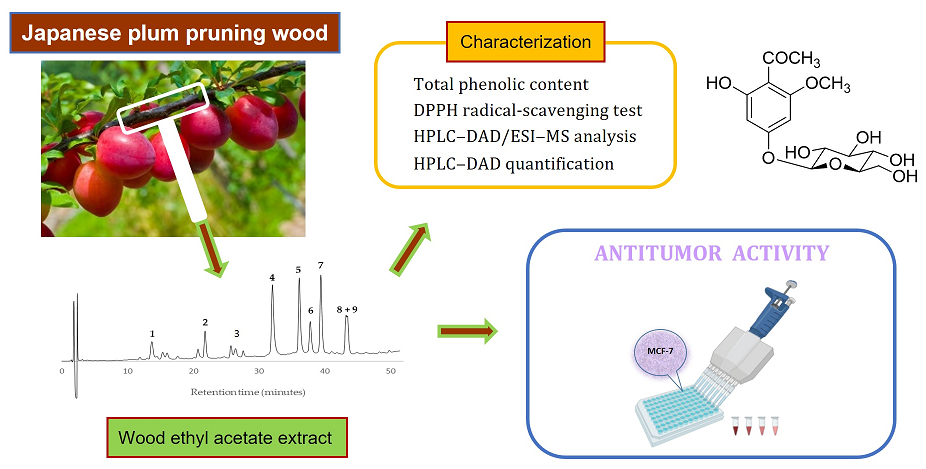
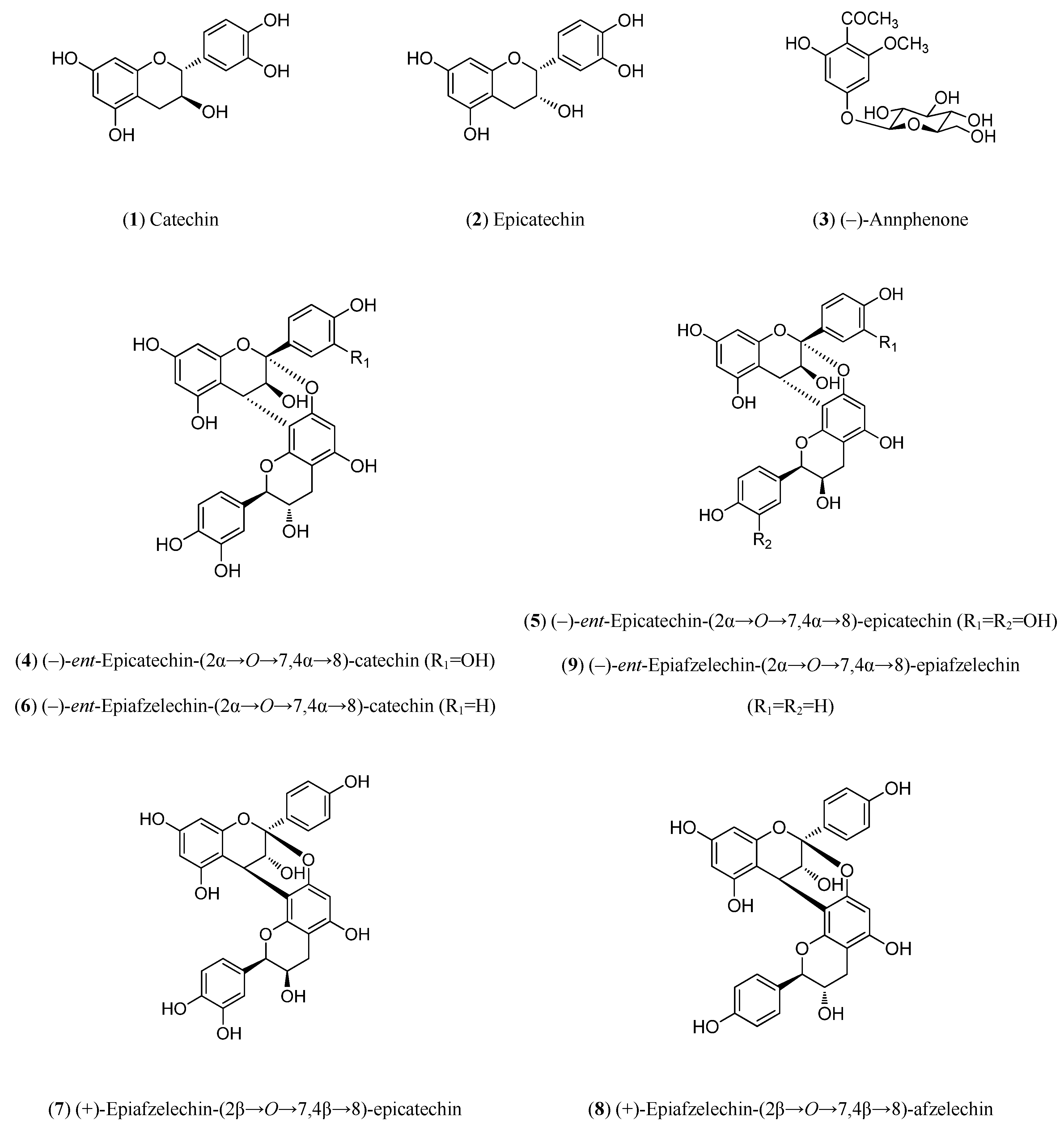1. Introduction
Historically, the use of natural preparations, like infusions from plants or ointments, to alleviate symptoms produced by human diseases has been closely linked with society. Later on, a wide number of studies have reported their effective effects to treat several diseases and, hence, to play an important role in drug discovery. This is evidenced by the large number of known drugs with a natural origin and proving the high interest about live organism as inexhaustible sources of bioactive compounds [
1,
2]. The agricultural activity produces tons of waste around the world, some of them really interesting as pruning. Currently, this agricultural by-product shows some uses as biomass in order to produce energy, organic fertilizers after a fermentation process and as vegetation cover to soil protection from erosion. In addition to above, this cheap and valuable raw material could be a source of bioactive molecules as has been reported before [
3]. Within this research topic, our group has shown some experience on valorisation of crops and pruning wood residues [
4,
5,
6,
7,
8,
9].
Japanese plum (
Prunus salicina Lindl.,
Rosaceae), notwithstanding the name, is considered to originate from China. However,
P. salicina was introduced to the USA from Japan adopting the above name [
10]. Japanese plum crops are located in temperate areas, and also subtropical, showing a worldwide commercial significance. Fresh plum production in the world exceeded 12 million metric tons in 2021, according to FAO [
11], although these statistics show jointly production data of European plum (
Prunus domestica L.) and Japanese plum. China is the largest producer of plums in the world, well ahead of Romania, Serbia, Italy and Spain, which is among the largest plum producers in Europe [
12]. Numerous Japanese plum cultivars are currently known due to farming improvement, such as ‘Angeleno’, ‘Fortune’, ‘Red Beaut’ or ‘Friar’, amongst many others [
10].
Plums are a highly nutritive fruit containing carbohydrates, fiber, minerals, vitamins, carotenoids and phenolic compounds [
13,
14]. Among phenolic composition, there are hydroxycinnamic acid derivatives and flavonoids, such as anthocyanins, flavonols, flavanones, flavan-3-ols and B-type procyanidins [
15,
16,
17]. In addition, some authors have reported many healthy effects related with plum consumption, such as anticancer and antiinflammatory activity, cardiovascular protective or antiallergic action [
14,
18,
19]. Despite the high interest of fruit from this deciduous tree, the number of works focused on chemical composition from other organs, such as heartwood, bark or leaves [
20], is very limited.
Thus, the aims of this work were to carry out the chemical characterization of eight pruning wood samples of Prunus salicina Lindl, belonging to cultivars ‘Songold’, ‘Angeleno’, ‘Fortune’, ‘Red Beaut’, ‘Souvenir’ and ‘Showtime’ (by total phenolic content, antioxidant assays, HPLC–DAD and HPLC–DAD/ESI–MS analyses), and to evaluate the antitumor activity of their extracts and some pure compounds by in vitro assays. This is the first time that the wood of Japanese plum tree has been chemically studied and their extracts evaluated in terms of antiproliferative activity.
2. Results and Discussion
2.1. Sampling Collection and Extractions of Woods
Eight samples of pruning wood of Japanese plum (
P. salicina) of six cultivars were collected on the same day during pruning works in Southern Spain (
Table 1). Seven of which came from the experimental plot of Centro IFAPA “Las Torres-Tomejil”, Seville province, and another one from a farming plot located in Cordoba province. The extractions were carried out following an efficient method used previously in numerous occasions by us [
6]. It consists in two extractions at reflux for 2 hours, firstly, with dichloromethane (DCM) and, subsequently, with ethyl acetate (EtOAc). The purpose of the extraction with DCM is to eliminate non-polar compounds and not interesting in the work with phenolic antioxidants, prior to extraction with EtOAc. All samples showed yields with DCM (0.1–0.7%) lower than with EtOAc (0.6–1.5%) (
Table 1). Focusing on EtOAc extracts, the cultivar ‘Showtime’ (1.5%,
Ps8) showed the highest yield followed by ‘Red Beaut’ (1.2%,
Ps6). These extraction percentages were around other pruning samples previously studied by us [
6,
8,
21], but lower than a sample of European plum (
P. domestica L.), cultivar ‘De la Rosa’ [
7], which showed the highest value in our laboratory.
