1. Introduction
Onchocerciasis, a parasitic infection caused by the nematode
Onchocerca volvulus and transmitted by the small biting fly
Simulium, is a major public health problem due to the irreversible complications (blindness, debilitating itching) that result [
1]. It is one of the 20 neglected tropical diseases (NTDs) to be eliminated by 2030 [
1,
2]. According to the World Health Organization (WHO)[
4], the vast majority of the 26 million people infected with onchocerciasis worldwide (99%) live in sub-Saharan Africa [
3,
4].
The advent of vector control programs implemented since 1974 in West Africa, and later associated with mass treatment campaigns with Ivermectin implemented in the 1990s, have improved the socio-economic conditions of populations through the elimination of the disease as a public health problem in some countries [
4,
5]. This ivermectin-based treatment initially provided by mobile teams is now led by the community in an approach called community-directed treatment (CDTI) [
4].
However, some experts believe that it will not be possible to eliminate onchocerciasis in Africa with mass treatment with Ivermectin alone because of the more complex epidemiology in thios region compared with Latin America [
6]. Indeed, the outbreaks in Latin America were localized with low to moderate endemicity and the vectors were less effective in transmitting infection compared to those in Africa. This could explain why the results of several years of CDTI appear more effective in sub-Saharan Africa. For example, studies in Niger, Senegal, Nigeria and Ethiopia have shown that at least 15 years of mass treatment with Ivermectin were required to interrupt
O. volvulus transmission and achieve prevalence rates of zero [
7,
8,
9]. Similarly, other studies, in Cameroon and Togo, showed that more than 15 years of CDTI failed to interrupt onchocerciasis transmission[
10,
11,
12,
13]. Several hypotheses have been put forward, including insufficient coverage of the population eligible for CDTI and maintenance of the infectious focus through human or vector migration.
Almost the entire country of Burkina Faso was endemic to onchocerciasis, with a prevalence of 60-80%, except for the extreme north. [
14]. Onchocerciasis control was mainly based, as elsewhere in West Africa, on vector control using larvicides from 1974 until the 1990s. Ivermectin treatment was used in the Bougouriba basin in 1996 and in the Nakambé and Sissili basins from 1992 to 1998 with the aim of controlling resurgence [
4,
14]. Onchocerciasis control efforts thus brought the prevalence of
O. volvulus to a level where the disease was no longer a public health problem and a national onchocerciasis control program was set up in 1991 to maintain the gains made. In addition, starting in 2000, Burkina Faso implemented mass distribution of Ivermectin to control lymphatic filariasis which was scaled up starting in 2004 [
14], covering all health districts in the country.
A resurgence of onchocerciasis cases has been observed in two regions (Comoé, South West) located around several river basins in the 2010s [
14,
15,
16]. In accordance with WHO guidelines, for the management of resurgent cases, CDTI was implemented in the affected areas of Comoé and Sud-Ouest regions [
17,
18,
19]. Five years after the start of CDTI in these regions, epidemiological surveys were conducted to assess progress in controlling the resurgence of the disease. Taking advantage of the pre-CDTI survey and the last epidemiological survey, the objective of this study was to evaluate the effects of five years of CDTI on the parasitological indices of onchocerciasis. The study is unique in that it concerns CDTI conducted in the context of resurgence control, unlike other studies conducted in other sub-Saharan African countries that were based on initial disease control interventions [
8,
17,
18]. Furthermore, this resurgence occurred despite the use of Ivermectin for lymphatic filariasis control and therefore the efficacy of CDTI in this context is questionable.
2. Methods
2.1. Study Design
This was a before-and-after intervention study without control groups in the Cascades region (before, 2011, and after, 2016) and the Southwest region (before, 2013, and after, 2018) of Burkina Faso.
2.2. Study Setting
Burkina Faso, a country located in West Africa, borders Côte d’Ivoire, Mali, Ghana, Togo, Benin and Niger (see
Figure 1). It had a population of 20,487,979 in 2019, of which more than three-quarters live in rural areas and 40% below the poverty line [
20]. In addition, 56.2% of the population are farmers and/or skilled workers in agriculture, forestry or fishing [
21].
Climatically, Burkina Faso is divided into 3 zones: a Sahelian zone which is located in the north of the country, a Sudanese-Sahelian zone running from the east to the north and including the center of the country and a Sudanese zone located in the southwest [
22]. The vegetation is much more abundant in the Sudanian zone of the country where the two onchocerciasis endemic regions are located.
The country is made up of numerous rivers, the main ones being the Mouhoun, Nakambé and Nazinon. River Mouhoun, the main tributary of the Volta River, has a total area of 91,036 km2 which runs through Burkina Faso. Its main tributaries include the Poni, Bougouriba, Grand Balé, Vranso and Sourou rivers. River Nakanbé is also a tributary of the Volta River with 81,932 km2 of its basin surface area in Burkina Faso. Its main tributaries are the Sissili, Nazinon, Pendjari and Nouhao rivers. Other perennial rivers exist in Burkina Faso, including the river Comoé, which extends over Mali, Burkina Faso and Côte d’Ivoire. The Burkina Faso portion of the riverbasin spreads over the provinces of Comoé, Léraba, Houet, Kénédougou and Poni. It includes five sub-basins: Lerabah, Comoé, Kodoun, Baoué and Iringou.
Onchocerciasis endemic regions are crossed by some of these tributaries and basins. In the South-West region, the districts where onchocerciasis are endemic are crossed by the Mouhoun (Bas Mouhoun) and Bougouriba rivers. As for the Cascades region, the 2 endemic districts are crossed by the Comoe river.
