Submitted:
03 July 2024
Posted:
04 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Method
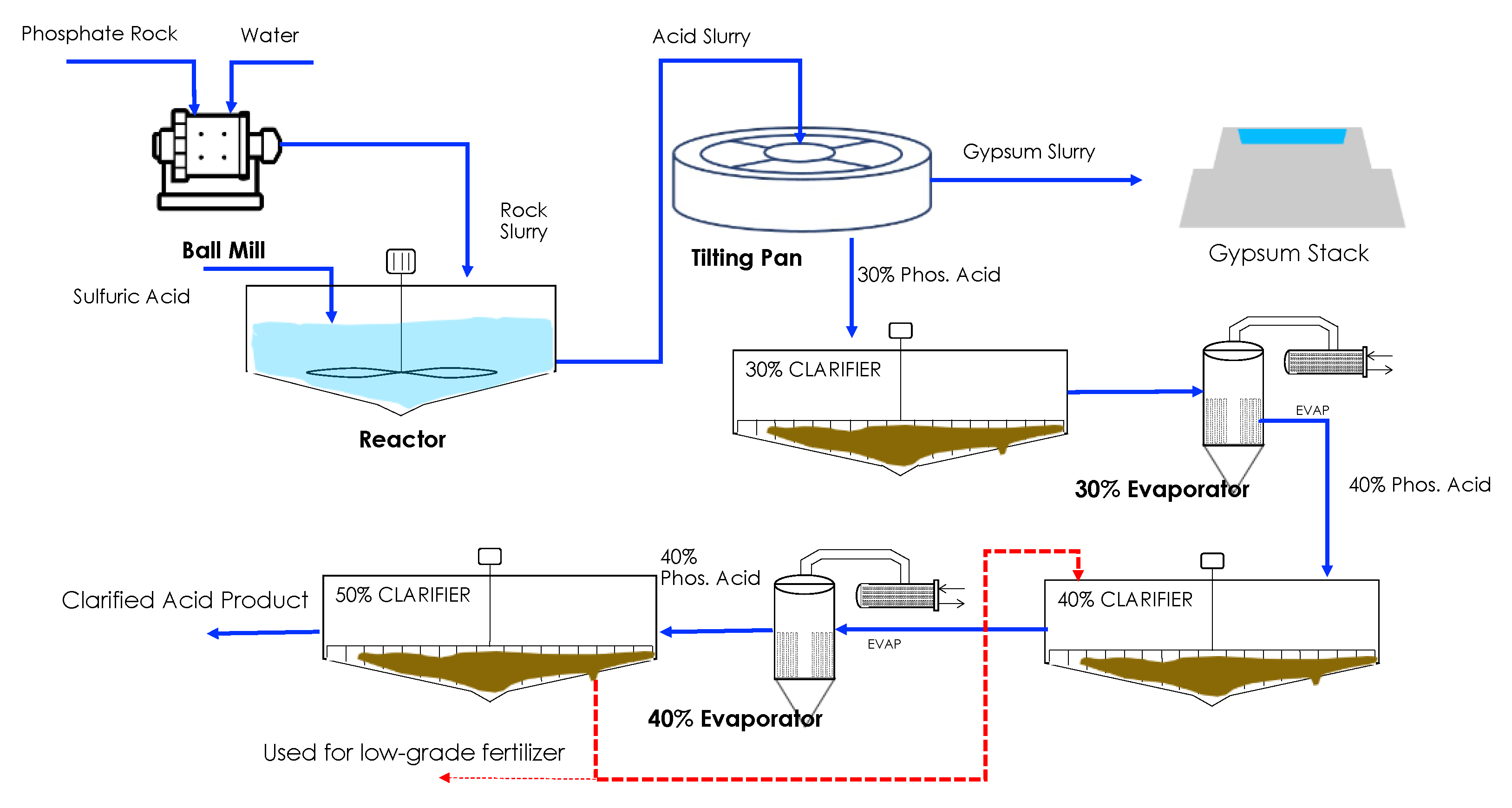
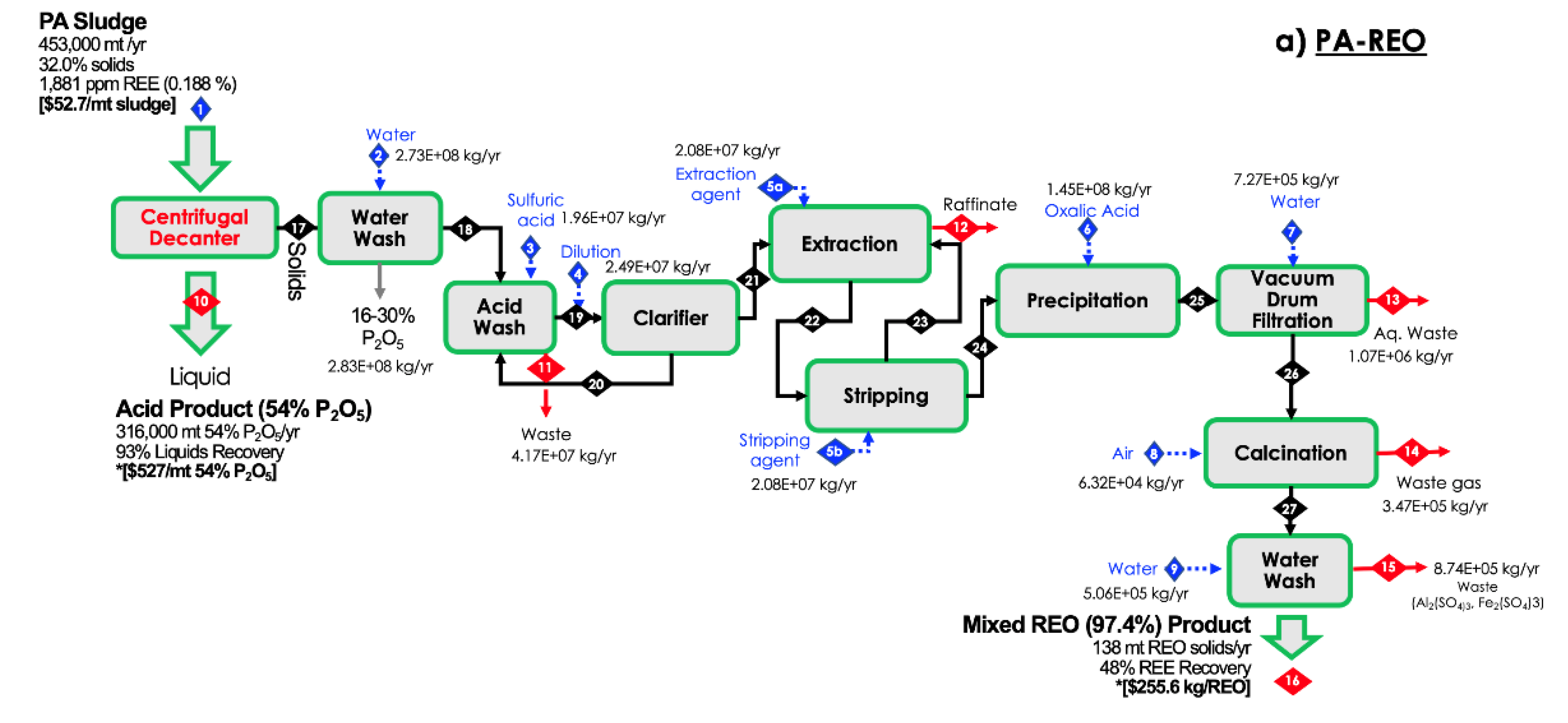
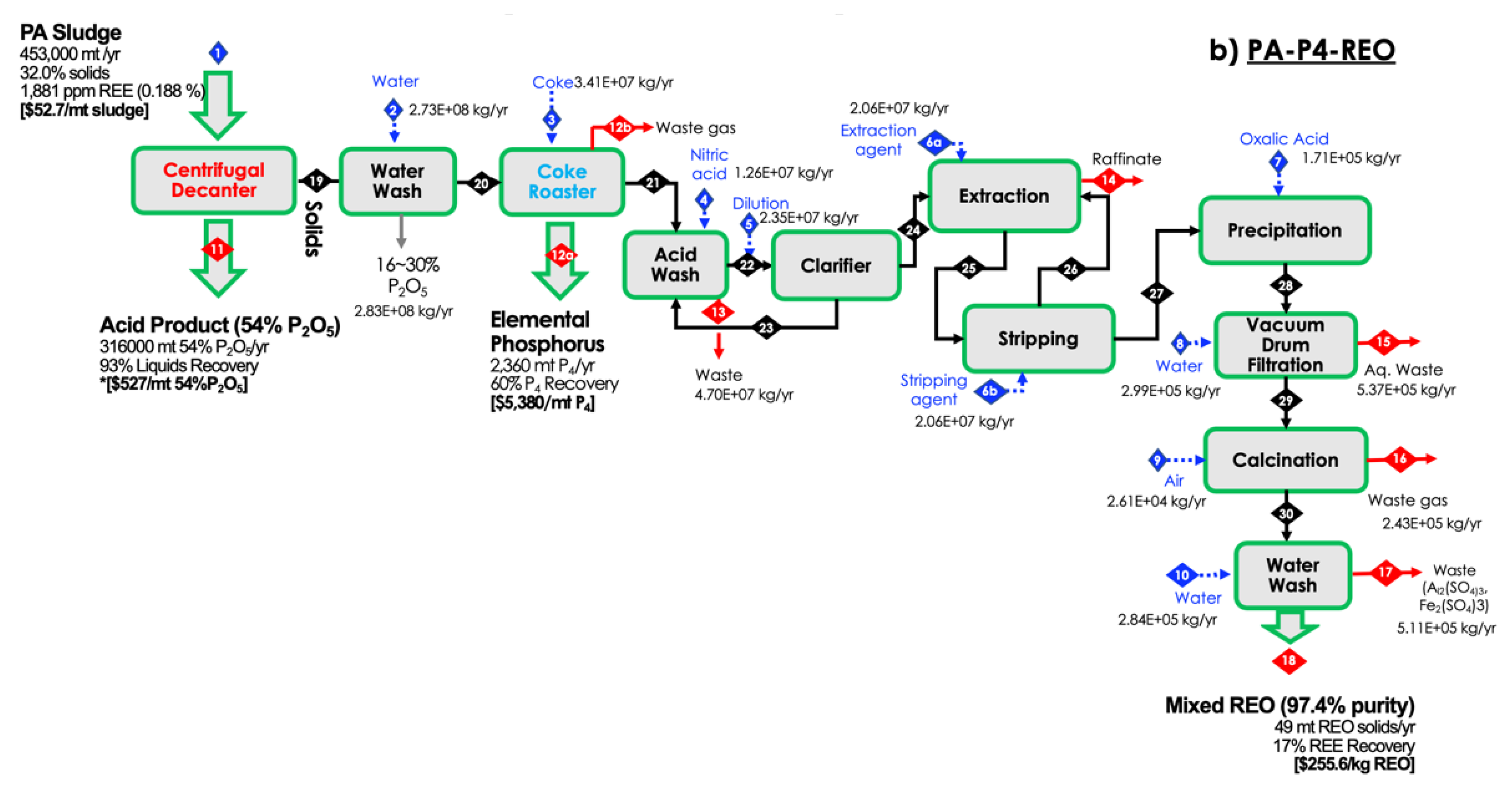
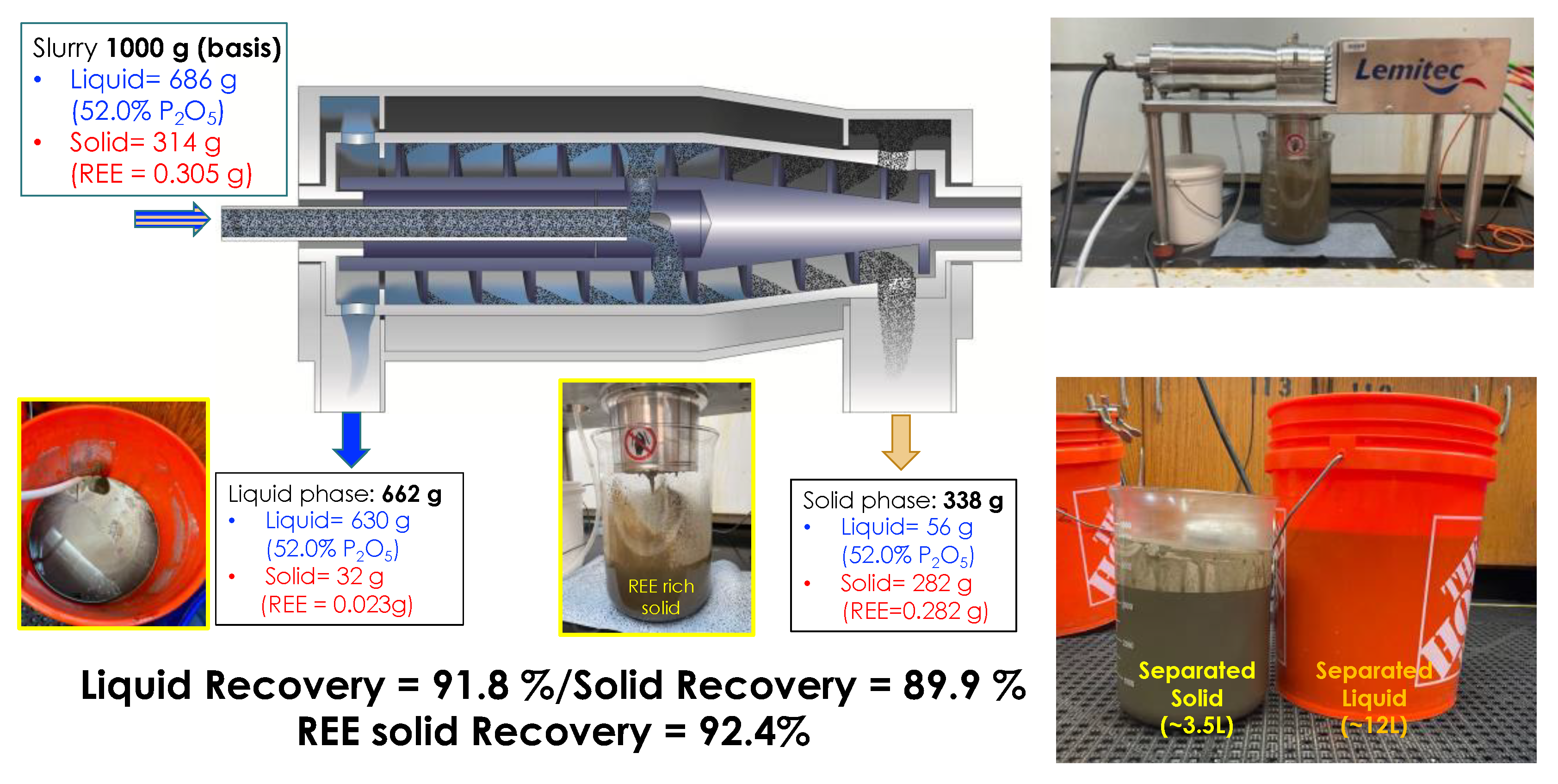
2.1. Solid/Liquid Separation
2.2. Technoeconomic Analysis
| Price Point: (100% P2O5) | Average Price ($/MT) | 2016-2023 AVG | Spot Price | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 | |||
| Brazil Quarterly Spot/Contract CFR | $756 | $665 | $781 | $746 | $683 | $1,128 | $1,773 | $1,197 | $980 | $1,034 |
| India Quarterly Contract CFR |
$626 | $566 | $732 | $691 | $628 | $1,073 | $1,541 | $975 | $849 | $850 |
| North Africa Quarterly Contract FOB | $699 | $602 | $764 | $745 | $687 | $1,114 | $1,617 | $1,073 | $906 | $887 |
| NW Europe (non-food grade) Quarterly Spot/Contract CFR | N/A | N/A | N/A | N/A | N/A | $1,254 | $1,712 | $1,218 | $1,427 | $1,034 |
| NW Europe Quarterly Spot/Contract CFR | $826 | $705 | $855 | $852 | $791 | $1,193 | $1,729 | $1,223 | $1,024 | $1,034 |
3. Results and Discussion
3.1. Process Validation
3.2. Technoeconomic Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Binnemans, K.; Jones, P.T.; Blanpain, B.; Van Gerven, T.; Yang, Y.X.; Walton, A.; Buchert, M. Recycling of rare earths: a critical review. J Clean Prod 2013, 51, 1–22. [Google Scholar] [CrossRef]
- Klaus, J.H.D.; Schulz, J., Jr.; Seal, R.R., II; Bradley, D.C. Critical mineral resources of the United States—Economic and environmental geology and prospects for future supply; U.S. Geological Survey: 2017.