2.2. Determination of Total Phenolic Content (TPC) and Antioxidant Activity of Prunus salicina Wood Ethyl Acetate Extracts
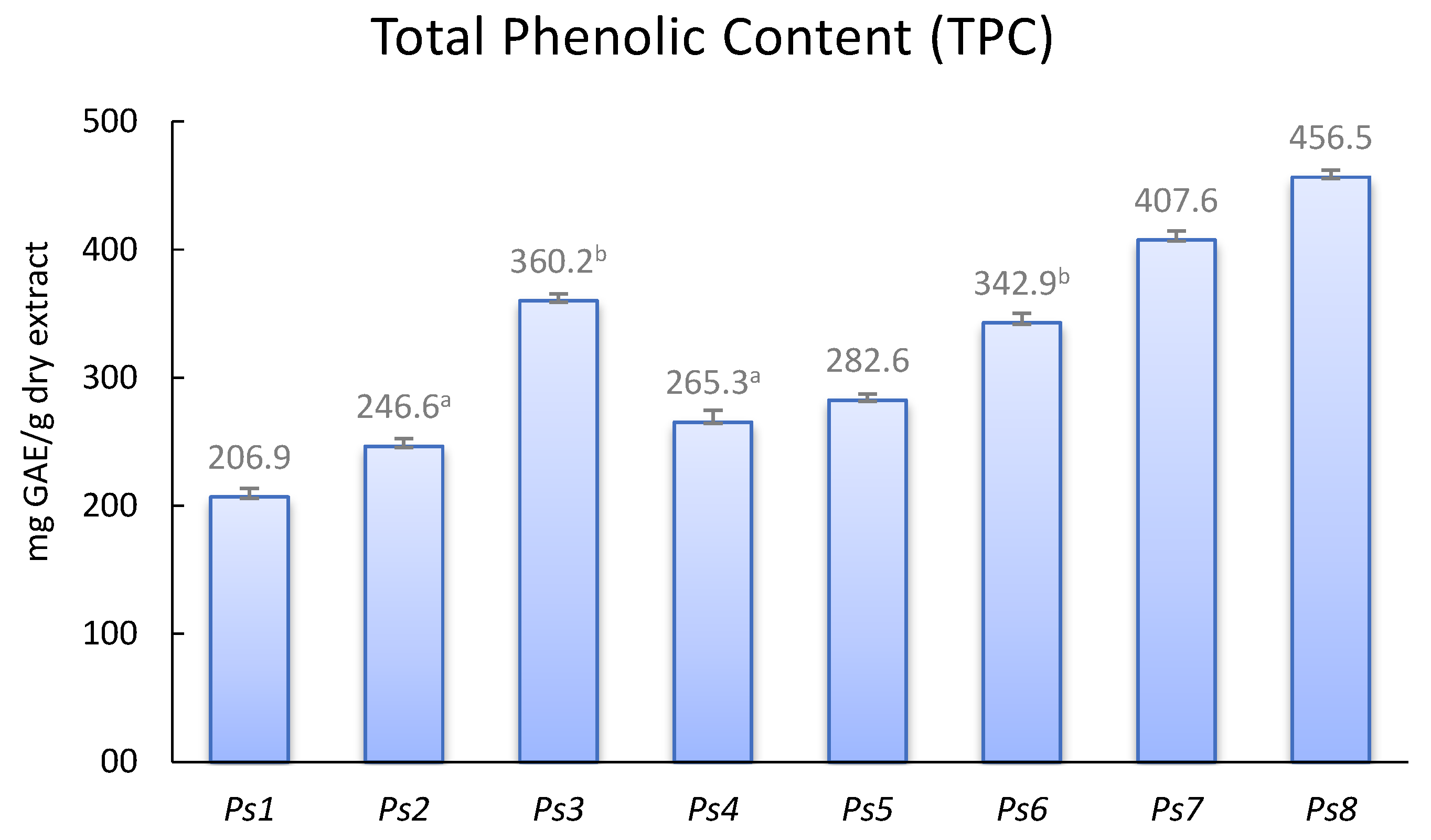
The evaluation of total phenolic content (TPC) and antioxidant activity of EtOAc extracts was carried out as a first approach to characterize the plum wood extracts. TPC was achieved using the Folin-Ciocalteu colorimetric methodology (
Figure 1). The highest TPC value (456.5 mg GAE/g dry extract) corresponded to cv. ‘Showtime’ (Ps8) and the lowest one to cv. ‘Songold’ (Ps1). Focusing on the three samples of cv. ‘Angeleno’, the highest TPC number (362.2 mg GAE/g dry extract) was shown by sample grown under organic management (Ps3), while Ps2 and Ps4, collected from a conventional farm, showed similar values (246.6 and 265.3 mg GAE/g dry extract, respectively). In addition, these values were in the order of those found in woods of the European species (Prunus domestica L.) analysed by us [
9]. Japanese plum woods also showed similar TPC than wood extracts obtained from apricot tree (Prunus armeniaca L.) [
22]. However, our extracts reveals higher TCP content that stem and wood extracts from Prunus avium L. [
23,
24].
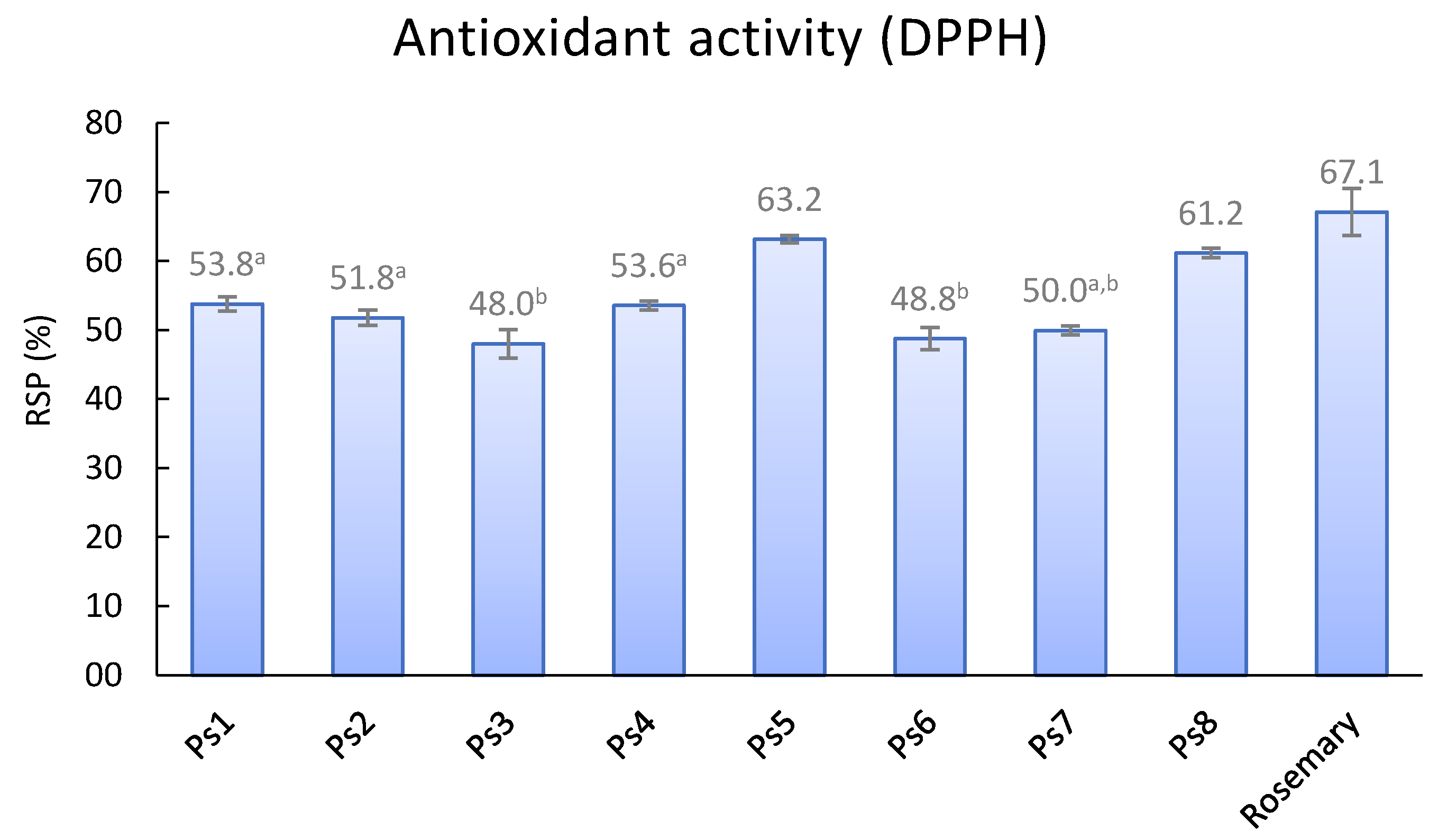
The antioxidant activity of wood EtOAc extracts was determined by the widely used DPPH-scavenging assay. The radical scavenging percentages (RSP) values from ethyl acetate extracts were ranged between 63.2% (Ps5) and 48.0% (Ps3) (
Figure 2). The RSP results for the three samples of cv. ‘Angeleno’ (Ps2, Ps3 and Ps4) showed similar antioxidant activity. A sample of a commercial rosemary (Rosmarinus officinalis L.) extract was evaluated (67.1%) for comparison purposes, showing that all wood extracts had lower activities. Comparing with our previous work on P. domestica wood [
9], Japanese plum samples were as antioxidant as European plum samples, except P. domestica cv. ‘De la Rosa’, which showed the highest percentage (72.9%) of all of them.
