Submitted:
03 July 2024
Posted:
04 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
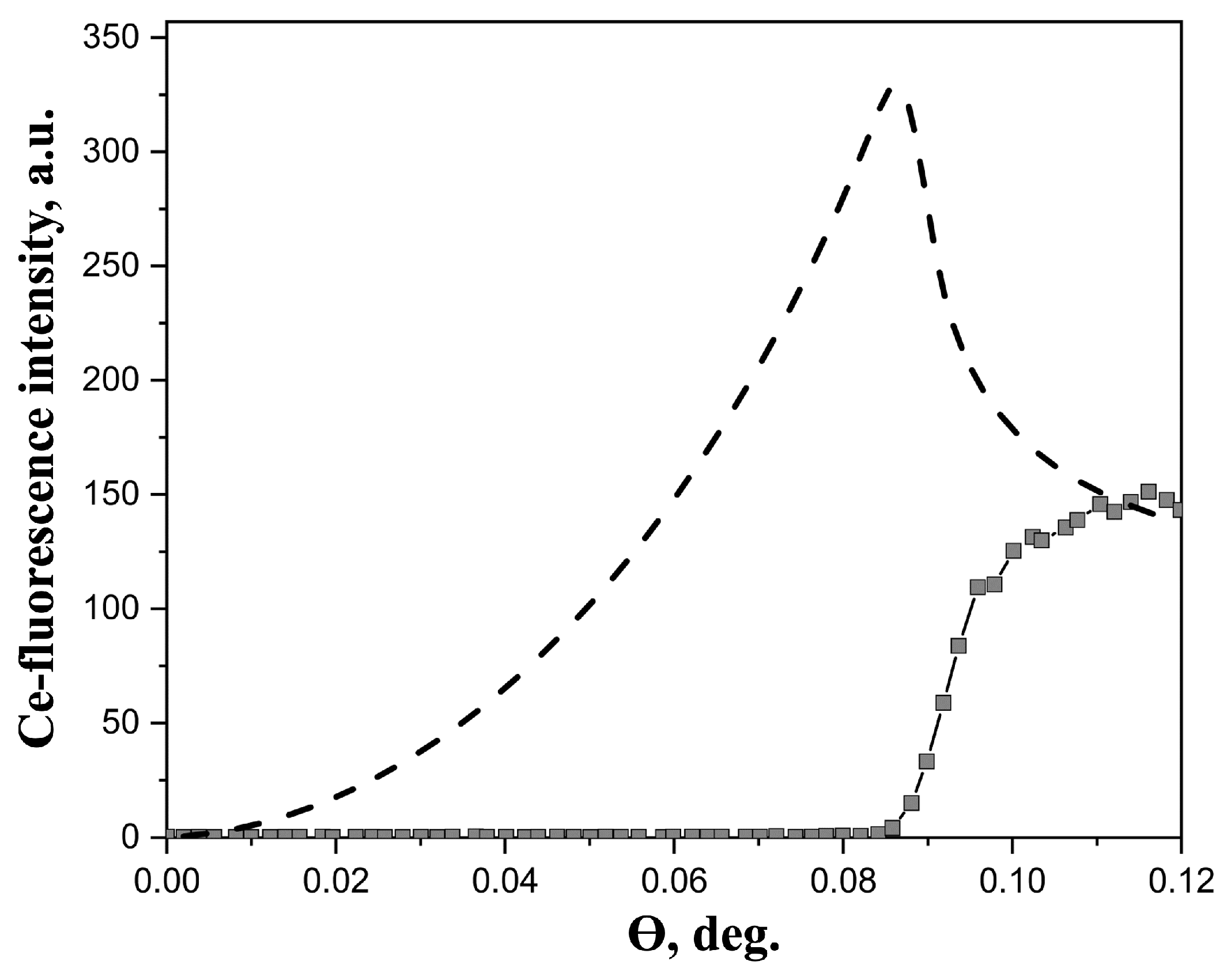
2.1. X-ray Standing Wave Studies
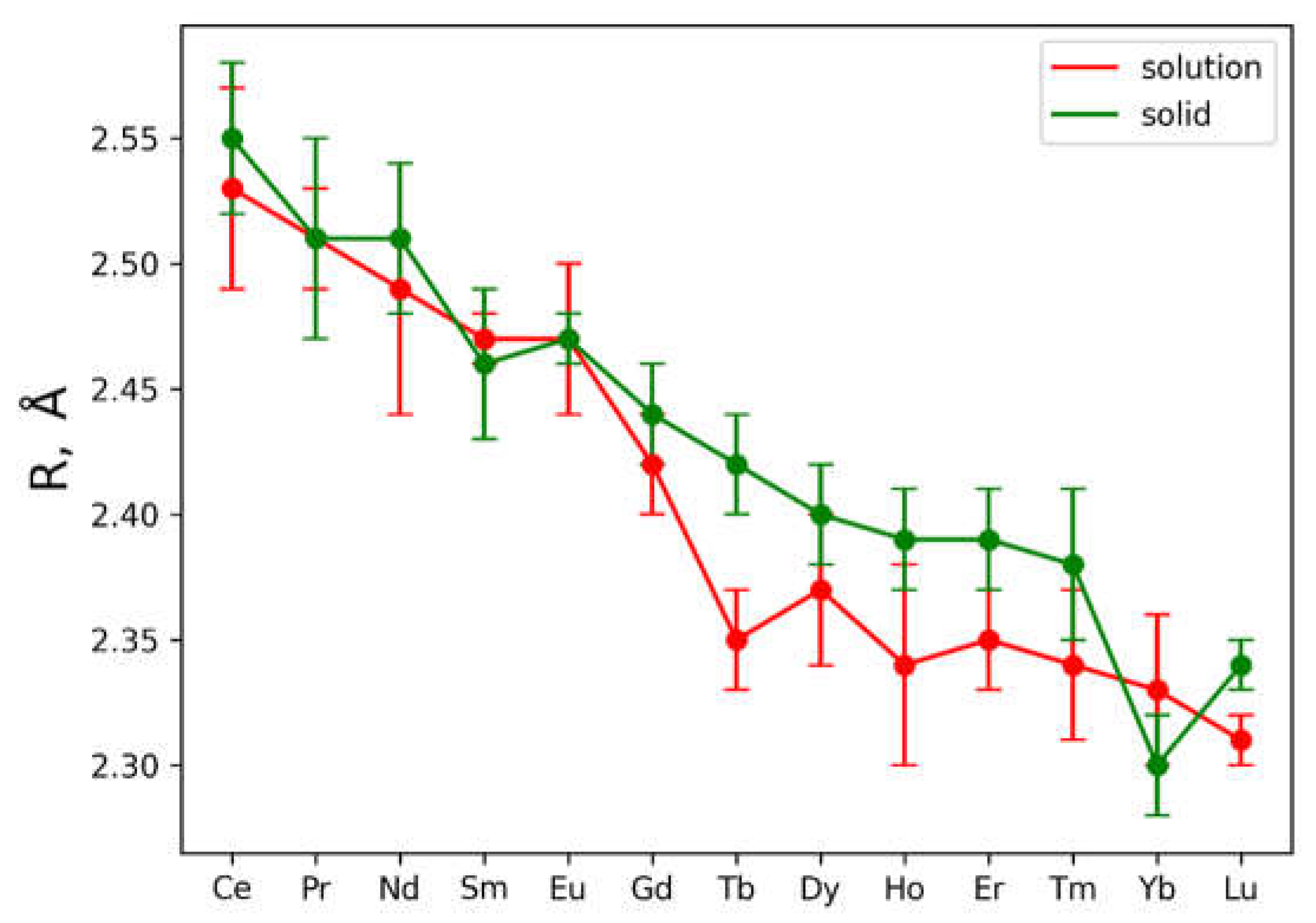
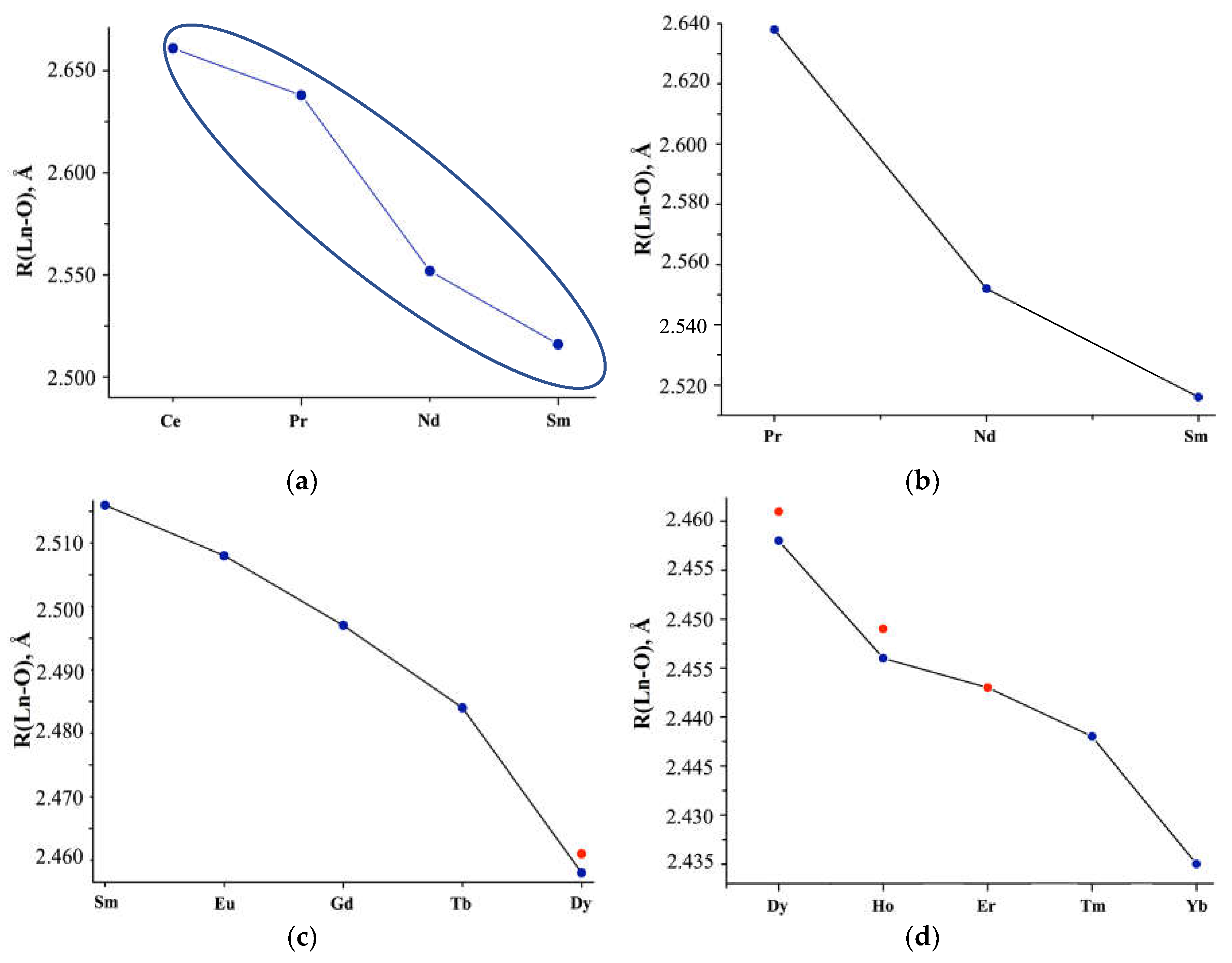
2.2. X-ray Absorption Spectroscopy Studies
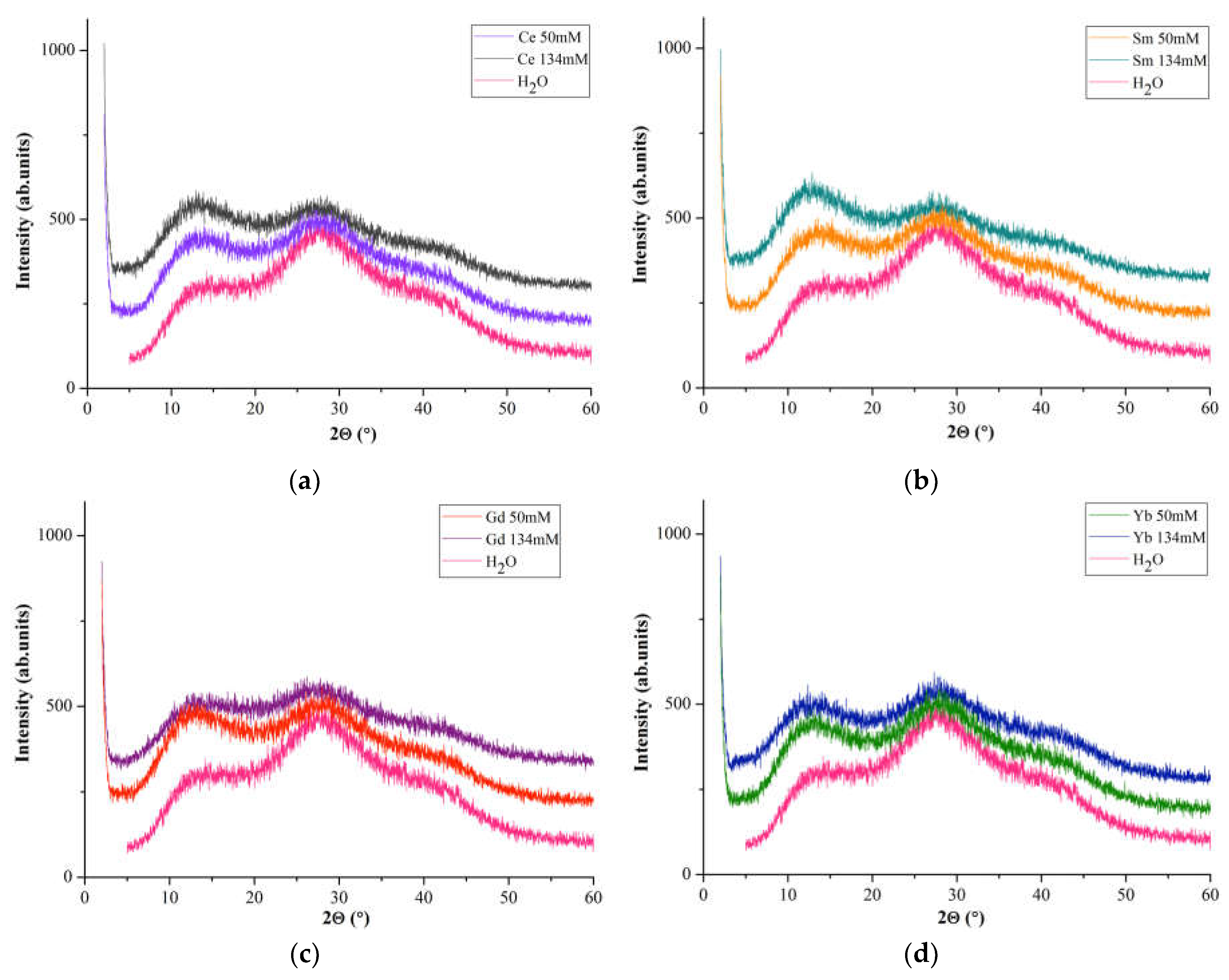
2.3. X-ray Diffraction
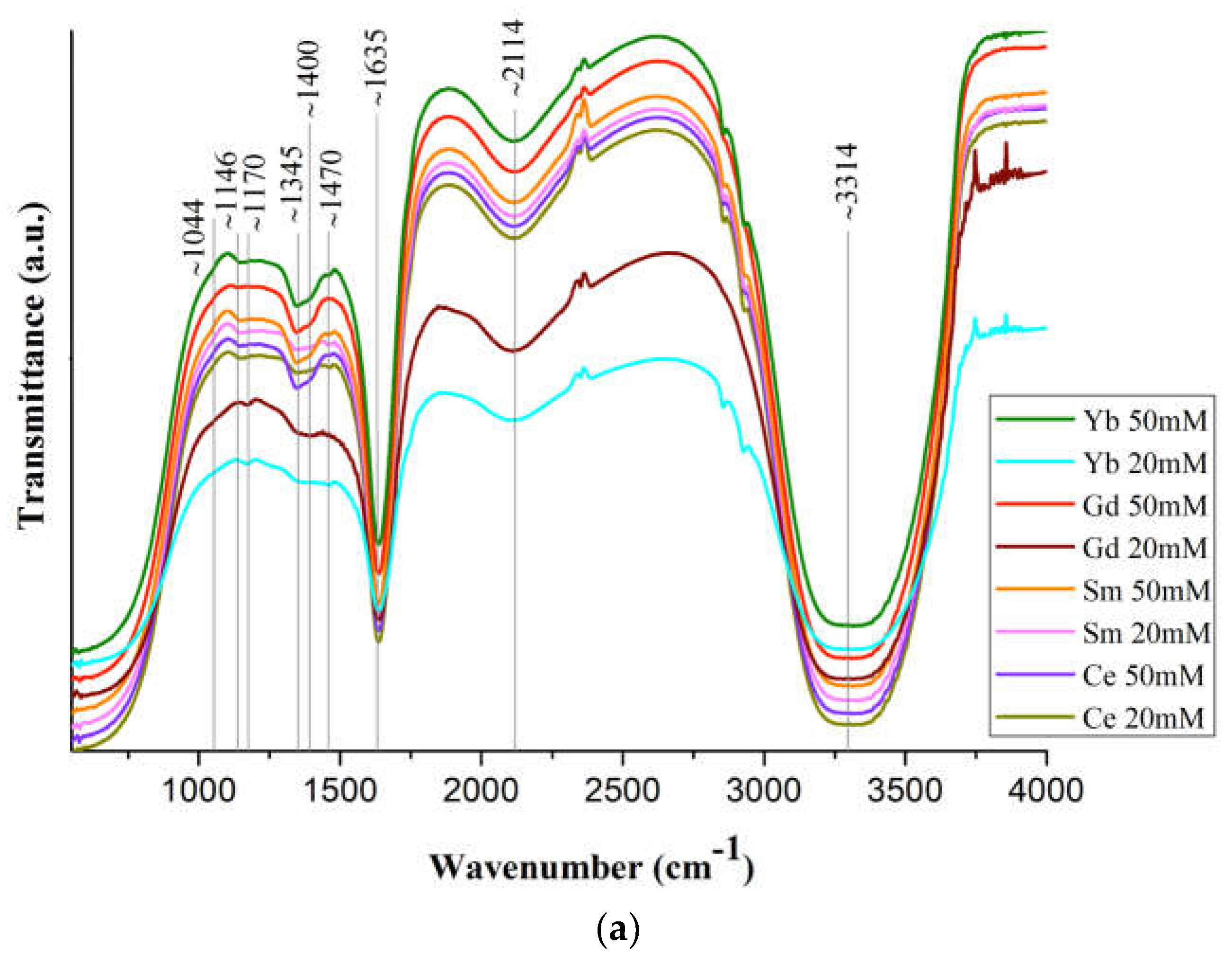
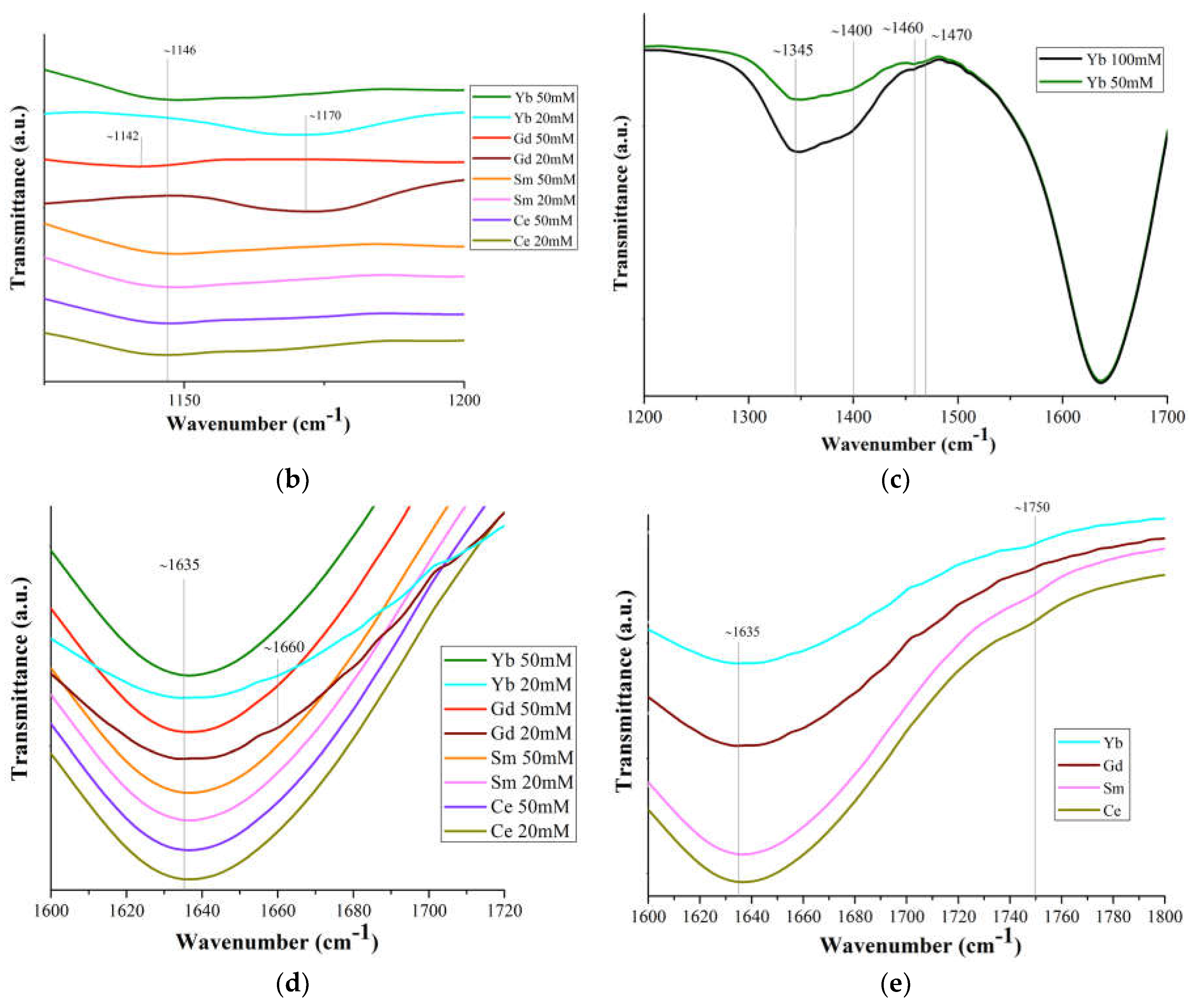
2.4. FT-IR Spectroscopy Data
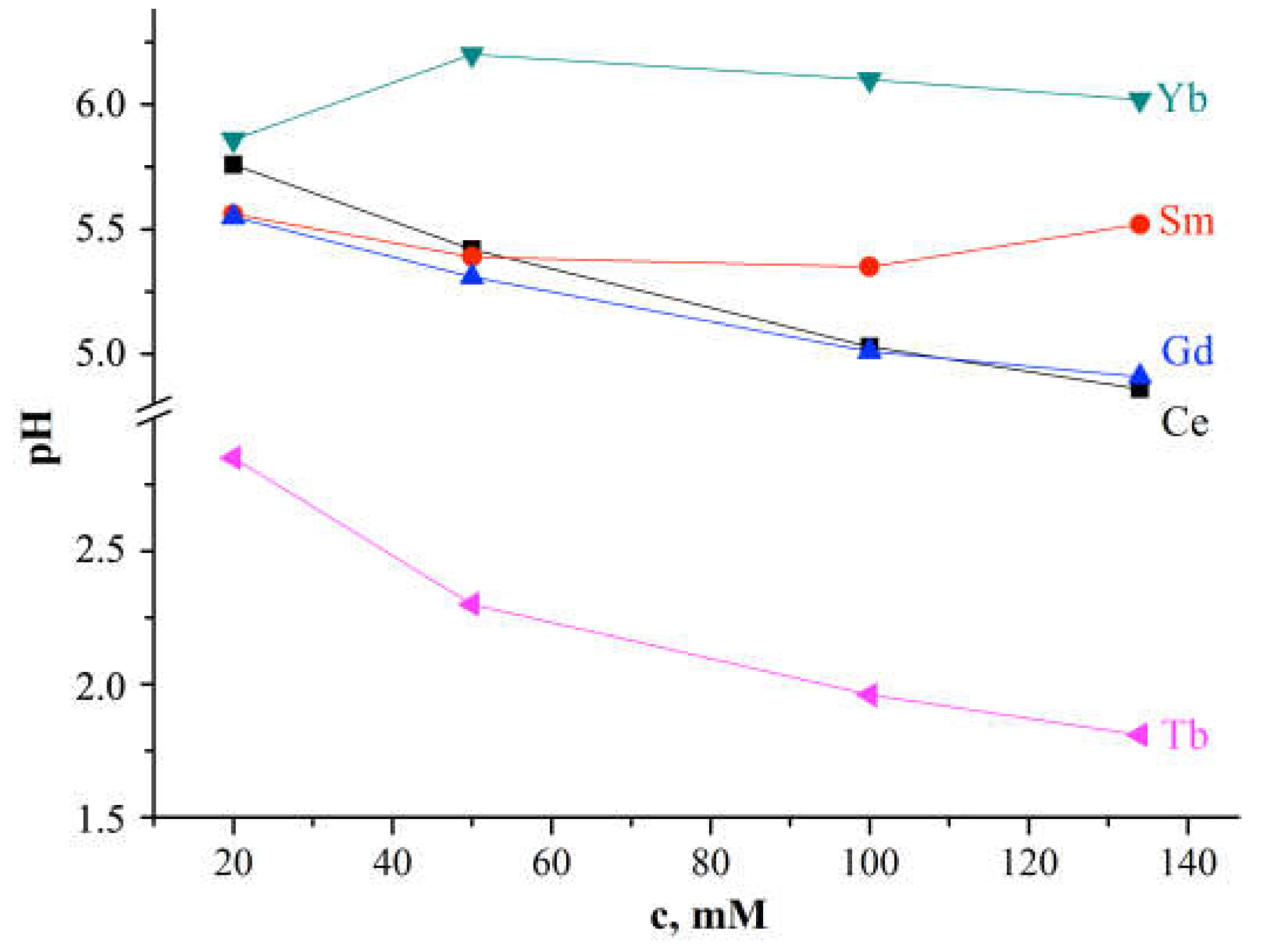
3. Discussion
4. Materials and Methods
4.1. X-ray Studies
4.1.1. X-ray Absorption Spectroscopy Measurements at Liquid Surface
4.1.2. X-ray Absorption Spectroscopy Measurements of Powder Samples
4.1.3. XRSW Measurements
4.2. EXAFS Data Analysis
4.4. FT-IR Spectroscopy
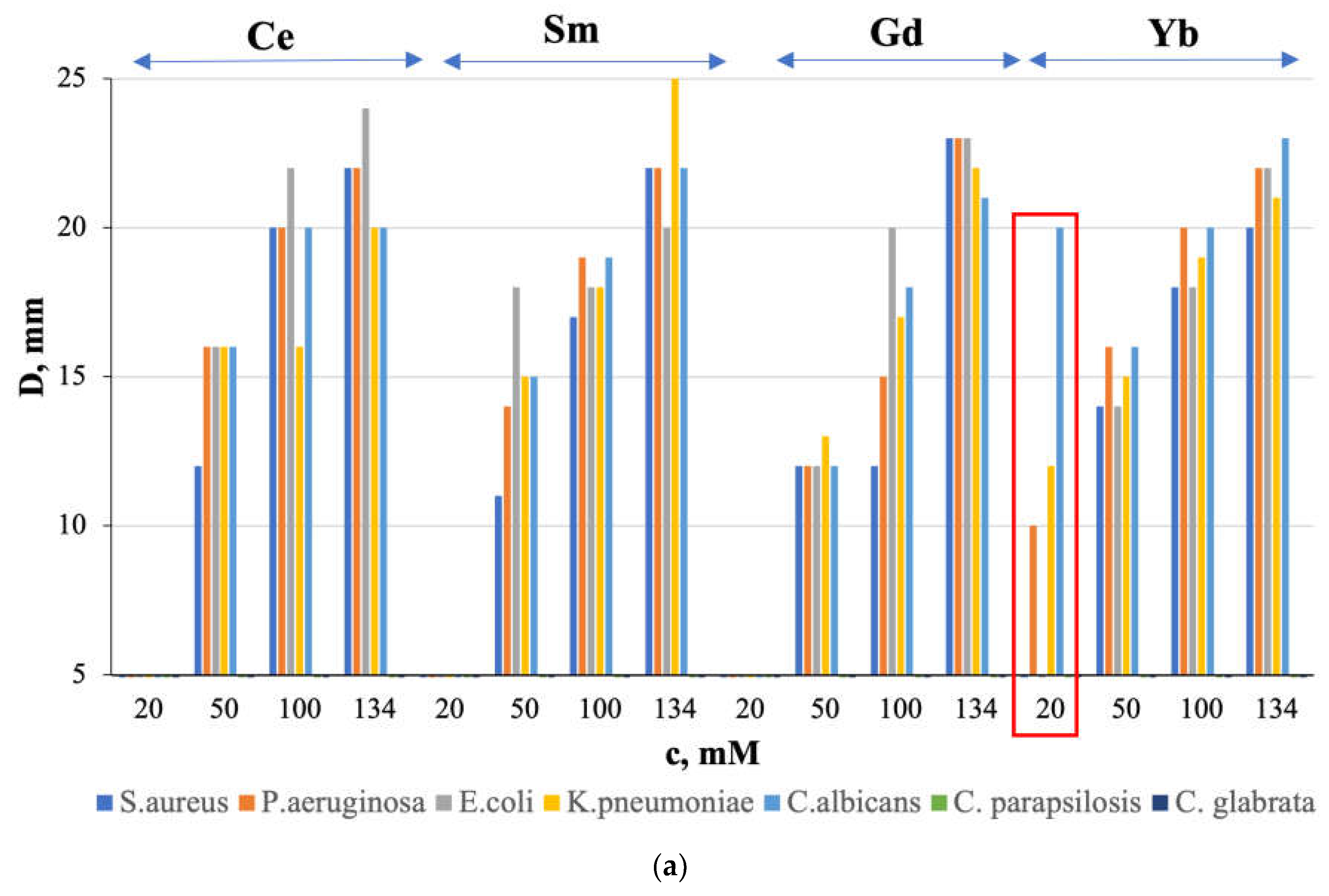
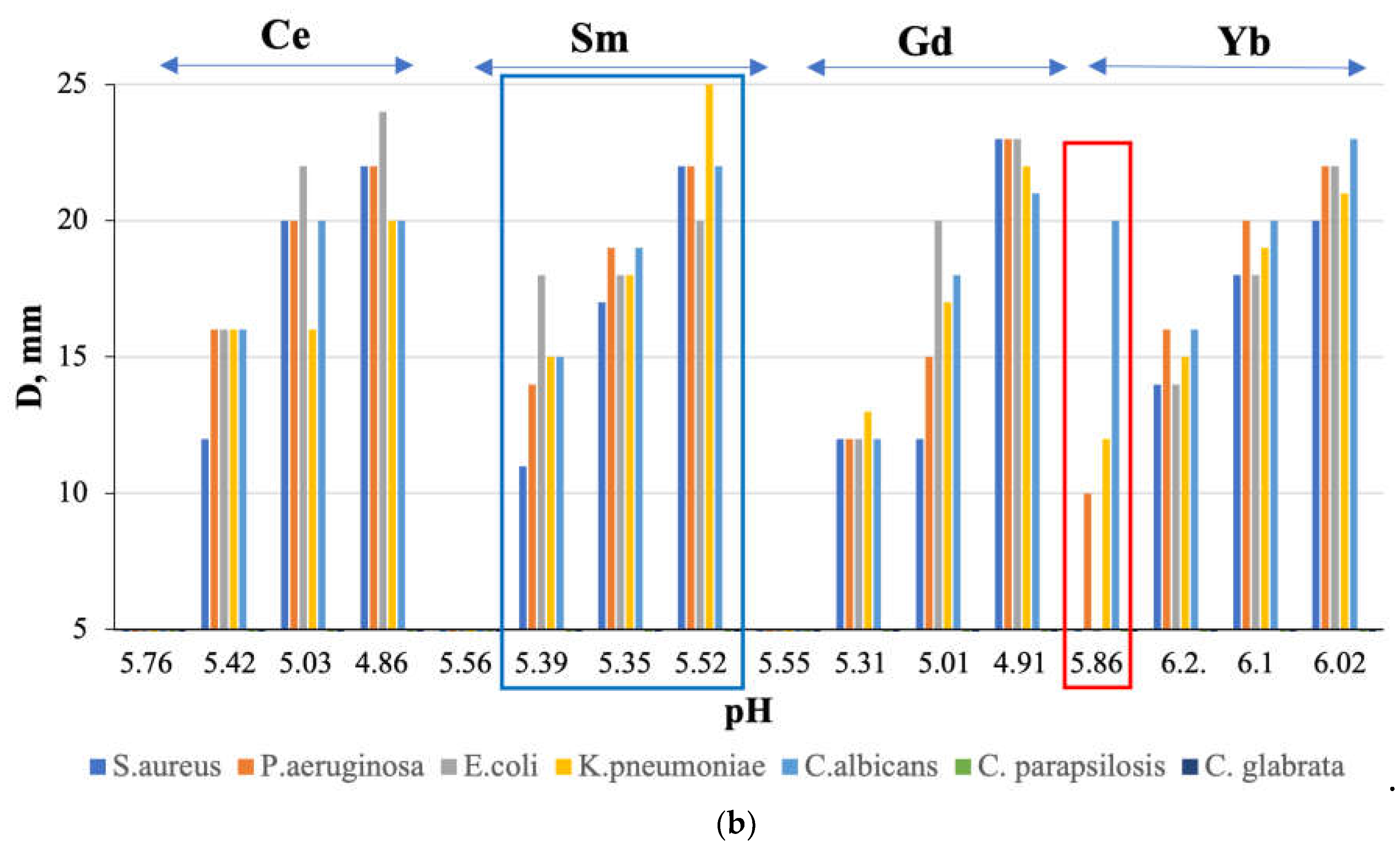
4.5. The Antimicrobial Activity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garner, J.P.; Heppell, P.S.J. Cerium nitrate in the management of burns. Burns 2005, 31, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Silva-Dias, A.; Miranda, I.M.; Branco, J.; Cobrado, L.; Monteiro-Soares, M.; Pina-Vaz, C.; Rodrigues, A.G. In vitro antifungal activity and in vivo antibiofilm activity of cerium nitrate against Candida species. J. Antimicrob Chemother. 2015, 70, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- Cota, I.; Marturano, V.; Tylkowski, B. Ln complexes as double faced agents: Study of antibacterial and antifungal activity. Coordin. Chem. Rev. 2019, 396, 49–71. [Google Scholar] [CrossRef]