- Critical Materials Supply Chain: A Situational White Paper U.S. Departement of Energy, Office of Energy Efficiency & Renewable Energy: 2020.
- Jang, G.G.; Ladshaw, A.; Keum, J.K.; Zhang, P.; Tsouris, C. Continuous-Flow Centrifugal Solid/Liquid Separation for the Recovery of Rare-Earth Elements Containing Particles from Phosphoric Acid Sludge. Ind Eng Chem Res 2020, 59, 21901–21913. [Google Scholar] [CrossRef]
- Jang, G.G.; Ladshaw, A.; Keum, J.K.; Thompson, J.A.; Zhang, P.T.; Tsouris, C. Continuous recovery of phosphoric acid and Rare-Earths containing particles from phosphoric acid sludge using a decanter centrifuge. Chem Eng J 2023, 458. [Google Scholar] [CrossRef]
- Wu, S.X.; Wang, L.S.; Zhao, L.S.; Zhang, P.; El-Shall, H.; Moudgil, B.; Huang, X.W.; Zhang, L.F. Recovery of rare earth elements from phosphate rock by hydrometallurgical processes - A critical review. Chem Eng J 2018, 335, 774–800. [Google Scholar] [CrossRef]
- <i>Mineral Commodity Summaries, 2019</i>; U.S. Department of the Interior, U.S. Mineral Commodity Summaries 2019; U.S. Department of the Interior, U.S. Geological Survey: 2019.
- Argus North American Fertilizer, (23-1) (2023). https://doi.org/www.argusmedia.com/argus-north-america-fertilizer.pdf.
- Das, S.; Gaustad, G.; Sekar, A.; Williams, E. Techno-economic analysis of supercritical extraction of rare earth elements from coal ash. J Clean Prod 2018, 189, 539–551. [Google Scholar] [CrossRef]
- Fritz, A.G.; Tarka, T.J.; Mauter, M.S. Technoeconomic Assessment of a Sequential Step-Leaching Process for Rare Earth Element Extraction from Acid Mine Drainage Precipitates. Acs Sustain Chem Eng 2021, 9, 9308–9316. [Google Scholar] [CrossRef]
- Broutin, P.; Briot, P.; Ehlers, S.; Kather, A. Benchmarking of the DMX (TM) CO2 Capture Process. 13th International Conference on Greenhouse Gas Control Technologies, Ghgt-13 2017, 114, 2561–2572. [Google Scholar] [CrossRef]
- Max, S. Peters, K.D.T., Ronald E. West. Plant Design and Economics for Chemical Engineers, 5th ed.; McGraw-Hill Professional: New York, NY, 2002. [Google Scholar]
- Liang, H.; Zhang, P.; Jin, Z.; DePaoli, D. Rare earths recovery and gypsum upgrade from Florida phosphogypsum. Miner Metall Proc 2017, 34, 201–206. [Google Scholar] [CrossRef]
- Liang, H.; Zhang, P.; Jin, Z.; DePaoli, D. Rare-earth leaching from Florida phosphate rock in wet-process phosphoric acid production. Miner Metall Proc 2017, 34, 146–153. [Google Scholar] [CrossRef]
- Lokshin, E.P.; Tareeva, O.A.; Elizarova, I.P. A study of the sulfuric acid leaching of rare-earth elements, phosphorus, and alkali metals from phosphodihydrate. Russ J Appl Chem+ 2010, 83, 958–964. [Google Scholar] [CrossRef]
- Rychkov, V.N.; Kirillov, E.V.; Kirillov, S.V.; Semenishchev, V.S.; Bunkov, G.M.; Botalov, M.S.; Smyshlyaev, D.V.; Malyshev, A.S. Recovery of rare earth elements from phosphogypsum. J Clean Prod 2018, 196, 674–681. [Google Scholar] [CrossRef]
- Walawalkar, M.; Nichol, C.K.; Azimi, G. Process investigation of the acid leaching of rare earth elements from phosphogypsum using HCl, HNO3, and H2SO4. Hydrometallurgy 2016, 166, 195–204. [Google Scholar] [CrossRef]
- Preston, J.S.; Cole, P.M.; Craig, W.M.; Feather, A.M. The recovery of rare earth oxides from a phosphoric acid by-product.1. Leaching of rare earth values and recovery of a mixed rare earth oxide by solvent extraction. Hydrometallurgy 1996, 41, 1–19. [Google Scholar] [CrossRef]
- Sulphuric acid Price Trend and Forecast. Available online: https://www.chemanalyst.com/Pricing-data/sulphuric-acid-70#:~:text=The%20cost%20of%20Sulphuric%20Acid,the%20end%20of%20the%20quarter.&text=In%20the%20second%20quarter%20of%202022%2C%20Sulphuric%20Acid%20prices%20were,in%20the%20Asia%20Pacific%20market (accessed on 23 May 2023).