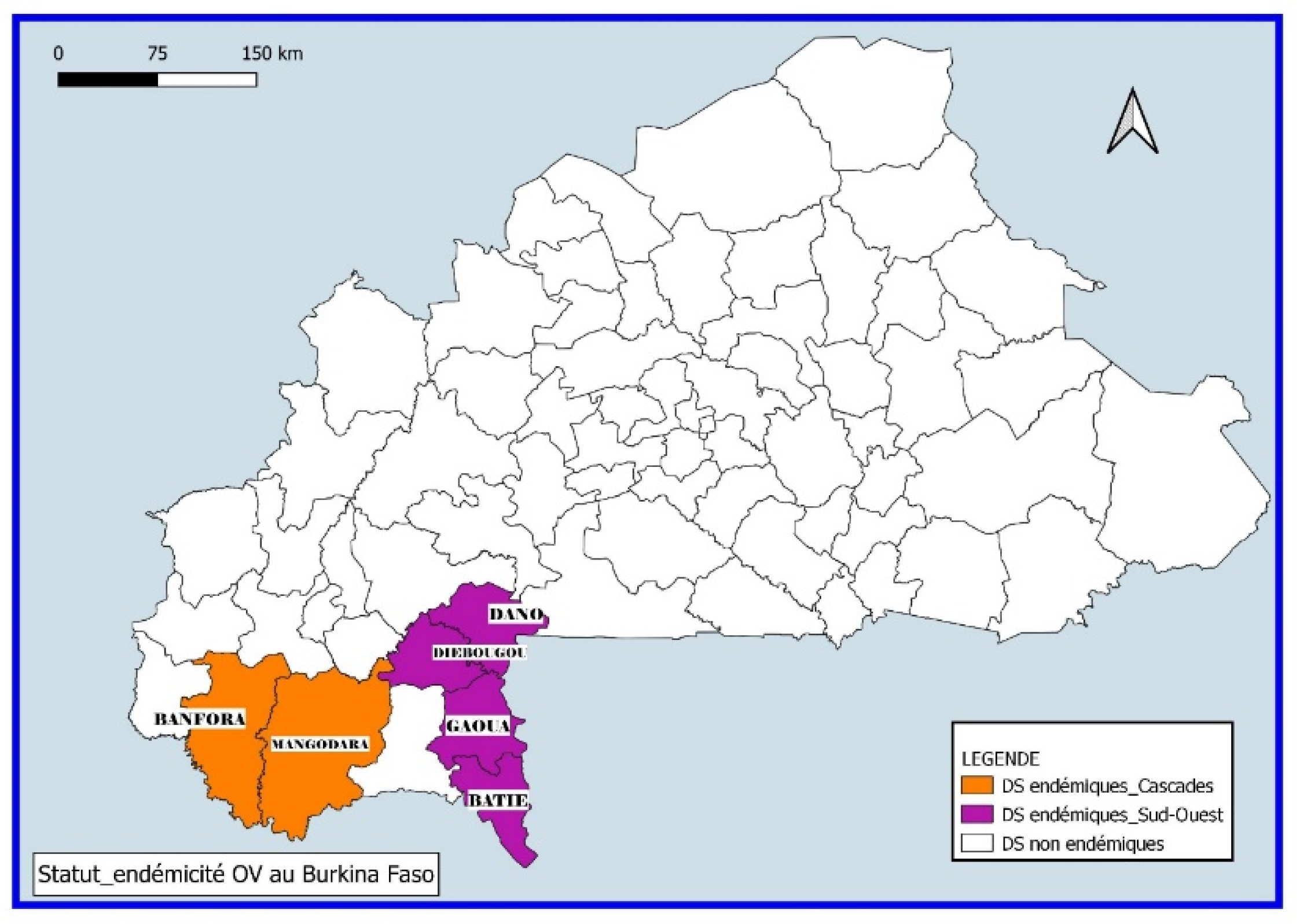
Only the South-West and Cascades regions out of the 13 regions of the country are endemic to onchocerciasis with districts having an endemicity threshold above 5% [
23]. The endemic districts are those of Gaoua, Batié, Diébougou and Dano (South-West region) and Banfora and Mangodara (Cascades region).
2.3. Specific Framework
2.3.1. Implementation of the CDTI by the National Program for the fight against NTDs in Burkina Faso
Implementation of treatment for onchocerciasis under community guidelines began in 2011 and 2013 for the Cascades and Southwest regions respectively (see
Figure 2).
In accordance with WHO guidelines, the primary strategy for onchocerciasis control is to interrupt the cycle of disease transmission by distributing ivermectin under community guidelines to individuals at risk of
Onchocerca volvulus infection. Administered orally, once or twice a year, ivermectin can reduce the burden of
Onchocerca volvulus microfilariae to levels below those required for effective transmission by the black fly
Simulium [
24].
Implementation of the CDTI was resumed in 2011 and 2013 in the Cascades and South-West regions respectively. After the 2010 (Cascades region) and 2011 (South-West region) epidemiological assessments showed a high prevalence of the disease, the CDTI was repeated in 2011 for the Cascades and in 2013 for the South-West region. CDTI is implemented biannually in both regions except in the years when an epidemiological survey is conducted (2016 for the Cascades and 2018 for the Southwest). For the study periods, 10 and 9 rounds of CDTI were conducted respectively in the endemic districts of the Cascades region and the Southwest region by the NTDP of the Ministry of Health.
The CDTI is a strategy implemented by communities and evaluated by community monitors. During CDTI campaigns, community-based distributors are responsible for administering treatment by name in all onchocerciasis-endemic villages. Experience has shown that this method works best if the communities themselves choose how they want to deliver the treatment and who will be responsible for it. Prior to the implementation of each CDTI campaign, the actors are trained in a cascade from the regional level to the community distributors (CDs). The administration of ivermectin is carried out by the CDs under the supervision of those responsible at the health facilities, the districts and the regional teams. Social mobilization is carried out at the regional, health district and community levels in order to encourage greater support from the population.
2.3.2. Epidemiological Surveys Implemented in the Framework of Onchocerciasis Surveillance
Epidemiological surveys are descriptive cross-sectional surveys that aim to determine the prevalence of onchocerciasis in communities. The diagnostic methods used to measure prevalence are direct observation of microfilaria in skin tissue (bloodless skin biopsy) and the search for parasite antigens using the
Onchocerca volvulus OV-16 antigen antibody test (OV-16). The use of rapid tests in epidemiological investigations is a component of the new WHO guidelines for the elimination of onchocerciasis as a public health problem [
24].
In the epidemiological surveys conducted before the resumption of CDTI in both regions in 2011 and 2013, the method used for the assessment of onchocerciasis prevalence was just the examination of exsanguinated skin biopsies. After the implementation of CDTI in both regions, both diagnostic methods (skin biopsy and antigen-antibody tests) were used in the epidemiological surveys.
The method for exsanguinated skin biopsy examination is as follows. A 1-2 mg skin flap is taken with Holtz forceps, including the superficial layers of the dermis, and is placed in distilled or physiological water. The emitted microfilariae are observed fresh under the microscope or with a binocular microscope. The reading of the biopsies with a magnifying glass is done 30 minutes after incubation in distilled water. The populations from whom skin biopsies are taken are from villages involved in the surveys with a sample size of 250-300. People are selected in order of arrival until the sample size is reached.
OV16 tests are performed in children aged 2 to 9 years. These tests are based on the detection of IgG4 antibodies reacting against the OV16 parasite antigen in a drop of blood taken from a fingertip. The OV16 tests are read after 20 minutes and again after 24 hours. A sample size of 3000 children are tested for OV16 in the entire region. This sample size is distributed among the villages in the survey area.
Uisng these methodologies, a total of four epidemiological surveys were implemented, two (one in each region) before the resumption of the CDTI and two after five years of CDTI implementation (2016 in Cascades and 2018 in the South West). The general objective of these surveys was to determine the epidemiological situation of onchocerciasis in the endemic villages.
2.4. Data Sources
Aggregate village data were extracted from the national database of epidemiological surveys conducted by the PNMTN in endemic districts. The variables sex and age were excluded from the database because they were not uniformly collected during the baseline epidemiological survey (before the intervention). Also, given that treatment coverage data by village was not availabledistrict therapeutic coverage was attributed to the study villages. To ensure data quality, only data collected according to the official CDTI reporting template set up by the PNMTN were considered for therapeutic coverage.