- Kuz’micheva, G. M.; Timaeva, O. I.; Novikova, N. N.; Yakunin, S. N.; Rogachev, A. V.; Svetogorov, R. D.; Pashkin, I. I.; Terekhovа, R. P. Antimicrobial Activity of Composite Hydrogels in the Poly(N-vinylpyrrolidone)–RE(NO3)3 · xH2O (RE Are Rare-Earth Ions) System. Crystallography Reports 2020, 65, 922–932. [Google Scholar] [CrossRef]
- Beuchat, С.; Hagberg, D.; Spezia, R.; Gagliardi, L. Hydration of Lanthanide Chloride Salts: A Quantum Chemical and Classical Molecular Dynamics Simulation Study. J. Phys. Chem. B 2010, 114, 15590–15597. [Google Scholar] [CrossRef]
- Rizkalla, E. N.; Choppin, G. R. Lanthanides and actinides hydration and hydrolysis. In Handbook on the Physics and Chemistry of Rare Earths; Gschneidner, K. A. Jr., Eying, L., Choppin, G. R., Lander, G. H., Eds.; Elsevier Science: Amsterdam, 1994; Volume 18, pp. 529–558. [Google Scholar]
- Yokoyama, H; Johansson G. Structures of Nitrate Complexes of Erbium in Aqueous Solutions. Acta Chemica Scandinavica 1990, 4, 567–573. [Google Scholar]
- Smirnov, P.R.; Grechin, O.V.; Trostin, V.N. Concentration Dependence of the Structure of Aqueous Solutions of Lutetium Nitrate According to X-ray Diffraction. Russian Journal of Physical Chemistry A 2014, 88, 250–253. [Google Scholar] [CrossRef]
- Butcher, T.A.; Formon, G.J.M.; Dunne, P.; Hermans, T.M.; Ott, F.; Noirez, L.; Coey, J.M.D. Neutron imaging of liquid-liquid systems containing paramagnetic salt solutions. Applied Physics Letters 2020, 116, 022405. [Google Scholar] [CrossRef]
- Yaita, T.; Narita, H.; Suzuki, S.; Tachimori, Sh. Structural study of lanthanides(III) in aqueous nitrate and chloride solutions by EXAFS. J Radioanal Nucl Chem. 1999, 239, 371–375. [Google Scholar] [CrossRef]
