1. Introduction
Spinal cord injury (SCI) leads to several severe symptoms, including motor deficits, sensory deficits, and autonomic nervous system dysfunction. SCI results in persistent disabilities for patients and has a notable socioeconomic impact. Historically, traumatic spinal cord injuries (tSCI) have been more prevalent among young adult men and women, primarily resulting from car accidents, falls from heights and sports-related incidents [
1]. Previous studies on the age distribution of tSCI showed a primary peak among individuals aged 10–29 years over three decades ago; however, since the 2000s, the age of this peak group has gradually increased. The reported incidence of tSCI varies widely between countries and even between regions within the same country [
2]. Recent data indicate that the incidence of tSCI in high-income countries ranges from 12.6 to 86 cases per million [
3,
4,
5,
6,
7,
8].
Understanding the up-to-date epidemiology and demographic characteristics of both, tSCI and non-traumatic SCI, is crucial for managing health care resources, as well as the understanding of the prognosis of neurological and functional outcomes after SCI is essential for responding to patients' questions regarding their potential functional capabilities. Moreover, it helps in determining the required resources for inpatient rehabilitation and post-discharge care. Additionally, having a thorough understanding of the trajectory and factors influencing the natural recovery of SCI has become a scientific necessity. This understanding is essential for assessing also the effectiveness of new pharmacological and rehabilitative treatments [
9].
Outcome prediction of neurorehabilitation is determined by prior knowledge of clinical and demographical factors, including functional and clinical scale scores, level of lesion, presence of comorbidities, as well as age and gender, useful for a rehabilitative prognosis. Classical statistical approaches are based on linear or binary regressions, taking into account many different variables assessed at admission into the rehabilitation hospital, and in some cases variables assessed during the hospitalization or at discharge. Over the past few years, many different prognostic factors have been identified.
In tSCI, several features such as age, the initial ISNCSCI evaluation [
10], the MRI characteristics of the lesion, the presence of comorbidity and the occurrence of complications (in particular pneumonia) [
11] have been correlated with the neurological and functional recovery. Consequently, interventions aimed at mitigating cognitive changes, post-injury pneumonia, and unhealthy body weight can enhance neurological improvement in SCI subjects. Regarding ambulation prognosis, a 2011 study introduced a straightforward and precise prediction rule for independent ambulation outcomes after tSCI considering age, motor scores of the myotomes L3 and S1 and light touch sensation of dermatomes L3 and S1[
12].
However, the relationships among demographical, clinical, biological and psychological factors and outcomes could be not linear and intertwined. For this reason, most conventional approaches may fail in revealing these complex relationships [
13,
14]. Artificial Neural Networks (ANNs) are recently emerging as an alternative approach more accurate for discriminating between various classes of potential prognostic factors useful for predicting outcomes in patients needing neurorehabilitation such as people who had a stroke or a traumatic brain injury [
13]. For patients with spinal cord injury, a recent systematic review of the literature highlighted that the use of artificial neural networks able to perform a machine learning has good potentialities in diagnosis, prognostication, management, rehabilitation planning, and risk prevention of chronic complications and mental illness[
15]. However, the same review identified to main problems in the studies concerning the use of ANNs for identifying the prognostic factors in individuals with SCI. The first one is the need to validate the ANN-based predictive scoring systems with respect to other approaches and with respect to the used algorithms that include the selection of the input variables. The other problem highlighted in that review was that most of the analyzed studies investigated a lot of input variables but with small datasets, going from only 9 patients up to a study with 862 patients (in mean: 165 patients for each study) [
15]. Another problem concerning the use of ANN as prognostic classifier is its reliability. In fact, the ANN could achieve the same level of prognostic accuracy assigning different weights to the synapses , and hence, suggesting different level of importance for the input variables as prognostic factors [
16].
Therefore this study has two aims: 1) to evaluate the clinical and demographic factors affecting the functional outcome of a large population with SCI by means of ANN, and 2) to compare the prognostic value of ANN with that or a more conventional statistical approach, and deeply analyzing also the problems of ANN for understanding the problems and identifying solutions.
3. Results
A database of 1256 patients with spinal cord injury was analyzed. The sample had a mean age of 51.5±18.4 years and was formed by 69% of males. A traumatic etiology of spinal cord injury was recorded in the 43% of the cases. The most common injury level was the thoracic one (47% of the individuals), and the SCI was mainly a sensory motor incomplete one (AIS C: 27%; AIS D: 35%). The 66% of patients underwent to a surgical intervention before neurorehabilitation. The mean SCIM score at admission was 26.2±20.6, whereas at discharge it was 61.3±26.3. Other data are reported in
Table 1.
