1. Introduction
Extracellular vesicles (EVs) are small lipid-bilayer nanospheres (about 40-400 nm diameter) secreted from cells belonging to the three domains of life [
1,
2,
3] and vary in their morphology, biogenesis, composition, and biological role [
2]. Although initially under-appreciated and considered cellular debris, biological fluids can contain large quantities of EVs that shuttle various molecules from parental cells to other cells, including proteins, genetic material and toxins. Eukarya EVs, and particularly those produced from mammalian cells, have then attracted great interest for their role in to struggle for infection, and in the control of normal physiological and disease processes [
2,
3,
4].
EVs fulfil a myriad of functions and are recognized as important vehicles of long-range intercellular communication, especially during stress conditions and host-pathogen interactions. Considering their ability to move into biological fluids, EVs are now considered promising biomarkers for disease diagnoses and therapeutical applications [
5,
6,
7].
In marine environments bacteria and cyanobacteria account for > 90% of the total oceanic biomass [
8]. Although largely unexplored, they provide a useful source of natural products, with a high-value biotechnological potential. During the last two decades, the scientific community has focused attention on bacterial extracellular vesicles (BEVs) [
9,
10,
11] involved in cell-to-cell interactions [
2,
3,
4], virulence [
12], horizontal gene transfer [
13], biofilm formation [
14], and quorum signaling [
15].
Interestingly, while for pathogenic [
12,
16] and gut [
17,
18,
19] bacteria, the secretion of BEVs and their functions have been investigated, still largely uncharted is the state of the art in the marine environment. Indeed, researchers are trying to shed new light on these structures' diverse roles in microbial ecology. One possibility is that in addition to that found for pathogens, the functions of marine BEVs could guarantee survival in an environment where the nutrients are poor [
20]. Another option is that the vesicles can carry a higher number of chemical effectors so that the cells have to produce a lower number of molecules. The final effect is less energy expenditure for the bacterial cell [
21]. This review will focus on marine BEVs since there is a growing interest in understanding their roles in biofouling, cellular defence, and horizontal gene transfer [
11,
12,
13,
14,
15,
22,
23]. Moreover, the BEVs’ motion over long distances implies that these structures are responsible for the marine carbon flux and may modulate the growth of heterotrophic communities. Beyond their ecological significance, vesicles produced by marine organisms hold promise for biotechnological applications. The molecular cargos encapsulated within these vesicles, including enzymes, metabolites, and genetic material, present opportunities for bioprospecting and biotechnological innovation.
2. Biogenesis of Bacterial Vesicles
BEVs are produced during normal growth, and stress can influence their production. In some cases, abiotic factors, including changes in temperature, nutrient availability, reactive oxygen species, and UV exposure, correlate with increased vesicle production. The release of EVs may also be induced by intracellular stimuli, such as the accumulation of peptidoglycan (PG) fragments and LPS [
24]. In
Cylindrospermopsis raciborskii vesicle formation is accompanied by phosphatidylserine exposure, a molecular event also observed in EV-secreting eukaryotic cells [
25].
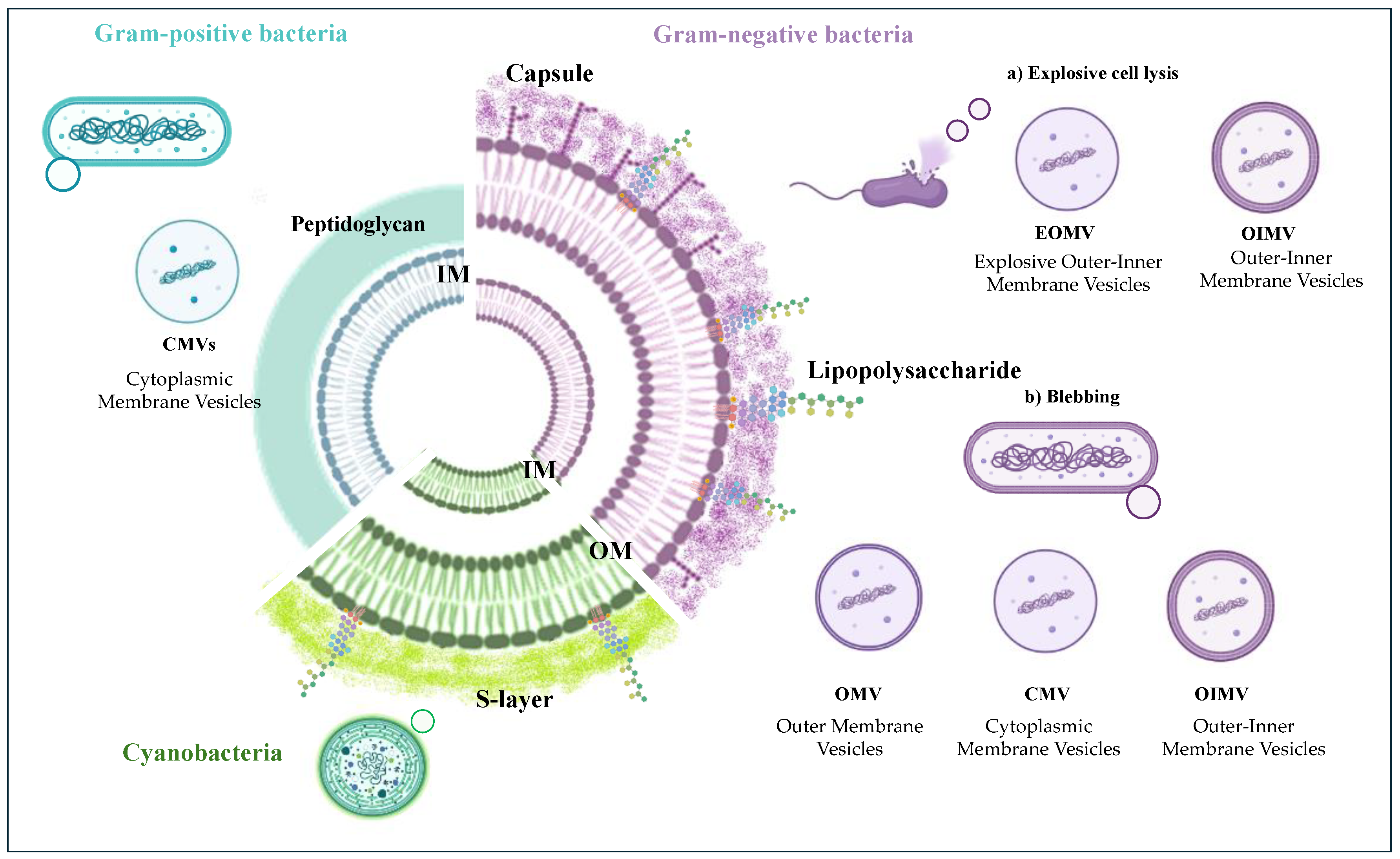
There are several types of BEVs depending on the microorganism’s type (Gram-positive or Gram-negative) and the way by which they are generated [
26].