2.3. Identification of Components in Prunus salicina Wood Ethyl Acetate Extracts
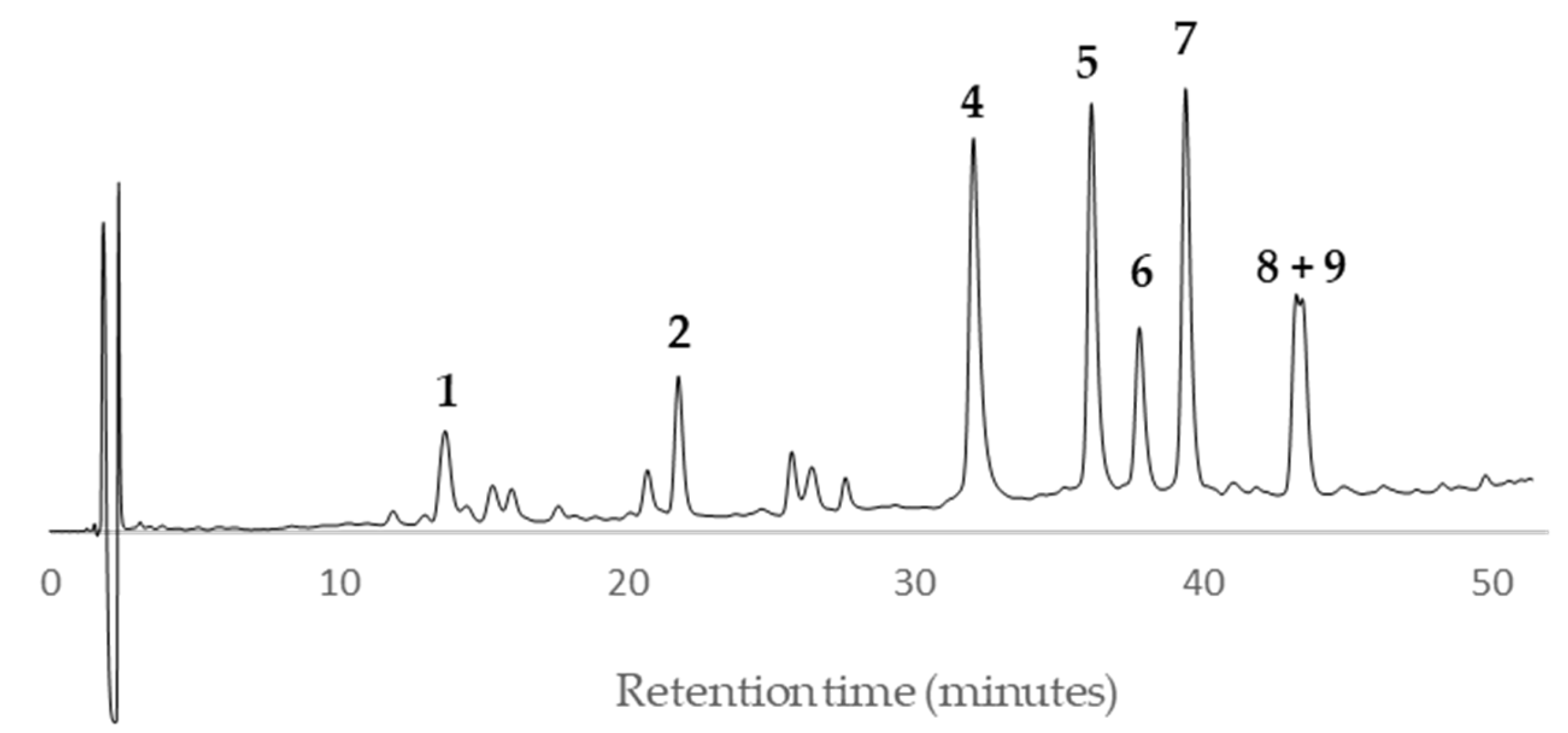
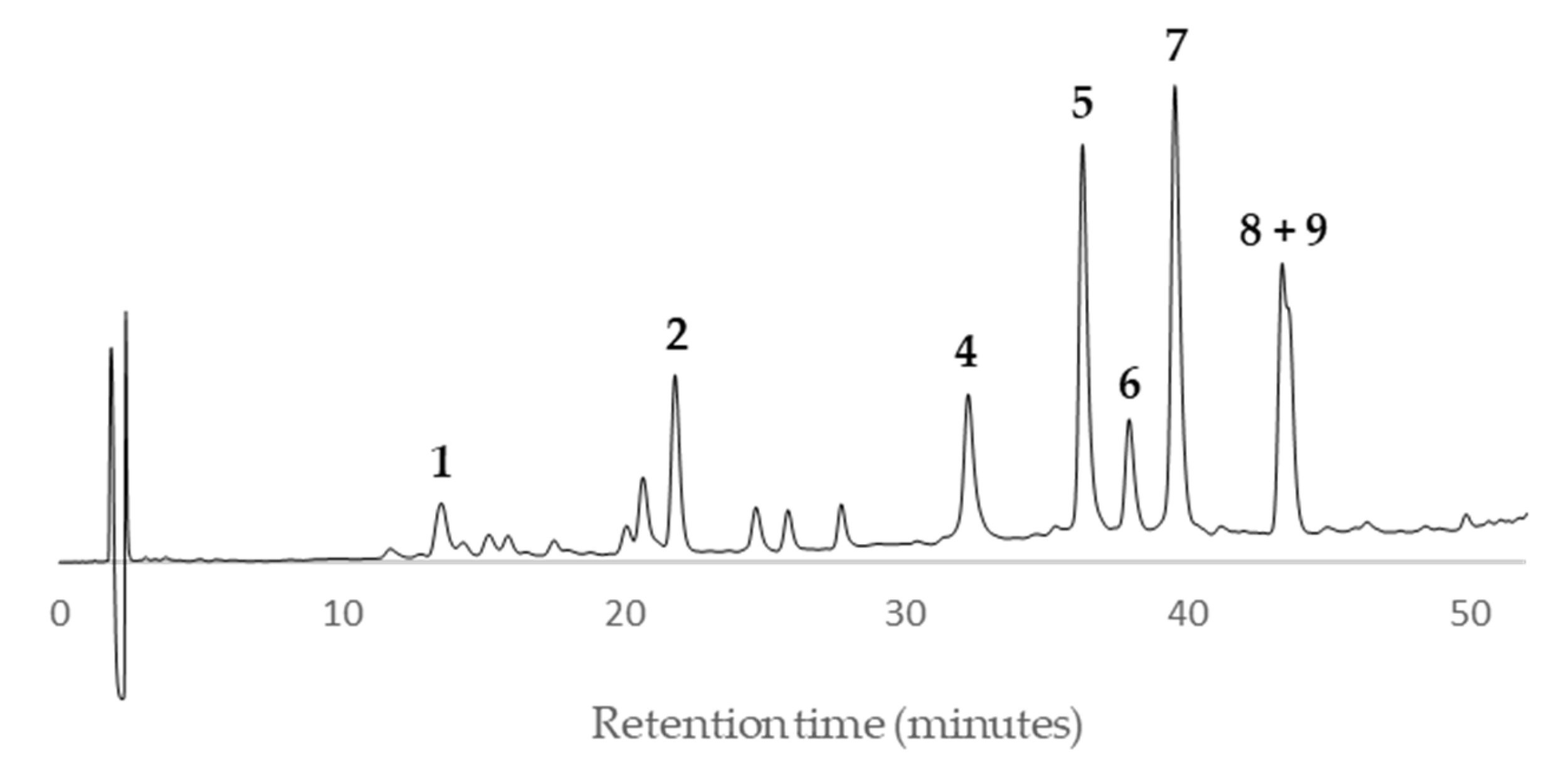
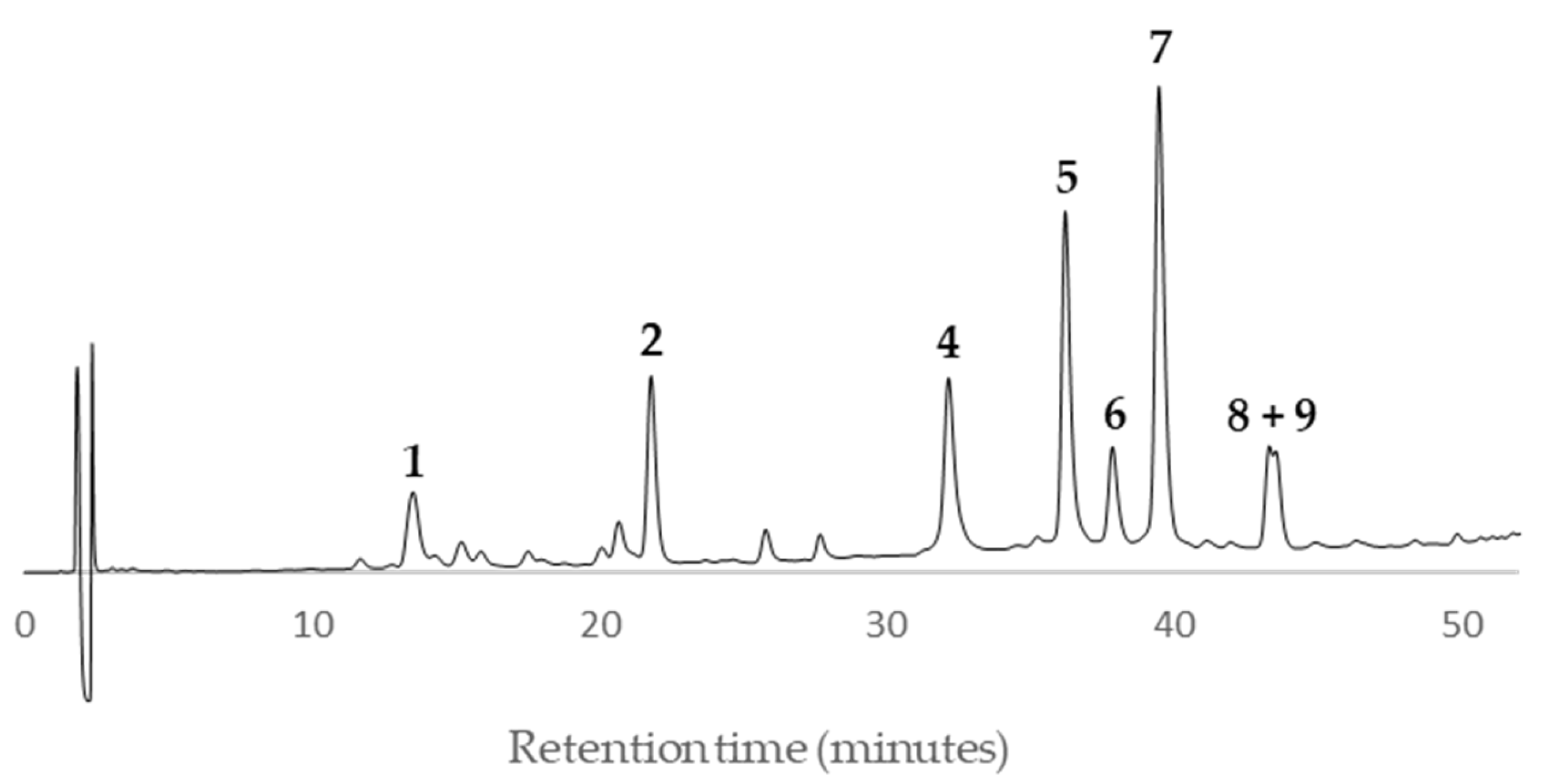
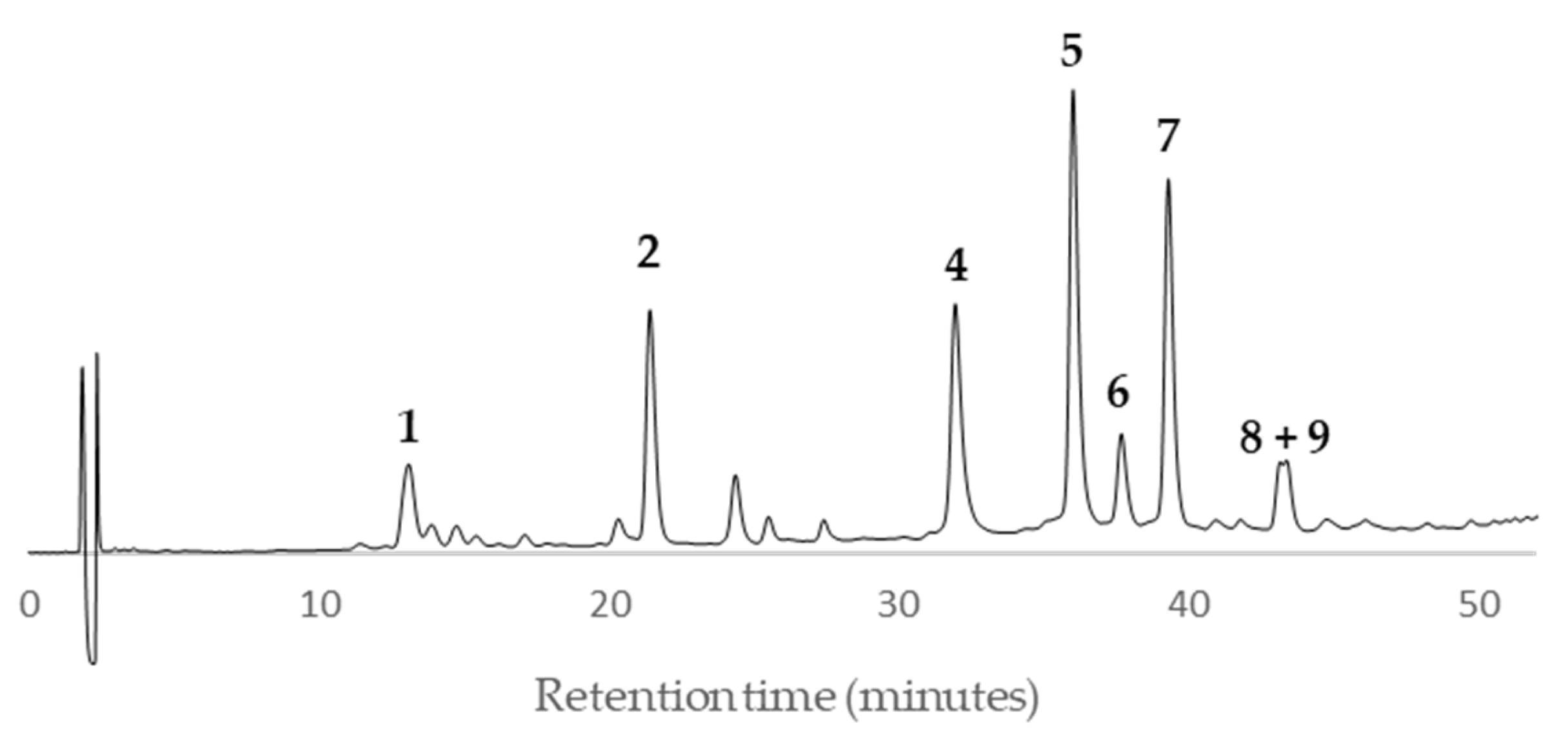
Peaks
1 and
2 showed a quasi-molecular ion peak [M–H]
– at
m/z 288.6 according to the flavan-3-ol (epi)catechin, which was confirmed comparing their HPLC retention time (13.8 and 21.4, respectively) and UV spectrum with standards of (–)-catechin and (–)-epicatechin (
Figure 11). The rest of main peaks (
4–
9) showed similar MS/MS fragmentation pattern, UV spectra and HPLC retention times (
Table 2) than compounds isolated and fully characterized by us from a wood EtOAc extract of European plum (
P. domestica L.) cv. ‘De la Rosa’ [
7]. It allowed us to identify two procyanidins (
4 and
5,
Figure 11) and four propelargonidins (
6–
9,
Figure 11) in present Japanese plum wood samples. In addition, a low quantity of the phenolic glucoside (–)-annphenone (
3) was identified in the studied extracts, which was isolated from
P. domestica L. as well [
7].