- Lutke, S.F.; Oliveira, M.L.S.; Waechter, S.R.; Silva, L.F.O.; Cadaval, T.R.S.; Duarte, F.A.; Dotto, G.L. Leaching of rare earth elements from phosphogypsum. Chemosphere 2022, 301. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.T.; Hong, W.X.; Zhou, Y.S.; Pu, B.S.; Gong, A.J.; Xu, T.; Yang, Q.S.; Li, F.K.; Qiu, L.N.; Zhang, W.W.; et al. Extraction and back-extraction behaviors of 14 rare earth elements from sulfuric acid medium by TODGA. J Rare Earth 2018, 36, 642–647. [Google Scholar] [CrossRef]
- Ansari, S.A.; Pathak, P.; Mohapatra, P.K.; Manchanda, V.K. Chemistry of Diglycolamides: Promising Extractants for Actinide Partitioning. Chem Rev 2012, 112, 1751–1772. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, M. Separation of Trace Concentrations of Tantalum from Niobium and Some Other Heavy-Metal Ions by Extraction with N-Oxide of Trioctylamine. J Radioanal Chem 1975, 27, 67–75. [Google Scholar] [CrossRef]
- Derek Brigham, L.D., David DePaoli Lipophilic diglycolamide compounds for extraction of rare earth metals from aqueous solutions. June 22, 2019.
- Nawab, A.; Yang, X.B.; Honaker, R. Parametric study and speciation analysis of rare earth precipitation using oxalic acid in a chloride solution system. Miner Eng 2022, 176. [Google Scholar] [CrossRef]