Gram-negative bacteria possess a cell wall of two phospholipids-enriched membranes spaced out by a thin peptidoglycan layer. The outer membrane (OM) comprises proteins, phospholipids, and lipopolysaccharides (LPSs) [
27], whereas the inner membrane (IM) is a fluid phospholipid bilayer. In Gram-positive cell walls, as much as 90% is a single type of molecule, the peptidoglycan, although teichoic acids are usually present in small amounts. Furthermore, both Gram-negative and Gram-positive cell walls can be decorated by a polysaccharide material forming a capsule.
The ways of generation for Gram-negative bacteria vesicles up to now identified are two: blebbing of the outer membrane [
28] and explosive cell lysis [
29]. The non-lytic biogenesis can produce different types of vesicles (
Figure 1), of which the Outer membrane vesicles (OMVs) are devoid of cytoplasmic components and only include membrane molecules. The blebbing biogenesis can also furnish vesicles including inner-membrane products (OIMVs) and cytoplasmic membrane vesicles (CMVs) containing cytoplasmic components [
24]. All these vesicles are the results of the stress on the cell wall such as antibiotics or environmental conditions [
24,
26,
30]. Conversely, the lytic mechanism generates two subtypes of vesicles, namely explosive outer-inner membrane vesicles (EOIMVs) and explosive outer-membrane vesicles (EOMVs), depending on the presence of the double-layered membrane [
24,
26,
28].
Gram-positive bacteria have been demonstrated to form vesicles through a different mechanism triggered by endolysin, rising to vesicles containing both membrane and cytoplasmic components (cytoplasmic membrane vesicles, CMVs) [
26,
31,
32]. Finally, although the production of EVs on the cell wall of cyanobacterial strains has been demonstrated by microscopy images, the detailed mechanism regarding EVs’ biogenesis in these microorganisms is still very limited [
11,
13].
3. Structure of the BEVs and Molecular Components
Membrane vesicles are small particles [
24] carrying many molecules inserted in a nanosystem surrounded by a double-layered membrane, the last being different depending on the type of microorganism and biogenesis. Following the most recent papers about the structure of membrane vesicles the following components are described.
3.1. Proteins
Proteins in vesicles have been demonstrated to be cytoplasmic, periplasmic, inner and outer membrane proteins (OMP) [
33,
34], virulence factors [
35], enzymes, and proteins involved in biofilm formation [
36].
Alteromonas macleodii KS62 has been reported to produce OMVs, the protein content of which is very rich in hydrolytic enzymes (30 % of the proteome) [
37]. This is not surprising since the hydrolytic enzymes are necessary for nutrient supply and colonization surfaces. Similarly, for
Bacteroides fragilis, a gut microbiota bacterium, it has been suggested that the EVs equipped with hydrolytic enzymes could facilitate the recruiting of the necessary nutrients for the entire microbiota bacterial community [
38].
The production of OMVs containing hydrolytic enzymes was also found for a pool of
Alteromonas macleodii strains [
39]. For all the examined strains, despite the presence of two different populations of OMVs due to their different sizes, the content of hydrolytic enzymes was high. In addition, the presence of proteins probably involved in bacterial adhesion processes was observed.
Many BEVs are composed not only of membrane proteins but also cytoplasmic and periplasmic ones. This is not true for the marine extremophile
Novosphingobium pentaromativorans, for which the proteomic analysis of the vesicles indicated most exclusively the presence of OMPs [
40]. Authors suggested that for this bacterium the possibility to load cytoplasmic cargo proteins in the vesicles could be hampered by high salts and low nutrients available in its natural environments. A family of marine Gram-negative bacteria of particular interest is
Vibrio. This comprises both pathogens and non-pathogenic bacteria. Among the former,
Vibrio cholerae has been found to produce a higher amount of vesicles after shifting from the aquatic environment to the infected host [
40]. It is possible that the vesiculation is augmented to eliminate outer membrane unfavourable compounds and for better colonizing the host environment. The bacterium regulates the protein expression (and the lipid A structure, see below) to adapt to different environments. The expression of porin OmpT in place of OmpU in Vibrio cholerae affects the pathogenesis mechanism, and promotes the resistance to bile, and the ability to colonize [
41]. In another paper,
Vibrio cholerae cells and vesicle proteomics have been compared [
42]. The study demonstrated that the vesicles were enriched in virulence factors with respect to the cells. The authors hypothesize that this enrichment points to the theory that the vesicles are not simply the product of membrane blebbing but a programmed way to vehicle molecules [
42].
The protein profile of the OMVs from
Pseudomonas syringae Lz4W, an Antarctic isolate, comprises OMPs, lipoproteins, ABC transporters, ribosomal proteins cytosolic enzymes, and many others [
43]. Kulkarni and co-authors underlined that OMVs from
P. syringae Lz4W are involved in antibiotic resistance and sensitivity. The mechanisms of action played by the vesicles seem to be environmental situation-dependent. In addition, since phospholipids and LPSs from cold-adapted bacteria are different from corresponding mesophiles, due to the higher amount of unsaturated fatty acids necessary for membrane fluidity at low temperatures, the packing parameters of the membrane are different for this bacterium.
The genus
Shewanella is prone to the production of OMVs, as revealed by the species
livingstonensis AC10 [
44],
vesiculosa M7 [
45,
46] and HM13 [
47]. All these strains are cold-adapted with a putative consideration for the secretory production of proteins in the extracellular. Proteomic studies have been performed for
S. vesiculosa M7 vesicles, revealing that this bacterium can produce a new type of vesicles named outer-inner membrane vesicles (EOIMVs, see
Figure 1). The last possesses a double-bilayered structure harboring cytoplasmic and plasma membrane proteins and can incorporate DNA [
46]. Unlike the M7 strain, the vesicles of HM13 have been carefully characterized for the presence of a cargo protein, named P49, for which the function is still unknown [
47]. Interestingly,
S. vesiculosa HM13 also produces a putative sensor protein involved in the suppression of biofilm formation [
48].
3.2. Nucleic Acids
Nucleic acids associated with vesicles play significant roles in marine microbial communities and ecosystem dynamics [
49]. The incorporation into vesicles occurs through passive encapsulation within the vesicle lumen as the vesicle forms from the budding of the cell membrane. Furthermore, marine bacteria and cyanobacteria employ molecular chaperones, RNA-binding proteins, and membrane-associated complexes that recognize and sort nucleic acids into vesicles. Finally, the interaction between marine bacteria, cyanobacteria, and viruses (phages) influences nucleic acid incorporation into vesicles. For example, cyanophages infecting Prochlorococcus cyanobacteria have been shown to package their DNA into vesicles released by infected cells, leading to the co-presence of host and viral nucleic acids [
13].