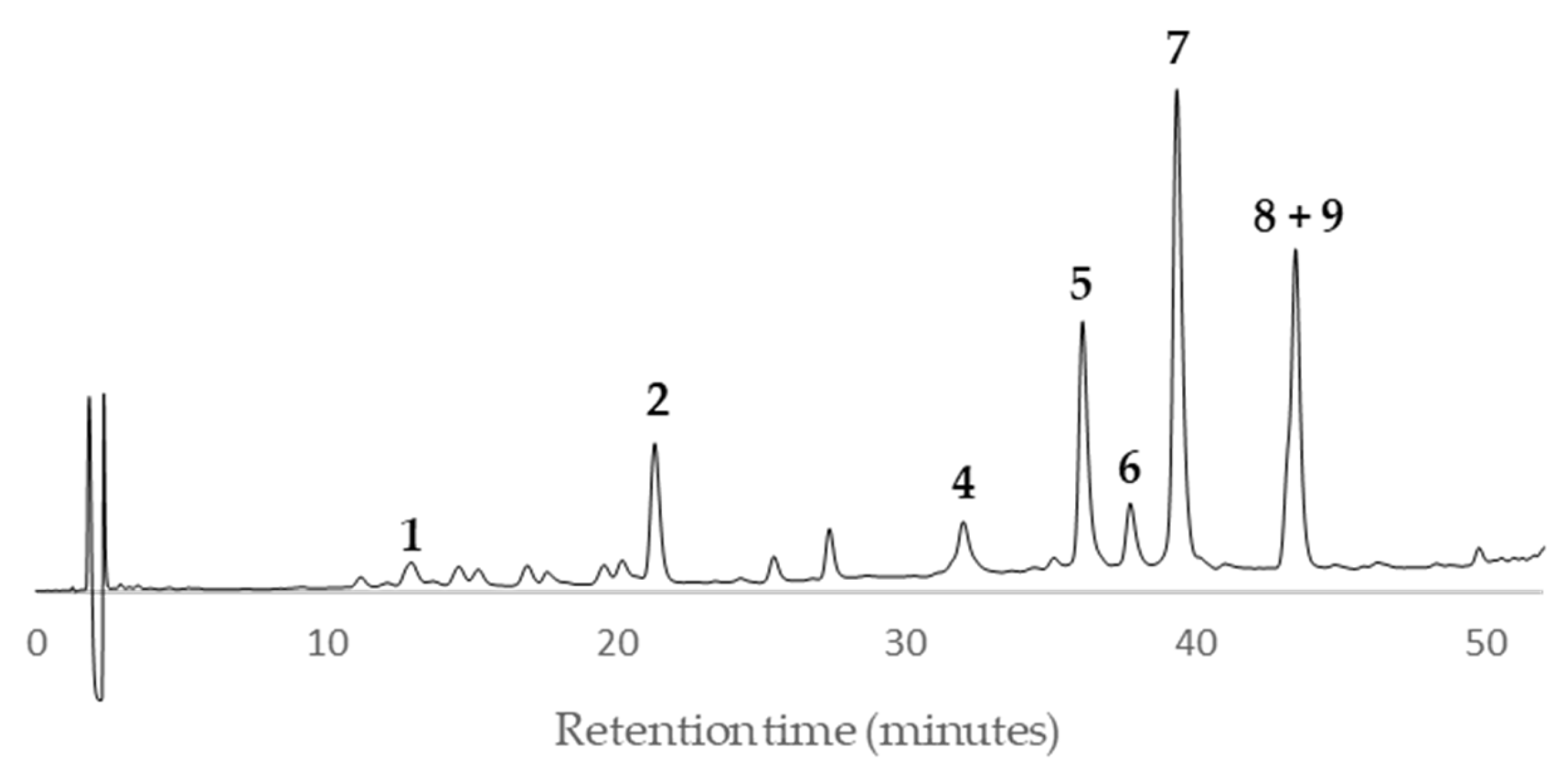
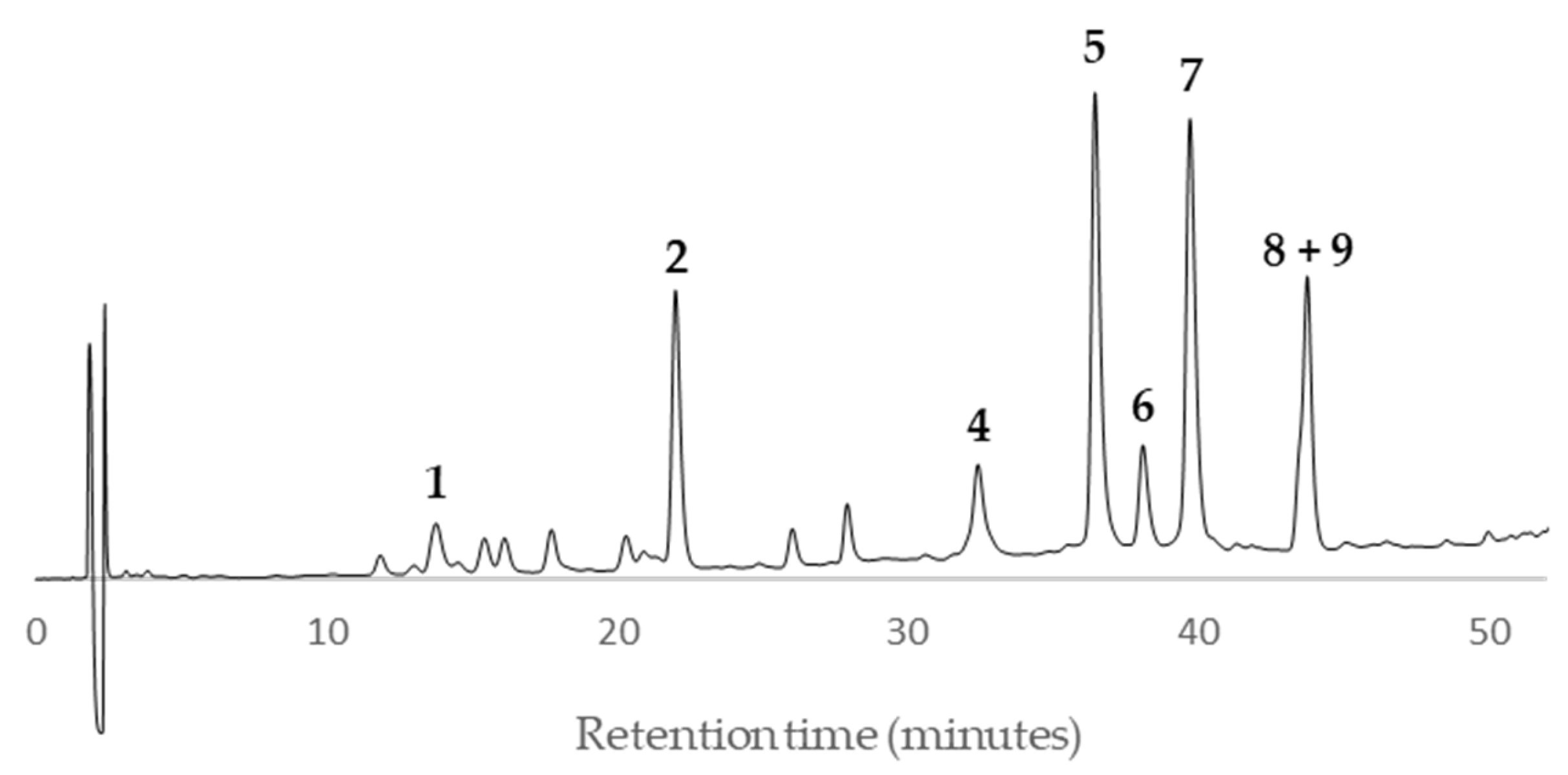
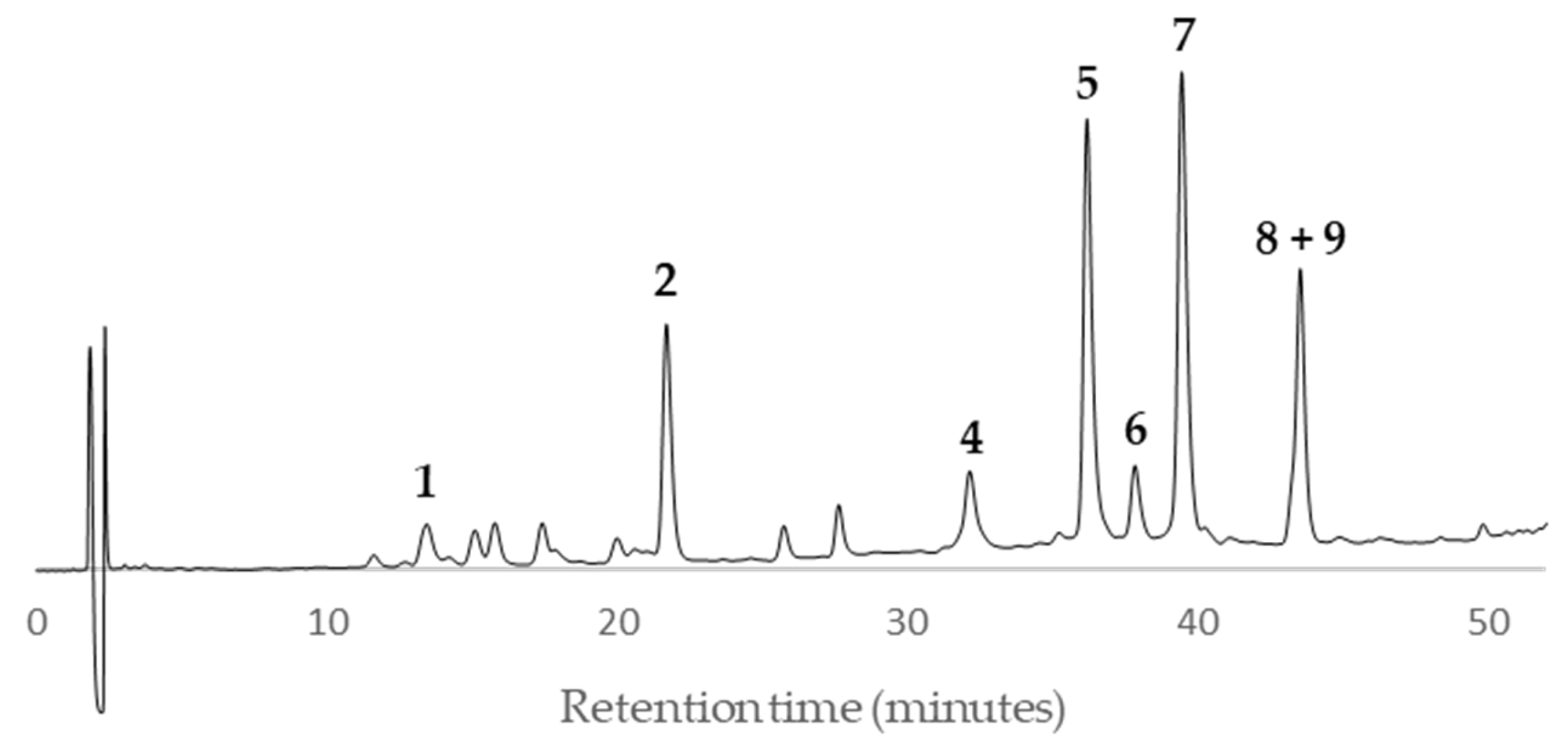
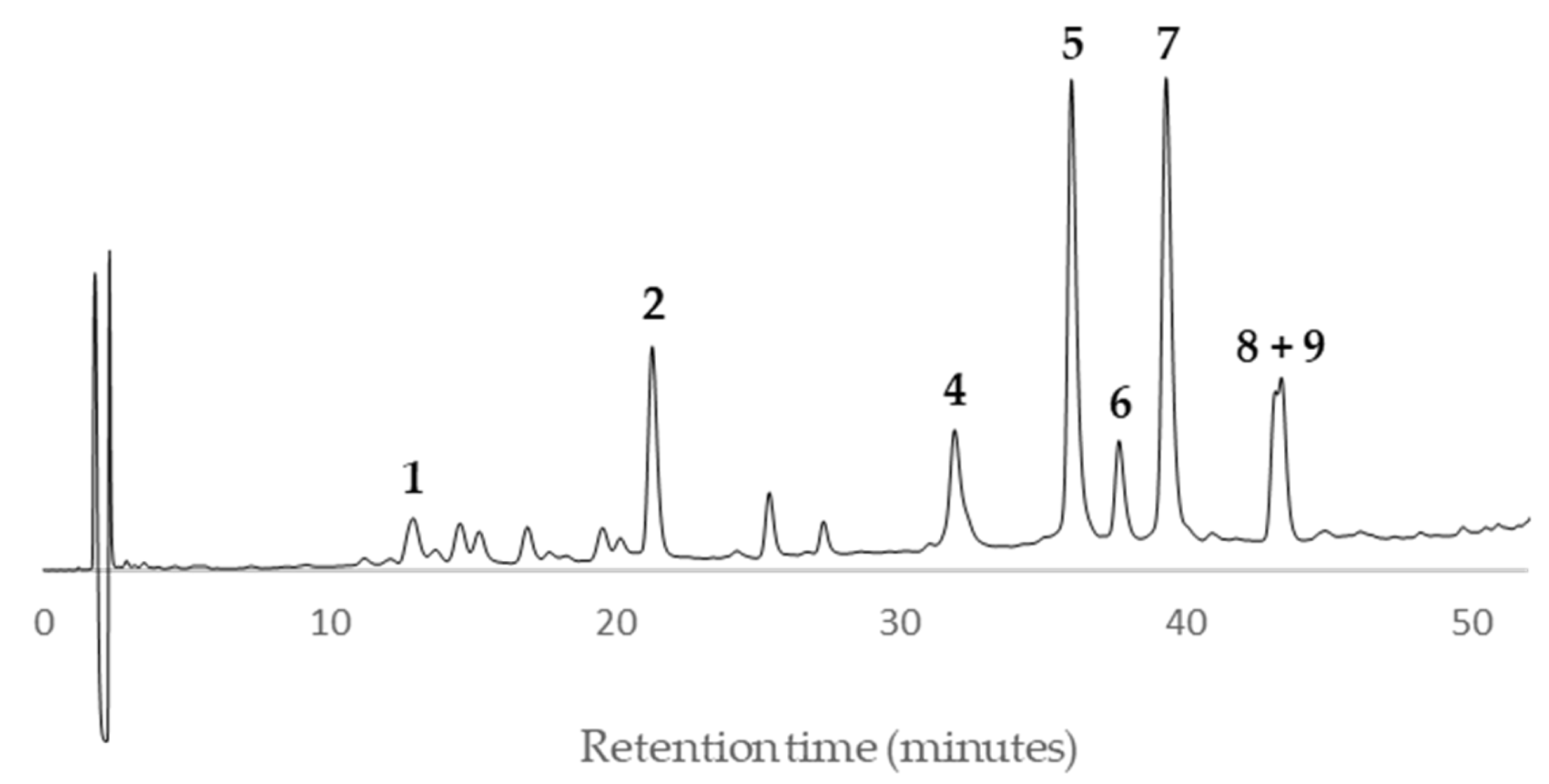
According to the HPLC profiles (
Figure 3,
Figure 4,
Figure 5,
Figure 6,
Figure 7,
Figure 8,
Figure 9 and
Figure 10), the major peaks in all extracts were
2,
5,
7,
8 and
9. In addition, cultivar ‘Songold’ (
Ps1) showed the peak
4 among the highest ones. On the other hand, a significant intensity of peak
1 only was detected in cultivars ‘Songold’ (
Ps1), ‘Red Beaut’ (
Ps6), ‘Souvenir’ (
Ps7) and ‘Showtime’ (
Ps8).
2.4. Antiproliferative Activity of Prunus salicina Wood Ethyl Acetate Extracts and Components
The antiproliferative activities of eight Japanese plum wood extracts (Ps1–Ps8) together with that of four pure compounds present in those extracts (
3–
6) were evaluated in MCF-7 cells after 48 h of induction (
Table 4). Both extracts and compounds seemed to inhibit cellular proliferation in a dose-response manner and just two of the compounds (
4,
6) did not affect the viability of tumor cells at 200 ppm. The most effective extracts at 200 ppm were Ps6 and Ps7, which decreased the cell population by 39.4% and 40.1%, respectively. The best antitumoral activities of pure compounds at 200 ppm corresponded to
3 and
