Submitted:
03 July 2024
Posted:
04 July 2024
Read the latest preprint version here
Abstract
Keywords:
Introduction
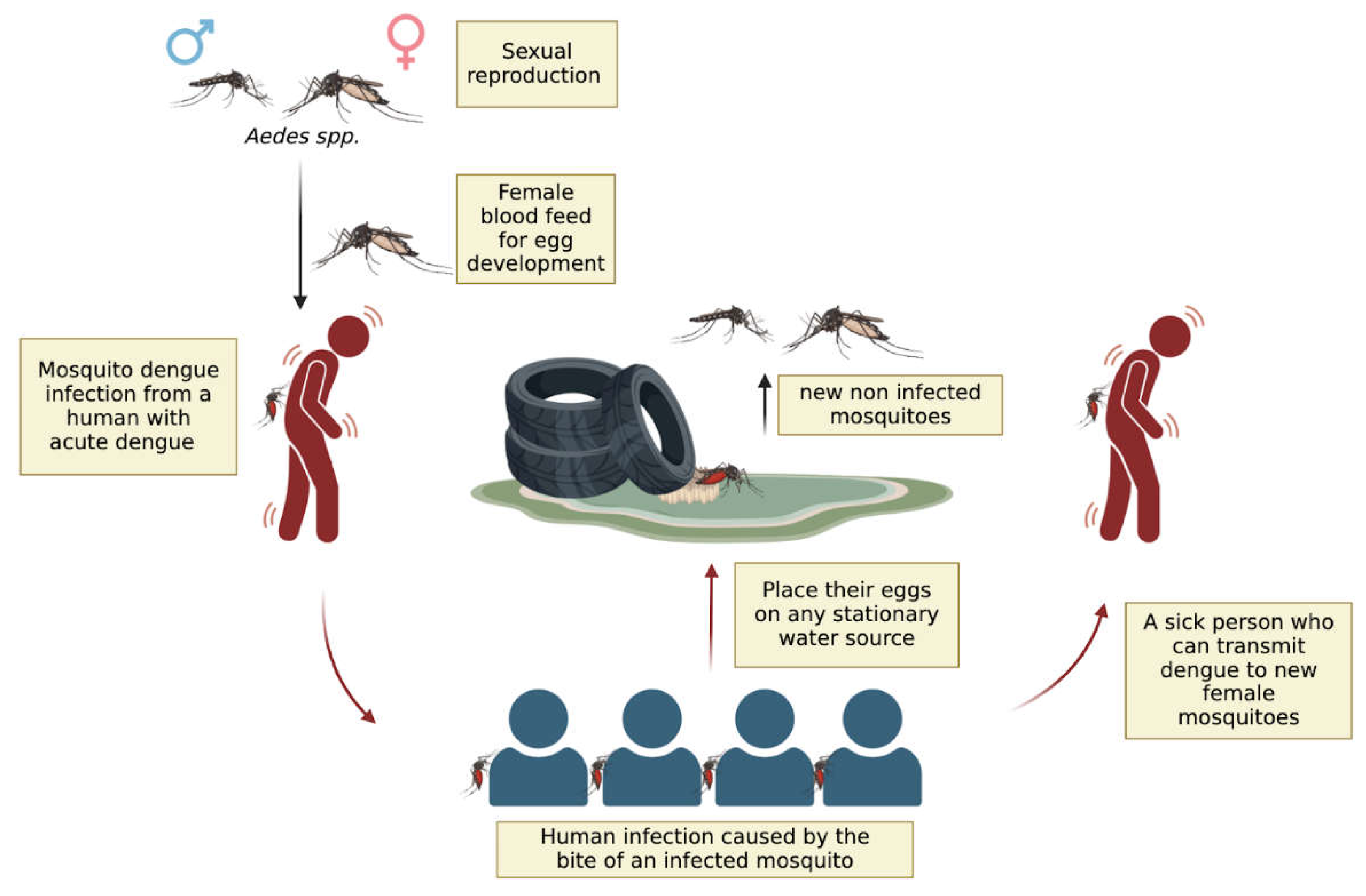
The Impact of Global Warming on Dengue Outbreaks in the Americas

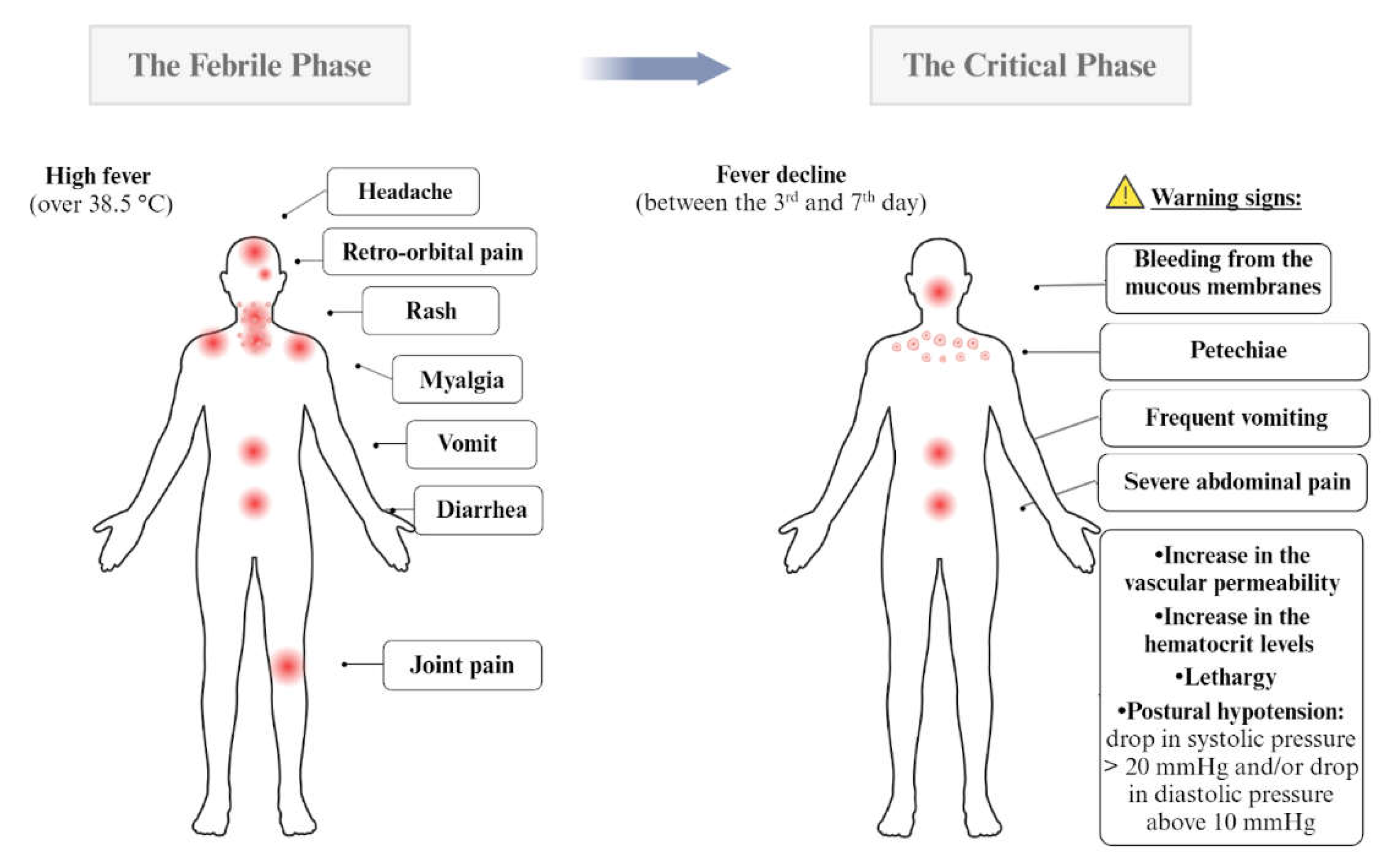
Dengue Symptoms and Determinants for Recurrence and Disease Severity
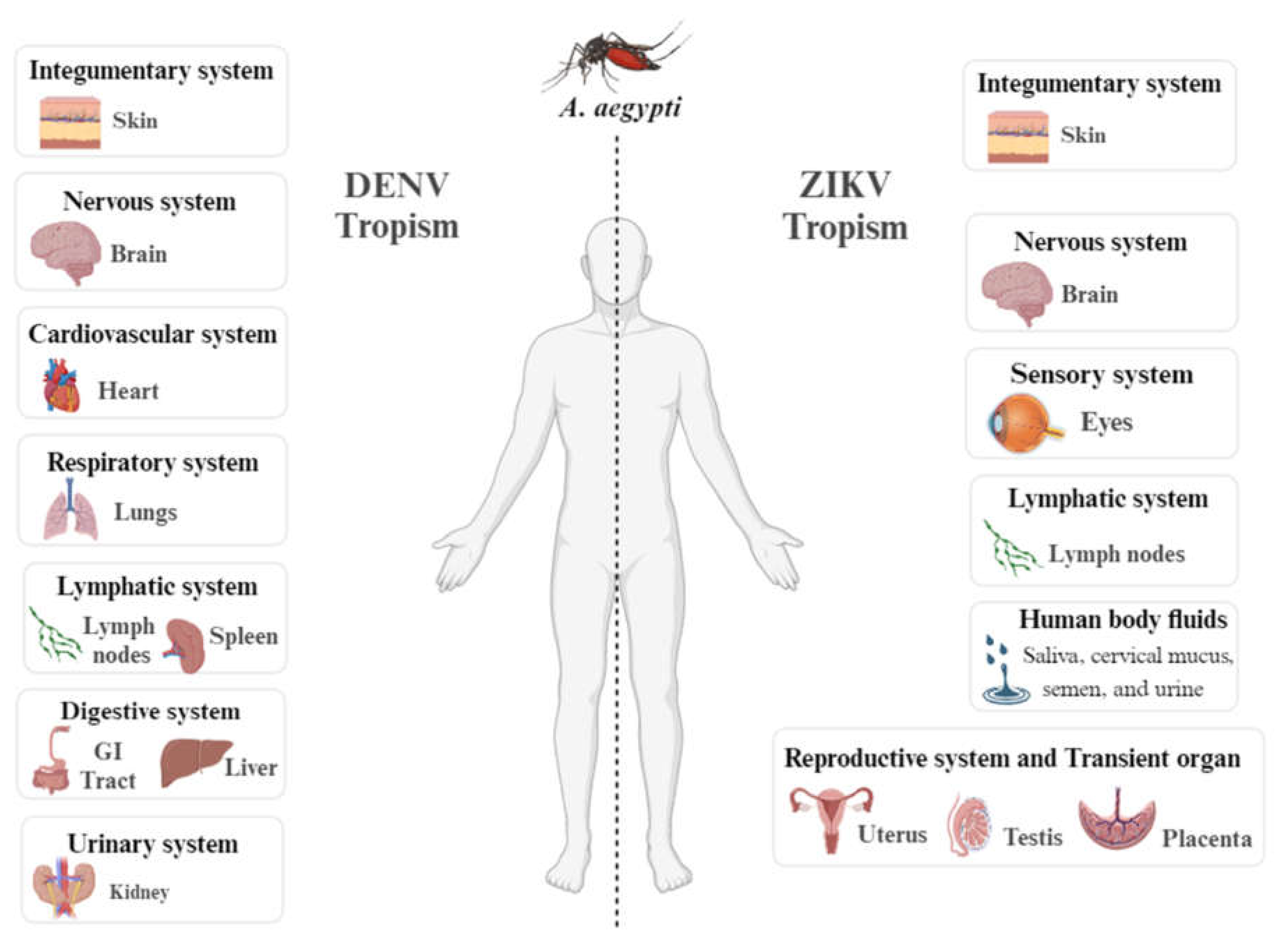
Clinical Management and Therapeutic Intervention in Dengue
| Signs of shock | Clinical approach | Reference | |
| Group A(Blue) | - |
|
[66,68,72,73] |
| Group B(Green) | Patients presenting two or more clinical signs of the acute phase in addition to spontaneous bleeding, which can be indicated by petechiae, gingival bleeding and ecchymosis. | The care occurs in a Secondary Health Care Unit equipped with an on-observation bed, where they are required to stay hospitalized for a minimum of 12 h. During this time, they receive oral or intravenous hydration and undergo a complete blood count to monitor their hematocrit levels. | [66,73] |