The incorporation of nucleic acids into vesicles significantly enriches their functional repertoire, contributing to various biological processes in marine microbial communities: serve as vectors for horizontal gene transfer (HGT), facilitating the dissemination of genetic material, including antibiotic resistance genes, metabolic pathways, and virulence factors, among microbial populations [
49,
50]. For instance, vesicles released by
Vibrio cholerae,
Pseudomonas aeruginosa,
Synechococcus, and
Shewanella genera contain both functional genes, facilitating HGT in marine environments and DNA-encoding bacteriocins that inhibit the growth of competing bacterial species [
51,
52]. Nucleic acids may encode regulatory elements, such as small regulatory RNAs (sRNAs), microRNAs (miRNAs), and transcription factors, which modulate gene expression and cellular responses to environmental cues ([
50,
53]. Vesicles released by the cyanobacteria belonging to
Synechococcus genus contain miRNAs involved in regulating photosynthesis and nitrogen metabolism in recipient cells [
53]. Vesicles released by
Vibrio parahaemolyticus and
Vibrio cholerae carry DNA fragments encoding virulence genes, enhancing the pathogenic potential of these bacteria [
52,
54].
Shewanella spp. vesicles contain DNA fragments encoding chemotaxis proteins involved in sensing environmental gradients [
50].
3.3. Phospholipids
Phospholipids in vesicles play a fundamental role in cargo selection and transport. Even if some sphingolipids have been demonstrated to be delivered for a long distance through vesicles in Bacteroides species [
55] the importance of characterization of lipid fraction in BEVs has been overlooked in many papers. Essential parts in the biogenesis of membrane vesicles are the structures of the fatty acids. The last ones are usually involved in maintaining the fluidity or the rigidity of the membrane, which is particularly important for microorganisms thriving in cold environments. A few papers describing phospholipid structure from bacterial membrane vesicles are devoted to studying cold-adapted bacteria. Antarctic
Pseudomonas syringae has been described as a producer of vesicles containing phospholipids with both saturated and unsaturated fatty acids [
43]. This was expected since the increase of membrane fluidity of cold-adapted bacteria necessary to survive at low T entails the biosynthesis of unsaturated fatty acids [
56]. In the case of another cold-adapted bacterium,
Pseudoalteromonas antarctica, only phosphatidylethanolamine and phosphatidylglycerol have been reported [
57].
An enhancement in the production of membrane vesicles has been observed for change in the phospholipids biogenesis with another Gram-negative bacterium named
Shewanella livingstonensis Ac10. A depletion of the gene for the biosynthesis of the eicosapentaenoic acid (EPA) induced a significative and quantitative increase in vesicle production [
58]. It was suggested that the lack of EPA fatty acid could alter the protein composition of the vesicles since in these conditions the transfer of a misfolded OmpC176 was facilitated.
Some marine bacteria can alter the molecular surface in response to different environments. The key case is represented by
Vibrio cholerae for which a change in the phospholipid composition moving from marine to host environment was observed. Zingl et al. [
59] reported that phospholipid accumulation on the membrane surface can be related to membrane vesicles release. After
Vibrio enters host cells it has been observed a change in the lipid moiety of the LPS (see below), with a consequent change in the asymmetry of the outer membrane and an accumulation of phospholipids [
31]. The different ratios of phospholipids/LPS are crucial to produce the vesicles. Among the factors regulating the increase of BEV production, is the repression of the VacJ/Yrb transporter influenced by the depletion of iron [
31] and sulfur [
60].
Vibrio species can produce CAI-1, a long-chain amino ketone, a signal molecule involved in the so-called quorum sensing, a way of communication among microorganism cells [
61,
62]. In some cases, the QS molecules can be associated also with vesicles, as has been reported for
Vibrio harveji strain MR17. The loading of this molecule is probably due to its lipophilic character that allows the interaction with phospholipids bilayer and LPS, facilitating its distribution among bacterial cells [
62].
3.4. Lipopolysaccharides
Lipopolysaccharides are the main components of the outer membrane of Gram-negative bacteria of which they constitute 75% of the outer leaflet [
63]. LPS is one of the most well-studied pathogen-associated molecular patterns (PAMPs) since it is a powerful activator of innate immune responses [
64,
65]. LPS binds to the proteins Toll-like receptor 4 (TLR4) and myeloid differentiation factor-2 (MD2) to activate pro-inflammatory signaling pathways. The TLR4–MD2 receptor complex is crucial for the host recognition of Gram-negative bacterial infection [
66]. These molecules are composed of three different domains, lipid A, embedded within the outer leaflet of the outer cell membrane, an oligosaccharide named “core”, and a polysaccharide mentioned as O-antigen that sticks out the extracellular environment [
67,
68,
69,
70]. Since the biogenesis of vesicles in Gram-negative bacteria is generated directly from the outer membrane, the lipopolysaccharides are particularly abundant in EVs. Nevertheless, their structures and the roles they eventually played in transportation have been only barely understood.
In some pathogenic Gram-negative bacteria, it has been demonstrated that the structure of the LPS components is involved in the vesiculation process.
P. aeruginosa is reported to produce two different O-chains, namely A and B-bands, respectively. The A-band is a hydrophobic D-rhamnan chain whereas the B-band displays negative charges due to the presence of acidic monosaccharides. The repulsion among the polysaccharide chains of the B-band could be responsible for a different curvature of the outer membrane thus releasing a higher number of vesicles [
71]. Differently from
Pseudomonas aeruginosa,
Salmonella enterica serovar Typhimurium is involved in a novel mechanism for OMV biogenesis where the lipid A modification is involved in a remodelling event caused by the induction of PagL enzyme [
72]. Feldman’s group has clarified that the LPS can play a role in the BEVs biogenesis [
73]. They proposed the presence of a peculiar cargo selection process in which the lack of some fatty acids on the lipid A moiety isolated from the
Porphyromonas gingivalis BEVs is responsible for the insertion of different proteins on the vesicles. Instead, for the same bacterium they demonstrated that there is no involvement of the O-chain in vesicle formation. Differently from
P. gingivalis, a study performed by the same research group on
Bacteroides fragilis, showed that there were no differences in the lipid A structures between cells and vesicles [
74].
Very few structures of LPS from marine EVs have been isolated and characterized. The molecular characterization of the LPS from both cells and EVs of
Shewanella vesiculosa HM13 has revealed the same structures [
75,
76]. The bacterium, classified as cold-adapted and isolated from the intestine of a fish, can produce abundant EVs carrying an unknown cargo protein named P49 [
47]. Even if many other
Shewanella strains have been reported to produce EVs [
77] no experiments to detect the chemical structures of the LPS from these strains have been performed [
45,
46]. Frias et al. observed a different amount of EVs for the marine
S. livingstonensis NF22T when the microorganism was grown at different temperatures. The lowering of temperature determines a higher amount of the recovered EVs [
77]. Other marine bacteria have been studied for the content of LPS in their produced EVs, such as
Pseudoalteromonas antarctica NF3, for which the LPS polymers from the cells and the vesicles have the same mobility on the SDS-PAGE [
57]. This bacterium was particularly interesting due to the presence of an additional band for both LPS samples near the top of the gel most probably due to a capsular polysaccharide. The negative stained TEM images of the vesicles and the observed fibrous fringe around the cells suggested the production of extracellular polysaccharides, thus confirming the above hypothesis.