In
Table 1 we also reported the raw and normalized importance of each prognostic factor as assessed by the ANN (on 951 patients having complete data). The reliability of ANN throughout the 10 runs was measured by the standard deviation of RI coefficients, which ranged between 0.7% for surgical intervention and 2.5% for motor completeness and SCIM and by the Cronbach's alpha computed on RI values that was 0.987. The linear regression identified 7 statistically significant variables that entered into the model, and also these results are reported in
Table 1.
As shown in
Table 1, the two approaches had in common the identification of the SCIM at admission as the most important predictor, followed by age, level of lesion, ASIA score and presence of pressure sores at admission. A role was also played by traumatic etiology in both the models. The ANN associated a high importance also to WISCI score at admission and to motor completeness of the lesion, complications and presence of deep vein thrombosis at admission. Linear regression also identified a significant role for gender, which was marginal for ANN.
The correlation between the SCIM scores at discharge and those predicted by ANN was R=0.75, just slightly superior to that with values predicted by classical regression R=0.73 (both were highly statistically significant, p<0.001). The mean absolute error with respect to the SCIM-score at discharge was 13±11 points for the values predicted by ANN and 15±10 for linear regression.
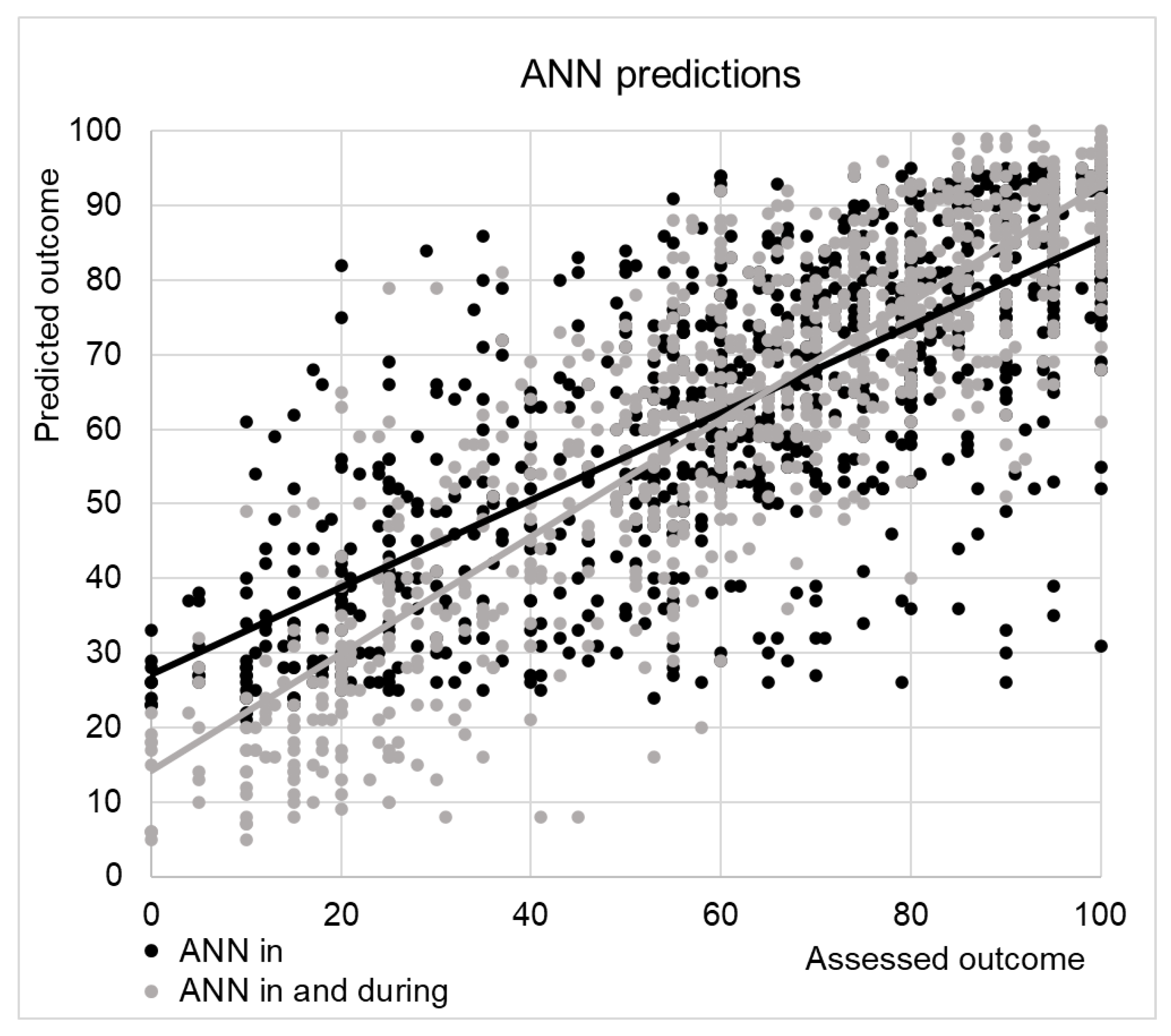
Figure 2 shows the predictions of ANN versus the actual SCIM score assessed at discharge. The linear regression had the disadvantage to predict also values out of the range of the SCIM (>100). Adjusting values for covering this range did not improve the performance of the linear regression predictions (R=0.73, MAE: 15±10). The ANN did not provide values out of the range, but it was evident that it overestimated the low score of SCIM, corresponding to the poor outcomes.