| Group C(Yellow) | Here symptoms may arise that may indicate the progression of the disease to a more serious clinical condition that includes lethargy, severe abdominal pain, postural hypotension, frequent vomiting, bleeding from the mucous membranes, progressive increase in the hematocrit levels and decrease of the platelets levels. |
|
[66,73] |
| Group D(Red) | Intended for patients presenting signs of shock: convergent blood pressure (Differential BP <20mm Hg), arterial hypotension, cyanosis, rapid pulse, and slow capillary refill. |
|
[66,72,73] |
Measures Adopted in Brazil to Mitigate the DENV Cases
Diagnosis Test Available in Brazil for Dengue Disease
| Diagnostic test timeline (days after symptom onset) | |||||
|---|---|---|---|---|---|
| Method principle | Primary Infection (PI) | Secondary Infection (SI) | Time to obtain the result | ||
| Capillary fragility | Tourniquet Test |
|
min | ||
| Virus or virus product detection | Virus isolation |
|
≥ one week | ||
|
|
||||
| RT-PCR or RT-qPCR |
|
around one day | |||
| NS1 protein detection | ELISA (serum) | - from 0 to 7 days
|
- from 0 to 3 days
|
around one day | |
| RDT (serum) | min | ||||
- from 0 to 3 days
| |||||
| Antibody detection | IgM detection | ELISA (serum) |
|
|
around one day |