The lipopolysaccharide from cells and OMVs of
Cellulophaga lytica, a marine Gram-negative bacterium, is involved in the process of metamorphosis for the marine worm
Hydroides elegans [
78]. A bioassay-guided fractionation of the molecular components of OMVs from
C. lytica indicated that LPS was responsible for the larva settlements. Authors hypothesized that the induction of larva settlement and metamorphosis is strain-specific due to the inherent structural variability of LPS.
3.5. Capsular Polysaccharide
Among the bacterial surface glycans, capsular polymers occupy an escalating position due to their involvement in many biological processes, such as engagement with biofilm formation [
79], pathogenesis mechanisms [
80], nutrients, involvement in biogeochemical cycling of elements in the oceans [
81]. Polysaccharides contribute to the formation of Extracellular Polymeric Substances (EPS) biofilm matrix, in which bacterial vesicles are entrapped [
82].
Capsular polysaccharides are strictly associated with the outer membrane of both Gram-positive and -negative bacteria [
83], the presence of which can be revealed by microscopy. The polysaccharide can be retained on the surface by a lipid moiety [
84] or by ionic interactions [
85] since most of these polymers are anionic. Bacteria can also produce exopolysaccharides, that are secreted into the surrounding environment [
86].
Since the generation of BEVs occurs through mechanisms involving the outer membrane it is reasonable to find a layer of capsular polysaccharide around the vesicles.
Capsular polysaccharides from pathogenic bacteria are classified as PAMPs and therefore they are among the preferred subjects for the construction of vaccines.
E.coli OMVs, used as a platform to deliver capsular polysaccharides against
Streptococcus pneumoniae, were found to induce a significant immune response [
87]. Also, engineered
E.coli were able to produce recombinant vesicles carrying the capsular PNAG, able to induce the formation of IgG antibodies after immunization in mice [
88].
Marine bacteria capable of producing cells covered by capsular polysaccharides have been reported for
Shewanella strains, and the presence of such polymers together with smooth LPS has been related to the surface strong adhesion capacity of members of this genus [
89]). In a paper from Mercade’s group, it has been reported that growths of cold-adapted bacteria belonging to various genera of class
Gamma proteobacteria revealed the presence of a large amount of extracellular material together with BEVs [
77]. It was speculated that the reasons for which extracellular matter was abundant could be to constitute a micro-environment for the survival of bacterial cells. In addition, when the temperature is low it has been demonstrated [
90,
91] that capsular polysaccharides can play a cryoprotectant role whereas the exopolysaccharides can protect from desiccation, enhance metal chelation, scavenge nutrients and small molecular compounds from solution, and aid cell motility and adherence [
92] Recently, a capsular polysaccharide isolated from both the cells and EVs of
Shewanella vesiculosa HM13 has been characterized [
93]. It is constituted by a pentasaccharide repeating unit containing three aminosugars, of which one is a new monosaccharide named shewanosamine. The structure of this capsule is peculiar since is characterized by a subtle equilibrium between hydrophilic and hydrophobic features. In the study Casillo et al. [
93] observed the formation of a “polysaccharide corona” on the surface of both synthetic polystyrene and liposome nanoparticles, thus demonstrating the strong adhesive properties of this polysaccharide. Capsular polysaccharides from some other cold-adapted and marine bacteria have been characterized [
90,
91,
94,
95,
96]. Intriguingly, all of them show the presence of aminosugars, hydrophobic moieties, and ionic groups. We could speculate that these features are necessary for adhesion on the biotic and abiotic surfaces. In addition, the sticky behaviour of these molecules is certainly exploited for biofilm formation. It has been reported that the MVs have a pivotal role in starting biofilm formation [
97,
98], and then the capsular polysaccharide may take an active part in this event.
Changes in the environment can create stress for the microorganisms [
99] and bacterial vesicles with their components are involved both as production amount and functional differences. However, a distinct role due to the presence of capsular polysaccharides on the MVs is far from being clarified.
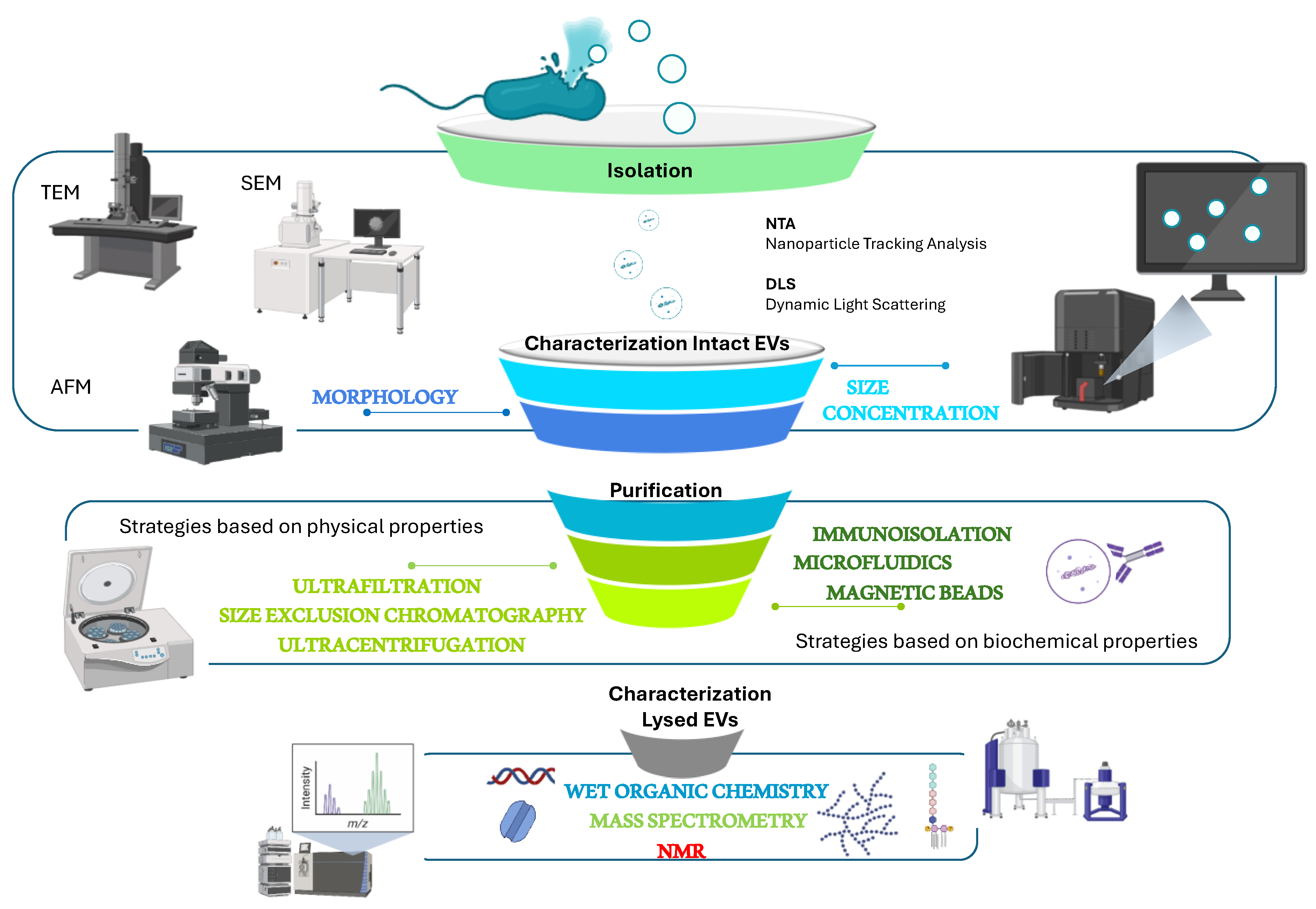
4. Conventional Techniques for BEV Isolation, Purification and Characterization
The isolation and purification of BEVs is a difficult task due to the possibility of recovering the vesicles together with non-EV materials, such as flagella, pili, phages, protein complexes, and DNA-protein complexes. For these reasons, shared protocols have been set up and published by the International Society for Extracellular Vesicles (2014 and 2018). The protocols have been regularly updated since 2014, and report both separation and characterization methods [
100].