To test if this overestimation was related to complications occurred during the recovery into the hospital, we have performed a new analysis with the ANN including other 8 variables (i.e. surgical intervention, presence of pressure sores, heterotopic ossifications, respiratory complications, pulmonary embolism, deep vein thrombosis, urologic complications or other complications) recorded during the recovery. The predicted values are shown in
Figure 2 with grey dots. The correlation increased to R=0.87, and the mean absolute error was reduced to 10±8. The factors most important for these predictions are reported in
Table 2.
The importance of variables at admission was reduced of 24.6%, covered by the variables assessed during hospitalization. In particular, pulmonary embolism, deep vein thrombosis or urologic complications occurred during hospitalization weighted for the 14.4% of the prediction.
4. Discussion
We investigated the feasibility of predicting the activity of daily life competency (SCIM Score) at discharge from rehabilitation, from demographic and clinical features at admission using data extracted from a single center, large database of individuals with traumatic and non-traumatic SCI who underwent inpatient treatment at a spinal center in Italy. To perform this analysis we used an ANN (ARIANNA), but we also performed a linear regression analysis to compare the two methodologies.
For both approaches, the level of independence (SCIM score) achieved at discharge from rehabilitation strictly depended on demographic (mainly age) and clinical (injury level, ASIA score, presence of presence of complications, in particular pressure ulcers, etiology of injury) variables. The ANN analysis also highlighted the significance of the WISCI score, motor completeness of the lesion, and, although marginal, a gender influence.
Findings about demographic and clinical variable corroborate existing literature. Starting from the former, research on the impact of age on SCI rehabilitation outcomes suggests that younger individuals generally achieve better recovery compared to older adults. This higher recovery in younger patients is often attributed to greater neural plasticity and a lower incidence of comorbid conditions, which enhance their ability to adapt to injury [
23]. Conversely, older adults with SCI face challenges due to age-related factors such as reduced muscle mass, decreased bone density, and the presence of comorbidities, all of which can impede the rehabilitation process and restrict functional recovery. Additionally, the slower neurological regeneration in older patients can limit the degree of possible improvement through rehabilitation efforts [
24]. Age data is intriguing considering that recently SCIs have seen a rise in cervical hyperextension injuries due to falls on level surfaces and low falls among the elderly [
24]. According to the World Health Organization the global population, which increased from 3.6 billion in 1970 to 5.3 billion in 1990, exploded to 8.0 billion by 2022. By 2050, the world’s population aged 60 years and older has been estimated to reach over 2.0 billion [
25] with the expectation that the average age at the time of injury will rise as the population ages [
26]. Furthermore, the heightened risk of reoccurring falls and subsequent fractures may increase the risk of mortality for those sustaining fall related SCIs [
27]. The falls are considered as a category of tSCI, as well as violence or and car/motorbike accidents with high hazard for mortality [
27]. Compared to tSCI, non-traumatic SCI patients were significantly older, numerous and mainly affected by incomplete lesion and associated with paraplegia. Despite no significant differences were found in the length of stay, tSCI patients showed greater improvement when discharged [
28]. Our data indicate an association between etiology of the injury and the level of independence, although weaker than the other relationships identified.