5 (28.8% and 19.2% of reduction, respectively) and regarding the IC
50, the lowest values corresponded to compound
3 and extract Ps1. In a similar manner, a study reported in the literature showed that plum (fruit) extracts were able to reduce the cell viability of two types of breast tumor cells, including MCF-7, and their effects were also concentration-dependent [
32].
3. Materials and Methods
3.1. Chemicals
Solvents as dichloromethane (DCM) and ethyl acetate (EtOAc) used for extractions, and acetonitrile (ACN) for high-performance liquid chromatography (HPLC) analyses, were purchased from VWR (Barcelona, Spain). Ultrapure water used for HPLC analyses was produced by a Milli-Q-Water (1.8 MΩ) equipment (Merck, KGaA, Darmstadt, Germany). (–)-Catechin (99% of purity by HPLC) used for quantification purposes was isolated from
Prunus avium L. wood [
6]. Procyanidin A-2 (98% of purity by HPLC) used as standard was purchased from Extrasynthese (Genay, France). 2,2-Diphenyl-1-picrylhydrazyl radical (DPPH
•) used for radical-scavenging activity was purchased from Sigma-Aldrich Chemie (Steinheim, Germany). Rosemary extract used as reference was purchased from Evesa (Cádiz, Spain). Folin-Ciocalteau reagent used in total phenolic content determination was purchased from Merck Chemicals (Darmsdadt, Germany).