2.5. Study Population
Inhabitants (aged ≥5 years) residing in endemic villages in the health districts of Banfora (20/23 villages) and Mangodara (5/6) in the Cascades region and Batié (8/10), Gaoua (2/4), Dano (3/6), and Diébougou (5/9) in the Southwest region constituted our study population. Thus, a total of 43 villages were involved in the study. The villages that were selected were those that participated in the epidemiological surveys before the implementation of CDTI (in 2010 and 2011) and after five years of treatment.
2.6. Study Variables
Variables included those of exposure to the intervention taking the value 0 for the period before and 1 for the period after the implementation of CDTI, number of CDTI rounds, average treatment coverage during the 5 years of treatment, distance between the village and the river (coded 1 if the distance <5 km and 0 if ≥5 km). The number of CDTI rounds was the number of CDTI rounds conducted in each area between the two epidemiological surveys. Average treatment coverage was the average treatment coverage of the different CDTI rounds in each district. The epidemiological survey databases provided information by village on the number of people surveyed, the prevalence of microfilarodermia, the standardized prevalence and the microfilaria burden in the community.
2.7. Data Analysis
Data in Excel format (version 2019) were processed and then imported into Stata V.15.2 software (Stata Corporation, CollegeStation, TX, USA) for analysis. We first plotted the observed trajectories of all villages. We then used whisker boxes to examine the observed pre- and post-CDTI prevalence and community microfilarial load (CMFLs) for the entire sample, by region, and by village distances from streams. To assess the effects of CDTI on the prevalence and the community microfilaria burden, we first used a paired t-test. We considered the effects to be different across regions and village distances to rivers. To test these interactions, we used linear random-effects models, which allowed us to adjust standard errors for the dependence of within-village observations. The structure of the residual covariances between the two periods was modeled using unstructured covariance. The analysis was conducted with the mixed command of Stata V.15.2 (StataCorp) and the Kroger method was used to account for the small sample size. Although the interactions were not significant, we presented the results unstratified and stratified by region and then by distance of villages from rivers because this lack of statistical significance could be explained by a lack of statistical power. Finally, we graphically presented the predicted estimates with 95% confidence intervals (CI).
2.8. Ethical Considerations
This study was a secondary analysis of data from the NTDP’s CDTI implementation activities. The data used were anonimized and did not include information that would allow for the identification of respondents. In addition, for all studies related to the elimination of onchocerciasis, the PNMTN had approval from the National Health Research Ethics Committee, signed in 2009. This approval has also been used for the publication of other studies [
25]. However, prior to the implementation of the surveys, the teams sensitized the populations on onchocerciasis and its consequences and on the need to monitor its endemicity. Following this, the participants in the survey gave oral or written consent prior to sampling. The database was kept on the principal investigator’s password-protected computer.
3. Results
3.1. Characteristics of the Villages Surveyed
A total of 43 villages in 6 health districts, i.e., 100% of the villages that participated in the epidemiological surveys before and after the implementation of CDTI in the Southwest and Cascade regions were included in the study. More than half (57%) of the villages were located in the districts of Banfora and Mangodara in the Cascades region. The Banfora district alone accounted for 47% of the villages surveyed.
3.2. Standardized Microfilarodermia Prevalence Rates after Five Years of Treatment in the Study Villages
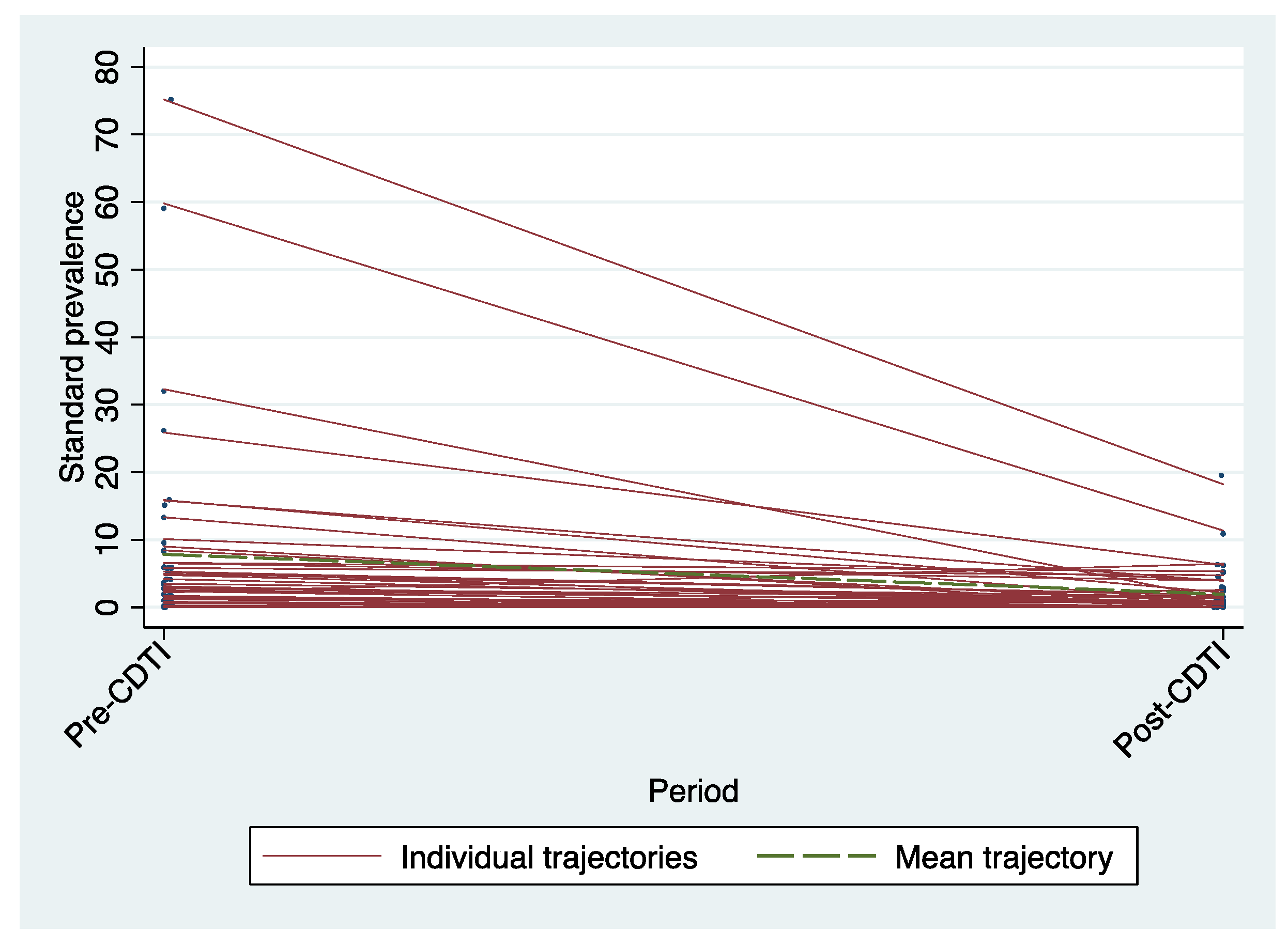
Standardized microfilarodermia prevalence rates by village decreased during the period between treatment resumption and after five years of annual and/or biannual CDTI implementation. The trajectories in the figure below (
Figure 3) showed a significant decline for some villages.