- Ohta, A.; Kagi, H.; Tsuno, H.; Nomura, M.; Kawabe, I. Influence of multi-electron excitation on EXAFS spectroscopy of trivalent rare-earth ions and elucidation of change in hydration number through the series. American Mineralogist, 2008; 93, 1384–1392. [Google Scholar]
- Nelson, D.L.; Irish, D.E. Interactions in Lanthanide Systems. I. A Raman and Infrared Study of Aqueous Gadolinium Nitrate. The journal of chemical physics. 1971, 54, 4479–4489. [Google Scholar] [CrossRef]
- Onghena, B.; Papagni, E.; Rezende Souza, E.; Banerjee, D.; Binnemans, K.; Hoogerstraete, T. Speciation of lanthanide ions in the organic phase after extraction from nitrate media by basic extractants. RSC Adv. 2018, 8, 32044–32054. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, W.W.; Irmer, G. ; On the Hydration of the Rare Earth Ions in Aqueous Solution. J Solution Chem. 2020, 49, 316–331. [Google Scholar] [CrossRef]
- Fratiello, A.; Kubo-Anderson, V.; Azimi, S.; Flores, T.; Martiinez, E.; Matejka, D.; Perrigan, R.; Vigil, M. A hydrogen-1, nitrogen-15, and chlorine-35 NMR coordination study of Lu(ClO4)3 and Lu(NO3)3 in aqueous solvent mixtures. J Solution Chem. 1990, 19, 811–829. [Google Scholar] [CrossRef]
- Dobler, M.; Guilbaud, P.; Dedieub, A.; Wipff, G. Interaction of trivalent lanthanide cations with nitrate anions: a quantum chemical investigation of monodentate/bidentate binding modes. New J. Chem. 2001, 25, 1458–1465. [Google Scholar] [CrossRef]