- Chi, R.; Xu, Z. A solution chemistry approach to the study of rare earth element precipitation by oxalic acid. Metall Mater Trans B 1999, 30, 189–195. [Google Scholar] [CrossRef]
- Zhang, H.G.; Wang, L.G.; Xu, Y.C. Design research of efficient vacuum-type rotary drum filter. Key Eng Mater 2011, 480-481, 1246–1250. [Google Scholar] [CrossRef]
- Weber, L.L., Tyler; and Alzubairi, Abdullah. An Economic Analysis of the Extraction of Rare Earth Elements from WPPA Sand Tailings Waste Stream; University of Tennessee, Knoxville: 2015.
- Miranda, M.M.; Bielicki, J.M.; Chun, S.; Cheng, C.M. Recovering Rare Earth Elements from Coal Mine Drainage Using Industrial Byproducts: Environmental and Economic Consequences. Environ Eng Sci 2022, 39, 770–783. [Google Scholar] [CrossRef] [PubMed]
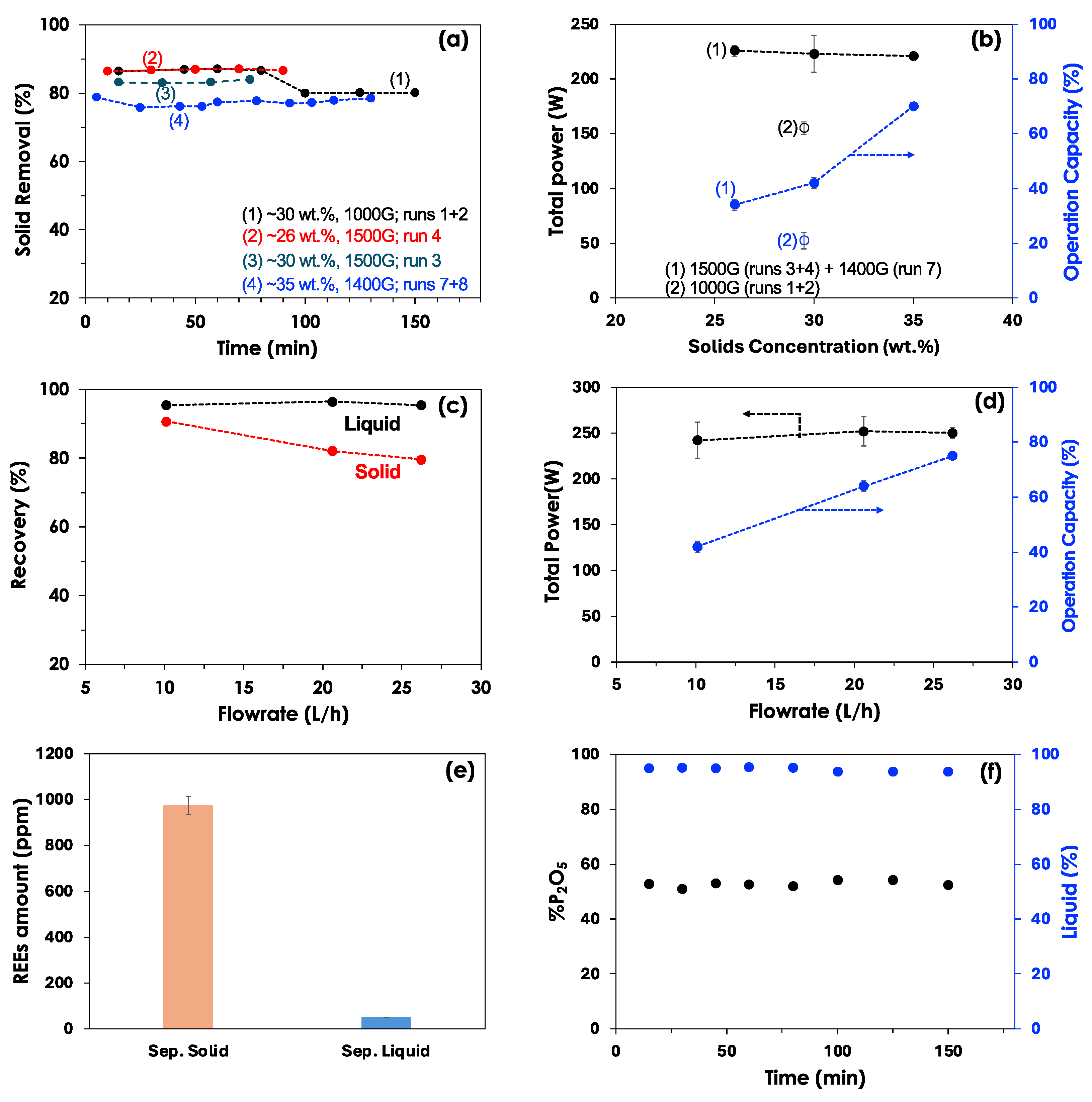
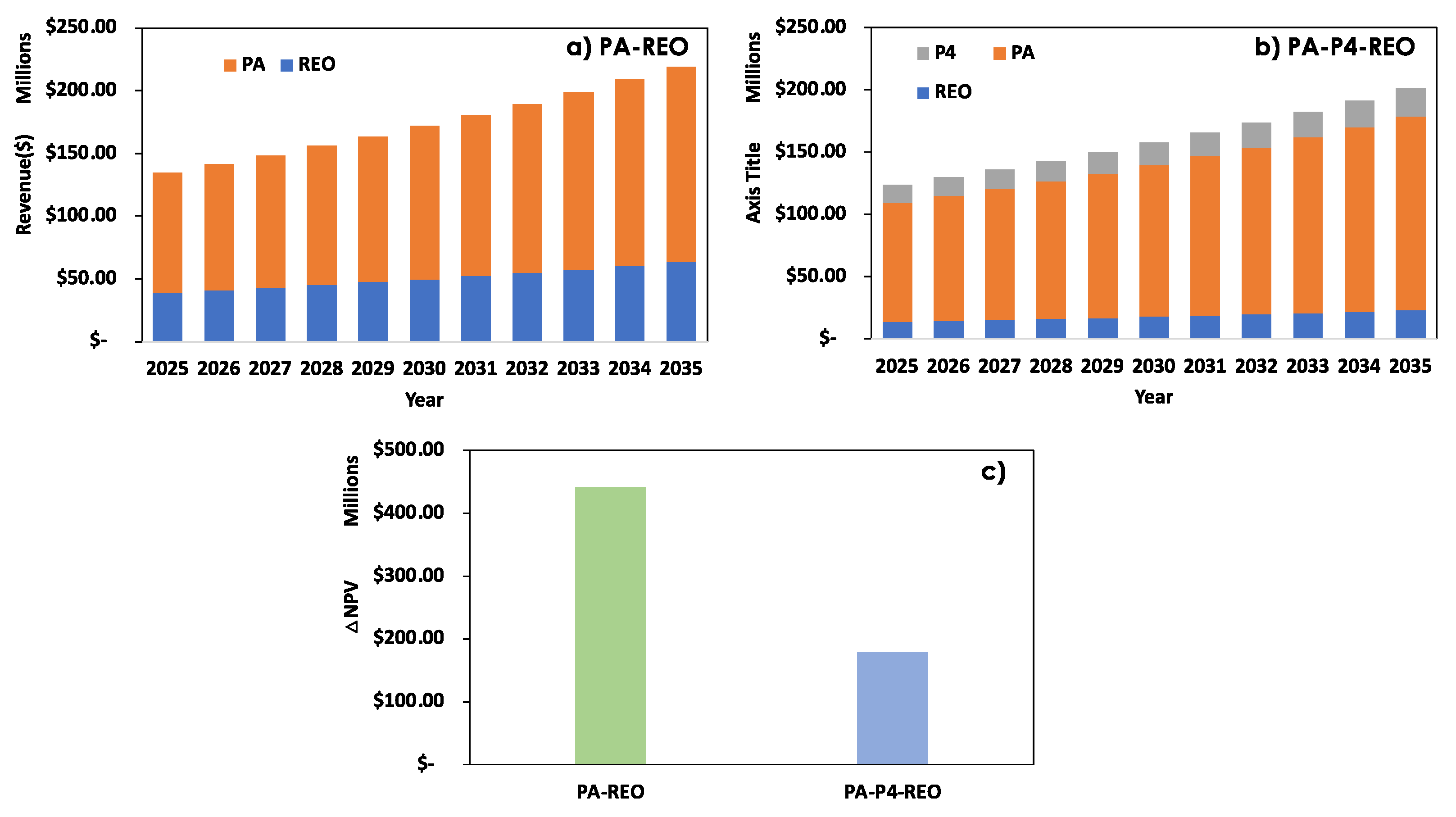
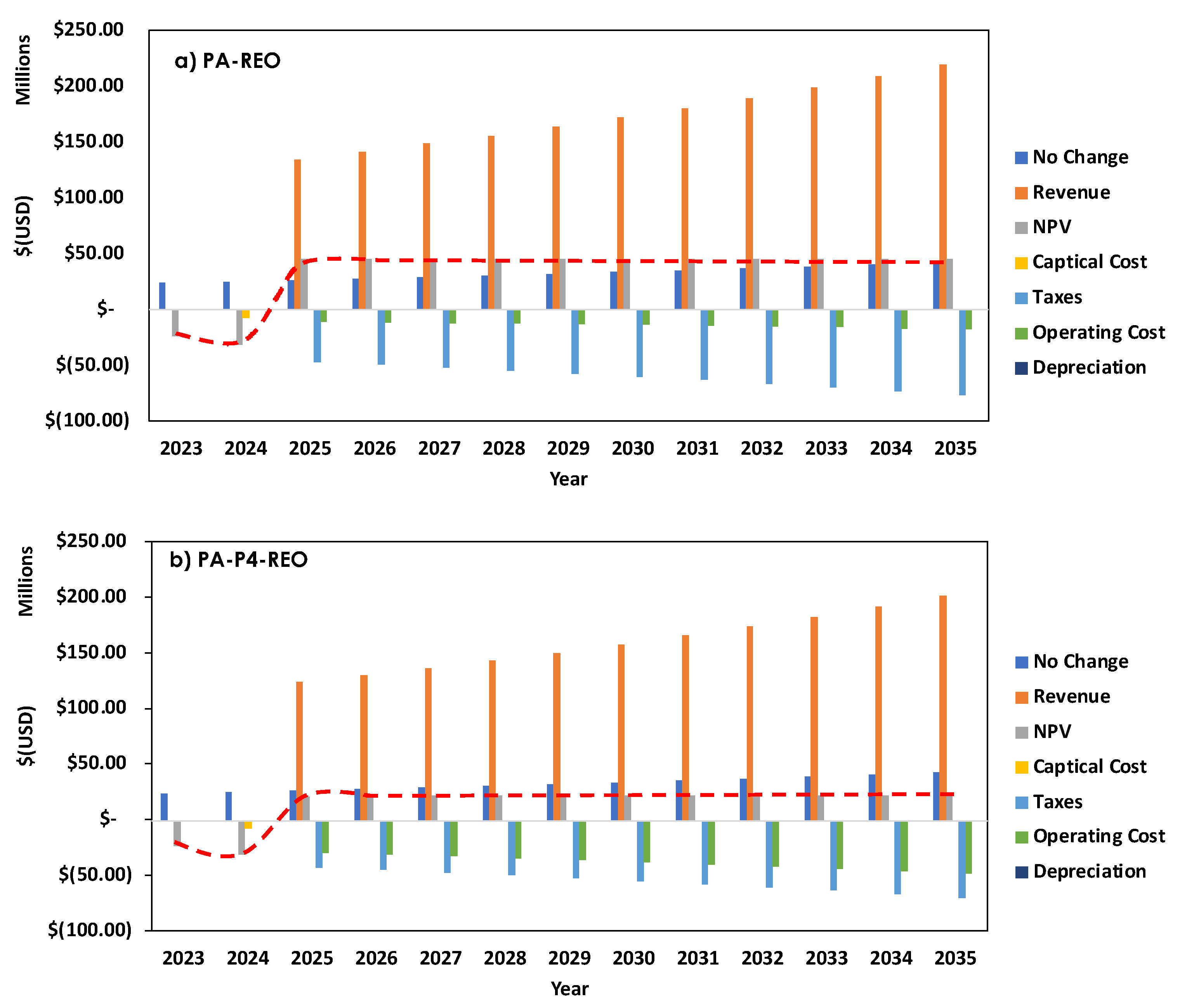
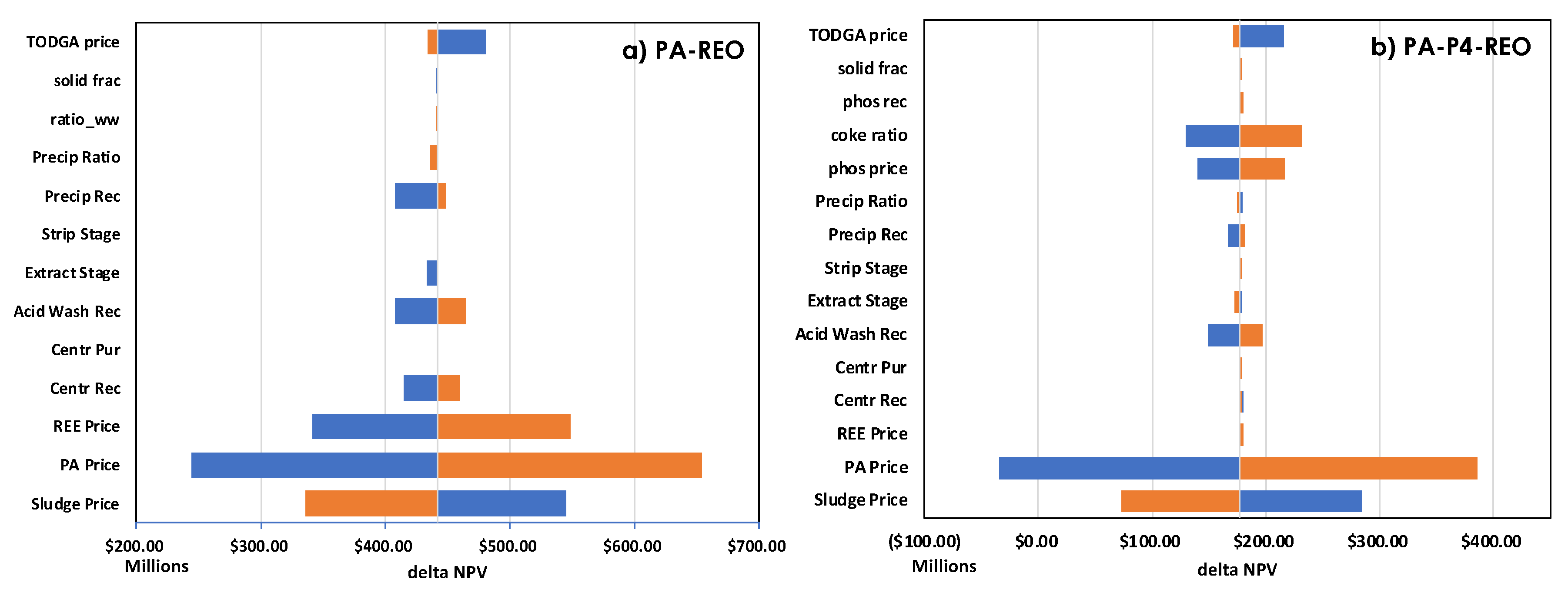
| Method | Source (REE amount, ppm) | Capacity | CAPEX ($) |
OPEX REO($/kg) | Value of REO ($/kg) | Economic Viability |
Critical Factor | Ref |
|---|---|---|---|---|---|---|---|---|
| Supercritical Extraction | Coal ash (270-1480) | - | - | 690-2181 | 6-557 | low | Price of REE (i.e., Sc) | [9] |
| Leaching/solvent extraction | Acid mine drainage precipitation (1103-1669) | 1 mt REE/yr | 2.6-3.4 million | 3400-5900 | - | Very low | Process improvement Market change |