3.2. Plant Material Collection and Extraction
Eight samples (Ps1–Ps8) of pruning wood from P. salicina Lindl. cultivars (‘Songold’, ‘Angeleno’, ‘Fortune’, ‘Red Beaut’, ‘Souvenir’ and ‘Showtime’) were collected in October 2015 during the pruning works of this fruit tree in the Centro IFAPA “Las Torres-Tomejil”, Alcalá del Río, province of Seville, Spain, and in a farming plot (“Finca La Veguilla”; N: 37º 49’ 13.94’’ W: 4º 54’ 12.38’’) located in Gualdalcázar, province of Córdoba, Spain. Both orchards were located in the Guadalquivir River Valley, Southern Spain. Collection works was supervised by Dr. Francisco T. Arroyo (Researcher at the Centro IFAPA “Las Torres-Tomejil”, Spain) who confirmed the identify and origin of each wood sample. They consisted in pieces of leafless wood (with a diameter of 0.8–1.4 cm). Plant material was dried at room temperature and chipped before using. Samples were extracted with DCM and, then, with EtOAc (
Scheme 1).
3.3. Total Phenolic Content (TPC) and Antioxidant Activity
The total phenolic content (TPC) value of each EtOAc extract was determined by the Folin-Ciocalteu colorimetric method [
33] using gallic acid as standard. The calibration curve (y = 0.0103x + 0.1393, r
2 = 0.9981) was performed recording absorbances at 760 nm from methanolic solutions (25–100 µg/mL) of gallic acid on a Varian Cary 4000 spectrophotometer [
9]. Briefly, 0.6 mL of an aqueous solution of Folin-Ciocalteu reagent (0.2 N), and 0.12 mL of methanolic solutions of each ethyl acetate extracts (at 10 μg/mL) were added to a 1-cm path length semi-micro cuvette. The mixture was incubated in the dark for 5 min at room temperature. Next, 0.48 mL of an aqueous solution of Na
2CO
3 (7.5 g/L) was added, shaken and incubated again for 1 h in the dark at room temperature. Finally, the absorbance at 760 nm was determined. Results were obtained as mg of Gallic acid equivalent (GAE) per gram of dry extract. Three replicates of each sample were measured.
The antioxidant activity of each EtOAc wood extract was achieved by the DPPH radical-scavenging method [
9]. Briefly, 0.4 mL of methanolic solutions (50 µg mL
–1) of EtOAc extracts were mixed with 0.8 mL of a methanolic solution of DPPH radical (7.09×10
–5 M) in semi-micro cuvettes, shaken and kept in dark for 15 minutes at room temperature. In the case of blank, the mixture was prepared with 0.8 mL of DPPH radical and 0.4 mL of MeOH. Next, the absorbance was measured at 515 nm on a Varian Cary 4000 spectrophotometer. A calibration curve (y = 0.1114x + 0.020, r
2 = 0.9919) was used to determine the concentration of DPPH radical in each solution. Three replicates of each sample were measured.
Antioxidant activity results were expressed as radical-scavenging percentage (RSP) and were determined using the following formula [
9]:
where A
B is the absorbance of the blank (t=0 min) and A
A is the absorbance of the evaluated solution (t=15 min).
3.4. Analyses by HPLC–DAD and HPLC–DAD/ESI–MS of EtOAc Extracts
All EtOAc extracts (Ps1–Ps8) were analysed by HPLC–DAD using the same instrument described by us [
6]. The mobile phase consisted in mixtures of ACN (solvent A) and H
2O (solvent B), both with 0.2% AcOH, at a flow of 0.7 mL/min. The optimal separation was achieved with the following gradient method: 0.0–40.0 min, 5–20% A; 40.0–45.0 min, 20–25% A; 45.0–60.0 min, 25–40% A; 60.0–62.0 min, 40–5% A. Ethyl acetate extracts were analysed by HPLC–DAD/ESI–MS, using an instrument and analysis conditions described previously by us [
7]. The mobile phase consisted in mixtures of ACN (solvent A) and H
2O (solvent B), both with 0.2% AcOH, at a flow of 0.25 mL/min. The optimal separation was achieved with the following gradient method: 0.0–22.5 min, 5–20% A; 22.5–27.5 min, 20–25% A; 27.5–32.5 min, 25–40% A; 32.5–33.5 min, 40–5% A. MS parameters were described before by us [
5].
3.5. Quantification of Phenolics Compounds in EtOAc Extracts
The quantification of identified compounds in EtOAc extracts was achieved by an external standard method using their HPLC peak areas at 230 nm as previously reported by us [
7]. Calibrations curves were made using solutions (0.1–1 mg/mL in methanol) of (–)-catechin [
6] and a commercial standard of procyanidin A-2, and correlating the area value of each peak with its concentration [
7]. The concentration of each compound was expressed as milligrams of compound per gram of dry wood (DW).
3.6. Cell Lines and Culture
The human breast adenocarcinoma line MCF-7 (ECACC 86012803) was obtained from Cell Cultures Unit of the University of Granada (Spain). Cells were cultured at 37 °C and 5% CO2 with humidified atmosphere, using DMEM supplemented with 10% FBS, 10 mL/L penicillin-streptomycin 100× and 2 mM L-glutamine.
3.7. In Vitro Antiproliferative Assays
MCF-7 cells were seeded in sterile 96-well plates (Thermo Fisher Scientific, Denmark) at high density (1.5 x 10
4 cells/well) and incubated for 24 h to allow cell adhesion. Increasing concentrations of all extracts and compounds were added in the corresponding wells and incubated for 48 h. The effect on cells viability was evaluated using a colorimetric technique with sulforhodamine-B (SRB) [
34]. Optical density values were determined in a microplate reader (Multiskan EX, Thermo Electron Corporation) at 450 nm. The assessment of absorbance was obtained using "SkanIt" RE 5.0 for Windows v.2.6 (Thermo Labsystems, USA) and a regression analysis was carried out with the Statgraphics software (Statistical Graphics Corp, 2000, USA). The IC
50 values were calculated from the semi-logarithmic dose–response curve by linear interpolation. All assays were performed in duplicate.
3.8. Statistical Analysis
The results are expressed as mean values ± SD. A statistical analysis of the data was carried out using GraphPad Prism version 5.00 for Windows (GraphPad Software, La Jolla, California, USA). Statistical significative differences among measurements were determined by a one-way analysis of variance (ANOVA) with Tukey’s test (p<0.05).
4. Conclusions
Pruning wood of Japanese plum tree (Prunus salicina Lindl.) could be an excellent natural source of A-type proanthocyanidins, the cultivar ‘Showtime’ showing the higher amount among all studied samples. The characterization of the wood extracts of six plum cultivars was performed by total phenolic content and DPPH radical-scavenging assays and by HPLC-DAD and HPLC-DAD/ESI-MS analyses. It allowed for the identification of flavan-3-ols catechin (1) and epicatechin (2), the phenolic glucoside annphenone (3), and six A-type proanthocyanidins (4–9). After quantification of components by an external standard method, proanthocyanidins 5 and 7 were the major components in most of the plum cultivars. Regarding the antiproliferative activity in MCF-7 breast tumor cells activity of both wood extracts and pure compounds 3–6, most of the samples evaluated were able to achieve a reduction of up to 40% in the cell population with concentrations of 200 ppm. These results highlight the potential use of pruning wood from P. salicina as a renewable, cheap and abundant source of bioactive compounds.
