1. Introduction
Penile cancer (PeCa) is a rare urological malignancy with significant geographic variations in incidence and mortality [
1]. Squamous cell carcinoma (SCC) accounts for approximately 95% of PeCa; however, sarcoma, melanoma, and basal cell carcinoma are also reported [
1,
2]. Due to its rarity and the consequent lack of randomized trials, current therapy is based on retrospective studies and small prospective trials. In addition, the diagnostic pathways and therapeutic management options are heterogeneous and vary significantly between poor and well-developed countries [
1,
3,
4]. However, over the past decades, the overall survival (OS) of PeCa has increased, mainly due to significant improvements in imaging and risk stratification [
5].
The presence and extent of regional lymph nodes determine the prognosis of PeCa. Thus, early detection and treatment of lymph node (LN) metastasis is paramount to ensure better outcomes [
4]. Noninvasive N and M staging currently relies on ultrasonography, computed tomography (CT), and magnetic resonance imaging (MRI). These imaging techniques, however, are not sufficiently robust in detecting inguinal metastases, with 20–25% of cN0 patients harboring occult metastasis [
4,
5]. Positron emission tomography (PET) combined with CT may provide additional anatomic and metabolic information for staging and assessing treatment response. It is currently used in the management of several malignancies, such as lung or head-neck cancers, that are mainly SCC, similar to PeCa [
6,
7]. PET imaging using
18F-fluorodeoxyglucose (FDG) relies on the increased cellular uptake of glucose and FDG, particularly in malignant cells and other tissues characterized by heightened glycolytic activity [
8].
The role of PET imaging in penile cancer has not been adequately explored. Our aim is to assess the current role of PET/CT imaging in the management of patients with PeCa.
2. Material and Method
An extensive non-systematic literature search of the Scopus, PubMed/MEDLINE, and Web of ScienceTM databases was conducted to identify recent manuscripts published between 2013 and 2023 related to PET/CT imaging of primary PeCa. Key search words included “penile cancer, “penile squamous cell carcinoma,” “PET CT,” “18 FDG,” “lymph nodes,” “clinically node negative,” “clinically node positive,” and “enlarged nodes.” There were no filters for language. Studies that evaluated pediatric/congenital malignancies, secondary penile cancers, case series with <5 patients, or not exclusive to primary PeCa were excluded (N=28). The manual investigation of citations within identified articles was also performed (N=4). The systemic standards, including impact factor and citation frequency, were considered when citing other review studies.
3. Results
3.1. Role of PET-CT for T Staging in Penile Cancer
Physical examination, including palpation, has traditionally been and remains the cornerstone for evaluating the extent of the primary tumor (T stage). In addition, imaging plays a crucial role when considering penile preservation surgery. In the detection of corpora cavernosa invasion, ultrasound exhibits a sensitivity of 97% and a specificity of 99%, whereas MRI shows a sensitivity of 74% and a specificity of 99% [
9].
In a Brazilian study that included 53 patients, a higher pSUVmax was observed in more advanced tumors (pT1b and above) and with poorer differentiation compared to less aggressive lesions (p < 0.019). Several pathological features, such as dartos infiltration, lamina propria, or perineural invasion, were associated with increased FDG uptake [
10]. Although almost all PeCa present a certain degree of FDG uptake, small lesions can be missed due to limited spatial resolution and urine leakage that can mask the tumor [
11]. Due to these limitations, PET/CT cannot be recommended for the initial T stage.
In summary, when considering organ-sparing treatment for PeCa patients, ultrasound with Doppler emerges as the preferred method for detecting corporal invasion. MRI can also be considered as an alternative [
11] de Vries et al. In surgically treated patients, an uptake appearing within the foreskin or shaft may be regarded as a residual or recurrent tumor as opposed to an artifact, which may be amenable for surgery [
11]. In a small case series of 13 patients, Musi et al demonstrated that local recurrences can be safely treated with salvage surgery, either by local excision or laser ablation, without compromising oncological control of the disease [
12]. However, the data is insufficient to establish the role of PET/CT in assessing the primary tumor in initial and recurrent scenarios.
