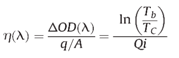1. Introduction
Metal oxides are widely studied with respect to their electrochromic behavior for applications such as display devices and smart windows. To decrease the absorbed heat in buildings, electrochromic films have been used as smart windows. Electrochromic materials have been applied in energy-effective glazing, automobile sunroofs, smart windows, and mirrors. The structure of a smart window contains an electrochromic material layer (usually metal oxide) sandwiched between a transparent conductive layer and some solid electrolyte. Recently, energy efficiency has been affected and focused on energy solution strategies for utilizing this important field.
The electrochromic process is based on a reversible redox process and characterized by the coloration efficiency (CE). The energy-saving windows (smart window), energy storage systems: such as electro-chromic (EC), photochromic and thermochromic have been designed on the same mechanism and both have sandwich device structures, and it was based on the elec-trochemical reaction of the electrode materials.
Transition metal (Titanium, Tungsten, Nickel, Vanadium, Molybdenum and others) oxide films are the most interesting and most widely studied materials for this purpose. Conventional thin film preparation methods include for instance: chemical methods (spin coating, sol–gel deposition, chemical bath deposition, Langmuir–Blodgett technique, etc.), chemical and physical vapor deposition, electrochemical methods (anodization, plating), see ref. [
1] and references therein.
The Ti oxide (TiO
2) was extensively studied prepared by the different methods: sputtering [
2,
3], chemical vapor deposition [
4] and spray pyrolysis [
5,
6], various wet chemical techniques [
7,
8], and anodization [
9,
10,
11].
The most widely studied EC oxide is Tungsten oxide (WO
3), and films of this material have been prepared by several different methods. Examples for physical vapor deposition: thermal evaporation [
12,
13,
14,
15,
16], sputtering [
17,
18], pulsed laser deposition [
19,
20] and spray pyrolysis [
21].
Electrochromic Mo oxide (MoO
3) shows similar behaviour to W oxide, and widespread studies used films prepared by evaporation [
22,
23], chemical vapor deposition [
24], and wet chemical techniques [
25,
26].
Regarding V-pentoxide-based materials with intermediate one of the EC properties as CE, we note films prepared by vacuum evaporation [
27], sputter deposition [
28], spray pyrolysis [
29], chemical techniques [
30,
31,
32,
33], electrodeposition [
34,
35] and inkjet printing [
36].
Nickel oxide films (NiO) were rather considered in hydrous nickel oxide form as EC material [
37,
38].
Nevertheless, relatively few publications studied the possible advantages (higher coloration efficiency) of the mixtures of different metal-oxides as electrochromic material. The electrochromic effectiveness (the change of light absorption for the same electric charge) can be higher in mixed metal-oxide layers.
Earlier, we performed experiments with mixed metal-oxides and found positive effect in electrochromic behavior. Ismaeel et al [
39] determined the optimal composition of reactive magnetron-sputtered combinatorial mixed layers of Titanium oxide and Tin oxide (TiO
2-SnO
2) for electrochromic purposes. The maximum enhancement in light absorption was found at (30%) TiO
2– (70%) SnO
2 composition.
In other experiments, also combinatorial material synthesis approach has been applied for the binary MoO
3/WO
3 system. By using organic propylene carbonate electrolyte cells in a conventional three-electrode configuration, electrochromic redox reactions have been made. Coloration efficiency data has been evaluated from the primary data plotted against the composition displayed a characteristic maximum at around 60% MoO
3. The localization of the maximum at 5% accuracy has been allowed in that combinatorial approach [
40].
We found only a few publications about the EC behavior of Ti-Mo mixed oxide. Mahajan et al [
41] reported the positive effect of doping of Ti (0, 3, 6, 9 at%) in MoO
3 thin films prepared by spray pyrolysis technique. Shrestha et al [
42] successfully fabricated self-organized TiO
2–MoO
3 composite oxide nano-tubes with tunable dimensions by anodization. These nano-tube layers exhibited a significantly enhanced electrochromic color contrast compared with plain TiO
2 nano-tubes. Haiyan Yu et al [
43] prepared MoO
3-TiO
2 composite core/shell nanorod films by the combination of hydrothermal and electrodeposition method. They attributed the improved electrochromic properties mainly to the porous space among the nanorods array, which makes the ion diffusion easier.
The present study is considered new since its objective was to investigate the electrochromic effectiveness of TiO2-MoO3 mixed layers in a wide compositional range by determination of CE as a function of composition. This paper also aims to assess the results of investigations of such materials showing enhanced electrochromic behavior compared to the pure materials. One can expect that using metal atoms with different diameters in the layers can enhance the CE.
2. Results
The main criterion of the EC device performance is CE. Transmittance changes were directly measured during the coloration process, while the charge was calculated from the integral of the current vs. time data, and the electrolyte wetted area of the sample.