To be sure that a bacterium produces membrane vesicles methods for their visualization are necessary (
Figure 2). The large majority of marine BEVs have been visualised by negative stained TEM (Transmission Electron Microscopy) [
37,
40,
47,
57,
77,
78,
101,
102,
103,
104,
105,
106] SEM (Scansion Electron Microscopy), epifluorescence microscopy, Atomic Force Microscopy (AFM). FE-SEM (Field Emission-Scanning Electron Microscopy) analysis has been used for
Shevanella vesiculosa HM13 for observing the surface morphology of the cells secreting BEVs thus demonstrating the absence of cell lysis while producing vesicles [
47]. Finally, super-resolution microscopy (Cryo-EM) the cryo-electron micrographs, allowed the visualization of a large periplasm, a protrusion of the cytoplasm and tubular appendages [
105].
The characterization of BEVs is done by taking into account the physical state of the sample, both native vesicles and lysed vesicles, by considering their shape, size distribution, concentration, surface, or internal contents (
Figure 2). The preliminary optical analytical approaches that can be used are usually related to the physical state of the isolated vesicles. The intact EVs can be analysed in the form of a dynamic suspension by using NTA, which gives information about particle number and size distribution [
107,
108], dynamic light scattering [
109], but also fluorescence correlation spectroscopy (FCS) and high-resolution flow cytometry [
11], flow cytometry, fluorescence anisotropy, live microscopy, or captured on a surface employing immunomagnetic beads, arrays, microfluidics, microscopy on fixed samples. Instead, the analyses of lysed EVs generally require molecular analyses [
110,
111]. Generally, because none of these approaches can yield comprehensive data about MVs, a panel of these approaches is usually used [
112].
The main activity for the purification of BEVs is reported to be differential ultracentrifugation, integrated with various other techniques, such as precipitation, filtration, density gradients, gel filtration chromatography, and immunoisolation [
111,
113,
114,
115]. Preliminary information about BEV composition can be obtained through the quantification of suitable markers such as proteins and phospholipids. The protein concentration can be measured by classical colourimetric methods such as Lowry or Bradford, together with stained gel-electrophoresis analysis showing at least membrane or outer membrane proteins [
107,
113] whereas the lipids can be measured through a fluorescent probe with a fluorometer [
113].
The purification of vesicles is mandatory for the consecutive analyses for proteomic [
116,
117], lipids [
118], nucleic acids [
119], and carbohydrate analyses [
120,
121].
5. Functional Significance and Biotechnology Application of Vesicles
The extracellular vesicles are implicated in various biological processes. One of the most recognized functions of BEVs is transmitting information between bacterial and eukaryotic cells. Furthermore, material vehicled through BEVs rather than excretion directly into the environment may be advantageous for the bacterium. Indeed, BEVs are more suitable for delivering microbial molecules at higher distances than a surface secretion system [
122]. The human microbiota
Bacteroides fragilis has been found to deliver to the host immune system an inflammatory molecule such as the PSA polysaccharide, where the transportation takes advantage of the vesicle system [
123]. Vesicles are also involved in cell signaling, since they contain quorum-sensing molecules, secondary messengers, and other signaling compounds, thus facilitating cell-cell and cell-environment communication. Vesicles can serve as vehicles for transporting nutrients such as carbon, nitrogen, and phosphorus. Indeed, in nutrient-limited environments, this mechanism allows cyanobacterial populations to efficiently scavenge and share scarce resources, enhancing their collective fitness and resilience [
124].
Extracellular vesicles may carry antimicrobial compounds, toxins, and defensive proteins that help protect cells from predation, competition, and environmental stressors. Additionally, they can serve as vehicles for horizontal gene transfer facilitating the exchange of genetic information between cells and potentially contributing to the evolution and diversification of microbial populations. Finally, they are involved in environmental interactions.
Studies have shown that
Prochlorococcus EVs can interact with diverse microbial cells, suggesting a potential role in mediating microbial interactions and ecosystem dynamics in marine environments [
13,
125]. They can be taken up by other microbial cells, including bacteria, archaea, and eukaryotes, influencing their physiology, metabolism, and behaviour. Additionally, vesicles released by cyanobacteria can impact the structure and function of microbial communities, shaping ecosystem dynamics and biogeochemical cycling in marine environments.
While the role of vesicles in bacterial pathogens is actively studied, the role of vesicles in the marine environment is poorly understood. Membrane vesicles were previously observed in the cyanobiont that colonizes the sporocarp of the water fern
Azolla microphylla [
126]. The authors hypothesized that vesicles could deliver soluble sugars and material for biofilm development.
Recently the applications of vesicles from marine bacteria have increased in various fields as they offer a great diversity of cargo molecules (
Figure 3 and
Table 1). Vesicle-associated nucleic acids hold significant biotechnological potential for various applications, including environmental monitoring, bioremediation, and biopharmaceutical production [
126]. The utilization of vesicle-derived DNA for metagenomic analysis has emerged as a powerful tool for studying microbial diversity and functional potential in marine ecosystems [
13].
The use of nanoparticles (NPs) for drug delivery has been extensively exploited [
132]. When released in circulation, NPs are immediately exposed to high protein concentrations, thus determining the formation of a protein layer on their surface, altering their identity and producing its so-called ‘biological identity’. Conversely, the surface of BEVs is often decorated by complex glycans that reduce the adsorption of proteins, thus maintaining the same composition. The BEVs are hence considered attractive for use as drug nanocarriers, due to their high biocompatibility and ability to enter cells [
133]. BEVs can also be considered a therapeutic platform due to their capacity to load and deliver active molecules [
134].
