 2016, 287, 585–592. [Google Scholar] [CrossRef]
- Zhou, J.; You, Z.; Xu, W.; Su, Z.; Qiu, Y.; Gao, L.; Yin, C.; Lan, L. Microwave irradiation directly excites semiconductor catalyst to produce electric current or electron-holes pairs. Sci Rep 2019, 9, 5470. [Google Scholar] [CrossRef] [PubMed]
- Pawar, S.P.; Gandi, M.; Bose, S. High performance electromagnetic wave absorbers derived from PC/SAN blends containing multiwall carbon nanotubes and Fe3O4 decorated onto graphene oxide sheets. RSC Advances 2016, 6, 37633–37645. [Google Scholar] [CrossRef]
- Menéndez, J.A.; Arenillas, A.; Fidalgo, B.; Fernández, Y.; Zubizarreta, L.; Calvo, E.G.; Bermúdez, J.M. Microwave heating processes involving carbon materials. Fuel Processing Technology 2010, 91, 1–8. [Google Scholar] [CrossRef]
- Li, C.; Xia, H.; Zhang, L.; Wang, S.; Peng, J.; Cheng, S.; Shu, J.; Jiang, X.; Zhang, Q. Analysis of dielectric characterization and microwave adsorbing properties in organism-contained spent carbon: An efficient regeneration method via microwave-assisted ultrasound. Chemical Engineering and Processing - Process Intensification 2018, 125, 74–86. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, R.; Huang, W.; Kong, L.; Guo, S.; Cheng, L. Morphology Design of Co-electrospinning MnO-VN/C Nanofibers for Enhancing the Microwave Absorption Performances. ACS Appl Mater Interfaces 2020, 12, 13208–13216. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Xi, J.; Zhou, E.; Peng, L.; Chen, Z.; Gao, C. Porous Graphene Microflowers for High-Performance Microwave Absorption. Nanomicro Lett 2018, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, F.; Lu, H.; Guo, W.; He, X.; Yuan, Y. Heterogeneous rod-like Ni@C composites toward strong and stable microwave absorption performance. Carbon 2021, 181, 358–369. [Google Scholar] [CrossRef]
- Wang, B.; Wu, Q.; Fu, Y.; Liu, T. A review on carbon/magnetic metal composites for microwave absorption. Journal of Materials Science & Technology 2021, 86, 91–109. [Google Scholar] [CrossRef]
- Hidaka, H.; Saitou, A.; Honjou, H.; Hosoda, K.; Moriya, M.; Serpone, N. Microwave-assisted dechlorination of polychlorobenzenes by hypophosphite anions in aqueous alkaline media in the presence of Pd-loaded active carbon. J Hazard Mater 2007, 148, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Dong, B.; Cao, M.; Wei, B.; Hu, C. Hierarchical Dendrite-Like Magnetic Materials of Fe3O4, γ-Fe2O3, and Fe with High Performance of Microwave Absorption. Chemistry of Materials 2011, 23, 1587–1593. [Google Scholar] [CrossRef]
- Ren, Y.; Zhu, C.; Zhang, S.; Li, C.; Chen, Y.; Gao, P.; Yang, P.; Ouyang, Q. Three-dimensional SiO2@Fe3O4 core/shell nanorod array/graphene architecture: synthesis and electromagnetic absorption properties. Nanoscale 2013, 5, 12296–12303. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Bhaskar, R.; Narayanan, K.B.; Kumar, A.; Debnath, K. Development of silicon carbide (SiC)-based composites as microwave-absorbing materials (MAMs): A review. Journal of the European Ceramic Society 2024, 44, 7411–7431. [Google Scholar] [CrossRef]