[10] |
| Nano-fluid extraction | Geothermal Brine (0.25) |
6800 gal brine/ min |
6.8 million |
468 | - | low | REE conc. /price, electromagnet price Need co-production other valuable material |
[11] |
| Our study (PA-REO) |
Solid in PA sludge (1000-3000) |
431,000 mt/yr |
7.1 million |
71 | 256 | Promising | Co-production of PA | |
| Our study (PA-P4-REO) |
Solid in PA sludge (1000-3000) |
431,000 mt/yr |
7.5 million |
551 | 256 | Promising | Co-production of PA and elemental P |
| PA-REO ($) | PA-P4-REO ($) | |
|---|---|---|
| Centrifugal Decanter | 756,594 | 756,594 |
| Roster | - | 882,480 |
| Acid Washer | 1,088,849 | 562,587 |
| Clarifier | 161,910 | 158,047 |
| Extractor | 1,670,663 | 1,638,071 |
| Stripper | 464,497 | 472,427 |
| Precipitator | 29,587 | 27,826 |
| Drum Filter | 373,052 | 352,104 |
| Calciner | 22,415 | 21,648 |
| Total Module Cost | 4,567,565 | 4,871,798 |
| Installation | 685,135 | 730,770 |
| Piping/Materials | 685,135 | 730,770 |
| Buildings | 685,135 | 730,770 |
| Engineering | 456,756 | 487,180 |
| Total Capital Cost | 7,079,725 | 7,551,286 |
| PA-REO | PA-P4-REO | |||||
| Category | Stream | Operating Cost ($/yr) | Category | Stream | Operating Cost ($/yr) | |
| Centrifuge Decanter | Electricity | 1 | 43,843 | Electricity | 1 | 43,518 |