6. Conclusions and Future Perspectives
The study of BEVs offers valuable insights into microbial ecology, biogeochemistry, and biotechnology. Further research is needed to unravel the mechanisms underlying EV production, decipher the functional roles of vesicles in marine ecosystems, and explore their potential applications in biotechnology. Understanding the intricate interplay between marine microorganisms, their vesicles, and the surrounding environment holds promise for advancing our knowledge of microbial life and harnessing its potential for the benefit of society and the environment. Their natural propensity to serve as vehicles for delivering bioactive compounds [
135], combined with the recent advances in synthetic biology for engineering vesicles with tailored cargos, makes these natural nanoparticles a promising strategy for specific biotechnological purposes, opening new avenues for bioprospecting and innovation [
136].
Author Contributions
Writing—original draft preparation, A.C., M.M.C; writing—review and editing, A.C., R.D.A, R.L., M.M.C.; supervision, M.M.C; funding acquisition, M.M.C.
Acknowledgments
M.M.C acknowledges the support of NBFC to the University of Naples Federico II, Department of Chemical Sciences. Project code CN_00000033, Concession Decree No. 1034 of 17 June 2022 adopted by the Italian Ministry of University and Research, CUP E63C22000990007, Project title “National Biodiversity Future Center –NBFC”. M.M.C. acknowledges the MUR the project PNRR Mission 4 component 2, investment 3.1. Founded by the European Union-NextGenerationEU; Award number: Project code IR0000035, CUP C63C22000570001 Project title: “Unlocking the potential for health and food from the seas-EMBRC UP”.
References
- Deatherage, B.L.; Brad, T.C. Membrane vesicle release in bacteria, eukaryotes, and archaea: a conserved yet underappreciated aspect of microbial life. Infect. Immun. 2012, 80, 1948–1957.
- Gill, S.; Catchpole, R.; Forterre, P. Extracellular membrane vesicles in the three domains of life and beyond. FEMS Microbiol. Rev. 2018, 43, 273–303, . [CrossRef]
- Buzas, E.I. The roles of extracellular vesicles in the immune system. Nat. Rev. Immunol. 2023, 23, 236–250, . [CrossRef]
- Couch, Y.; Buzàs, E.I.; Di Vizio, D.; Gho, Y.S.; Harrison, P.; Hill, A.F.; Lötvall, J.; Raposo, G.; Stahl, P.D.; Théry, C.; Witwer, K.W.; Carter, D.R.F.; et al. A brief history of nearly EV-erything–The rise and rise of extracellular vesicles. JEV 2021, 10.14, e12144.
- Weiwei, H.; et al. Development of novel nanoantibiotics using an outer membrane vesicle-based drug efflux mechanism. J. control. release 2020; 317, 1–22.
- Somiya, M.; Yoshioka, Y.; Ochiya, T. Drug delivery application of extracellular vesicles; insight into production, drug loading, targeting, and pharmacokinetics. AIMS Bioeng. 2017, 4, 73–92, . [CrossRef]
- Chen, Q.; Bai, H.; Wu, W.; Huang, G.; Li, Y.; Wu, M.; Tang, G.; Ping, Y. Bioengineering Bacterial Vesicle-Coated Polymeric Nanomedicine for Enhanced Cancer Immunotherapy and Metastasis Prevention. Nano Lett. 2019, 20, 11–21, . [CrossRef]
- Bar-On, Y.M.; Milo, R. The Biomass Composition of the Oceans: A Blueprint of Our Blue Planet. Cell 2019, 179, 1451–1454, . [CrossRef]
- Toyofuku, M.; Nomura, N.; Eberl, L. Types and origins of bacterial membrane vesicles. Nat. Rev. Microbiol. 2019, 17, 13–24, doi:10.1038/s41579-018-0112-2.
- Zlatkov, N.; Nadeem, A.; Uhlin, B.E.; Wai, S.N. Eco-evolutionary feedbacks mediated by bacterial membrane vesicles. FEMS Microbiol. Rev. 2021, 45, . [CrossRef]
- Biller, S.J.; Lundeen, R.A.; Hmelo, L.R.; Becker, K.W.; Arellano, A.A.; Dooley, K.; Heal, K.R.; Carlson, L.T.; Van Mooy, B.A.S.; Ingalls, A.E.; et al. Prochlorococcus extracellular vesicles: molecular composition and adsorption to diverse microbes. Environ. Microbiol. 2022, 24, 420–435, . [CrossRef]
- Kadurugamuwa, J.L.; Beveridge, T.J. Virulence factors are released from Pseudomonas aeruginosa in association with membrane vesicles during normal growth and exposure to gentamicin: a novel mechanism of enzyme secretion. J. Bacteriol. 1995, 177, 3998–4008, . [CrossRef]
- Biller, S.J.; Schubotz, F.; Roggensack, S.E.; Thompson, A.W.; Summons, R.E.; Chisholm, S.W. Bacterial Vesicles in Marine Ecosystems. Science 2014, 343, 183–186, . [CrossRef]
- Puca, V.; Marinacci, B.; Pellegrini, B.; Campanile, F.; Santagati, M.; Grande, R. Biofilm and bacterial membrane vesicles: recent advances. Expert Opin. Ther. Patents 2024, 34, 475–491, . [CrossRef]
- Manning, A.J.; Kuehn, M.J. Contribution of bacterial outer membrane vesicles to innate bacterial defense. BMC Microbiol. 2011, 11, 258–258, . [CrossRef]
- Pérez-Cruz, C.; Delgado, L.; López-Iglesias, C.; Mercade, E. Outer-Inner Membrane Vesicles Naturally Secreted by Gram-Negative Pathogenic Bacteria. PLOS ONE 2015, 10, e0116896–e0116896, . [CrossRef]
- Meng, R.; Zeng, M.; Ji, Y.; Huang, X.; Xu, M. The potential role of gut microbiota outer membrane vesicles in colorectal cancer. Front. Microbiol. 2023, 14, 1270158, . [CrossRef]
- Badi, S.A.; Moshiri, A.; Fateh, A.; Jamnani, F.R.; Sarshar, M.; Vaziri, F.; Siadat, S.D. Microbiota-Derived Extracellular Vesicles as New Systemic Regulators. Front. Microbiol. 2017, 8, 1610, . [CrossRef]
- Arntzen, M..; Várnai, A.; Mackie, R.I.; Eijsink, V.G.H.; Pope, P.B. Outer membrane vesicles from Fibrobacter succinogenes S85 contain an array of carbohydrate-active enzymes with versatile polysaccharide-degrading capacity. Environ. Microbiol. 2017, 19, 2701–2714, . [CrossRef]
- Abe, K.; Nomura, N.; Suzuki, S. Biofilms: hot spots of horizontal gene transfer (HGT) in aquatic environments, with a focus on a new HGT mechanism. FEMS Microbiol. Ecol. 2020, 96, . [CrossRef]
- Schatz, D.; Vardi, A. Extracellular vesicles—new players in cell–cell communication in aquatic environments. Current opinion in microbiology, 2018, 43, 148-154.