3.2. Role of PET-CT in the Management of Clinically Node-Negative PeCa
The presence and extent of regional inguinal lymph nodes (ILN) metastases are regarded as the most important prognostic indicator for determining long-term survival in men with invasive PeCa [
4,
5,
13]. Furthermore, node-negative (cN0) patients still have a 13—16% chance of harboring occult metastases, whereas node-positive (cN+) patients have a 20-40% chance of being metastasis-free [
14].
Several studies assessed the use of FDG PET/CT to detect inguinal involvement in PeCa patients with non-palpable lymph nodes initially classified as node-negative (cN0). Salazar et al used PET/CT imaging in a mixed cN0 and cN1 cohort of 53 patients. With an SUV
max cut-off of 6.5, they reported a sensitivity of 77% and a specificity of 78% when compared to histopathologic results after inguinal lymph node dissection (ILND) [
1]0). A recent study investigated 18F-FDG PET/CT for ILN staging in cN0-only patients (n= 41) and reached a patient-based sensitivity and specificity of 80% and 68%, respectively, similar to the results of Salazar et al. [
15]. Although the sensibility of these new studies is higher than that reported by a meta-analysis of seven trials conducted by Sadeghi et al and colleagues in 2012 of only 57% per groin, none of these studies explicitly compared FDG PET/CT with other imaging modalities, such as ultrasound, that present a similar accuracy to PET/CT in this clinical scenario [
16].
Jakobsen et al evaluated the diagnostic accuracy of sentinel node biopsy (SNB) combined with preoperative 18F-FDG PET/CT in a cohort of 61 cN0 PeCa patients. They reported a combined FDG PET/CT-SNB sensitivity of 94.4% (95% confidence interval [CI] 81-99%) per groin and a false-negative rate of 5.6% (95% CI 1-19%) per groin [
17]. Despite promising results and a low rate of adverse events after SNB, the false-negative SNB is still relatively high, suggesting that more trials are needed to demonstrate the clinical utility of this combined diagnostic approach. The incorporation of fluorescence imaging into the SNB procedure utilizing the hybrid fluorescence and radioactive tracer indocyanine green (ICG)-99mTc-nanocolloid has been demonstrated to improve intraoperative localization of sentinel nodes in PeCa [
18]. As observed in other cancers, a high 18F-FDG uptake in the primary tumor frequently leads to decreased uptake in regional nodes, suggesting that PET imaging assessment in node-negative patients should be conducted post-primary tumor removal to enhance the likelihood of LN detection. However, this approach is hindered by elevated costs and an increased risk of false positive rates due to postoperative inflammation [
19].
In conclusion, in cN0, although FDG PET/CT reveals a significant negative predictive value (NPV), it cannot replace the invasive nodal staging due to its inadequate sensitivity (particularly regarding micrometastasis) and specificity (i.e., in discriminating between (post)inflammatory and metastatic lymph nodes).