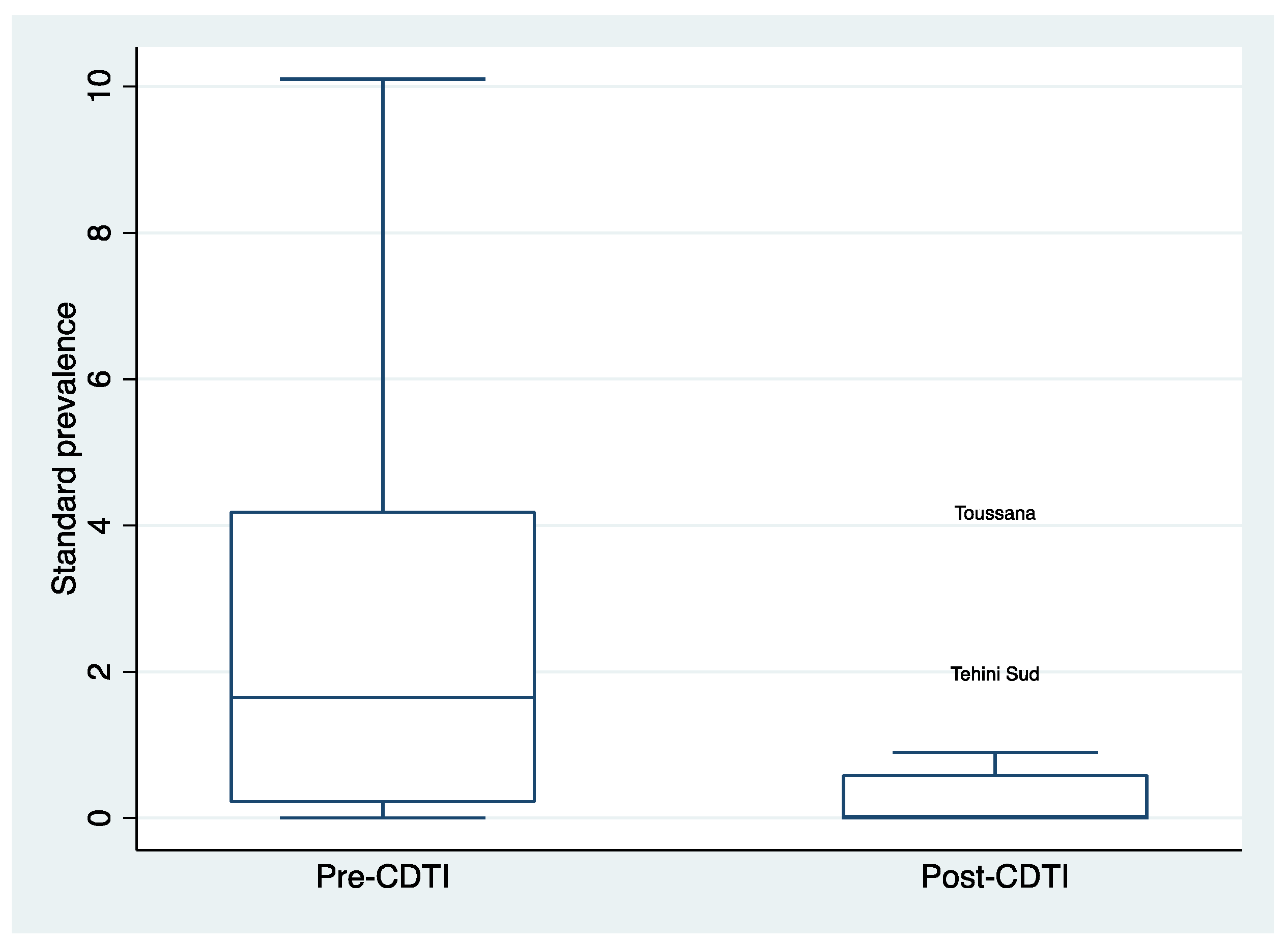
3.3. Distribution of Standardized Microfilaria Prevalence Rates after 5 Years of CDTI by Region
The decline in standardized microfilaria prevalence was also observed in each of the study regions. The distribution of standardised prevalence in villages in each region between the pre-CDTI period and after five years of CDTIimplementation also declined significantly (
Figure 4 and
Figure 5).
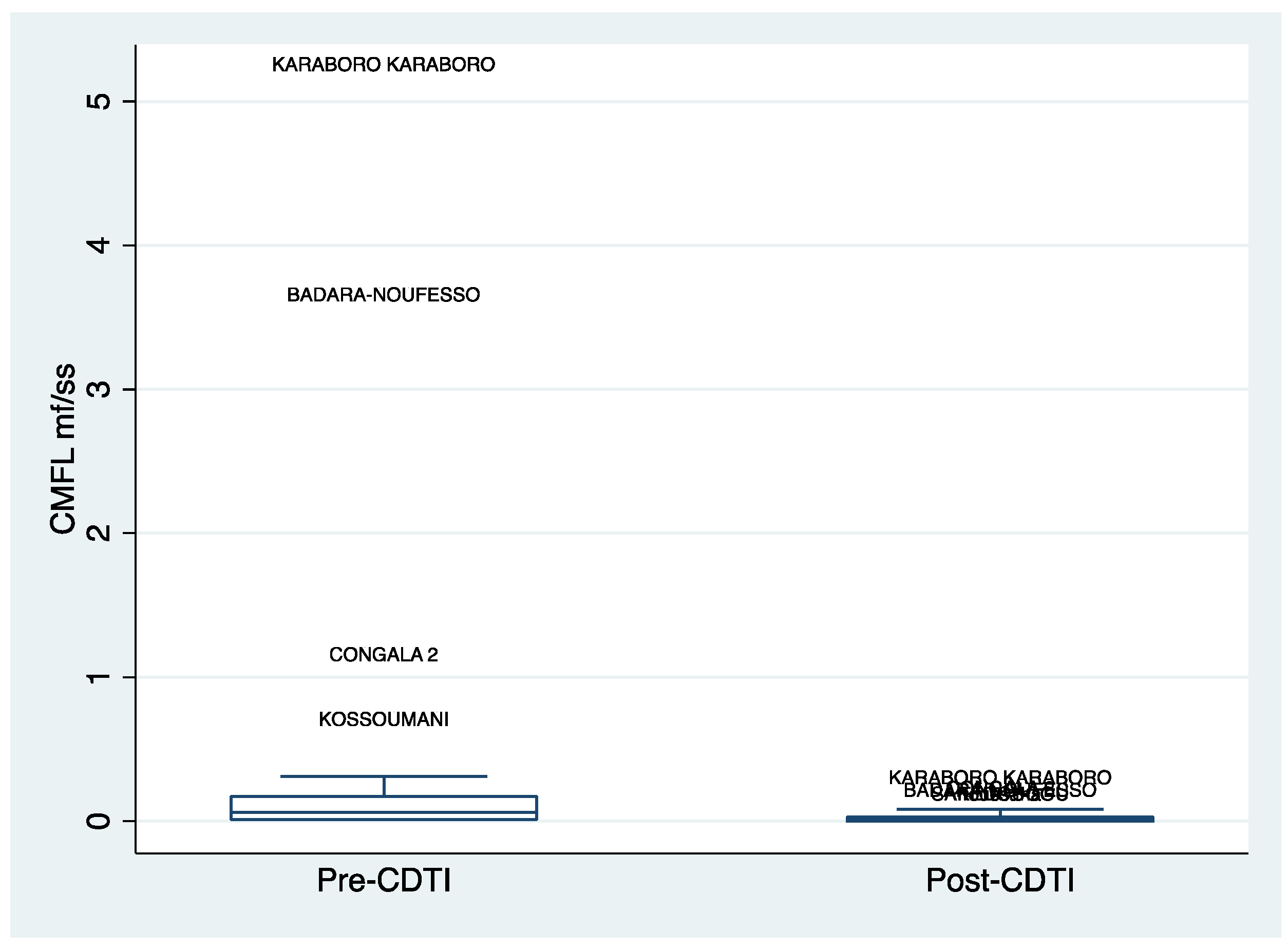
3.4. Distribution of Community Micofilarial Load (CMFL) after 5 Years of CDTI
Similar to the standardized microfilaria prevalence rates, the distribution of the Community Microfilarial Load (CMFL) decreased after the implementation of CDTI (see
Figure 6). This decline was observed in both the Cascades and Southwest regions.