- Wang, J.; Zhang, W.; Wang, X.; Hu, X.; Peng, L.; Yang, J.-L.; Liang, X. Mussel settlement mediated by bacterial VgrG proteins via extracellular outer membrane vesicles. Int. Biodeterior. Biodegradation 2023, 180, . [CrossRef]
- Linney, M.D.; Eppley, J.M.; Romano, A.E.; Luo, E.; DeLong, E.F.; Karl, D.M. Microbial Sources of Exocellular DNA in the Ocean. Appl. Environ. Microbiol. 2022, 88, e0209321, . [CrossRef]
- Toyofuku, M.; Schild, S.; Kaparakis-Liaskos, M.; Eberl, L. Composition and functions of bacterial membrane vesicles. Nat. Rev. Microbiol. 2023, 21, 415–430, . [CrossRef]
- Zarantonello, V.; Silva, T.P.; Noyma, N.P.; Gamalier, J.P.; Mello, M.M.; Marinho, M.M.; Melo, R.C.N. The Cyanobacterium Cylindrospermopsis raciborskii (CYRF-01) Responds to Environmental Stresses with Increased Vesiculation Detected at Single-Cell Resolution. Front. Microbiol. 2018, 9, 272, . [CrossRef]
- Toyofuku, M.; Nomura, N.; Eberl, L. Types and origins of bacterial membrane vesicles. Nat. Rev. Microbiol. 2019, 17, 13–24, doi:10.1038/s41579-018-0112-2.
- Beveridge, T.J. Structures of Gram-Negative Cell Walls and Their Derived Membrane Vesicles. J. Bacteriol. 1999, 181, 4725–4733, . [CrossRef]
- Schwechheimer, C.; Kuehn, M.J. Outer-membrane vesicles from Gram-negative bacteria: biogenesis and functions. Nat. Rev. Microbiol. 2015, 13, 605–619, . [CrossRef]
- Turnbull, L.; Toyofuku, M.; Hynen, A.L.; Kurosawa, M.; Pessi, G.; Petty, N.K.; Osvath, S.R.; Cárcamo-Oyarce, G.; Gloag, E.S.; Shimoni, R.; et al. Explosive cell lysis as a mechanism for the biogenesis of bacterial membrane vesicles and biofilms. Nat. Commun. 2016, 7, 11220–11220, . [CrossRef]
- Juodeikis, R.; Carding, S.R. Outer Membrane Vesicles: Biogenesis, Functions, and Issues. Microbiol. Mol. Biol. Rev. 2022, 86, e0003222, . [CrossRef]
- Roier, S.; Zingl, F.; Cakar, F.; Schild, S. Bacterial outer membrane vesicle biogenesis: a new mechanism and its implications. Microb. Cell 2016, 3, 257–259, . [CrossRef]
- Briaud, P.; Carroll, R.K. Extracellular Vesicle Biogenesis and Functions in Gram-Positive Bacteria. Infect. Immun. 2020, 88, . [CrossRef]
- Gasperini, G.; Biagini, M.; Arato, V.; Gianfaldoni, C.; Vadi, A.; Norais, N.; Bensi, G.; Delany, I.; Pizza, M.; Aricò, B.; et al. Outer Membrane Vesicles (OMV)-based and Proteomics-driven Antigen Selection Identifies Novel Factors Contributing to Bordetella pertussis Adhesion to Epithelial Cells. Mol. Cell. Proteom. 2018, 17, 205–215, . [CrossRef]
- Ruiz-Palma, M.d.S.; Avila-Calderón, E.D.; Aguilera-Arreola, M.G.; López-Merino, A.; Ruiz, E.A.; Morales-García, M.d.R.; López-Villegas, E.O.; Gomez-Lunar, Z.; Arellano-Reynoso, B.; Contreras-Rodríguez, A. Comparative proteomic analysis of outer membrane vesicles from Brucella suis, Brucella ovis, Brucella canis and Brucella neotomae. Arch. Microbiol. 2021, 203, 1611–1626, . [CrossRef]
- Chen, Q.; Ma, B.; Xu, M.; Xu, H.; Yan, Z.; Wang, F.; Wang, Y.; Huang, Z.; Yin, S.; Zhao, Y.; et al. Comparative proteomics study of exosomes in Vibrio harveyi and Vibrio anguillarum. Microb. Pathog. 2023, 181, 106174, . [CrossRef]
- McMillan, H.M.; Kuehn, M.J. Proteomic Profiling Reveals Distinct Bacterial Extracellular Vesicle Subpopulations with Possibly Unique Functionality. Appl. Environ. Microbiol. 2023, 89, e0168622, . [CrossRef]
- Naval, P.; Chandra, T. Characterization of membrane vesicles secreted by seaweed associated bacterium Alteromonas macleodii KS62. Biochem. Biophys. Res. Commun. 2019, 514, 422–427, . [CrossRef]
- Elhenawy, W.; Debelyy, M.O.; Feldman, M.F. Preferential Packing of Acidic Glycosidases and Proteases into Bacteroides Outer Membrane Vesicles. mBio 2014, 5, e00909–14, . [CrossRef]
- Fadeev, E.; Bastos, C.C.; Hennenfeind, J.H.; Biller, S.J.; Sher, D.; Wietz, M.; Herndl, G.J. Characterization of membrane vesicles in Alteromonas macleodii indicates potential roles in their copiotrophic lifestyle. microLife 2023, 4, uqac025, . [CrossRef]
- Yun, S.H.; Lee, S.-Y.; Choi, C.-W.; Lee, H.; Ro, H.-J.; Jun, S.; Kwon, Y.M.; Kwon, K.K.; Kim, S.-J.; Kim, G.-H.; et al. Proteomic characterization of the outer membrane vesicle of the halophilic marine bacterium Novosphingobium pentaromativorans US6-1. J. Microbiol. 2017, 55, 56–62, . [CrossRef]
- Provenzano, D.; Klose, K. E. Altered expression of the ToxR-regulated porins OmpU and OmpT diminishes Vibrio cholerae bile resistance, virulence factor expression, and intestinal colonization. Proc. Nat. Acad. Sci. 2000, 97(18), 10220-10224.