3.3. Role of PET-CT in the Management of Clinically Node-Positive PeCa
Palpable lymphadenopathy warrants prompt evaluation, as the risk of metastasis is high (45-80%) [
4]. Although early treatment of lymph node involvement has been shown to positively impact survival, clinically nodal disease at diagnosis does not warrant an immediate ILND since ~50% of patients present inflammatory swelling instead of metastatic spread [
4,
20]. PET/CT demonstrates a strong diagnostic performance in this context, as shown in
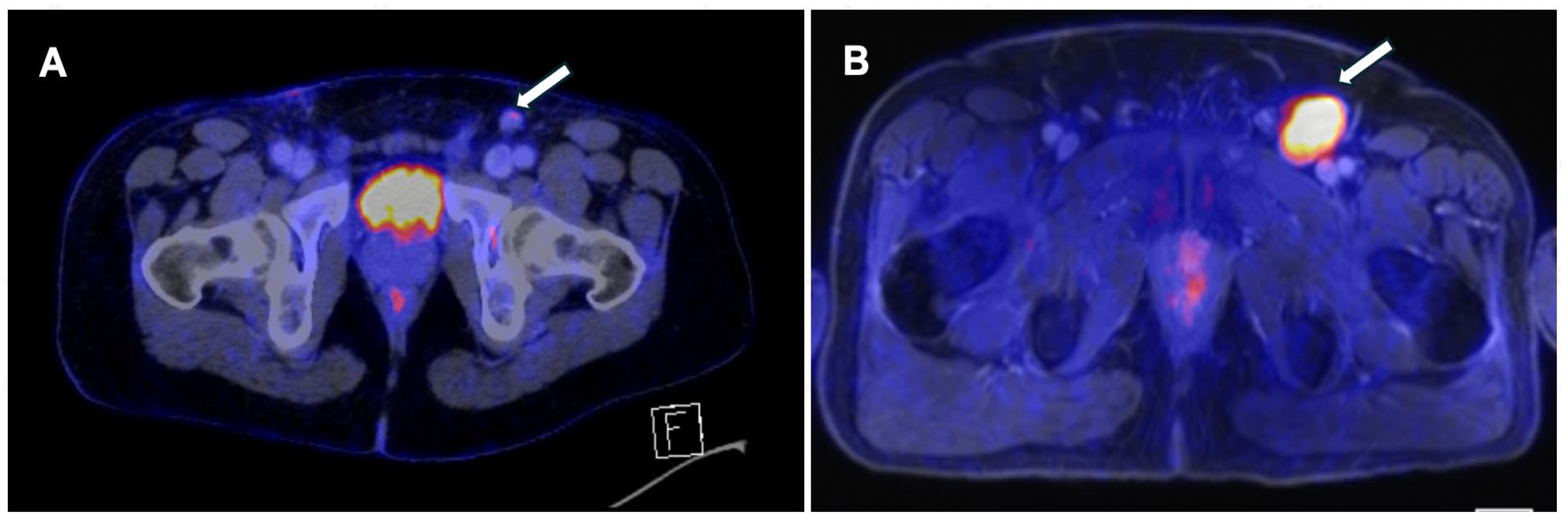
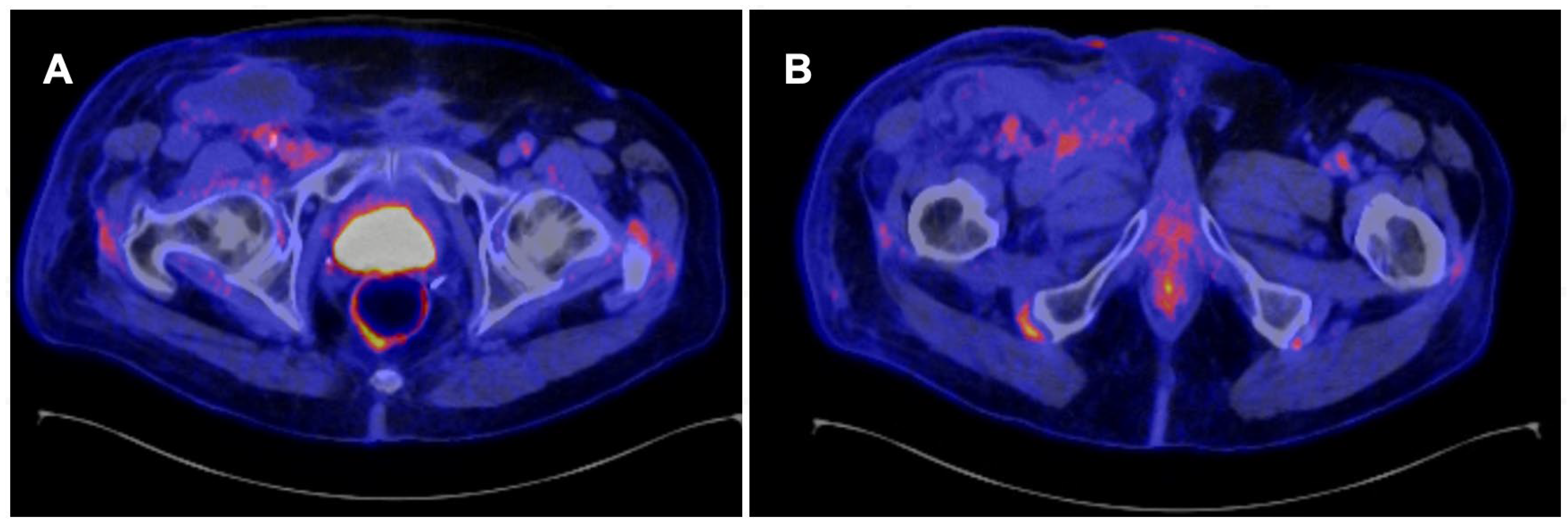
Figure 1 and
Figure 2.