- Duvail, M.; Ruas, A.; Venault, L.; Moisy, P.; Guilbaud, P. Molecular Dynamics Studies of Concentrated Binary Aqueous Solutions of Lanthanide Salts: Structures and Exchange Dynamics. Inorg. Chem. 2010, 49, 519–530. [Google Scholar] [CrossRef]
- Migliorati, V.; Serva, A.; Sessa, F.; Lapi, A.; D’Angelo, P. Influence of Counterions on the Hydration Structure of Lanthanide Ions in Dilute Aqueous Solutions. J. Phys. Chem. B. 2018, 122, 2779–2791. [Google Scholar] [CrossRef] [PubMed]
- Bonal, C. ; Morel, J-P.; Morel-Desrosiers, N. Interactions between lanthanide cations and nitrate anions in water Part 2. Microcalorimetric determination of the Gibbs energies, enthalpies and entropies of complexation of Y3‘ and trivalent lanthanide cations. J. Chem. Soc., Faraday Trans, 1998; 94, 1431–1436. [Google Scholar]
- Rao, L.; Tian, G. Complexation of Lanthanides with Nitrate at Variable Temperatures: Thermodynamics and Coordination Modes. Inorg. Chem. 2009, 48, 964–970. [Google Scholar] [CrossRef]
- Allen, P.G.; Bucher, J.J.; Shuh, D.K.; Edelstein, N.M.; Craig, I. Coordination Chemistry of Trivalent Lanthanide and Actinide Ions in Dilute and Concentrated Chloride Solutions. Inorg. Chem. 2000, 39, 595–601. [Google Scholar] [CrossRef]
- Bera, M.K.; Luo, G.; Schlossman, M.L.; Soderholm, L.; Lee, S.; Antonio, M.R. Erbium(III) Coordination at the Surface of an Aqueous Electrolyte. J. Phys. Chem. B. 2015, 119, 8734–8745. [Google Scholar] [CrossRef]
- Shiery, R.C.; Fulton, J.L.; Balasubramanian, M, Nguyen, M. -T., Lu, J.-B., Li, J., Rousseau, R., Glezakou, V.-A., Cantu, D.C. Coordination Sphere of Lanthanide Aqua Ions Resolved with Ab Initio Molecular Dynamics and X-ray Absorption Spectroscopy. Inorg. Chem. 2021, 60, 3117–3130. [Google Scholar] [CrossRef]