3.5. Parasitological Indices after 5 Years of CDTI Implementation
For all the villages involved in the study, a decrease in the prevalence of standardized and CMFL was observed. However, there was a high standard deviation for villages in the Cascade region and villages that were less than 5 km from rivers, indicating a wide variation in prevalence rates between villages, especially for the baseline survey (
Table 1).
3.6. The Effects of CDTI on Standardized Microfilaria Prevalence and CMFL
The evaluation of the effects of CDTI showed that the significant reduction in standardized prevalence and CMFL could be attributed to the five years of CDTI implementation in both regions. This CIeduction in parasitological indices was observed independently of the distance between the village and the river and was significant. For the average of the two regions, the results for standardized microfilaria prevalence were -5.91 with a 95% confidence interval of (-9.67; -2.16) and for CMFL were -0.29 with a 95% confidence interval of (-0.57; -0.01) (see
Table 2).
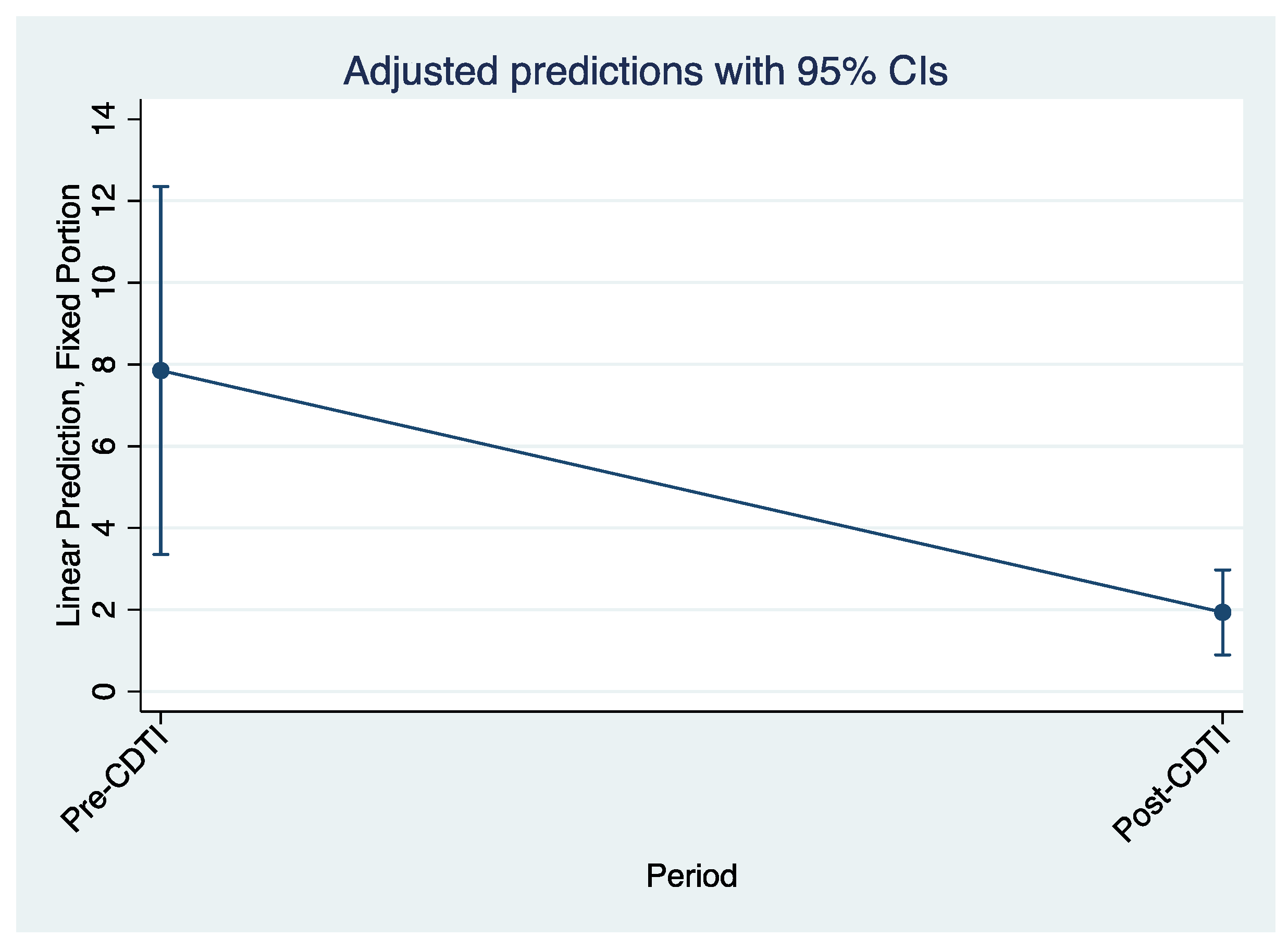
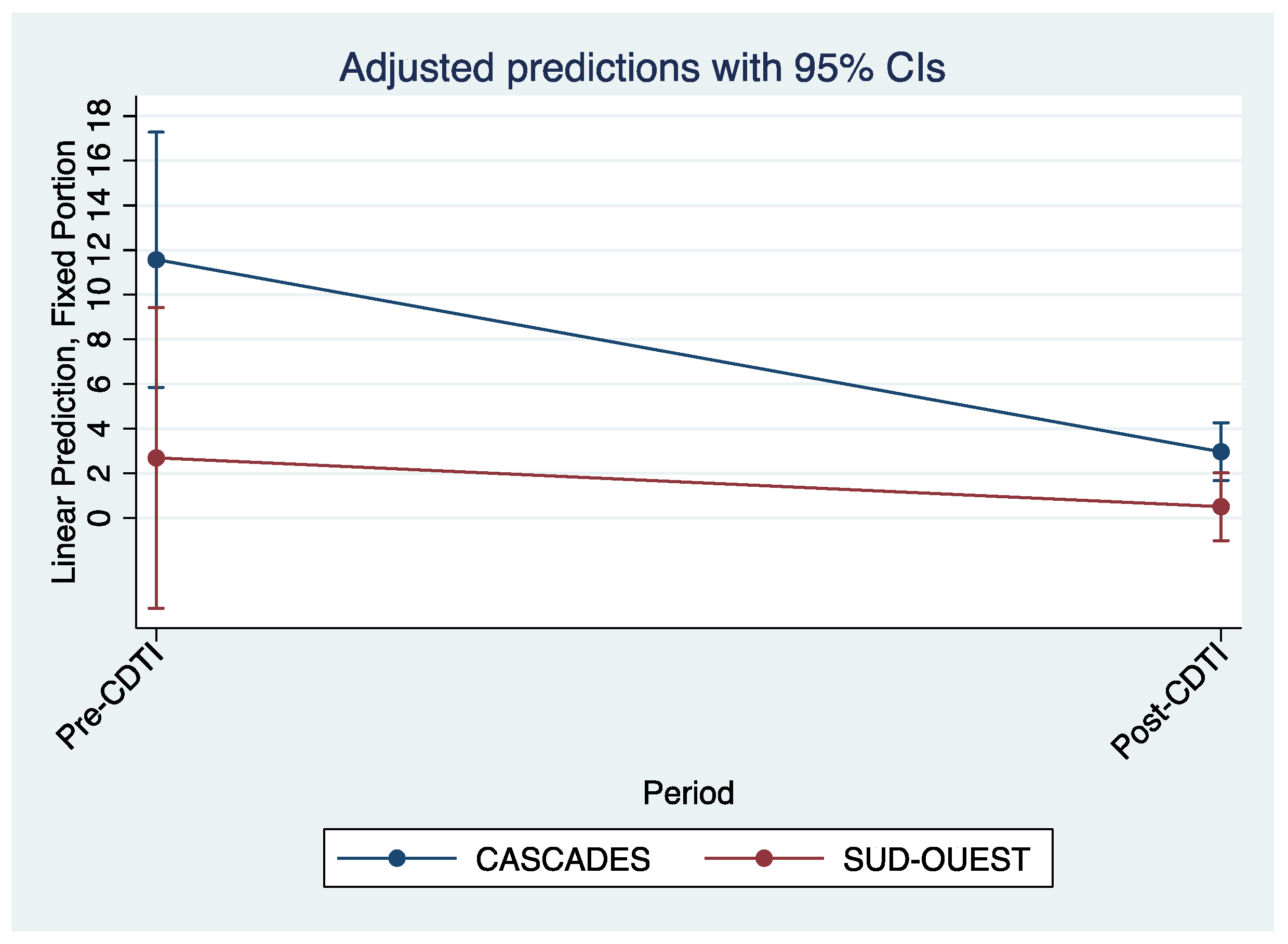
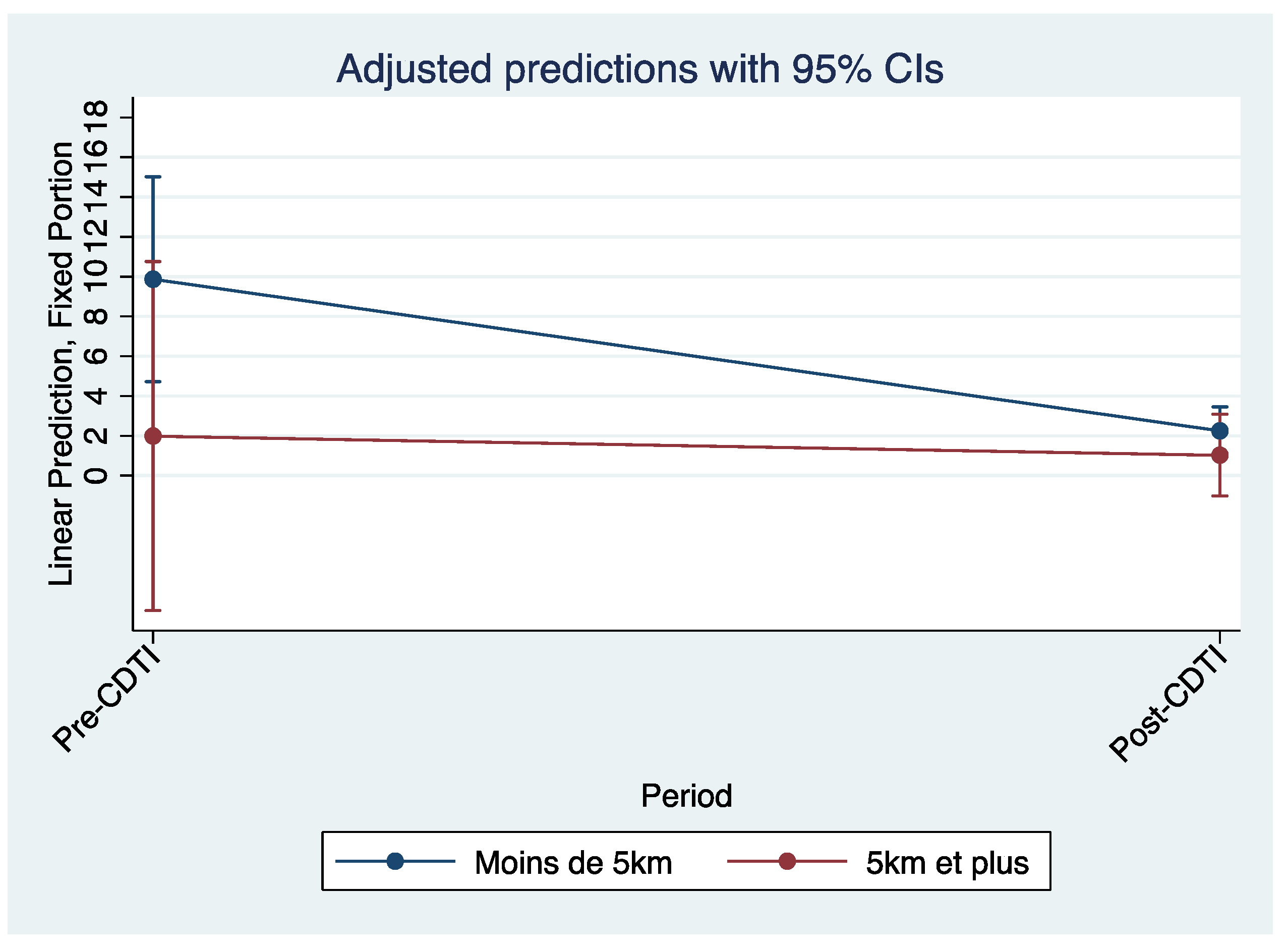
Figure 7 and
Figure 8, which are predictions adjusted to a 95% confidence interval, also illustrate the decline in biopsy microfilaria prevalence after 5 years of CDTI for all regions and by region, respectively.