In a recently published meta-analysis that evaluated 12 studies [
4]79 patients), the pooled sensitivity of 18F-FDG PET/CT was 0.87 (95% confidence interval [CI], 0.79-0.92), and the pooled specificity was 0.88 (95% CI, 0.79-0.93) [
21]. These results are better than those reported in the meta-analysis conducted by Sadeghi et al in 2012 [
16]. These differences could be related to the use of SUV cutoff points since this methodology was not used in the previous analysis, which was based exclusively on qualitative analysis [
10].
Salazar et al demonstrated that the semiquantitative PET/CT parameters (pSUVmax and nSUVmax) may have a prognostic value. Their cohort reported that a pSUVmax of 16.6 was the best predictor for OS (p =0.0001), followed by a nSUVmax of 6.5 (p = 0.019) [
10]. Although these PET parameters are already used in other squamous cancers, these results should be carefully considered, with further evaluations being necessary to consolidate these findings in this clinical setting [
22,
23].
In cases where an inguinal biopsy is not always feasible due to patient refusal or the risk of damaging the femoral vessels, FDG PET/CT can be a useful tool in assessing the nature of a suspicious lesion. Zhang and colleagues reported that in a cohort of 48 cN1 patients, FDG PET/CT detected more malignant diseases than CT or MRI in 33% of cases and treatment was changed after PET/CT results in 57% of patients [
24].
Primary surgery is not recommended in the case of fixed, bilateral, or large nodal illness due to the poor prognosis [
4]. A multimodal approach, including neoadjuvant chemotherapy followed by consolidative surgery, is recommended in cases with complete or partial response. In this setting, the impact of neoadjuvant treatments may be assessed by PET/CT. Ottenhof et al reported the safety and efficacy of chemoradiotherapy (CRT) as the primary treatment for nonmetastatic patients with large/inoperable primary tumors and large palpable nodes in a prospective, single-center, single-arm study [
25]. In their cohort of 33 patients, the impact of CRT was assessed using 18F-FDG PET/CT. Thirteen patients (39%) achieved a complete response, whereas 73% exhibited a partial response, enabling 27% of patients to undergo salvage surgery. Despite the authors reporting only short-term outcomes (2-year OS of 46%), these results are promising, considering the reported less than 20% 5-year survival rate for this cohort with a poor prognosis [
4,
20]. Nevertheless, the elevated FDG uptake in most primary penile tumors and LN metastases indicates that FDG PET/CT is a valuable tool for staging. penile cancer [
5].
In order to increase the diagnostic accuracy of PET/CT, Mascia et al. reported the results of 64(II)dichloride (64Cu(II)Cl2) as a new PET tracer for evaluation of nodal disease in PeCa in a phase 2 clinical trial [
26]. The authors had a small sample size of only 6 PeCa patients; the detection rate was 83.3% (5/6) with an area under the curve of 0.775. Although 64Cu(II)Cl2 is an effective and well-tolerated tracer, more trials are needed to confirm its utility in the management of node-positive PeCa patients. Moreover, in a pilot study aimed at preoperative assistance, 11 histologically confirmed PeCa patients underwent staging [68Ga]Ga-FAPI-46 PET/CT before surgery. The histologically confirmed lymph node regions exhibit significantly elevated FAPI uptakes (SUVmax 17.9 (16.4 – 23.5)), with no instances of false-positive FAPI uptake, potentially enabling the detection of occult LN metastases [
27].