| RDT (serum) | - from 0 to 3 days - from 4 days onwards | min | |||
| IgG detection | RDT (serum) |
|
|
min | |
Dengue's Emergence in Europe: a Changing Epidemiological Landscape
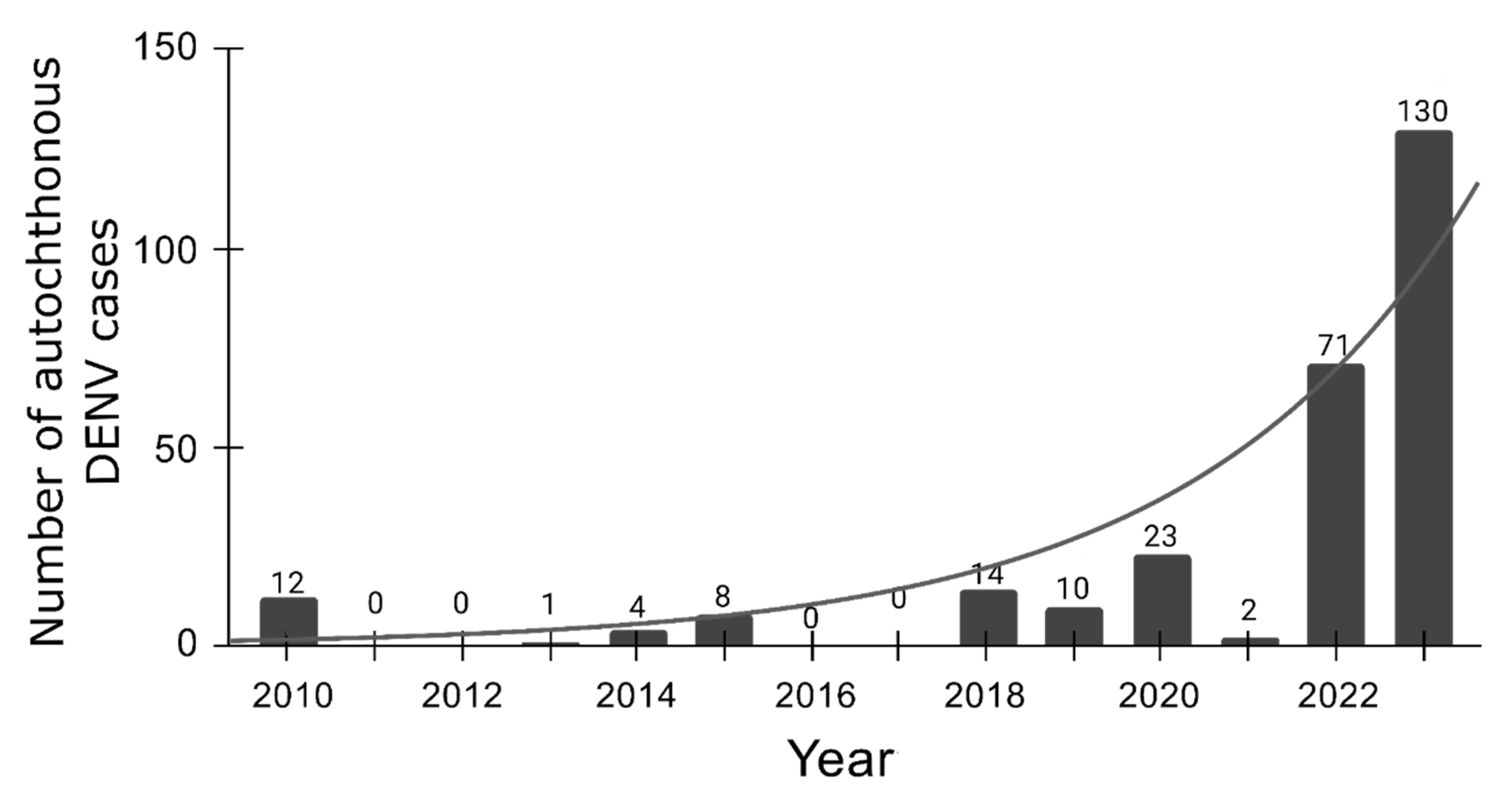
Conclusions
Informed Consent Statement
Data Availability Statement
Acknowledgment
Conflicts of Interest
References
- FAO-WHO Expert Committee on Zoonoses. Zoonoses: Report of the FAO-WHO Expert Committee on Zoonoses, 3rd, Geneva, 1966. (1967).
- Harapan, H., Michie, A., Sasmono, R. T. & Imrie, A. Dengue: A Minireview. Viruses 12, (2020).
- Arthropod-borne and rodent-borne viral diseases. Report of a WHO Scientific Group. World Health Organ. Tech. Rep. Ser. 719, 1–116 (1985).
- Young, P. R. Arboviruses: A Family on the Move. Adv. Exp. Med. Biol. 1062, 1–10 (2018).
- Go, Y. Y., Balasuriya, U. B. R. & Lee, C.-K. Zoonotic encephalitides caused by arboviruses: transmission and epidemiology of alphaviruses and flaviviruses. Clin. Exp. Vaccine Res. 3, 58–77 (2014).
- Hollidge, B. S., González-Scarano, F. & Soldan, S. S. Arboviral encephalitides: transmission, emergence, and pathogenesis. J. Neuroimmune Pharmacol. 5, 428–442 (2010).