- Persson, I.; D'Angelo, P.; De Panfilis, S.; Sandström, M.; Erikssonet, L. Hydration of Lanthanoid (III) Ions in Aqueous Solution and Crystalline Hydrates Studied by EXAFS Spectroscopy and Crystallography: The Myth of the “Gadolinium Break”. Chem.-Eur. J. 2008, 14, 3056–3066. [Google Scholar] [CrossRef] [PubMed]
- Solera, J. A.; García, J.; Proietti, M. G. Multielectron excitations at the L edges in rare-earth ionic aqueous solutions. Phys. Rev. B. 1995, 51, 2678. [Google Scholar] [CrossRef] [PubMed]
- Plakhova, T.; Romanchuk, A.; Yakunin, S.; Dumas, T.; Demir, S.; Wang, S.; Minasian, S.; Shuh, D.; Tyliszczak, T.; Shiryaev, A.; Egorov, A.; Ivanov, V.; Kalmykov, S. Solubility of Nanocrystalline Cerium Dioxide: Experimental Data and Thermodynamic Modeling. J. Phys. Chem. C. 2016, 120, 22615–22626. [Google Scholar] [CrossRef]
- Zegenhagen, J.; Kazimirov, A. X-ray Standing Wave Technique, Principles and Applications, World Scientific Publishing, Singapore, 2013; 556 p.
- Parratt, L.G. Surface Studies of Solids by Total Reflection of X-Rays. Phys. Rev. 1954, 95, 359–369. [Google Scholar] [CrossRef]
- Rizkalla, E.N.; Choppin, G.R. Hydration and hydrolysis of lanthanides. In Handbook on the Physics and Chemistry of Rare; Gschneidner, K.A.; Eyring, L., Eds.; Elsevier, Amsterdam, 1991, Volume 15, pp. 393-443.
- Rizkalla, E.N.; Choppin, G.R. Hydration of lanthanides and actinides in solution. Journal of Alloys and Compounds 1992, 180, 325–336. [Google Scholar] [CrossRef]