3.4. Role of PET CT in the Management of Pelvic Lymph Nodes and Distant Metastasis
The literature regarding the impact of PET CT on the detection and management of pelvic LN and metastasis is scarce and mainly comprised of case presentations or retrospective series. In the aforementioned study by Zhang and collaborators, the sensitivity and specificity of FDG PET/CT were 85% and 86%, respectively, for all metastatic sites, including lymph nodes, lung, liver, bone, brain, using histopathology or follow-up imaging as a reference [
24]. Ottenhof et al reported that in their cohort of 61 high-risk PeCa patients, PET/CT demonstrated a sensitivity of 85% for pelvic LN staging (specificity of 75%, NPV 90%, and a PPV of 65%) and a PPV of 93% for the detection of distant metastases, suggesting that FDG PET/CT imaging should be used in the initial diagnosis of PeCa to avoid futile treatment of patients with distant metastases [
6].
3.5. Role of PET CT in Follow-Up After Treatment
Local or nodal recurrences usually occur within 2–3 yr of primary treatment. This suggests a rigorous follow-up schedule for the first two years, followed by less intense follow-up for at least five years [
4,
11],. Current guidelines recommend that PET/CT may be an alternative to CT or MRI for imaging follow-up after definitive treatment [
4,
28]. In comparison to other imaging tests, PET/CT is usually more costly, and its availability may be a limiting factor in some countries [
29]. Another drawback of PET/CT for systemic staging is that it is frequently conducted without intravenous contrast, which makes measuring the extent of visceral metastases difficult [
20].
4. Conclusions
18F-FDG PET imaging plays a pivotal role in the management of clinically node-positive penile cancer patients and may serve as an independent prognostic factor for survival in this population. In addition, PET/CT can assist in surgical planning and evaluating chemotherapy response. Integrating 18F-FDG PET/CT into future staging algorithms has the potential to guide more precise and stage-appropriate therapeutic strategies.
Author Contributions
Study conception and design: Mirvald, Garaz, Surcel, Tsaur; Acquisition of data: Mirvald, Surcel, Labanaris, Garaz; Analysis and interpretation of data: Mirvald, Surcel, Sinescu, Labanaris, Garaz; Drafting of manuscript: Mirvald, Garaz, Sinescu, Labanaris, Yossepowitch, Tsaur, Surcel; Critical revision: Surcel, Sinescu, Yossepowitch, Tsaur, Labanaris.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fu, L.; Tian, T.; Yao, K.; Chen, X.-F.; Luo, G.; Gao, Y.; Lin, Y.-F.; Wang, B.; Sun, Y.; Zheng, W.; et al. Global Pattern and Trends in Penile Cancer Incidence: Population-Based Study. JMIR Public Heal. Surveill. 2022, 8, e34874, . [CrossRef]
- Moch, H.; Cubilla, A.L.; Humphrey, P.A.; Reuter, V.E.; Ulbright, T.M. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. Eur. Urol. 2016, 70, 93–105. [CrossRef]
- Hogan, D.; Norton, S.M.; Patterson, K.; Murphy, A.; O'Neill, B.; Daly, P.; Cullen, I.M. Phallus preservation and reconstruction: 5-year outcomes of national penile cancer centralisation in the Republic of Ireland. Surg. 2024, . [CrossRef]
- Brouwer, O.R.; Albersen, M.; Parnham, A.; Protzel, C.; Pettaway, C.A.; Ayres, B.; Antunes-Lopes, T.; Barreto, L.; Campi, R.; Crook, J.; et al. European Association of Urology-American Society of Clinical Oncology Collaborative Guideline on Penile Cancer: 2023 Update. Eur. Urol. 2023, 83, 548–560, . [CrossRef]
- Ottenhof SR, Leone AR, Horenblas S, Spiess PE, Vegt E. Advancements in staging and imaging for penile cancer. Curr Opin Urol. 2017;27[6]):612-20.