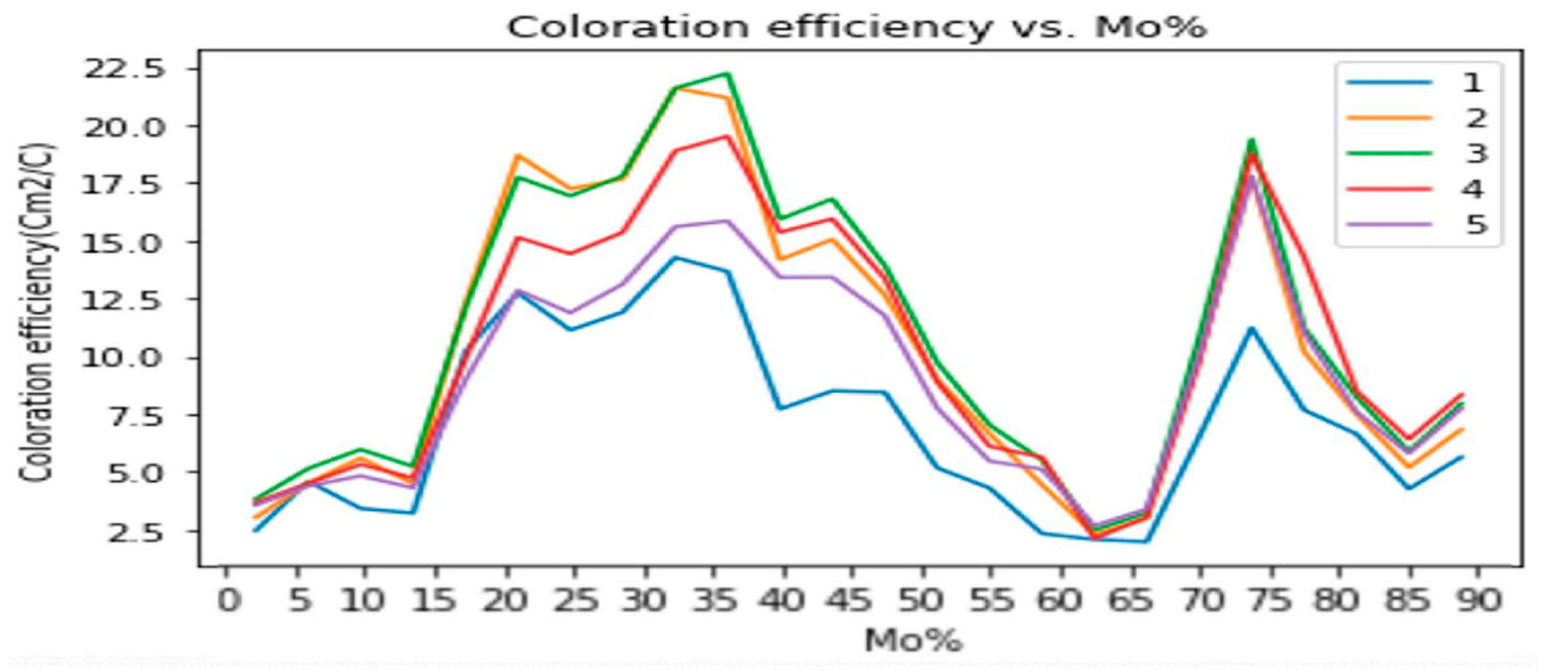
Figure 1 shows the calculated CE data as a function of MoO
3 fraction of the layer (individual color-coded curves represent different wavelengths), while
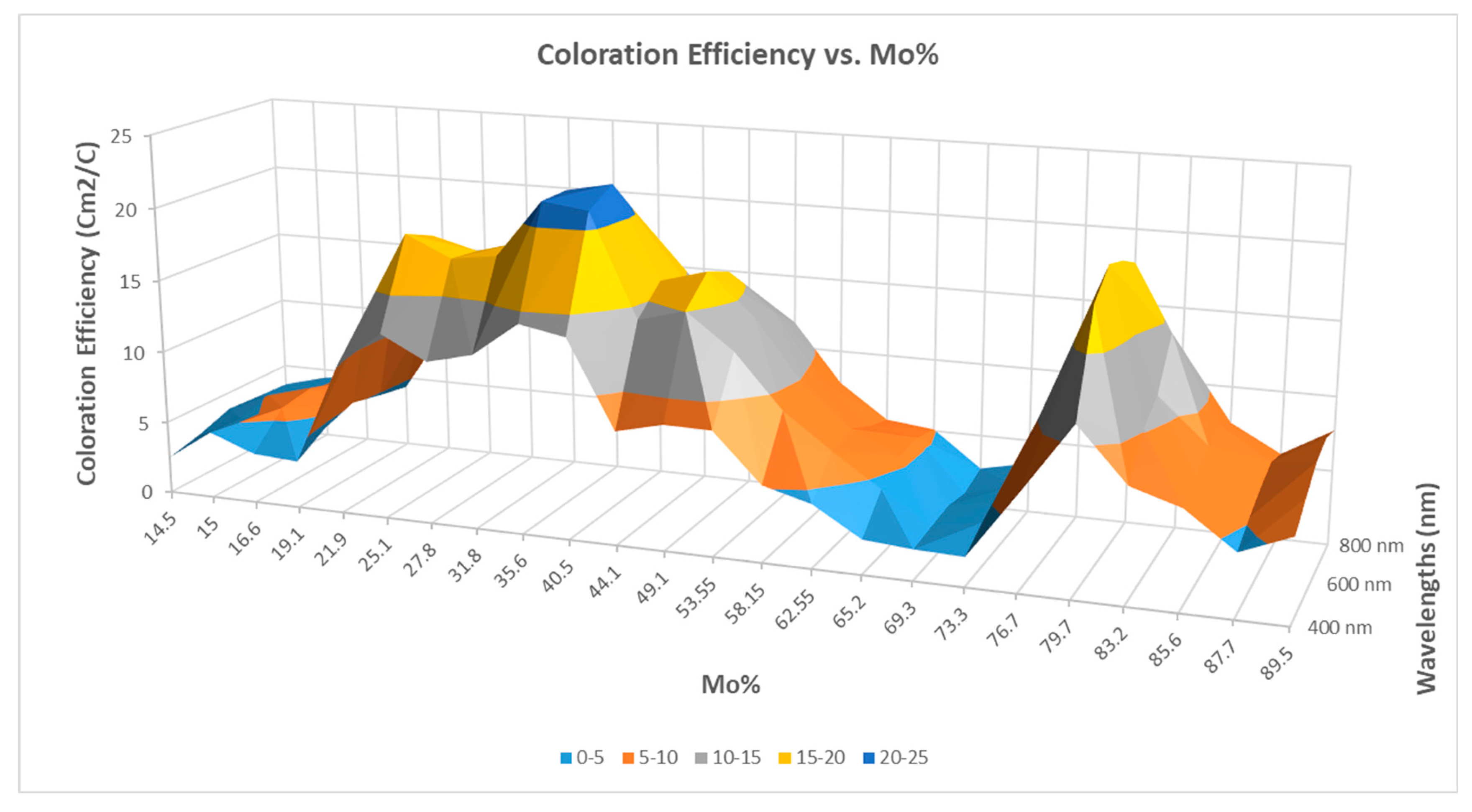
Figure 2 is a 3D diagram of the data. Individual points were calculated from the average of three independent measurements. Error is estimated as 3 %, calculated based on the accuracy of sample positioning in the measuring cell and the spot size of the optical beam. The calculated data are given in
Table 1 according to the equation (1).