- Smirnov, P.R.; Trostin, V.N. Structural parameters of the immediate environment of ions in aqueous solutions of inorganic electrolytes. Ivanovo Publishing house, Russia, 2011; 400 p. (RU).
- Kanno, Н.; Hiraishi, J. Raman study of aqueous rare earth nitrate solutions in liquid and glassy states. J. Phys. Chem. 1984, 88, 2787–2792. [Google Scholar] [CrossRef]
- Choppin, G. R.; Strazik, W. F. Complexes of Trivalent Lanthanide and Actinide Ions. I. Outer-Sphere Ion Pairs. Inorg. Chem. 1965, 4(9), 1250–1254.
- Klimov, V.D.; Chudinov, E.G. Application of infrared spectroscopy to study bonds in complexes of rare earth nitrates with alkylammonium nitrates. Moscow: Order-of-Lenin I. V. Kurchatov Institute of Atomic Energy, Russia, 1974. (RU).
- Milinski, N.; Ribar, B.; Sataric, M. Pentaaquatrinitratocerium(III) monohydrate, Ce(H2O)5(NO3)3 · H2O. Crystal Structure Communication 1980, 9, 473–477. [Google Scholar]
- Klein, W. Crystal structures of the penta- and hexahydrate of thulium nitrate. Acta Cryst. 2020, E76, 1863–1867. [Google Scholar] [CrossRef] [PubMed]
- Taha, Z.A.; Ajlouni, A.; Hijazi, A.K.; Kühn, F.E.; Herdtweck, E. Redetermination of [Gd(NO3)3(H2O)4]·2H2O. Acta Cryst. 2012, E68, i56–i57. [Google Scholar] [CrossRef]
- Kawashima, R.; Sasaki, M.; Satoh, S.; Isoda, H.; Kino, Y.; Shiozaki, Y. Report on Temperature Dependence of Crystal Structure for Samarium Nitrate Having Metastable Phenomena. J. Phys. Soc. Jpn. 2000, 69, 3297–3303. [Google Scholar] [CrossRef]
- Decadt, R.; Van Der Voort, P.; Van Driessche, I.; Van Deun, R.; Van Hecke, K. Redetermination of [Pr(NO3)3(H2O)4]_2H2O. Acta Cryst. 2012, E68, i59–i60. [Google Scholar]