- Weaver, S. C. & Reisen, W. K. Present and future arboviral threats. Antiviral Res. 85, 328–345 (2010). [CrossRef]
- Liu, Y., Guan, W. & Liu, H. Subgenomic Flaviviral RNAs of Dengue Viruses. Viruses 15, (2023).
- Murugesan, A. & Manoharan, M. Dengue Virus. in Emerging and Reemerging Viral Pathogens 281–359 (Elsevier, 2020). [CrossRef]
- Drugs targeting structural and nonstructural proteins of the chikungunya virus: A review. Int. J. Biol. Macromol. 262, 129949 (2024). [CrossRef]
- Caicedo, E.-Y. et al. The epidemiology of Mayaro virus in the Americas: A systematic review and key parameter estimates for outbreak modelling. PLoS Negl. Trop. Dis. 15, e0009418 (2021).
- Martins-Filho, P. R., Soares-Neto, R. F., de Oliveira-Júnior, J. M. & Alves Dos Santos, C. The underdiagnosed threat of oropouche fever amidst dengue epidemics in Brazil. Lancet Reg Health Am 32, 100718 (2024).
- Cattarino, L., Rodriguez-Barraquer, I., Imai, N., Cummings, D. A. T. & Ferguson, N. M. Mapping global variation in dengue transmission intensity. Sci. Transl. Med. 12, (2020).
- Kularatne, S. A. & Dalugama, C. Dengue infection: Global importance, immunopathology and management. Clin. Med. 22, 9–13 (2022).