The Coloration Efficiency η is given by following equation:
where Qi is the electronic charge inserted into the electrochromic material per unit area, ΔOD is the change of optical density, Tb is the transmittance in the bleached state, and Tc is the transmittance in the colored state. The unit of a Coloration Efficiency is cm2 C-1.
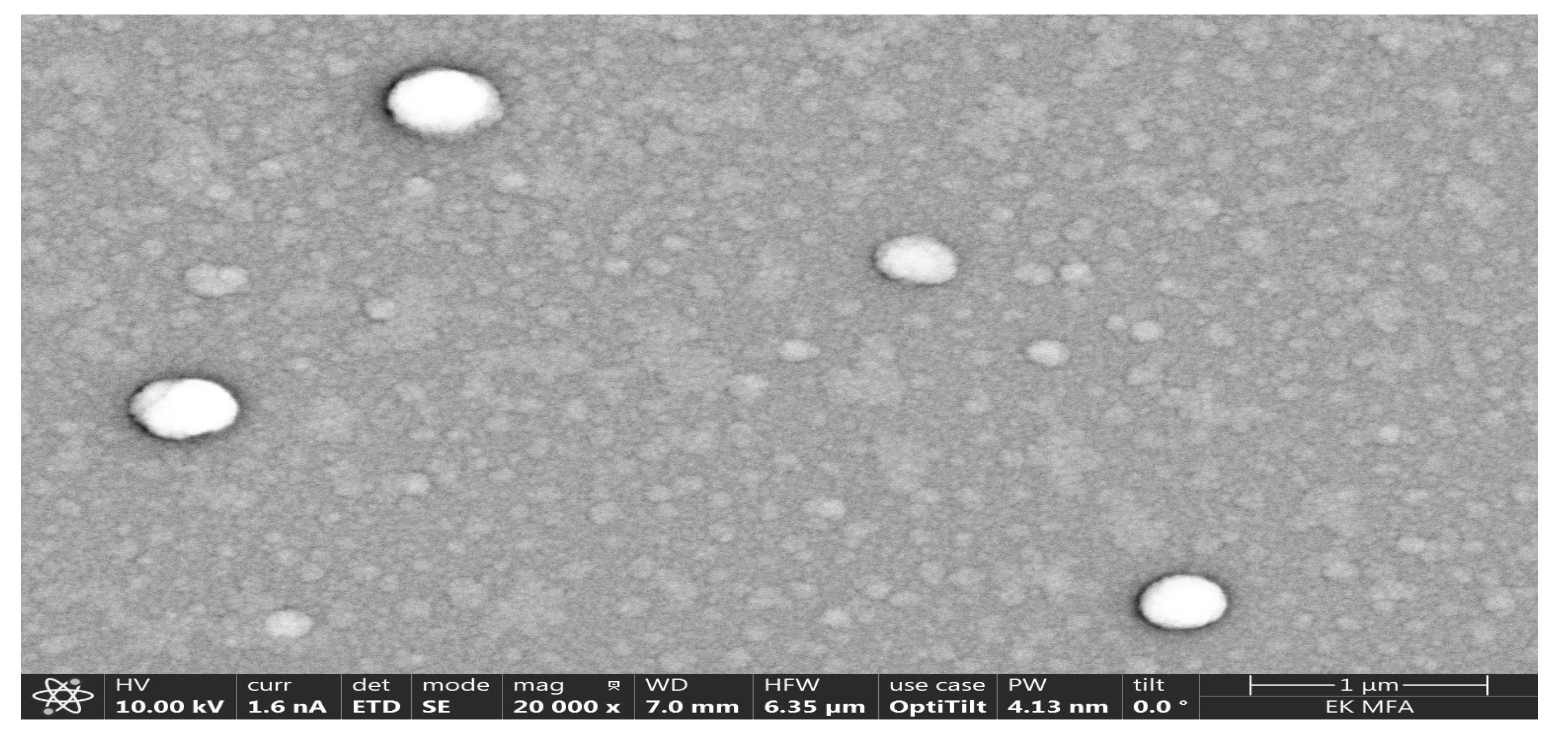
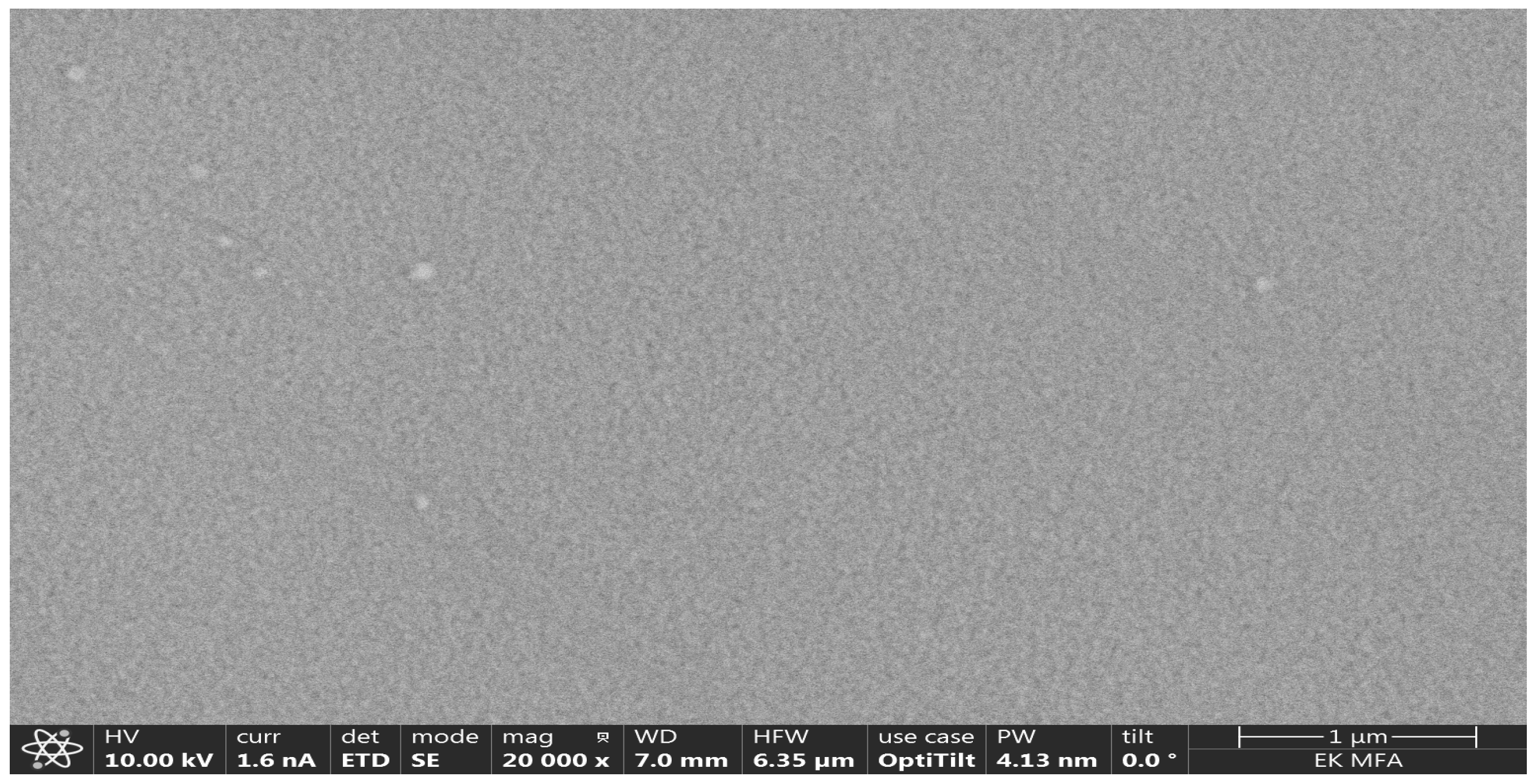
Figure 3 and
Figure 4. Showed SEM-photos from the Ti-rich side and Mo-rich side.
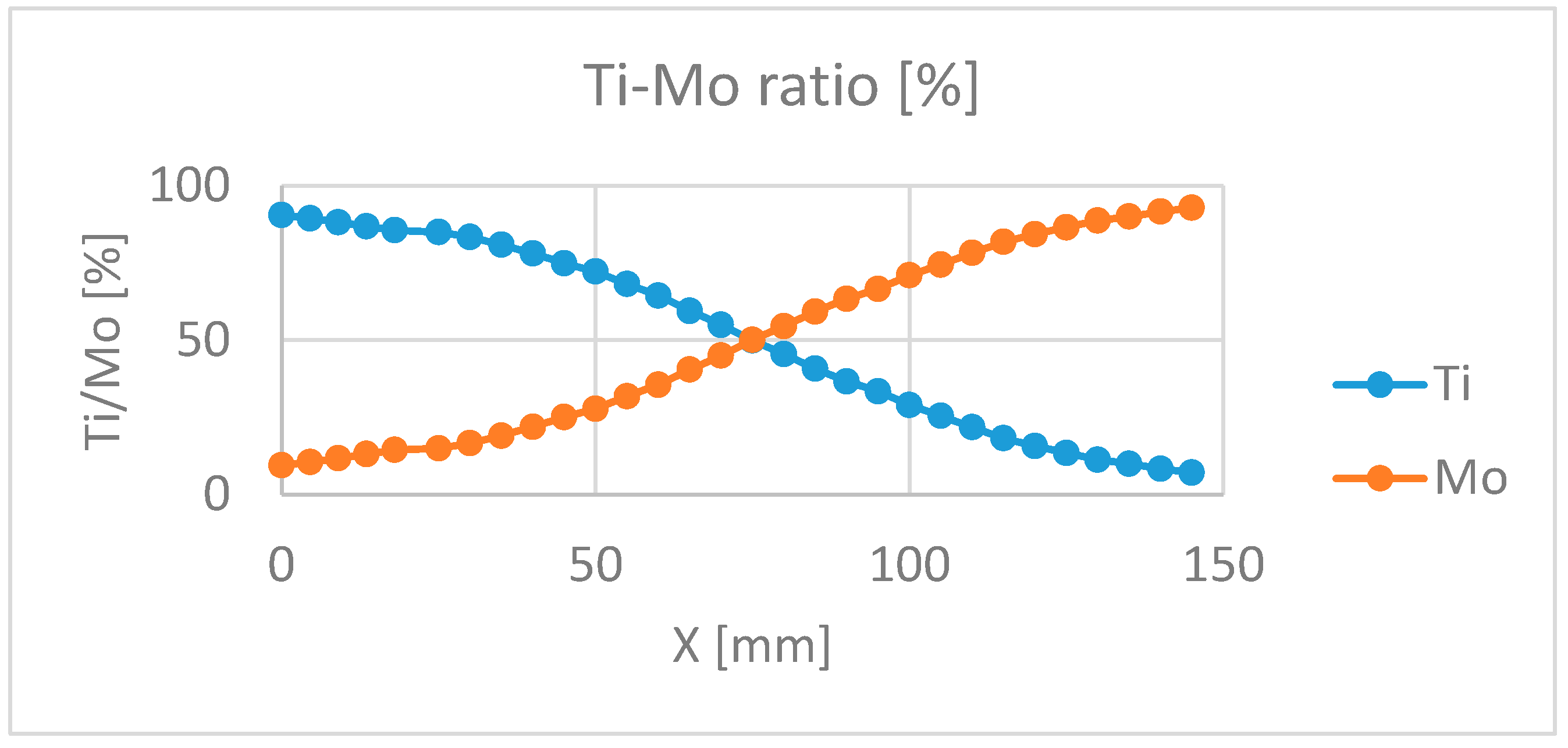
The precision of the Ti/Mo ratio is 2 %, while the precision of the position is 1 mm. see
Figure 5.