- Stumpf, T.; Bolte, M. Tetraaquatrinitratoeuropium (III) dehydrate. Acta Cryst. 2001, E57, i10–i11. [Google Scholar]
- Moret, E.; Bunzli, J-C. G.; Schenk, K.J. Structural and luminescence study of europium and terbium nitrate hexahydrates. Inorg. Chim. Acta 1990, 178, 83–88. [Google Scholar] [CrossRef]
- Rogers, D.J.; Taylor, N.J.; Toogood, G.E. Tetraaquatrinitratoneodymium(III) dihydrate, [Nd(NO3)3(H2O)4].2H2O. Acta Cryst. 1983, C39, 939–941. [Google Scholar] [CrossRef]
- Junk, P.C.; Kepert, D.L.; Skelton, B.W.; White, A.H. Structural Systematics of Rare Earth Complexes. XIII. ("Maximally") Hydrated (Heavy) Rare Earth Nitrates. Aust. J. of Chem. 1999, 52, 497–505. [Google Scholar]
- Rincke, C.; Schmidt, H.; Voigt, W. Rebuttal of the Existence of Solid Rare Earth Bicarbonates and the Crystal Structure of Holmium Nitrate Pentahydrate. Zeitschrift Für Anorganische Und Allgemeine Chemie, 2017; 643, 437–442. [Google Scholar]
- Klein, W. Crystal structure of tetraaqua-tris(nitrato-κ2O,O′) erbium(III) monohydrate, Er(NO3)3·5H2O, H10ErN3O14. Zeitschrift fur Kristallographie - New Crystal Structures, 2022; 237, 265–266. [Google Scholar]
- Setyaeva, E.A.; Glushko, A.A.; Kuzmicheva, G.M.; Neznanov, A.A.; Terekhova, R.P.; Svetogorov, R.D. Application of information technologies for the selection of salts and solutions RE(NO3)3·xH2O (RE=La-Lu,Y, Sc) with optimal structural characteristics and biocidal and neutron studies. In Book of reports of the Kurchatov Forum of Synchrotron and Neutron Research, Moscow, Russia, 24-27 October 2023. (RU).