- Ottenhof SR, Djajadiningrat RS, Versleijen MWJ, Donswijk ML, van der Noort V, Brouwer OR, et al. F-18 Fluorodeoxyglucose Positron Emission Tomography with Computed Tomography Has High Diagnostic Value for Pelvic and Distant Staging in Patients with High-risk Penile Carcinoma. Eur Urol Focus. 2022;8[1]):98-104.
- Reske SN, Kotzerke J. FDG-PET for clinical use. Results of the 3rd German Interdisciplinary Consensus Conference, "Onko-PET III", 21 July and 19 September 2000. Eur J Nucl Med. 2001;28[1]1):1707-23.
- Fletcher, J.W.; Djulbegovic, B.; Soares, H.P.; Siegel, B.A.; Lowe, V.J.; Lyman, G.H.; Coleman, R.E.; Wahl, R.; Paschold, J.C.; Avril, N.; et al. Recommendations on the Use of 18F-FDG PET in Oncology. J. Nucl. Med. 2008, 49, 480–508, . [CrossRef]
- Bozzini, G.; Provenzano, M.; Otero, J.R.; Margreiter, M.; Cruz, E.G.; Osmolorskij, B.; Verze, P.; Pavan, N.; Sanguedolce, F.; Buffi, N.; et al. Role of Penile Doppler US in the Preoperative Assessment of Penile Squamous Cell Carcinoma Patients: Results From a Large Prospective Multicenter European Study. Urology 2016, 90, 131–135, . [CrossRef]
- Salazar, A.; Júnior, E.P.; Salles, P.G.O.; Silva-Filho, R.; Reis, E.A.; Mamede, M. 18F-FDG PET/CT as a prognostic factor in penile cancer. Eur. J. Nucl. Med. 2019, 46, 855–863, . [CrossRef]
- Ottenhof SR, Vegt E. The role of PET/CT imaging in penile cancer. Transl Androl Urol. 2017;6[5]):833-8.
- Musi, G.; Molinari, F.; Mistretta, F.A.; Piccinelli, M.L.; Guzzo, S.; Tozzi, M.; Lievore, E.; Blezien, O.; Fontana, M.; Cioffi, A.; et al. Penile-Sparing Surgery for Tumour Recurrence after Previous Glansectomy/Partial Penectomy: Treatment Feasibility and Oncological Outcomes. Cancers 2023, 15, 4807, . [CrossRef]
- De Vries, H.M.; Brouwer, O.R.; Heijmink, S.; Horenblas, S.; Vegt, E. Recent developments in penile cancer imaging. Curr. Opin. Urol. 2019, 29, 150–155, . [CrossRef]
- Hughes, B.; Leijte, J.; Shabbir, M.; Watkin, N.; Horenblas, S. Non-invasive and minimally invasive staging of regional lymph nodes in penile cancer. World J. Urol. 2008, 27, 197–203, . [CrossRef]
- Drager, D.L.; Heuschkel, M.; Protzel, C.; Erbersdobler, A.; Krause, B.J.; Hakenberg, O.W.; Schwarzenbock, S.M. [F-18]FDG PET/CT for assessing inguinal lymph nodes in patients with penile cancer - correlation with histopathology after inguinal lymphadenectomy. 2018, 57, 26–30, . [CrossRef]
- Sadeghi R, Gholami H, Zakavi SR, Kakhki VR, Horenblas S. Accuracy of 18F-FDG PET/CT for diagnosing inguinal lymph node involvement in penile squamous cell carcinoma: systematic review and meta-analysis of the literature. Clin Nucl Med. 2012;37[5]):436-41.