- Ferreira-de-Lima, V. H. & Lima-Camara, T. N. Natural vertical transmission of dengue virus in Aedes aegypti and Aedes albopictus: a systematic review. Parasit. Vectors 11, 77 (2018).
- Tauil, P. L. [Critical aspects of dengue control in Brazil]. Cad. Saude Publica 18, 867–871 (2002).
- Paz-Bailey, G., Adams, L. E., Deen, J., Anderson, K. B. & Lc., K. Website. Lancet 403, 667–682 (2024).
- Xu, Z. et al. Projecting the future of dengue under climate change scenarios: Progress, uncertainties and research needs. PLoS Negl. Trop. Dis. 14, e0008118 (2020).
- Morin, C. W., Comrie, A. C. & Ernst, K. Climate and dengue transmission: evidence and implications. Environ. Health Perspect. 121, 1264–1272 (2013).
- Combined effects of hydrometeorological hazards and urbanisation on dengue risk in Brazil: a spatiotemporal modelling study. The Lancet Planetary Health 5, e209–e219 (2021).
- Dengue overview: An updated systemic review. J. Infect. Public Health 16, 1625–1642 (2023).
- Chen, R. & Vasilakis, N. Dengue--quo tu et quo vadis? Viruses 3, 1562–1608 (2011).
- Bashyam, H. S., Green, S. & Rothman, A. L. Dengue virus-reactive CD8+ T cells display quantitative and qualitative differences in their response to variant epitopes of heterologous viral serotypes. J. Immunol. 176, 2817–2824 (2006).
- Parveen, S. et al. Dengue hemorrhagic fever: a growing global menace. J. Water Health 21, 1632–1650 (2023).
- Brathwaite Dick, O. et al. The history of dengue outbreaks in the Americas. Am. J. Trop. Med. Hyg. 87, 584–593 (2012).
- Juan, C. Dengue Fever: Strategies for Preventing Dengue and Bite Transmission Via Mosquitoes. (Independently Published, 2024).
- Cristodulo, R. et al. Dengue Myocarditis: A Case Report and Major Review. Glob. Heart 18, 41 (2023).
- Aedes albopictus - current known distribution: October 2023. European Centre for Disease Prevention and Control https://www.ecdc.europa.eu/en/publications-data/aedes-albopictus-current-known-distribution-october-2023 (2023).
- Schaefer, T. J., Panda, P. K. & Wolford, R. W. Dengue Fever. in StatPearls (StatPearls Publishing, Treasure Island (FL), 2024).
- Gamez, S., Antoshechkin, I., Mendez-Sanchez, S. C. & Akbari, O. S. The Developmental Transcriptome of , a Major Worldwide Human Disease Vector. G3 10, 1051–1062 (2020).
- Weerakoon, K. G. et al. Histopathological diagnosis of myocarditis in a dengue outbreak in Sri Lanka, 2009. BMC Res. Notes 4, 268 (2011).
- Sinha, S. et al. Dengue virus pathogenesis and host molecular machineries. J. Biomed. Sci. 31, 43 (2024).
- Madhry, D. et al. Role of non-coding RNAs in Dengue virus-host interaction. Front. Biosci. 13, 44–55 (2021).
- Guzman, M. G., Gubler, D. J., Izquierdo, A., Martinez, E. & Halstead, S. B. Dengue infection. Nature Reviews Disease Primers 2, 1–25 (2016).
- Gubler, D. J., Ooi, E. E., Vasudevan, S. & Farrar, J. Dengue and Dengue Hemorrhagic Fever, 2nd Edition. (CABI, 2014).
- Kok, B. H. et al. Dengue virus infection - a review of pathogenesis, vaccines, diagnosis and therapy. Virus Res. 324, 199018 (2023).
- Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control: New Edition. (World Health Organization, Geneva, 2009).
- Zerfu, B., Kassa, T. & Legesse, M. Epidemiology, biology, pathogenesis, clinical manifestations, and diagnosis of dengue virus infection, and its trend in Ethiopia: a comprehensive literature review. Trop. Med. Health 51, 11 (2023).
- Kalayanarooj, S. Clinical Manifestations and Management of Dengue/DHF/DSS. Trop. Med. Health 39, 83–87 (2011).
- Verma, R. et al. Neurological complications of dengue fever: Experience from a tertiary center of north India. Ann. Indian Acad. Neurol. 14, 272–278 (2011).
- Marengo, J. A. Água e mudanças climáticas. Estud. av. 22, 83–96 (2008).
- Southern Brazil has seen an increase of up to 30% in average annual rainfall over the last three decades. Planalto https://www.gov.br/planalto/en/latest-news/2024/05/southern-brazil-has-seen-an-increase-of-up-to-30-in-average-annual-rainfall-over-the-last-three-decades (2024).
- Diallo, D. et al. Dengue vectors in Africa: A review. Heliyon 8, e09459 (2022).
- BRASIL. Painel de Monitoramento das Arboviroses. Ministério da Saúde https://www.gov.br/saude/pt-br/assuntos/saude-de-a-a-z/a/aedes-aegypti/monitoramento-das-arboviroses/painel (2024).
- Statistica. Dengue cases in Latin America & the Caribbean 2016-2024. Statista https://www.statista.com/statistics/1099801/latin-america-number-dengue-cases/ (2024).