Author Contributions
Conceptualization, S.S. and J.A.; methodology, J.O.-V., J.M.T. and N.M.-S.; formal analysis, S.S. and J.A.; investigation, J.O.-V.; resources, J.O.-V. and N.M.-S.; writing—original draft preparation, J.O.-V. and N.M.-S.; writing—review and editing, S.S., J.M.T. and J.A.; supervision, S.S. and J.A.; project administration, S.S. and J.A.; funding acquisition, S.S. and J.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially supported by the Centro de Instrumentación Científico-Técnica (CICT) of the University of Jaén, Spain.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Authors wish to thank Dr. Francisco T. Arroyo (Researcher at the Centro IFAPA “Las Torres-Tomejil”, Seville, Spain) for providing most of the plant material used in this work.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Atanasov, A. G.; Zotchev, S. B.; Dirsch, V. M.; Supuran, C. T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Aliaño-González, M.J.; Gabaston, J.; Ortiz-Somovilla, V.; Cantos-Villar, E. Wood waste from fruit trees: Biomolecules and their applications in agri-food industry. Biomolecules 2022, 12, 238. [Google Scholar] [CrossRef]
- Pérez-Bonilla, M.; Salido, S.; van Beek, T.A.; Altarejos, J. Radical-scavenging compounds from olive tree (Olea europaea L.) wood. J. Agric. Food Chem. 2014, 62, 144–151. [Google Scholar] [CrossRef]
- Alejo-Armijo, A.; Tello-Abolafia, A.; Salido, S.; Altarejos, J. Phenolic compounds in laurel wood: A new source of proanthocyanidins. J. Wood Chem. Technol. 2019, 39, 436–453. [Google Scholar] [CrossRef]
- Ortega-Vidal, J.; Cobo, A.; Ortega-Morente, E.; Gálvez, A.; Alejo-Armijo, A.; Salido, S.; Altarejos, J. Antimicrobial and antioxidant activities of flavonoids isolated from wood of sweet cherry tree (Prunus avium L.). J. Wood Chem. Technol. 2021, 41, 104–117. [Google Scholar] [CrossRef]
- Ortega-Vidal, J.; Cobo, A.; Ortega-Morente, E.; Gálvez, A.; Martínez-Bailén, M.; Salido, S.; Altarejos, J. Antimicrobial activity of phenolics isolated from the pruning wood residue of European plum (Prunus domestica L.). Ind. Crops Prod. 2022, 176, 114296. [Google Scholar] [CrossRef]
- Alejo-Armijo, A.; Ortega-Vidal, J.; Salido, S.; Altarejos, J. Recovery and seasonal variation of cinnamtannin B-1 from laurel (Laurus nobilis L.) pruning wood wastes. Chem. Biodiversity 2022, 19, 1–13. [Google Scholar] [CrossRef]
- Ortega-Vidal, J.; Ruiz-Martos, L.; Salido, S.; Altarejos, J. Proanthocyanidins in pruning wood extracts of four European plum (Prunus domestica L.) cultivars and their hLDHA inhibitory activity. Chem Biodivers. 2023, 20, e202200931. [Google Scholar] [CrossRef]
- Topp, B.L.; Russell, D.M.; Neumüller, M.; Dalbó, M.A.; Liu, W. Plum. In Fruit Breeding, Handbook of Plant Breeding; Badenes, M.L., Byrne, D.H., Eds.; Springer: New York, 2012; Volume 8, pp. 571–621. [Google Scholar]
- FAOSTAT, FAO. Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/faostat/es/#data/QCL (accessed on 6 May 2024).
- FAOSTAT, FAO. Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/faostat/es/#rankings/countries_by_commodity (accessed on 6 May 2024).
- Poonam, V.; Kumar, G.; Reddy, C. S.; Jain, R.; Sharma, S. K.; Prasad, A. K.; Parmar, V. S. Chemical constituents of the genus Prunus and their medicinal properties. Curr. Med. Chem. 2011, 18, 3758–3824. [Google Scholar] [CrossRef]
- Igwe, E. O.; Charlton, K. E. ; A systematic review on the health effects of plums (Prunus domestica and Prunus salicina). Phytother. Res. 2016, 30, 701–31. [Google Scholar] [CrossRef]
- Jaiswal, R.; Karaköse, H.; Rühmann, S.; Goldner, K.; Neumüller, M.; Treutter, D.; Kuhnert, N. Identification of phenolic compounds in plum fruits (Prunus salicina Lindl. and Prunus domestica L.) by high-performance liquid chromatography/tandem mass spectrometry and characterization of varieties by quantitative phenolic fingerprints. J. Agric. Food Chem. 2013, 61, 12020–12031. [Google Scholar] [CrossRef]
- Venter, A.; Joubert, E.; De Beer, D. Characterisation of phenolic compounds in South African plum fruits (Prunus salicina Lindl.) using HPLC coupled with diode-array, fluorescence, mass spectrometry and on-line antioxidant detection. Molecules 2013, 18, 5072–5090. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Li, W.; You, B.; Yang, S.; Xian, W.; Deng, Y.; Huang, W.; Yang, R. Phenolic profiles, bioaccessibility and antioxidant activity of plum (Prunus Salicina Lindl). Food Res. Int. 2021, 143, 110300. [Google Scholar] [CrossRef]
- Liu, W.; Nan, G.; Nisar, M. F.; Wan, C. Chemical constituents and health benefits of four Chinese plum species. J. Food Qual. 2020, 2020, 842506. [Google Scholar] [CrossRef]
- Bahrin, A. A.; Moshawih, S.; Dhaliwal, J. S.; Kanakal, M. M.; Khan, A.; Lee, K. S.; Goh, B. H.; Goh, H. P.; Kifli, N.; Ming, L. C. Cancer protective effects of plums: A systematic review. Biomed. Pharmacother. 2022, 146, 112568. [Google Scholar] [CrossRef]
- Delgado-Adámez, J.; Fernández-León, M. F.; Velardo-Micharet, B.; González-Gómez, D. In vitro assays of the antibacterial and antioxidant activity of aqueous leaf extracts from different Prunus salicina Lindl. cultivars. Food Chem. Toxicol. 2012, 50, 2481–2486. [Google Scholar] [CrossRef]
- Salido, S.; Pérez-Bonilla, M.; Adams, R. P.; Altarejos, J. Phenolic components and antioxidant activity of wood extracts from 10 main Spanish olive cultivars. J. Agric. Food Chem. 2015, 63, 6493–6500. [Google Scholar] [CrossRef] [PubMed]
- Bruno, M.R.; Russo, D.; Faraone, I.; D'Auria, M.; Milella, L.; Todaro, L. Orchard biomass residues: Chemical composition, biological activity and wood characterization of apricot tree (Prunus armeniaca L.). Biofuels, Bioprod. Bioref. 2021, 15, 377–391. [Google Scholar] [CrossRef]
- Sanz, M.; Cadahía, E.; Esteruelas, E.; Muñoz, A. M.; Fernández De Simón, B.; Hernández, T.; Estrella, I. Phenolic compounds in cherry (Prunus avium) heartwood with a view to their use in cooperage. J. Agric. Food Chem. 2010, 58, 4907–14. [Google Scholar] [CrossRef] [PubMed]
- Ademović, Z.; Hodžić, S.; Halilić-Zahirović, Z.; Husejnagić, D.; Džananović, J.; Šarić-Kundalić, B.; Suljagić, J. Phenolic compounds, antioxidant and antimicrobial properties of the wild cherry (Prunus avium L.) stem. Acta Period. Technol. 2017, 48, 1–13. [Google Scholar] [CrossRef]
- Ramos, P.A.B.; Pereira, C.; Gomes, A.P.; Neto, R.T.; Almeida, A.; Santos, S.A.O.; Silva, A.M.S.; Silvestre, A.J.D. Chemical characterisation, antioxidant and antibacterial activities of Pinus pinaster Ait. and Pinus pinea L. bark polar extracts: Prospecting forestry by-products as renewable sources of bioactive compounds. Appl. Sci. 2022, 12, 784. [Google Scholar] [CrossRef]
- Monagas, M.; Garrido, I.; Lebrón-Aguilar, R.; Bartolome, B.; Gómez-Cordovés, C. Almond (Prunus dulcis (Mill.) D.A. Webb) skins as a potential source of bioactive polyphenols. J Agric Food Chem. 2007, 21, 8498–507. [Google Scholar] [CrossRef]
- Ma, Y.; Kosińska-Cagnazzo, A.; Kerr, W. L.; Amarowicz, R.; Swanson, R. B.; Pegg, R. B. Separation and characterization of phenolic compounds from dry-blanched peanut skins by liquid chromatography-electrospray ionization mass spectrometry. J. Chromatogr. A 2014, 1356, 64–81. [Google Scholar] [CrossRef]
- Neilson, A. P.; O'Keefe, S. F.; Bolling, B. W. High-molecular-weight proanthocyanidins in foods: Overcoming analytical challenges in pursuit of novel dietary bioactive components. Annu. Rev. Food Sci. Technol. 2016, 7, 43–64. [Google Scholar] [CrossRef] [PubMed]
- Howell, A. B. Bioactive compounds in cranberries and their role in prevention of urinary tract infections. Mol. Nutr. Food Res. 2007, 51, 732–737. [Google Scholar] [CrossRef]
- Maffei, M.E.; Salata, C.; Gribaudo, G. Tackling the future pandemics: Broad-spectrum antiviral agents (BSAAs) based on A-type proanthocyanidins. Molecules 2022, 27, 8353. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Zhao, L.; Wang, K.; Renard, C.M.G.C.; Le Bourvellec, C.; Hu, Z.; Liu, X. A-Type proanthocyanidins: Sources, structure, bioactivity, processing, nutrition, and potential applications. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13352. [Google Scholar] [CrossRef]
- Vizzotto, M.; Porter, W.; Byrne, D.; Cisneros-Zevallos, L. Polyphenols of selected peach and plum genotypes reduce cell viability and inhibit proliferation of breast cancer cells while not affecting normal cells. Food Chem 2014, 164, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Jr Rossi, J.A. Colorimetry to total phenolics with phosphomolybdic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–58. [Google Scholar] [CrossRef]
- Vichai, V.; Kirtikara, K. Sulforhodamine B Colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).