- Jakobsen, J.K.; Alslev, L.; Ipsen, P.; Costa, J.C.; Krarup, K.P.; Sommer, P.; Nerstrøm, H.; Toft, B.G.; Høyer, S.; Bouchelouche, K.; et al. DaPeCa-3: promising results of sentinel node biopsy combined with 18F-fluorodeoxyglucose positron emission tomography/computed tomography in clinically lymph node-negative patients with penile cancer – a national study from Denmark. BJU Int. 2016, 118, 102–111, . [CrossRef]
- KleinJan, G.H.; van Werkhoven, E.; Berg, N.S.v.D.; Karakullukcu, M.B.; Zijlmans, H.J.M.A.A.; van der Hage, J.A.; van de Wiel, B.A.; Buckle, T.; Klop, W.M.C.; Horenblas, S.; et al. The best of both worlds: a hybrid approach for optimal pre- and intraoperative identification of sentinel lymph nodes. Eur. J. Nucl. Med. 2018, 45, 1915–1925, . [CrossRef]
- van Westreenen, H.; Westerterp, M.; Bossuyt, P.; Pruim, J.; Sloof, G.; van Lanschot, J.; Groen, H.; Plukker, J. Systematic Review of the Staging Performance of18F-Fluorodeoxyglucose Positron Emission Tomography in Esophageal Cancer. J. Clin. Oncol. 2004, 22, 3805–3812, . [CrossRef]
- Peyraud, F.; Allenet, C.; Gross-Goupil, M.; Domblides, C.; Lefort, F.; Daste, A.; Yacoub, M.; Haaser, T.; Ferretti, L.; Robert, G.; et al. Current management and future perspectives of penile cancer: An updated review. Cancer Treat. Rev. 2020, 90, 102087, . [CrossRef]
- Lee, S.W.; Kim, S.-J. Diagnostic Performance of 18F-FDG PET/CT for Lymph Node Staging in Penile Cancer. Clin. Nucl. Med. 2022, 47, 402–408, . [CrossRef]
- Chu KP, Murphy JD, La TH, Krakow TE, Iagaru A, Graves EE, et al. Prognostic value of metabolic tumor volume and velocity in predicting head-and-neck cancer outcomes. Int J Radiat Oncol Biol Phys. 2012;83[5]):1521-7.
- Yoo Ie R, Chung SK, Park HL, Choi WH, Kim YK, Lee KY, et al. Prognostic value of SUVmax and metabolic tumor volume on 18F-FDG PET/CT in early stage non-small cell lung cancer patients without LN metastasis. Biomed Mater Eng. 2014;24[6]):3091-103.
- Zhang, S.; Li, W.; Liang, F. Clinical value of fluorine-18 2-fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography in penile cancer. Oncotarget 2016, 7, 48600–48606, . [CrossRef]
- Ottenhof SR, de Vries HM, Doodeman B, Vrijenhoek GL, van der Noort V, Donswijk ML, et al. A Prospective Study of Chemoradiotherapy as Primary Treatment in Patients With Locoregionally Advanced Penile Carcinoma. Int J Radiat Oncol Biol Phys. 2023;117[1]):139-47.
- Mascia M, Villano C, De Francesco V, Schips L, Marchioni M, Cindolo L. Efficacy and Safety of the 64Cu(II)Cl2 PET/CT for Urological Malignancies: Phase IIa Clinical Study. Clin Nucl Med. 2021;46[6]):443-8.
- Eismann, L.; Ledderose, S.T.; Enzinger, B.; Berg, E.; Westhofen, T.; Rodler, S.; Schulz, G.B.; Toms, J.; Holzgreve, A.; Gildehaus, F.J.; et al. [68Ga]Ga-FAPI-46 PET/CT for penile cancer – a feasibility study. Eur. J. Nucl. Med. 2024, 1–4, . [CrossRef]
- Clark PE, Spiess PE, Agarwal N, Biagioli MC, Eisenberger MA, Greenberg RE, et al. Penile cancer: Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2013;11[5]):594-615.
- Campbell, R.A.; Slopnick, E.A.; Ferry, E.K.; Zhu, H.; Kim, S.P.; Abouassaly, R. Disparity between pre-existing management of penile cancer and NCCN guidelines. Urol. Oncol. Semin. Orig. Investig. 2017, 35, 531.e9–531.e14, . [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).