3. Discussion
To check the position dependent composition, Scanning Electron Microscopy (SEM) with Energy-Dispersive X-ray Spectroscopy (EDS) has been used, see
Figure 5. To explain the two maximums in CE (
Figure 1 and
Figure 2) we show here SEM-photos from the Ti-rich side and Mo-rich side, see
Figure 3 and
Figure 4. These are the proof that the material at the Ti-side is polycrystalline (several hundred nm) and the Mo-side is amorphous or nanocrystalline. We think that the Ti-rich side was at significantly (several hundred C degree) higher temperature (plasma power 4.2 kW) during the deposition process, so the Ti-rich oxide is polycrystalline (several hundred nm grainsize) compared to the Mo-rich side where the oxide remains amorphous or nanocrystalline. This microstructure difference can be the reason for the two peaks in
Figure 1 and
Figure 2 of the CE curves.
CE values were determined at every composition from the measured relative transmission vs. input charge curves. We used eq. 1 to determine CE at 5 wavelengths (400-800 nm visible range) for every compositional position using the relative transmission curves and fitted exponential curves. The determined results, CE vs. composition and vs. wavelength are shown in
Figure 1 and
Figure 2.
4. Materials and Methods
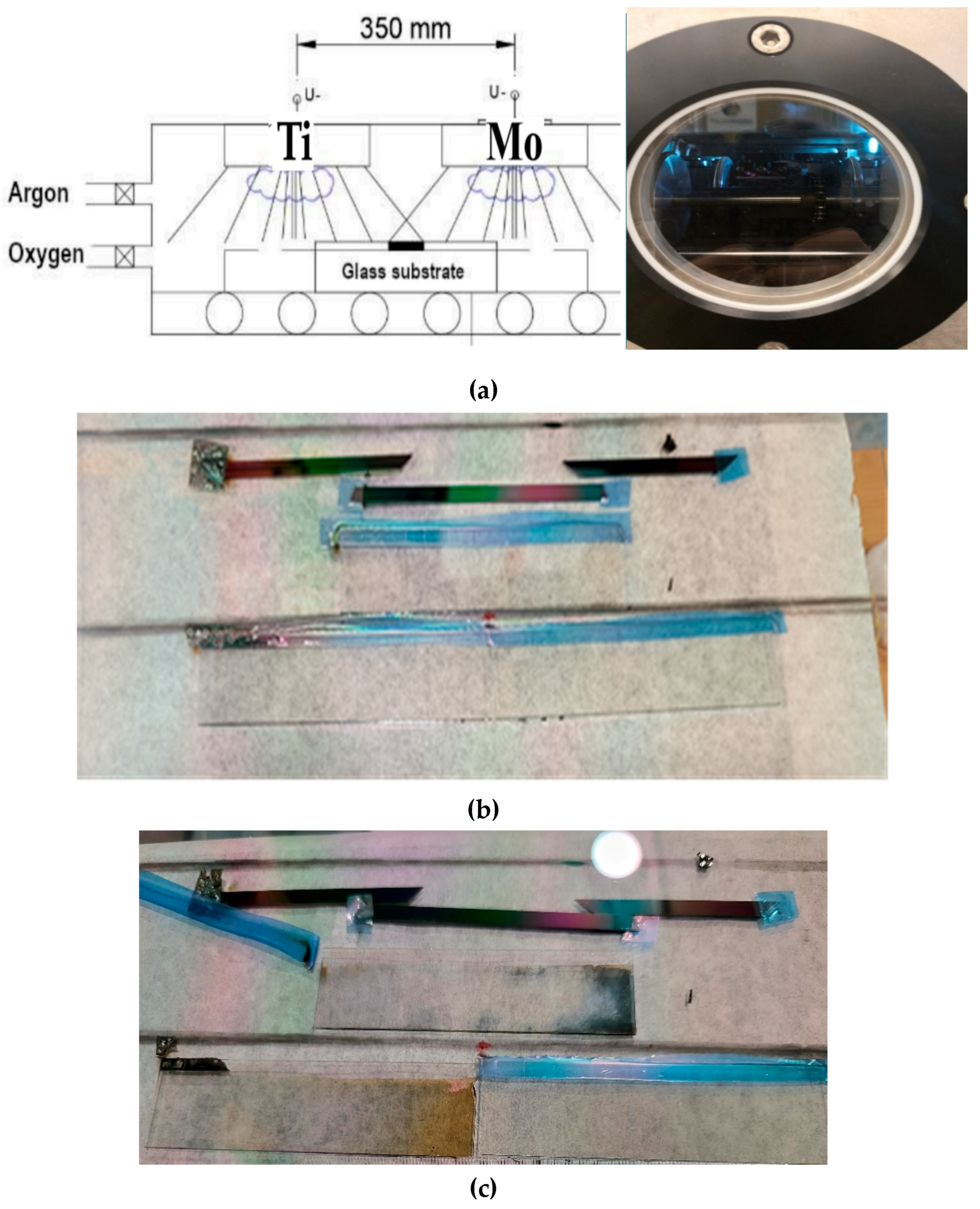
Optimal composition of reactive magnetron-sputtered have determined the combinatorial mixed layers of Titanium oxide and Molybdenum oxide (Mo
xTi
1-x) oxide thin film system, where 0 < x < 1) for electrochromic purposes. Ti and Mo targets were put separately from each other, and the ITO-covered glass and Si-probes on a glass substrate (30 cm × 30 cm) were moved under the two separated targets (Ti and Mo) in a reactive Argon-Oxygen (Ar-O
2) gas mixture, see
Figure 6a. Titanium-Molybdenum-Oxide layers were deposited onto Indium-Tin-Oxide (ITO) covered 100x25 mm size glass surfaces. Layer depositions were made by reactive sputtering in an (Ar + O
2) gas mixture at ~2 × 10
−4 Pa base pressure and at ~10
−1 Pa process pressure. The target - substrate working distance was 6 cm. 30 sccm/s Ar and 70 sccm/s O
2 volumetric flow rates were applied in the magnetron sputtering chamber. The plasma powers of the Ti and Mo metal targets were selected as (4200 and 1500) W respectively. Samples were moved back and forth at 25 cm/s of walking speed between the Mo and Ti targets and a mixed oxide film was deposited onto the ITO surface, see
Figure 6b. 5 min cooling interrupt was applied after every 50 walking cycles.