| Water washing | Process Water | 2 | 92,715 | Process water | 2 | 93,893 |
| Roster | - | - | - | Coke/Air | 3 | 8,405,691 |
| Acid Wash | Sulfuric acid/ Electricity | 3/4 | 2,846,754 /69,410 |
Nitric acid/ Electricity |
4/5 | 11,903,503 /36,435 |
| Extraction | TODGA /Trioctylamine |
5a | 4,348,734 /113 |
TODGA/ Trioctylamine |
6a | 4,298,506 /112 |
| Exxal 13 /Isopropanol |
5b | 6,049 /6,600 |
Exxal 13 /Isopropanol |
6b | 5,979 /6,535 |
|
| Precipitation | Oxalic acid | 6 | 441,637 | Oxalic acid | 7 | 314,582 |
| Drum filter | Process water & Electricity | 7 | 2,681 | Process water & Electricity | 8 | 2,310 |
| Calcination | Air & Power | 8 | 21,175 | Air & Power | 9 | 14,669 |
| Total | 7,879,711 | 25,125,734 | ||||
| Maintenance & Repairs |
15% Capital | 685,135 | 730,770 | |||
| Operating Supplies | 15% Maint. Rep. | 102,770 | 109,615 | |||
| Charges | 15% Op. Suppl. | 15,415 | 16,442 | |||
| Labor | Operator Salary = $60k | 309,240 | 302,884 | |||
| Overhead | 695,582 | 717,115 | ||||
| Taxes | 91,351 | 97,436 | ||||
| Insurance | 45,676 | 48,718 | ||||
| Total Operating Costs | 9,824,880 | 27,148,714 | ||||
| PA-REO | PA-P4-REO | |
|---|---|---|
| 54% PA | $0.9/mt | $0.9/mt |
| P4 | - | $4,104.8/mt |
| Mixed REO (97.4%) | $71.0/kg | $550.6/kg |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