- PAHO. Situation Report No 20 - Dengue Epidemiological Situation in the Region of the Americas - Epidemiological Week 20, 2024. Pan American Health Organization (PAHO) https://www.paho.org/en/documents/situation-report-no-20-dengue-epidemiological-situation-region-americas-epidemiological (2024).
- BRASIL. Centro de Operações de Emergências (COE). Ministério da Saúde https://www.gov.br/saude/pt-br/assuntos/saude-de-a-a-z/a/arboviroses/informe-semanal/informe-semanal-no-02-coe (2024).
- Khanam, A., Gutiérrez-Barbosa, H., Lyke, K. E. & Chua, J. V. Immune-Mediated Pathogenesis in Dengue Virus Infection. Viruses 14, (2022).
- Waickman, A. T. et al. Evolution of inflammation and immunity in a dengue virus 1 human infection model. Sci. Transl. Med. 14, eabo5019 (2022).
- Dengue. Ministério da Saúde https://www.gov.br/saude/pt-br/assuntos/saude-de-a-a-z/d/dengue/dengue.
- Yung, C.-F. et al. Dengue serotype-specific differences in clinical manifestation, laboratory parameters and risk of severe disease in adults, singapore. Am. J. Trop. Med. Hyg. 92, 999–1005 (2015).
- Begam, N. N. et al. Management of dengue with co-infections: an updated narrative review. Drug Discov. Ther. 15, 130–138 (2021).
- Henrique Ferreira Sucupira, P. et al. Serotype-associated immune response and network immunoclusters in children and adults during acute Dengue virus infection. Cytokine 169, 156306 (2023).
- Rowe, E. K. et al. Challenges in dengue fever in the elderly: atypical presentation and risk of severe dengue and hospital-acquired infection [corrected]. PLoS Negl. Trop. Dis. 8, e2777 (2014).
- Lin, C.-H. et al. Dengue outbreaks in high-income area, Kaohsiung City, Taiwan, 2003-2009. Emerg. Infect. Dis. 18, 1603–1611 (2012).
- Paz-Bailey, G. et al. Predominance of Severe Plasma Leakage in Pediatric Patients With Severe Dengue in Puerto Rico. J. Infect. Dis. 226, 1949–1958 (2022).
- Hober, D. et al. Serum levels of tumor necrosis factor-alpha (TNF-alpha), interleukin-6 (IL-6), and interleukin-1 beta (IL-1 beta) in dengue-infected patients. Am. J. Trop. Med. Hyg. 48, 324–331 (1993).
- Soo, K.-M., Khalid, B., Ching, S.-M. & Chee, H.-Y. Meta-Analysis of Dengue Severity during Infection by Different Dengue Virus Serotypes in Primary and Secondary Infections. PLoS One 11, e0154760 (2016).
- Estofolete, C. F. et al. Influence of previous Zika virus infection on acute dengue episode. PLoS Negl. Trop. Dis. 17, e0011710 (2023).
- Musso, D., Ko, A. I. & Baud, D. Zika Virus Infection - After the Pandemic. N. Engl. J. Med. 381, 1444–1457 (2019).
- Zhang, Y. et al. Oropouche virus: A neglected global arboviral threat. Virus Res. 341, 199318 (2024).
- Mohapatra, R. K., Mishra, S., Satapathy, P., Kandi, V. & Tuglo, L. S. Surging Oropouche virus (OROV) cases in the Americas: A public health challenge. New Microbes New Infect 59, 101243 (2024).
- Public Health risk assessment related to Oropouche virus (OROV) in the region of the Americas, 9 February 2024. ReliefWeb https://reliefweb.int/report/world/public-health-risk-assessment-related-oropouche-virus-orov-region-americas-9-february-2024.
- Febre do Oropouche. Ministério da Saúde https://www.gov.br/saude/pt-br/assuntos/saude-de-a-a-z/f/febre-do-oropouche.
- Sakkas, H., Bozidis, P., Franks, A. & Papadopoulou, C. Oropouche Fever: A Review. Viruses 10, (2018).
- B A Seixas, J., Giovanni Luz, K. & Pinto Junior, V. [Clinical Update on Diagnosis, Treatment and Prevention of Dengue]. Acta Med. Port. 37, 126–135 (2024).
- Rajapakse, S., de Silva, N. L., Weeratunga, P., Rodrigo, C. & Fernando, S. D. Prophylactic and therapeutic interventions for bleeding in dengue: a systematic review. Trans. R. Soc. Trop. Med. Hyg. 111, 433–439 (2017).
- Jasamai, M., Yap, W. B., Sakulpanich, A. & Jaleel, A. Current prevention and potential treatment options for dengue infection. J. Pharm. Pharm. Sci. 22, 440–456 (2019).
- Silva, N. M., Santos, N. C. & Martins, I. C. Dengue and Zika Viruses: Epidemiological History, Potential Therapies, and Promising Vaccines. Trop Med Infect Dis 5, (2020).
- Miner, J. J. & Diamond, M. S. Zika Virus Pathogenesis and Tissue Tropism. Cell Host Microbe 21, 134–142 (2017).
- Begum, F., Das, S., Mukherjee, D., Mal, S. & Ray, U. Insight into the Tropism of Dengue Virus in Humans. Viruses 11, (2019).