Regarding the other factors, the ANN identifies gender as a marginal prognostic factor compared to others. Gender was statistically significant as a prognostic factor, but it has the smallest weight among those identified. This finding could align with the conflicting data present in the literature, related to a small, sometimes significant, effect. To date, there is no universal consensus on the gender-prognosis correlation for recovery. Authors previously demonstrated on 281 patients that gender does not seem to influence rehabilitation outcomes despite the fact that men and women showed significant epidemiological differences. Particularly, female patients had a lower frequency of traumatic lesions, a lower frequency of complications at admission and a higher frequency of incomplete lesions (ASIA impairment C) [
29]. On the contrary other research suggested that the severity of the injury as well as the ultimate recovery of motor function after SCI is significantly influenced by gender, being remarkably better in females probably due to the mechanism(s) of neuroprotection in females, although not yet elucidated, associated with the effects of estrogen on pathophysiological processes [
30].
The observed correlations between higher SCIM scores at discharge and lesion at lumbar or thoracic spinal cord (i.e. less severe lesions) and younger age are consistent with already published data. Both the level and severity of spinal cord injury (SCI) greatly influence rehabilitation outcomes, with literature emphasizing the importance of the anatomical location of the injury in determining functional recovery and quality of life [
31]. Individuals with cervical SCI typically face greater rehabilitation challenges than those with thoracic or lumbar injuries, largely because of the more significant effects on upper limb function and overall mobility [
32]. Furthermore, the severity of the lesion, scored with ASIA impairment scale, correlates with the degree of functional impairment and the likelihood of meaningful recovery [
33]. The individuals classified as AIS A, B, and C show better improvements in functional independence if the SCI is localized at a caudal level compared to those with a cervical injury. Similarly, the individuals with complete SCI (AIS A) have higher scores in the SCIM if they have an injury at a caudal level [
33]. Furthermore, recently has been reported that the rehabilitative goals selected by the Physical Neurorehabilitation in SCI management during recovery, are strictly influenced by the AIS score and the lesion level [
34]. Consequently, it is crucial in the neurorehabilitation pathway to tailor ad hoc the rehabilitation strategies considering both lesion level and severity, as well as the greater difficulties of an elderly patient, to better enhance outcomes and independence in the Activities of Daily Living. Besides the significant differences in rehabilitation needs and recovery potential among individuals with cervical, thoracic, and lumbar injuries it should be considered that non-traumatic lesions could have minor benefits after rehabilitation therapy if compared with traumatic ones cause their presentation is often insidious and slow the timing of diagnosis and the admission to a rehabilitation unit. This data reinforce that a different planning of rehabilitation intervention in the different categories of spinal cord injury can be useful. In particular elaborating a specific rehabilitation plan for individuals more compromised (e.g., older subjects, non-traumatic SCI, …) could lead to a better functional recovery of these patients.
With regard to the impact of complications at admission and during hospitalization, mainly the pressure sores, our study is in agreement with previous ones demonstrating that the presence of complications has a negative impact on patients’ functional status at discharge [
35,
36,
37]. To gain a deeper understanding of this relationship, various factors must be considered, particularly the concept of "immobility" required for healing complications. This is especially pertinent for pressure ulcers, as this approach may delay attaining a sitting position, utilizing a wheelchair, and ultimately achieving comprehensive rehabilitation goals [
38]. It is also important to consider that complications, particularly pressure ulcers, induce a chronic inflammatory state characterized by anemia, low serum iron, hypoproteinemia, and hypoalbuminemia [
39]. These conditions can significantly diminish the functional potential of patients [
40]. Irrespective of the previously mentioned factors influencing recovery. Furthermore patients experiencing complications tend to have extended hospital stays, receiving fewer hours of rehabilitation or less intensive rehabilitation compared to individuals without complications [
38].
The second aim of the study was to compare the prognostic value of ANN with that of a more conventional statistical approach, considering that ANNs may manage large databases and providing accurate predictions of outcomes of neurorehabilitation. Previous studies showed their accuracy was higher than that of conventional statistical approaches such as linear regression [
21], [
22]. However, it was not always true, especially for the artificial neural networks developed many years ago [
41], or when the results of ANN have been compared to specific statistical methods such as cluster analysis [
21].
A recent study conducted on 210 patients with spinal cord injury compared different ANNs for predicting the SCIM-score at outcome [
14]. The MAE was in mean 8.6, slightly lower than that of our study (that was on average of 13 points). Despite the authors performed a linear regression on their data, they did not compare the accuracy of the two approaches in that study. Dietz et al. reported as main problems of ANN-based predictive scoring systems the necessity of validate this approach with respect to other approaches, the small datasets in mean 165 patients for each study and the reliability of using ANN as prognostic classifier [
15]. In our study we analyzed the data by overcoming these problems with a sample of 1256 patients, a comparison with conventional statistical regression analysis method and by analyzing the reliability measured by the standard deviation of RI coefficients. Our analysis, performed on an extensive dataset of 1256 individuals with SCI, underpins that for both approaches, the R value exceeds 0.70, indicating that they can be considered reliable predictors of the SCIM- score at discharge. Additionally, the nuanced higher R-value obtained with ANN suggests that this new approach is not less valid than linear regression, but slightly superior to linear regression in predicting SCIM output.