In addition, the drop in the trajectory of the curve representing the predicted standardized microfilaria prevalence of villages located within 5 km of the streams was greater than that of villages located more than 5 km from the streams (
Figure 9).
4. Discussion
This study was conducted to analyse the effects of CDTI implementation on changes in the prevalence of O. Volvulus in two regions in Burkina Faso that had seen a recrudescence.
The key findings were that standardized microfilaria prevalence, skin biopsy prevalence and CMFL decreased significantly after the implementation of CDTI in both regions. There was no significant difference between the decline in parasitological data within the two regions. A greater decline in the trajectory of prevalence was observed for villages located less than 5 km from the watercourse compared to villages located more than 5 km from the watercourse.
We conducted an extensive analysis to determine the effects of CDTI on parasitological indices of onchocerciasis in these two areas and we also analyzed the data in relation to the location of the village in relation to the river to see if this was a factor in the decrease in prevalence. This in-depth analysis allowed us to obtain satisfactory results despite the small size of our sample as one of the limitations of our study was the small sample. We were unable to obtain individual data from the epidemiological studies and had to work on the basis of villages as our unit of measure. In addition, we had to assign district therapeutic coverage rates to villages due to the lack of coverage data at the village level.
Our results showed a decline in standardized microfilaria prevalence and CMFL in all villages involved in the study. This was shown by the trajectories and the distribution by villages before the implementation of CDTI and after five years of treatment. In addition, the reduction in standard microfilaria prevalence and CMFL could be attributed to the effects of CDTI. For example, for standardized prevalence, the reduction was nearly -6 with a 95% confidence interval of [-9.6 to -2.2]. Previous research has found that Ivermectin is an effective treatment for onchocerciasis microfilariae to stop the transmission of onchocerciasis. This discovery allowed vector control for the elimination of onchocerciasis to be suspended in order to strengthen efforts to implement Ivermectin treatment [
26]. The findings also support the view that mass treatment with Ivermectin is better in several respects compared to vector control. It has an impact on morbidity and it helps to reduce disease transmission [
27]. The significant decline in prevalence after five years of treatment in the two regions of Burkina Faso suggests that the implementation of CDTI could lead to a cessation of onchocerciasis transmission within the 15-year time frame recommended in the studies. Indeed, according to mathematical models, in the event of a resurgence, CDTI conducted for 15 years with a coverage rate of at least 65% would be sufficient to stop the recrudescence and spread of onchocerciasis [
4].
Although the decline in standard and biopsy prevalence rates and CMFL appeared to be greater in the Cascades region than in the Southwest region, this was not statistically significant. This could be due to the small sample size which did not allow for statistical power. Studies have shown area differences in the time taken to total elimination of onchocerciasis but these were usually done at the country level with large samples. For example, in Abu Hamed, Sudan, onchocerciasis was declared eliminated after 14 years of annual and then biannual treatment with Ivermectin [
28]. In Geba Valley, Guinea-Bissau, it took just six years of treatment [
4]. In our study we dealt with two regions of the same country that were located in the same geographical area. These two regions have the same type of climate and vegetation with people who have the same habits in terms of professional activities (mainly agriculture, livestock and fishing), culture and migration. This could also explain the lack of significant difference in the decline in prevalence. In addition, the implementation of CDTI in both regions was coordinated by the same team at the national level. The strategies used to achieve adherence and treatment goals were probably the same in both regions, as evidenced by the satisfactory treatment coverage.
We also did not observe a significant difference in the decrease in standardized prevalence and CMFL of villages located less than 5 km from the rivers and those located more than 5 km away. However, we know that the amount of dermal microfilariae is a function of the number of adult filariae and hence the number of infective larvae transmitted to the subject by the bites of infective female simulium black flies [
27]. The proximity of villages to watercourses leads to greater simulidean nuisance and hence higher transmission.
The small size of our sample was a limitation of our study. It did not allow us to demonstrate the effect of proximity to waterways on the reduction in the prevalence of onchocerciasis, despite extensive analysis. Another limitation was the absence of data on the therapeutic coverage of the villages concerned by the study, which obliged us to attribute the therapeutic coverage of the districts to the villages.
However, the results obtained show that the implementation of CDTI in the two regions has produced satisfactory results, and the reduction in the endemicity of onchocerciasis will certainly lead to other programmatic decisions such as the implementation of preSTop and full stop surveys in these two regions.
5. Conclusion
Our study showed the effects of CDTI on the decrease of standardized microfilaria prevalence, biopsy prevalence and CMFL in all the villages concerned. CDTI was implemented for at least five years between the first and second epidemiological assessments, with satisfactory treatment coverage in all districts. Our results thus show that the implementation of effective CDTI could stop the transmission of O. volvulus in these two regions. The main challenge for stopping transmission could be the migration of populations to neighboring countries. It was observed that most of the people who tested positive during the epidemiological surveys after the five years of treatment had stayed outside Burkina Faso for a longer or shorter period of time. Effective strategies should be put in place to track these individuals as soon as they return to the country in order to prevent them from transmitting onchocerciasis to populations that are regularly treated. In addition to population migration, migration of the vector from one country to another (as Burkina Faso shares some river basins with neighboring countries) may also delay the cessation of transmission. Future studies are needed to assess the impact of these migration patterns on preventing O. Volvulus transmission.
Author Contributions
Bertrand Meda and Karifa Kourouma participated in the finalization of the methodology and provided support for the data analysis. Dieudonné Naré proofread the article Clarisse Bougouma and Justin Compaoré provided the baseline data for the study and proofread the document Seni Kouanda finalized the article and translated it into English.
Financing
The program was funded by: the World Health Organization’s Special Programme for Research and Training in Tropical Diseases (WHO/TDR) as well as covering the costs of open access publication. The donor had no role in the study design, data collection and analysis, decision to publish, or writing of the manuscript.
Availability of Data and Materials
The data used in this study belong to the PNMTN in Burkina Faso. They are available upon reasonable request.
Acknowledgments
This research was conducted as part of the Structured Operational Training and Research Initiative (SORT IT), a global partnership coordinated by the World Health Organization’s Special Programme for Research and Training in Tropical Diseases (WHO/TDR). The training model, adapted to French and neglected tropical diseases (NTDs), is based on the one developed jointly by the International Union Against Tuberculosis and Lung Disease (The Union) and Médecins Sans Frontières (MSF). The specific SORT IT program adapted to NTDs that resulted in this publication was implemented by the African Institute of Public Health (IASP), Ouagadougou, Burkina Faso. Coordination of the online training sessions and email exchanges were provided by the African Institute of Public Health (IASP), Ouagadougou, Burkina Faso. Mentoring/facilitation was provided by researchers from IASP and the Centre de Formation et de Recherche en Santé Rurale de Maferinyah (CNFRSR), Forréariah, Guinea. Our thanks also go to the coordination of the PNMTN, which allowed us to access the data and thus facilitate the implementation of this study.Finally, we would like to thank our hierarchy for allowing us to participate in this operational research course and for their unstinting efforts to accompany us.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Elimination of human onchocerciasis: progress report, 2021. [cited 19 Dec 2022]. Available: https://www.who.int/publications/i/item/who-wer9746-591-598.