- Singh, S.; Maurya, A.K.; Gupta, R.; Kumar, A.; Singh, D. Improved microwave absorption behavioral response of Ni/SiC and Ni/SiC/graphene composites: A comparative insight. Journal of Alloys and Compounds 2020, 823. [Google Scholar] [CrossRef]
- Wang, Y.; Di, X.; Chen, J.; She, L.; Pan, H.; Zhao, B.; Che, R. Multi-dimensional C@NiCo-LDHs@Ni aerogel: Structural and componential engineering towards efficient microwave absorption, anti-corrosion and thermal-insulation. Carbon 2022, 191, 625–635. [Google Scholar] [CrossRef]
- Hu, P.; Dong, S.; Li, X.; Chen, J.; Hu, P. Flower-like NiCo2S4 Microspheres Based on Nanosheet Self-Assembly Anchored on 3D Biomass-Derived Carbon for Efficient Microwave Absorption. ACS Sustainable Chemistry & Engineering 2020, 8, 10230–10241. [Google Scholar] [CrossRef]
- Liu, L.; Yang, S.; Hu, H.; Zhang, T.; Yuan, Y.; Li, Y.; He, X. Lightweight and Efficient Microwave-Absorbing Materials Based on Loofah-Sponge-Derived Hierarchically Porous Carbons. ACS Sustainable Chemistry & Engineering 2018, 7, 1228–1238. [Google Scholar] [CrossRef]
- Li, H.; Gao, S.; Tong, H.; Liu, Y.; Wu, A.; Hao, H. The capacitive loss of microwave energy in Ni@SiC@C core/bi-shell nanoparticles. Chemical Engineering Journal 2022, 434. [Google Scholar] [CrossRef]
- Candace, K. Chan, R.N.P., Michael J. O’Connell, Brian A. Korgel, and Yi Cui. Solution-Grown Silicon Nanowires for Lithium-Ion Battery Anodes. ACS Nano 2010, 4, 1443–1450. [Google Scholar]
- Liu, Y.; Liu, Y.; Choi, W.C.; Chae, S.; Lee, J.; Kim, B.-S.; Park, M.; Kim, H.Y. Highly flexible, erosion resistant and nitrogen doped hollow SiC fibrous mats for high temperature thermal insulators. Journal of Materials Chemistry A 2017, 5, 2664–2672. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.; Lei, Y.; Wu, N.; Gou, Y.; Han, C.; Fang, D. Hierarchically porous SiC ultrathin fibers mat with enhanced mass transport, amphipathic property and high-temperature erosion resistance. J. Mater. Chem. A 2014, 2, 20873–20881. [Google Scholar] [CrossRef]
- Huo, Y.; Tan, Y.; Zhao, K.; Lu, Z.; Zhong, L.; Tang, Y. Enhanced electromagnetic wave absorption properties of Ni magnetic coating-functionalized SiC/C nanofibers synthesized by electrospinning and magnetron sputtering technology. Chemical Physics Letters 2021, 763. [Google Scholar] [CrossRef]
- Li, T.; Luo, G.; Liu, K.; Li, X.; Sun, D.; Xu, L.; Li, Y.; Tang, Y. Encapsulation of Ni3Fe Nanoparticles in N-Doped Carbon Nanotube–Grafted Carbon Nanofibers as High-Efficiency Hydrogen Evolution Electrocatalysts. Advanced Functional Materials 2018, 28. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Z.; Zhou, S.; Yu, F.; Yu, M.; Chiang, C.Y.; Zhou, W.; Zhao, J.; Qiu, J. Metal-Organic-Framework-Derived Hybrid Carbon Nanocages as a Bifunctional Electrocatalyst for Oxygen Reduction and Evolution. Adv Mater 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Wang, D.P.; Rui, X.; Liu, B.; Zhou, K.; Law, A.W.; Yan, Q.; Wei, J.; Chen, Z. In-situ formation of hollow hybrids composed of cobalt sulfides embedded within porous carbon polyhedra/carbon nanotubes for high-performance lithium-ion batteries. Adv Mater 2015, 27, 3038–3044. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Salunkhe, R.R.; Liu, J.; Torad, N.L.; Imura, M.; Furukawa, S.; Yamauchi, Y. Thermal conversion of core-shell metal-organic frameworks: a new method for selectively functionalized nanoporous hybrid carbon. J Am Chem Soc 2015, 137, 1572–1580. [Google Scholar] [CrossRef]