Spectroscopic Ellipsometry (SE) is an optical characterization technique with high-accuracy [
44]. Several researchers have used SE for combinatorial or pure materials investigation [
45,
46,
47,
48,
49]. The combinatorial approach used to investigate mixed metal oxides has several advantages, Fried et al. [
49] have used SE (which is a fast, cost-effective, and non-destructive method) for the investigation and mapping of WO
3-MoO
3 mixed layers after sputtering. Different optical models, such as EMA and 2T–L, have been used to achieve the composition map and thickness map of the sample layers. We used SE similar manner to determine the composition map and thickness map of our Ti-Mo combinatorial layers.
The best determination of the optimal composition as a part of electrochromic (EC) properties, the layers were deposited onto ITO-covered glass. The composition map and thickness map were measured on the Si-probes, see
Figure 6a,b. We checked the resulted compositional map on the Si-probes
Figure 6b,c by using Scanning Electron Microscopy (SEM) with Energy-Dispersive X-ray Spectroscopy (EDS), see
Figure 5.
The Coloration Efficiency (CE) has been determined in a transmission electrochemical cell, see
Figure 7. The cell was filled with 1M lithium perchlorate (LiClO
4) / propylene carbonate electrolyte. A 5 mm width masked (Ti-Mo oxide-free) stripe of the slides remained above the liquid level allowing direct electric contact onto the ITO layer. A Pt wire counter electrode was placed into the electrolyte alongside with a reference electrode. This arrangement was a fully functional electrochromic cell. The applied current was controlled through the cell using a Farnell U2722 Source Measurement Unit (SMU). Constant current was registered through coloration and bleaching cycles of the electrochromic layer. Simultaneous spectral transmission measurements were performed by using the Woollam M2000 spectroscopic ellipsometer into transmission mode.
5. Conclusions
We could enhance the coloration efficiency of mixed Titanium oxide and Molybdenum oxide (TiO2-MoO3) layer deposited by reactive magnetron sputtering. We prepared combinatorial samples by moving the samples under the Ti and Mo sputtering targets in a reactive Argon-Oxygen (Ar-O2) gas mixture.
By using this combinatorial process, all the compositions (from 0 to 100%) were achieved in the same sputtering chamber after one sputtering. The mixed metal oxides showed better CE as a part of EC properties than the pure oxides, because the electrochromic effectiveness can be higher in mixed metal-oxide layers and mixing metal atoms with different diameters in the layers can enhance the CE.
The Ti-rich side was at significantly higher temperature during the deposition process, so the Ti-rich oxide is polycrystalline compared to the Mo-rich side where the oxide remains amorphous or nanocrystalline.
Coloration Efficiency has been considered an important parameter in this study. The maximum value of the CE is 22.2 cm2 C−1 at the wavelength 600 nm at ~ 60% - 40 % Ti-Mo ratio on the Ti-rich polycrystalline material, while CE is 19.8 cm2 C−1 at the wavelength 600 nm at ~ 20% - 80 % Ti-Mo ratio on the Mo-rich amorphous (or nanocrystalline) material.
Author Contributions
Z.L. and M.F.: conceptualization; methodology; Z.L.: investigation (sputtering); N. T. I., Z.L. and M.F.: investigation (CE and SE); N. T. I.: computing; M.F.: resources, funding acquisition; N. T. I., Z.L. and M.F.: writing—original draft preparation. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by NKFIH OTKA K 143216 and 146181 projects and Project TKP2021-EGA-04 has been implemented with the support provided by the Ministry of Innovation and Technology of Hungary from the National Research, Development, and Innovation Fund, financed under the TKP2021 funding scheme.
Acknowledgments
Authors are grateful to Noemi Szasz for the SEM–EDS measurements. This work was supported and funded by NKFIH OTKA NN 131269 (VOC-DETECT M-ERA.NET Transnational Call 2018) and NKFIH OTKA K 143216 and 146181 projects. Project TKP2021-EGA-04 has been implemented with the support provided by the Ministry of Innovation and Technology of Hungary from the National Research, Development, and Innovation Fund, financed under the TKP2021 funding scheme. The work in frame of the 20FUN02 ‘‘POLight’’ project has received funding from the EMPIR programme, co-financed by the Participating States and from the European Union’s Horizon 2020 research and innovation programme. Noor Taha Ismaeel is grateful for the Stipendium Hungaricum scholarship.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Granqvist, C.G. Electrochromics for smart windows: oxide-based thin films and devices. Thin Solid Films 2014, 564, 1–38. [Google Scholar] [CrossRef]
- Cantao, M.P.; Cisneros, J.I.; Torresi, R.M. Electrochromic behavior of sputtered titanium oxide thin films. Thin Solid Film 1995, 259, 70–74, https://www.academia.edu/10589451/Electrochromic_behaviour_of_sputtered_titanium_oxide_thin_films, SSDI 0040-6090(94)06401-6. [Google Scholar] [CrossRef]