- BRASIL. Diretrizes nacionais para a prevenção e controle de epidemias de dengue. Brasília, DF: MS, 2009. Ministério da Saúde http://bvsms.saude.gov.br/bvs/publicacoes/diretrizes_nacionais_prevencao_controle_dengue (2024).
- Stanley, S. M., Khera, H. K., Chandrasingh, S., George, C. E. & Mishra, R. K. A comprehensive review of dengue with a focus on emerging solutions for precision and timely detection. Int. J. Biol. Macromol. 254, 127613 (2024).
- BRASIL. Ministério da Saúde-Saúde de A a Z-Dengue. Ministério da Saúde https://www.gov.br/saude/pt-br/assuntos/saude-de-a-a-z/d/dengue/dengue (2024).
- OPAS. Alerta Epidemiológico-Aumento de casos de dengue na Região das Américas. Organização Pan-Americana da Saúde (OPAS) https://www.paho.org/pt/documentos/alerta-epidemiologico-aumento-casos-dengue-na-regiao-das-americas-16-fevereiro-2024 (2024).
- BRASIL. Ministério da Saúde entrega nova remessa de vacinas da dengue. Ministério da Saúde https://www.gov.br/saude/pt-br/assuntos/noticias/2024/maio/ministerio-da-saude-entrega-nova-remessa-de-vacinas-da-dengue (2024).
- BRASIL. Ministério da Saúde elabora plano para enfrentamento da dengue 2024/2025. Ministério da Saúde https://www.gov.br/saude/pt-br/assuntos/noticias/2024/maio/ministerio-da-saude-elabora-plano-para-enfrentamento-da-dengue-2024-2025 (2024).
- BRASIL. Combate ao mosquito. Ministério da Saúde https://www.gov.br/saude/pt-br/campanhas-da-saude/2023/combate-ao-mosquito/combate-ao-mosquito (2023).
- Conselho Federal de Farmácia. Orientações sobre o uso de repelentes. Conselho Federal de Farmácia https://site.cff.org.br/noticia/noticias-do-cff/01/03/2024/orientacoes-sobre-o-uso-de-repelentes (2024).
- BRASIL. Saiba como é utilizado o fumacê no combate ao mosquito da dengue. Ministério da Saúde https://www.gov.br/saude/pt-br/assuntos/noticias/2024/marco/saiba-como-e-utilizado-o-fumace-no-combate-ao-mosquito-da-dengue (2024).
- BRASIL. Você sabe o que é o Método Wolbachia? Ministério da Saúde https://www.gov.br/saude/pt-br/assuntos/saude-com-ciencia/noticias/2024/maio/voce-sabe-o-que-e-o-metodo-wolbachia (2024).
- World Mosquito Program. Sobre o Método Wolbachia. World Mosquito Program https://www.worldmosquitoprogram.org/sobre-o-metodo-wolbachia (2024).
- BRASIL. Ministério da Saúde incorpora vacina contra a dengue no SUS. Ministério da Saúde https://www.gov.br/saude/pt-br/assuntos/noticias/2023/dezembro/ministerio-da-saude-incorpora-vacina-contra-a-dengue-no-sus (2023).
- Butantan, I. Instituto Butantan-Portal do Butantan. Tira Dúvida- Vacina da dengue deve proteger de todos os subtipos da doença; entenda a complexidade do ensaio clínico. https://butantan.gov.br/covid/butantan-tira-duvida/tira-duvida-noticias/vacina-da-dengue-deve-proteger-de-todos-os-subtipos-da-doenca--entenda-a-complexidade-do-ensaio-clinico (2022).
- Muller, D. A., Depelsenaire, A. C. I. & Young, P. R. Clinical and Laboratory Diagnosis of Dengue Virus Infection. J. Infect. Dis. 215, S89–S95 (2017).
- Gregory, C. J. et al. Utility of the tourniquet test and the white blood cell count to differentiate dengue among acute febrile illnesses in the emergency room. PLoS Negl. Trop. Dis. 5, e1400 (2011).
- Kalayanarooj, S. et al. Early clinical and laboratory indicators of acute dengue illness. J. Infect. Dis. 176, 313–321 (1997).
- Montes y Gómez, M., Escalante, H. J., Segura, A. & de Dios Murillo, J. Advances in Artificial Intelligence - IBERAMIA 2016: 15th Ibero-American Conference on AI, San José, Costa Rica, November 23-25, 2016, Proceedings. (Springer, 2016).
- Furlan, N. B., Tukasan, C., Estofolete, C. F., Nogueira, M. L. & da Silva, N. S. Low sensitivity of the tourniquet test for differential diagnosis of dengue: an analysis of 28,000 trials in patients. BMC Infect. Dis. 16, 627 (2016).
- Mayxay, M. et al. Predictive diagnostic value of the tourniquet test for the diagnosis of dengue infection in adults. Trop. Med. Int. Health 16, 127–133 (2011).
- St John, A. L. & Rathore, A. P. S. Adaptive immune responses to primary and secondary dengue virus infections. Nat. Rev. Immunol. 19, 218–230 (2019).
- Tang, K. F. & Ooi, E. E. Diagnosis of dengue: an update. Expert Rev. Anti. Infect. Ther. 10, 895–907 (2012).