- Li, D.; Liao, H.; Kikuchi, H.; Liu, T. Microporous Co@C Nanoparticles Prepared by Dealloying CoAl@C Precursors: Achieving Strong Wideband Microwave Absorption via Controlling Carbon Shell Thickness. ACS Appl Mater Interfaces 2017, 9, 44704–44714. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Fan, S.; Li, X.; Qu, X.; Tian, Y.; Zhang, X.; Zhang, Z.; Dong, X.; Cao, T. Enhanced dielectric and conductivity properties of carbon-coated SiC nanocomposites in the terahertz frequency range. Nanotechnology 2021, 32, 265705. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Ren, P.; Deng, D.; Yu, L.; Yang, F.; Bao, X. Highly active and durable non-precious-metal catalysts encapsulated in carbon nanotubes for hydrogen evolution reaction. Energy Environ. Sci. 2014, 7, 1919–1923. [Google Scholar] [CrossRef]
- Cui, X.; Ren, P.; Deng, D.; Deng, J.; Bao, X. Single layer graphene encapsulating non-precious metals as high-performance electrocatalysts for water oxidation. Energy & Environmental Science 2016, 9, 123–129. [Google Scholar] [CrossRef]
- Cai, Z.; Su, L.; Wang, H.; Xie, Q.; Gao, H.; Niu, M.; Lu, D. Hierarchically assembled carbon microtube@SiC nanowire/Ni nanoparticle aerogel for highly efficient electromagnetic wave absorption and multifunction. Carbon 2022, 191, 227–235. [Google Scholar] [CrossRef]
- Di, X.; Wang, Y.; Lu, Z.; Cheng, R.; Yang, L.; Wu, X. Heterostructure design of Ni/C/porous carbon nanosheet composite for enhancing the electromagnetic wave absorption. Carbon 2021, 179, 566–578. [Google Scholar] [CrossRef]
- Li, D.; Guo, K.; Wang, F.; Wu, Z.; Zhong, B.; Zuo, S.; Tang, J.; Feng, J.; Zhuo, R.; Yan, D.; et al. Enhanced microwave absorption properties in C band of Ni/C porous nanofibers prepared by electrospinning. Journal of Alloys and Compounds 2019, 800, 294–304. [Google Scholar] [CrossRef]
- Ye, X.; Zhang, J.; Chen, Z.; Xiang, J.; Jiang, Y.; Xie, F.; Ma, X. Microwave absorption properties of Ni/C@SiC composites prepared by precursor impregnation and pyrolysis processes. Defence Technology 2023, 21, 94–102. [Google Scholar] [CrossRef]
- Wu, F.; Liu, Z.; Wang, J.; Shah, T.; Liu, P.; Zhang, Q.; Zhang, B. Template-free self-assembly of MXene and CoNi-bimetal MOF into intertwined one-dimensional heterostructure and its microwave absorbing properties. Chemical Engineering Journal 2021, 422. [Google Scholar] [CrossRef]
- Huang, B.; Wang, Z.; Hu, H.; Xiu, Z.; Huang, X.; Yue, J.; Wang, Y. Enhancement of the microwave absorption properties of PyC-SiCf/SiC composites by electrophoretic deposition of SiC nanowires on SiC fibers. Ceramics International 2020, 46, 9303–9310. [Google Scholar] [CrossRef]
- Li, W.; Guo, F.; Wei, X.; Du, Y.; Chen, Y. Preparation of Ni/C porous fibers derived from jute fibers for high-performance microwave absorption. RSC Adv 2020, 10, 36644–36653. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