- Controlling neglected tropical diseases to achieve the sustainable development goals: a sustainability framework for action on neglected tropical diseases 2021-2030. [cited 20 Dec 2022]. Available: https://www.who.int/fr/publications-detail/9789240019027.
- World Health Organization. Integrating neglected tropical diseases into the global health and development agenda: the fourth WHO report on neglected tropical diseases. Policy brief. Retrieved from Geneve. 2017.
- Dadzie Y, Amazigo U v., Boatin BA, Sékétéli A. Is onchocerciasis elimination in Africa feasible by 2025: A perspective based on lessons learned from the African control programmes. Infect Dis Poverty. 2018;7: 1-11. [CrossRef]
- Ed Cupp MSVCMEPJL and TRU. Elimination of onchocerciasis in Africa by 2025: the need for a broad perspective. Infect Dis Poverty.
- Dadzie Y, Neira M, Hopkins D. Final report of the Conference on the eradicability of Onchocerciasis. Filaria J. 2003;2: 1-141. [CrossRef]
- Diawara L, Traoré MO, Badji A, Bissan Y, Doumbia K, Goita SF, et al. Feasibility of onchocerciasis elimination with ivermectin treatment in endemic foci in Africa: First evidence from studies in Mali and Senegal. PLoS Negl Trop Dis. 2009;3. [CrossRef]
- Gebrezgabiher G, Mekonnen Z, Yewhalaw D, Hailu A. Status of parasitological indicators and morbidity burden of onchocerciasis after years of successive implementation of mass distribution of ivermectin in selected communities of Yeki and Asosa districts, Ethiopia. BMC Public Health. 2020;20. [CrossRef]
- Tekle AH, Zouré HGM, Noma M, Boussinesq M, Coffeng LE, Stolk WA, et al. Progress towards onchocerciasis elimination in the participating countries of the African Programme for Onchocerciasis Control: epidemiological evaluation results. Infect Dis Poverty. 2016;5: 66. [CrossRef]
- Eisenbarth A, Achukwi MD, Renz A. Ongoing Transmission of Onchocerca volvulus after 25 Years of Annual Ivermectin Mass Treatments in the Vina du Nord River Valley, in North Cameroon. PLoS Negl Trop Dis. 2016;10: e0004392. [CrossRef]
- Forrer A, Wanji S, Obie ED, Nji TM, Hamill L, Ozano K, et al. Why onchocerciasis transmission persists after 15 annual ivermectin mass drug administrations in South-West Cameroon. BMJ Glob Health. 2021;6: e003248. [CrossRef]
- Hendy A, Krit M, Pfarr K, Laemmer C, de Witte J, Nwane P, et al. Onchocerca volvulus transmission in the Mbam valley of Cameroon following 16 years of annual community-directed treatment with ivermectin, and the description of a new cytotype of Simulium squamosum. Parasit Vectors. 2021;14: 1-14. [CrossRef]
- Komlan K, Vossberg PS, Gantin RG, Solim T, Korbmacher F, Banla M, et al. Onchocerca volvulus infection and serological prevalence, ocular onchocerciasis and parasite transmission in northern and central Togo after decades of Simulium damnosum s.l. vector control and mass drug administration of ivermectin. PLoS Negl Trop Dis. 2018;12: e0006312. [CrossRef]
- Nikièma AS, Koala L, Post RJ, Paré AB, Kafando CM, Drabo F, et al. Onchocerciasis prevalence, human migration and risks for onchocerciasis elimination in the Upper Mouhoun, Nakambé and Nazinon river basins in Burkina Faso. Acta Trop. 2018;185: 176-182. [CrossRef]
- Koala L, Nikiema A, Post RJ, Paré AB, Kafando CM, Drabo F, et al. Recrudescence of onchocerciasis in the Comoé valley in Southwest Burkina Faso. Acta Trop. 2017;166: 96-105. [CrossRef]
- Koala L, NAS, PAB, DF, TLD, BAMG, DRK. Entomological assessment of the transmission following recrudescence of onchocerciasis in the Comoé Valley, Burkina Faso. Parasits Vectors. 2019;12: 34.
- Samuel A, Belay T, Yehalaw D, Taha M, Zemene E, Zeynudin A. Impact of six years community directed treatment with ivermectin in the Control of Onchocerciasis, western Ethiopia. PLoS One. 2016;11. [CrossRef]
- Opara KN, Fagbemi BO, Atting IA, Oyene UE, Okenu DMN. Status of forest onchocerciasis in the Lower Cross River Basin, Nigeria: change in clinical and parasitological indices after 6 years of ivermectin intervention. Public Health. 2007;121: 202-207. [CrossRef]
- Gebrezgabiher G, Mekonnen Z, Yewhalaw D, Hailu A. Status of parasitological indicators and morbidity burden of onchocerciasis after years of successive implementation of mass distribution of ivermectin in selected communities of Yeki and Asosa districts, Ethiopia. BMC Public Health. 2020;20: 1-15. [CrossRef]
- Human Development Report 2021/2022 Presentation. 2022.
- Fifth general population and housing census of Burkina Faso. Ouagadougou; 2019.
- (PDF) ETUDE DE L’ÉVOLUTION CLIMATIQUE AU BURKINA FASO DE 1983 A 2012 : CAS DES VILLES DE BOBO DIOULASSO, OUAGADOUGOU ET DORI. [cited 20 Dec 2022]. Available: https://www.researchgate.net/publication/323068909_ETUDE_DE_L’EVOLUTION_CLIMATIQUE_AU_BURKINA_FASO_DE_1983_A_2012_CAS_DES_VILLES_DE_BO_DIOULASSO_OUAGADOU_ET_DORI.
- Burkina Faso Ministry of Health. Strategic plan for the control of neglected tropical diseases 2016-2020, Burkina Faso. 2016.
- World Health Organization. Guidelines for stopping mass drug administration and verifying elimination of human onchocerciasis. World Health Organization;
- Nikièma AS, Koala L, Sondo AK, Post RJ, Paré AB, Kafando CM, et al. The impact of ivermectin on onchocerciasis in villages co-endemic for lymphatic filariasis in an area of onchocerciasis recrudescence in Burkina Faso. PLoS Negl Trop Dis. 2021;15. [CrossRef]
- Cupp EW, Sauerbrey M, Richards F. Elimination of human onchocerciasis: History of progress and current feasibility using ivermectin (Mectizan®) monotherapy. Acta Trop. 2011;120: S100-S108. [CrossRef]
- Boatin B. The onchocerciasis Control Program in West Africa (OCP). Ann Trop Med Parasitol,102 suppl 1. 2008;102, Suppl 1: 13-17.
- Zarroug IMA, Hashim K, ElMubark WA, Shumo ZAI, Salih KAM, ElNojomi NAA, et al. The First Confirmed Elimination of an Onchocerciasis Focus in Africa: Abu Hamed, Sudan. Am J Trop Med Hyg. 2016;95: 1037-1040. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).