- Avrami, S., Hoffman, T., Meltzer, E., Lustig, Y. & Schwartz, E. Comparison of clinical and laboratory parameters of primary vs secondary dengue fever in travellers. J. Travel Med. 30, (2023).
- Fisher, R., Lustig, Y., Sklan, E. H. & Schwartz, E. The Role of NS1 Protein in the Diagnosis of Flavivirus Infections. Viruses 15, (2023).
- Lanciotti, R. S., Calisher, C. H., Gubler, D. J., Chang, G. J. & Vorndam, A. V. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J. Clin. Microbiol. 30, 545–551 (1992).
- Iani, F. C. de M. et al. Dengue diagnostics: serious inaccuracies are likely to occur if pre-analytical conditions are not strictly followed. Mem. Inst. Oswaldo Cruz 115, e200287 (2021).
- Cecchetto, J., Fernandes, F. C. B., Lopes, R. & Bueno, P. R. The capacitive sensing of NS1 Flavivirus biomarker. Biosens. Bioelectron. 87, 949–956 (2017).
- Rastogi, M., Sharma, N. & Singh, S. K. Flavivirus NS1: a multifaceted enigmatic viral protein. Virol. J. 13, 131 (2016).
- Peeling, R. W. et al. Evaluation of diagnostic tests: dengue. Nat. Rev. Microbiol. 8, S30–8 (2010).
- Andries, A.-C. et al. Evaluation of the performances of six commercial kits designed for dengue NS1 and anti-dengue IgM, IgG and IgA detection in urine and saliva clinical specimens. BMC Infect. Dis. 16, 1–9 (2016).
- Lanciotti, R. S. et al. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg. Infect. Dis. 14, 1232–1239 (2008).
- Changal, K. H. et al. Differentiating secondary from primary dengue using IgG to IgM ratio in early dengue: an observational hospital based clinico-serological study from North India. BMC Infect. Dis. 16, 1–7 (2016).
- Bäck, A. T. & Lundkvist, A. Dengue viruses - an overview. Infect. Ecol. Epidemiol. 3, (2013).
- Ayukekbong, J. A., Oyero, O. G., Nnukwu, S. E., Mesumbe, H. N. & Fobisong, C. N. Value of routine dengue diagnosis in endemic countries. World J Virol 6, 9–16 (2017).
- Thomas, S. J. et al. Dengue plaque reduction neutralization test (PRNT) in primary and secondary dengue virus infections: How alterations in assay conditions impact performance. Am. J. Trop. Med. Hyg. 81, 825–833 (2009).
- Antunes, A. C. et al. Evaluation of the diagnostic value of the tourniquet test in predicting severe dengue cases in a population from Belo Horizonte, State of Minas Gerais, Brazil. Rev. Soc. Bras. Med. Trop. 46, 542–546 (2013).
- Grande, A. J., Reid, H., Thomas, E., Foster, C. & Darton, T. C. Tourniquet Test for Dengue Diagnosis: Systematic Review and Meta-analysis of Diagnostic Test Accuracy. PLoS Negl. Trop. Dis. 10, (2016).
- Jarman, R. G. et al. Factors Influencing Dengue Virus Isolation by C6/36 Cell Culture and Mosquito Inoculation of Nested PCR-Positive Clinical Samples. Am. J. Trop. Med. Hyg. 84, 218 (2011).
- Monitoring and improving the sensitivity of dengue nested RT-PCR used in longitudinal surveillance in Thailand. J. Clin. Virol. 63, 25–31 (2015).
- Tricou, V. et al. Comparison of two dengue NS1 rapid tests for sensitivity, specificity and relationship to viraemia and antibody responses. BMC Infect. Dis. 10, 1–8 (2010).
- Luvira, V. et al. Diagnostic Performance of Dengue NS1 and Antibodies by Serum Concentration Technique. Tropical Medicine and Infectious Disease 8, (2023).
- Laboratory diagnosis of primary and secondary dengue infection. J. Clin. Virol. 31, 179–184 (2004).
- Jang, W. S. et al. Comparative evaluation of three dengue duo rapid test kits to detect NS1, IgM, and IgG associated with acute dengue in children in Myanmar. PLoS One 14, e0213451 (2019).
- Dengue- Global situation. https://www.who.int/emergencies/disease-outbreak-news/item/2023-DON498#:~:text=Dengue%20is%20not%20endemic%20in,%2C%20Italy%2C%20Portugal%20and%20Spain.
- Aedes invasive mosquitoes - current known distribution: October 2023. European Centre for Disease Prevention and Control https://www.ecdc.europa.eu/en/publications-data/aedes-invasive-mosquitoes-current-known-distribution-october-2023 (2023).
- Prudhomme, J. et al. The native European mosquito species can transmit chikungunya virus. Emerg. Microbes Infect. 8, 962–972 (2019).
- LaTourrette, K. & Garcia-Ruiz, H. Determinants of Virus Variation, Evolution, and Host Adaptation. Pathogens 11, (2022).
- Autochthonous vectorial transmission of dengue virus in mainland EU/EEA, 2010-present. European Centre for Disease Prevention and Control https://www.ecdc.europa.eu/en/all-topics-z/dengue/surveillance-and-disease-data/autochthonous-transmission-dengue-virus-eueea (2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