- Sorar, I.; Pehlivan, E.; Niklasson, G.A.; Granqvist, C.G. Electrochromism of DC magnetron sputtered TiO2 thin films Role of deposition parameters. Sol. Energy Mater. Sol. Cells 2013, 115, 172–180. [Google Scholar] [CrossRef]
- Khalifa, S.; Lin, H.; Shah, S.I. Structural and electrochromic properties of TiO2 thin films prepared by metallorganic chemical vapor deposition. Thin Solid Films 2010, 518, 5457. [Google Scholar] [CrossRef]
- Shinde, P.S.; Deshmukh, H.P.; Mujawar, S.H.; Inamdar, A.I.; Patil, P.S. Spray deposited titanium oxide thin films as passive counter electrodes. Electrochim. Acta 2007, 52, 3114. [Google Scholar] [CrossRef]
- Zelakowska, E.; Rysiakiewicz-Pasek, E. Thin TiO2 films for an electrochromic system, Opt. Mater. 2009, 31, 1802–1804. [Google Scholar] [CrossRef]
- Lin, S.-Y.; Chen, Y.-C.; Wang, C.-M.; Liu, C.-C. Effect of heat treatment on electrochromic properties of TiO2 thin films. J. Solid State Electrochem 2008, 12, 1481-1486. https://link.springer.com/article/10. 1007. [Google Scholar]
- Wang, C.-M.; Lin, S.-Y.; Chen, Y.-C. Electrochromic properties of TiO2 thin films prepared by chemical solution deposition method. J. Phys. Chem. Solids 2008, 69, 451–455. [Google Scholar] [CrossRef]
- Hahn, R.; Ghikov, A.; Tsuchiya, H.; Macak, J.M.; Muñoz, A.G.; Schmuki, P. Lithium-ion insertion in anodic TiO2 nanotubes resulting in high electrochromic contrast, Phys. Stat. Sol. A 2007, 204, 1281–1285. [Google Scholar] [CrossRef]
- Paramasivam, I.; Macak, J.M.; Ghikov, A.; Schmuki, P. Enhance photochromism of Ag loaded self-organized TiO2 nanotube layers. Chem. Phys. Lett. 2007, 445, 233. [Google Scholar] [CrossRef]
- Berger, S.; Ghicov, A.; Nah, Y.-C.; Schmuki, P. Transparent TiO2 Nanotube Electrodes via Thin Layer Anodization: Fabrication and Use in Electrochromic Devices. Langmuir 2009, 25, 4841. [Google Scholar] [CrossRef]
- Liao, C.-C.; Chen, F.-R.; Kai, J.-J. Annealing effect on electrochromic properties of tungsten oxide nanowires. Sol. Energy Mater. Sol. Cells 2007, 91, 1258. [Google Scholar] [CrossRef]
- Barbosa, P.C.; Silva, M.M.; Smith, M.J.; Gonçalves, A.; Fortunato, E. . Optical Devices Performance with Poly (trim ethylene carbonate) based Electrolytes. Thin Solid Films 2008, 516, 1480. [Google Scholar] [CrossRef]
- Beydaghyan, G.; Renaud, J.-L.; Bader, G.; Ashrit, P.V. Enhanced electrochromic properties of heat-treated nanostructured tungsten trioxide thin films. J. Mater. Res. 2008, 23, 274–280. [Google Scholar] [CrossRef]
- Joraid, A.A. Comparison of electrochromic amorphous and crystalline electron beam deposited WO3 thin films. Curr. Appl. Phys. 2009, 9, 73–79. [Google Scholar] [CrossRef]
- Krishna, K.H.; Hussain, O.M.; Julien, C.M. Electrochromic properties of nanocrystalline WO3 thin films grown on flexible substrates by plasma-assisted evaporation technique. Appl. Phys. A 2010, 99, 921–929. [Google Scholar] [CrossRef]
- Sauvet, K.; Sauques, L.; Rougier, A. IR electrochromic WO3 thin films: From optimization to devices. Sol. Energy Mater. Sol. Cells 2009, 93, 2045–2049. [Google Scholar] [CrossRef]
- Sato, R.; Kawamura, N.; Tokumaru, H. Relaxation mechanism of electrochromism of tungsten-oxide film for ultra-multilayer optical recording depending on sputtering conditions. Jpn. J. Appl. Phys. 2007, 46, 3958. [Google Scholar] [CrossRef]
- Sauvet, K.; Rougier, A.; Sauques, L. Electrochromic WO3 thin films active in the IR region. Sol. Energy Mater. Sol. Cells 2008, 92, 209–215. [Google Scholar] [CrossRef]
- Rougier, A.; Sauvet, K.; Sauques, L. Electrochromic materials from the visible to the infrared region: An example WO3, Ionics 2008, 14, 99-105. 14. [CrossRef]
- Sivakumar, R.; Gopinath, C.S.; Jayachandran, M.; Sanjeeviraja, C. An electrochromic device (ECD) cell characterization on electron beam evaporated MoO3 films by intercalating/deintercalating the H+ ions. Curr. Appl. Phys. 2007, 7, 76–86. [Google Scholar] [CrossRef]
- Patil, R.S.; Uplane, M.D.; Patil, P.S. Electrosynthesis of Electrochromic Molybdenum Oxide Thin Films with Rod-Like Features. Int. J. Electrochem. Soc. 2008, 3, 259–265. [Google Scholar] [CrossRef]
- Gesheva, K.A.; Cziraki, A.; Ivanova, T.; Szekeres, A. Crystallization of chemically vapor deposited molybdenum and mixed tungsten/molybdenum oxide films for electrochromic application. Thin Solid Films 2007, 515, 4609–4613. [Google Scholar] [CrossRef]
- Dhanasankar, M.; Purushothaman, K.K.; Muralidharan, G. Effect of tungsten on the electrochromic behavior of sol–gel dip coated molybdenum oxide thin films. Mater. Res. Bull. 2010, 45, 542–545. [Google Scholar] [CrossRef]
- Hsu, C.-S.; Chan, C.-C.; Huang, H.-T.; Peng, C.-H.; Hsu, W.-C. Electrochromic properties of nanocrystalline MoO3 thin films, Thin Solid Films 2008, 516, 4839-4844. 516. [CrossRef]
- Laurinavichute, V.K.; Vassiliev, S.Y.; Plyasova, L.M.; IMolina, Y.; Khokhlov, A.A.; Pugolovkin, L.V.; Borzenko, M.I.; Tsirlina, G.A. Cathodic electrocrystallization and electrochromic properties of doped rechargeable oxotungstates. Electrochim. Acta 2009, 54, 5439–5448. [Google Scholar] [CrossRef]
- Scarminio, J.; Catarini, P.R.; Urbano, A.; Gelamo, R.V. Li diffusion and electrochromism in amorphous and crystalline vanadium oxide thin film electrodes. Journal of the Brazilian Chemical Society 2008, 19, 788–794. [Google Scholar] [CrossRef]