In terms of prognostic factors, both approaches agree on the importance of age and some clinical variables discussed above. The main discrepancies between the two approaches, were related to the WISCI score and motor completeness that were not taken into account by the linear regression, whereas weighted for 21% on the ANN prediction. Conceivably, it was due to the fact that linear regression aims to find the smallest number of possible predictors, and these two factors are strictly connected with SCIM-score at admission. In fact, the linear regression identified only 7 statistically significant variables that entered into the model, whereas ANN took into account all the input variables. It means that covarying variables can be both associated to a high level of prognostic weight by the ANN, but not by the linear regression.
ANN was more complicated than linear regression, and the small improvements in its performance seemed to do not justify its use. This improvement resulted small because the ANN tended to overestimated the patients who had a poor outcome. As shown in our secondary analysis, it could be explained by the fact that these subjects often had complications during the hospitalization. These complications weighted for about one fourth of the outcome, and they were difficult to be predicted at admission in the neurorehabilitation hospital. Another notable element is that the ANN analysis highlights the predictive role of complications, both those present at admission (see
Table 1) and those that arise during hospitalization (see
Table 2). Among the latter, the most important are the possibility of developing pulmonary embolism, deep vein thrombosis or urological complications (normalized importance score ranging from 24,9 to 18,2).
This is very intriguing considering that a recent study [
42] highlighted that since 2010 the highest mortality rate after SCI is for respiratory diseases and that the overall age-standardized mortality rate was 3 times higher for individuals with SCI than the general population. This study also stressed that a key element for improving life expectancy after SCI is to reduce mortality rates from respiratory diseases. In line with these data, in our study, the ANN identifies respiratory issues that occurred during hospitalization as the most important complication affecting the recovery. This element, combined with the literature data, indicates that special attention should be paid not only to the characteristics of SCI mentioned above, but also to the respiratory complications that occur during hospitalization. This connection is even more evident when considering the strong relationship between respiratory complications and deep vein thrombosis. Venous thrombosis is known to be associated with considerable short-term morbidity and mortality: the mortality rate after venous thrombosis is about 20% within one year, and studies to date have suggested that the mortality rate is two to four times higher for patients with pulmonary embolism, of whom 10%–20% die within three months after the event [
43]. The third type of complication to consider for the prognosis that the ANN has highlighted are the urological complications. Recurrent urinary tract infections are mainly developed in most of all SCI patients, with an incidence peaked in the 1st and 10th 5-year intervals. Besides these infections, the most common complications were bladder stone, hydronephrosis, and vesicoureteral reflux. A greater risk of urologic complications may be seen with certain factors (male gender, cervical SCI, and condom catheter use); however, all patients with SCI are at risk of urinary complications over time. Thus, even long-term patients who are thought to be "stable" from the urological point of view require regular follow-up and surveillance [45].
The main use of artificial intelligence in predicting outcomes seems to be not simply the replication of classical statistical approaches with a small improvement in terms of accuracy against a complication of the model and a reduction of reliability. Artificial intelligence may instead provide a continuous monitoring and computation of the clinical parameters of the patient, identifying the potential role of complications in real-time on the outcome. Cerasa and colleagues [
13] already claimed that, at front of good performance of specific machine learning algorithms (such as ANN), there is a high heterogeneity in features extracted from low-dimensional clinical datasets that may reduce the enthusiasm for applying this powerful method in clinical practice. They also claimed the need to better capture and predict the dynamic changes in patients. Our findings about the role of hospitalization complications support this point of view. Dietz and colleagues [
15] also suggested a potential role of machine learning approaches for personalizing the rehabilitative care. Future studies should investigate other important outcomes, such as the length of stay and the destination after discharge from neurorehabilitation hospitals.
Author Contributions
Conceptualization, M.I., G.S.; methodology, M.I., G.S..; software, M.I.; formal analysis, M.I..; investigation, M.I., G.S., F.T.; resources, G.S.; data curation, G.S., M.I., F.T.; writing—original draft preparation, G.S., M.I., F.T.; writing—review and editing, G.S., M.I., F.T., E.L., M.M..; visualization, G.S., M.I., F.T.; supervision, G.S., M.I.; project administration, G.S.; funding acquisition, M.I., G.S. All authors have read and agreed to the published version of the manuscript.