- Grechin, O.V.; Smirnov, P.R.; Trostin, V.N. X-Ray Diffraction Study of Aqueous solutions of Lanthanum Chloride and Nitrate. Chemistry and Chemical Technology 2013, 56, 15–20 (RU). [Google Scholar]
- Smirnov, P.R.; Grechin, O.V.; Vashurin, A.S. Ion Coordination in Aqueous Lanthanum Chloride and Lanthanum Nitrate Solutions as Probed by X-Ray Diffraction. Russian Journal of Inorganic Chemistry 2022, 67, 382–387. [Google Scholar] [CrossRef]
- Rabadanov, K.Sh.; Gafurov, M.M.; Aliev, A.R. Study of vitrified homogeneous and heterophase nitrate systems using vibrational spectroscopy methods. In Proceedings of the XVII All-Russian Conference “Optics and Spectroscopy of Condensed Matter”, Krasnodar, Russia, 12-14 September 2011. (RU). [Google Scholar]
- Khakhalin, A.V.; Koroleva, A.V. Investigation of the temperature dependence for the spectra of supercooled water in the middle infrared. Moscow University Physics Bulletin. 2014, 1, 66–69. [Google Scholar] [CrossRef]
- Vratny, F. Infrared Spectra of Metal Nitrates. Applied Spectroscopy. 1959, 13(3), 59–70. [Google Scholar] [CrossRef]
- Egorov, N.B.; Shagalov, V.V. Infrared spectroscopy of rare and trace elements. Guidelines for conducting laboratory work in the course “Physico-chemical methods of analysis”; Tomsk: TPU Publishing House, Russia, 2012; 20 p. (RU). [Google Scholar]
- Sidyakin, P.V.; Karpov, V.L.; Egorov, B.N.; Egorova, Z.S. Radiation transformations in epoxy oligomers based on epichlorohydrin and n,n’-dioxydiphenylpropane. Polymer Science, Series A. 1971; 8, 2195–2206. [Google Scholar]
- Trivedi, M.K.; Branton, A.; Trivedi, D.; Jana, S. Spectroscopic Characterization of Disodium Hydrogen Orthophosphate and Sodium Nitrate after Biofield Treatment. Journal of Chromatography & Separation Techniques, 2015; 6, 2–5. [Google Scholar]
- Mihaylov, M.Y.; Zdravkova, V.R.; Ivanova, E.Z.; Aleksandrov, H.A.; Petko, P.S.; Vayssilov, G.N.; Hadjiivanov, K.I. Infrared Spectra of Surface Nitrates: Revision of the Current Opinions Based on the Case Study of Ceria. Journal of Catalysis. 2021, 394, 245–258. [Google Scholar] [CrossRef]
- Bentouhami, E.; Bouet, G.M.; Meullemeestre, J.; Verling, F.; Khan, M.A. Physicochemical study of the hydrolysis of Rare-Earth elements (III) and thorium (IV). Comptes Rendus Chimie. 2004, 7(5), 537–545. [Google Scholar] [CrossRef]
- Yakunin, S.N.; Novikova, N.N.; Rogachev, А.V.; Trigub, А.L.; Kuzmicheva, G.M.; Stepina, N.D.; Rozenberg, O.A.; Yurieva, E.A.; Kovalchuk, М. V. Spectral-Selective X-Ray Studies at the “Langmuir” Beamline of the Kurchatov Synchrotron Radiation Source. Crystallogr. Rep. 2022, 67, 799–812. [Google Scholar] [CrossRef]
- Chernyshov, A.A.; Veligzhanin, A.A.; Zubavichus, Y.V. Structural materials science end-station at the Kurchatov synchrotron radiation source: recent instrumentation up-grades and experimental results. Nucl. Instr. Methods Phys. Res. 2009, A603, 95–98. [Google Scholar] [CrossRef]
- Bunker, G. Introduction to XAFS: A Practical Guide to X-ray Absorption Fine Structure Spectroscopy; Cambridge University Press, UK, 2010.
- Aksenov, V.L.; Koval’chuk, M.V.; Kuz’min, A.Y. Purans, Yu.; Tyutyunnikov S.I. Development of methods of EXAFS spectroscopy on synchrotron radiation beams: Review. Crystallogr. Rep. 2006, 51, 908–935. [Google Scholar] [CrossRef]
- Ankudinov, A. L.; Ravel, B.; Rehr, J. J.; Conradson, S. D. RealSpace Multiple-Scattering Calculation and Interpretation of X-RayAbsorption Near-Edge Structure. Phys. Rev. B. 1998, 58, 7565. [Google Scholar] [CrossRef]
- Rehr, J.J.; Albers, R.C. Theoretical Approaches to X-Ray Absorption Fine Structure. Rev. Mod. Phys. 2000, 72, 621. [Google Scholar] [CrossRef]
- Hedin, L.; Lundqvist, B. I. Explicit Local Exchange-Correlation Potentials. J. Phys. C Solid State Phys. 1971, 4, 2064. [Google Scholar] [CrossRef]
- Newville, M.L. An Analysis Package for XAFS and Related Spectroscopies. J. Phys.: Conf. Ser. 2013, 430, 012007. [Google Scholar] [CrossRef]
- Fonda, E.; Andreatta, D.; Colavita, P.E.; Vlaica, G. EXAFS analysis of the L3 edge of Ce in CeO2: effects of multi-electron excitations and final-state mixed valence. J. Synchrotron Rad. 1999, 6, 34–42. [Google Scholar] [CrossRef]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Rad. 2005, 12, 537–541. [Google Scholar] [CrossRef]
- Blatun, L. A. Wounds and wound infections. The prof. B.M. Kostyuchenok journal 2015, 2, 36–44. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