- Lee, S.; Eom, J.; Kwon, H. Electrochemical properties of amorphous LixV2O5−y thin film deposited by r.f.-sputtering. J. Appl. Electrochem 2009, 39, p–241. [Google Scholar] [CrossRef]
- Patil, C.E.; Tarwal, N.L.; Jadhav, P.R.; Shinde, P.S.; Deshmukh, H.P.; Karanjkar, M.M.; Moholkar, A.V.; Gang, M.G.; Kim, J.H.; Patil, P.S. Electrochromic performance of the mixed V2O5–WO3 thin films synthesized by pulsed spray pyrolysis technique. Current Applied Physics 2014, 14, 389–395. [Google Scholar] [CrossRef]
- Avellaneda, C.O. Electrochromic performance of sol–gel deposited V2O5:Ta films. Materials Science and Engineering: B 2007, 138, Issue–2. [Google Scholar] [CrossRef]
- Yakovleva, D.S.; Malinenko, V.P.; Pergament, A.L.; Stefanovich, G.B. Electrical and optical properties of thin films of hydrated vanadium pentoxide featuring electrochromic effect. Tech. Phys. Lett. 2007, 33, p–1022. [Google Scholar] [CrossRef]
- Xiong, C.; Aliev, A.E.; Gnade, B.; Balkus, K.J., Jr. Fabrication of Silver Vanadium Oxide and V2O5 Nanowires for Electrochromics. ACS Nano 2008, 2, 293. [Google Scholar] [CrossRef]
- Benmoussa, M.; Outzourhit, A.; Bennouna, A.; Ihlad, A. Li+ ions diffusion into sol-gel V2O5 thin films: electrochromic properties. Eur. Phys. Journal Appl. Phys. 2009, 48, 10502. [Google Scholar] [CrossRef]
- Li, L.; Steiner, U.; Mahajan, S. Improved electrochromic performance in inverse opal vanadium oxide films. J. Mater. Chem. 2010, 20, 7131. [Google Scholar] [CrossRef]
- Scherer, M.R.; Li, L.; Cunha, P.M.S.; Scherman, O.A.; Steiner, U. Enhanced Electrochromism in Gyroid-Structured Vanadium Pentoxide. Adv. Mater. 2012, 24, 1217. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Pinheiro, C.; Henriques, I.; Laia, C.A.T. Electrochromic Properties of Inkjet Printed Vanadium Oxide Gel on Flexible Polyethylene Terephthalate/Indium Tin Oxide Electrodes. ACS Appl. Mater. Interfaces 2012, 8, 5266–5275. [Google Scholar] [CrossRef] [PubMed]
- Avendaño, E.; Azens, A.; Niklasson, G.A.; Granqvist, C.G. Proton Diffusion and Electrochromism in Hydrated NiOy and Ni1 − x Vx Oy Thin Films, J. Electrochem. Soc. 2005, 152. [Google Scholar] [CrossRef]
- Avendaño, E.; Rensmo, H.; Azens, A.; Sandell, A.; de M, G.; Siegbahn, H.; Niklasson, G.A.; Granqvist, C.G. Coloration Mechanism in Proton-Intercalated Electrochromic Hydrated NiOy and Ni1−xVxOy Thin Films. J. Electrochem. Soc. 2009, 56, 132. [Google Scholar] [CrossRef]
- Ismaeel, N.T.; Lábadi, Z.; Petrik, P.; Fried, M. Investigation of Electrochromic, Combinatorial TiO2-SnO2 Mixed Layers by Spectroscopic Ellipsometry Using Different Optical Models. Materials 2023, 16, 4204. [Google Scholar] [CrossRef] [PubMed]
- Zoltán Lábadi; Dániel Takács; Zsolt Zolnai; Péter Petrik and Miklós Fried. Compositional optimization of sputtered WO3/MoO3 films for high coloration efficiency. Materials 2024, 17, p–1000. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, S.S.; Mujawar, S.H.; Shinde, P.S.; Inamdar, A.I.; Patil, P.S. Structural, morphological, optical and electrochromic properties of Ti-doped MoO3 thin films, Sol. Energy Mater. Sol. Cells 2009, 93, 183–187. [Google Scholar] [CrossRef]
- Shrestha, N.K.; Nah, Y.-C.; Tsuchiya, H.; Schmuki, P. Self-organized nano-tubes of TiO2–MoO3 with enhanced electrochromic properties. Chem. Commun. [CrossRef]
- Haiyan Yu; Yajun Li; Lei Zhao; Guangmin Li; Junwei Li; Hui Rong; Zhifeng Liu. Novel MoO3-TiO2 composite nanorods films with improved electrochromic performance. Materials Letters 2016, 169, 65–68. [Google Scholar] [CrossRef]
- Fujiwara, H. Spectroscopic Ellipsometry Principles and Applications; John Wiley & Sons: Hoboken, NJ, USA 2007; ISBN 9780470016084. [Google Scholar]
- Zimmer, A.; Gilliot, M.; Broch, L.; Boulanger, C.; Stein, N. and Horwat, D. Morphological and chemical dynamics upon electrochemical cyclic sodiation of electrochromic tungsten oxide coatings extracted by in situ ellipsometry. Appl. Opt. 2020, 59, 3766–3772. [Google Scholar] [CrossRef]
- Aryal, P.; Pradhan, P.; Attygalle, D.; Ibdah, A.-R.; Aryal, K.; Ranjan, V.; Marsillac, S.; Podraza, N.J. and Collins, R.W. Real-time, in-line, and mapping spectroscopic ellipsometry for applications in Cu (in Ga) Se metrology. IEEE J. Photovolt. 2014, 4, 333–339. [Google Scholar] [CrossRef]
- Petrik, P.; Fried, M. Mapping and Imaging of Thin Films on Large Surfaces: A review. Phys. Status Solidi 2022, 219, 2100800. [Google Scholar] [CrossRef]
- Dahal, L.R.; Li, J.; Stoke, J.A.; Huang, Z.; Shan, A.; Ferlauto, A.S.; Wronski, C.R.; Collins, R.W. and Podraza, N.J. Applications of real-time and mapping spectroscopic ellipsometry for process development and optimization in hydrogenated silicon thin-film photovoltaics technology. Sol. Energy Mater. Sol. Cells 2014, 129, 32–56. [Google Scholar] [CrossRef]
- Fried, M.; Bogar, R.; Takacs, D.; Labadi, Z.; Horvath, Z.E. and Zolnai, Z. Investigation of Combinatorial WO3-MoO3 Mixed Layers by Spectroscopic Ellipsometry Using Different Optical Models. Nanomaterials 2022, 12, 2421. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).