- Langlete, P.; Krabberød, A.K.; Winther-Larsen, H.C. Vesicles From Vibrio cholerae Contain AT-Rich DNA and Shorter mRNAs That Do Not Correlate With Their Protein Products. Front. Microbiol. 2019, 10, 2708, . [CrossRef]
- Kulkarni, H.M.; Swamy, C.V.B.; Jagannadham, M.V. Molecular Characterization and Functional Analysis of Outer Membrane Vesicles from the Antarctic Bacterium Pseudomonas syringae Suggest a Possible Response to Environmental Conditions. J. Proteome Res. 2014, 13, 1345–1358, . [CrossRef]
- Yokoyama, F.; Kawamoto, J.; Imai, T.; Kurihara, T. Characterization of extracellular membrane vesicles of an Antarctic bacterium, Shewanella livingstonensis Ac10, and their enhanced production by alteration of phospholipid composition. Extremophiles 2017, 21, 723–731, . [CrossRef]
- Bozal, N.; Montes, M.J.; Miñana-Galbis, D.; Manresa, A.; Mercadé, E. Shewanella vesiculosa sp. nov., a psychrotolerant bacterium isolated from an Antarctic coastal area. Int. J. Syst. Evol. Microbiol. 2009, 59, 336–340, . [CrossRef]
- Pérez-Cruz, C.; Carri n, O., Delgado, L., Martinez, G., L pez-Iglesias, C., Mercade, E. New type of outer membrane vesicle produced by the Gram-negative bacterium Shewanella vesiculosa M7T: implications for DNA content. Appl. Environ. Microbiol. 2013, 79(6), 1874-1881.
- Chen, C.; Kawamoto, J.; Kawai, S.; Tame, A.; Kato, C.; Imai, T.; Kurihara, T. Isolation of a Novel Bacterial Strain Capable of Producing Abundant Extracellular Membrane Vesicles Carrying a Single Major Cargo Protein and Analysis of Its Transport Mechanism. Front. Microbiol. 2020, 10, 3001, . [CrossRef]
- Yokoyama, F.; Imai, T.; Aoki, W.; Ueda, M., Kawamoto, J., Kurihara, T. Identification of a putative sensor protein involved in regulation of vesicle production by a hypervesiculating bacterium, Shewanella vesiculosa HM13. Front. Microbiol. 2021, 12, 629023.
- Adams, R. D.; Smith, J. K., Brown, S. A., Johnson, L. M. Mechanisms of nucleic acid incorporation into vesicles produced by marine bacteria and cyanobacteria. Mar. Mol. Biol. Rev. 2019, 10(4), 205-220.
- Zhang, W., Liu, H., & Tian, L. Extracellular Vesicles in Marine Microbial Ecology: Diversity, Functions, and Applications. Front. Microbiol. 2021, 12, 663543.
- Chen, Y., Vojtech, L. N., Hong, J.; Norris, M. H. Infection-Induced pH and Voltage Changes Contribute to Vesicle Content Mobilization in Pseudomonas aeruginosa. mBio 2021, 12(1), e03329-20.
- Liu, Q., Zhao, X., Liu, Q., Xu, L., Yan, Q., Han, Y., & Li, Y.The Diversity of Resistome in the Microbial Community in the Colored Snow from the Fildes Peninsula, King George Island, Maritime Antarctica. Frontiers in Microbiology 2018, 9, 2846.
- Kim, Y. S., Lee, J. H., Kim, S. W., & Kim, S.. Exosomal microRNAs as potential biomarkers in diagnosis and prognosis of colorectal cancer. World Journal of Gastroenterology 2018, 23(7), 3117–3126.
- Langlete, P.; Krabberød, A.K.; Winther-Larsen, H.C. Vesicles From Vibrio cholerae Contain AT-Rich DNA and Shorter mRNAs That Do Not Correlate With Their Protein Products. Front. Microbiol. 2019, 10, 2708, . [CrossRef]
- Sartorio, M.G.; Valguarnera, E.; Hsu, F.-F.; Feldman, M.F. Lipidomics Analysis of Outer Membrane Vesicles and Elucidation of the Inositol Phosphoceramide Biosynthetic Pathway in Bacteroides thetaiotaomicron. Microbiol. Spectr. 2022, 10, e0063421, . [CrossRef]
- Corsaro, M.M.; Lanzetta, R.; Parrilli, E.; Parrilli, M.; Tutino, M.L.; Ummarino, S. Influence of Growth Temperature on Lipid and Phosphate Contents of Surface Polysaccharides from the Antarctic Bacterium Pseudoalteromonas haloplanktis TAC 125. J. Bacteriol. 2004, 186, 29–34, . [CrossRef]
- Nevot, M.; Deroncelé, V.; Messner, P.; Guinea, J.; Mercadé, E. Characterization of outer membrane vesicles released by the psychrotolerant bacterium Pseudoalteromonas antarctica NF3. Environ. Microbiol. 2006, 8, 1523–1533, . [CrossRef]
- Yokoyama, F.; Kawamoto, J.; Imai, T.; Kurihara, T. Characterization of extracellular membrane vesicles of an Antarctic bacterium, Shewanella livingstonensis Ac10, and their enhanced production by alteration of phospholipid composition. Extremophiles 2017, 21, 723–731, . [CrossRef]
- Zingl, F.G.; Kohl, P.; Cakar, F.; Leitner, D.R.; Mitterer, F.; Bonnington, K.E.; Rechberger, G.N.; Kuehn, M.J.; Guan, Z.; Reidl, J.; et al. Outer Membrane Vesiculation Facilitates Surface Exchange and In Vivo Adaptation of Vibrio cholerae. Cell Host Microbe 2020, 27, 225–237.e8, . [CrossRef]
- Baeza, N.; Delgado, L.; Comas, J.; Mercade, E. Phage-Mediated Explosive Cell Lysis Induces the Formation of a Different Type of O-IMV in Shewanella vesiculosa M7T. Front. Microbiol. 2021, 12, . [CrossRef]
- Ganin, H.; Danin-Poleg, Y.; Kashi, Y.; Meijler, M.M. Vibrio cholerae Autoinducer CAI-1 Interferes with Pseudomonas aeruginosa Quorum Sensing and Inhibits its Growth. ACS Chem. Biol. 2012, 7, 659–665, . [CrossRef]
- Brameyer, S.; Plener, L.; Müller, A.; Klingl, A.; Wanner, G.; Jung, K. Outer Membrane Vesicles Facilitate Trafficking of the Hydrophobic Signaling Molecule CAI-1 between Vibrio harveyi Cells. J. Bacteriol. 2018, 200, e00740-17, . [CrossRef]
- Lüderitz, O., Freudenberg, M. A., Galanos, C., Lehmann, V., Rietschel, E. T., & Shaw, D. H. Lipopolysaccharides of gram-negative bacteria. In Current topics in membranes and transport. Academic Press 1982(Vol. 17, pp. 79-151).
- Raetz, C. R. H., Whitfield, C. Lipopolysaccharide Endotoxins. Biochem. 2002, 71, 635– 700,.
- Simpson, B.W.; Trent, M.S. Pushing the envelope: LPS modifications and their consequences. Nat. Rev. Microbiol. 2019, 17, 403–416, . [CrossRef]
- Park, B.S.; Song, D.H.; Kim, H.M.; Choi, B.-S.; Lee, H.; Lee, J.-O. The structural basis of lipopolysaccharide recognition by the TLR4–MD-2 complex. Nature 2009, 458, 1191–1195, . [CrossRef]
- Holst, O.; Brade, H. Chemical structure of the core region of lipopolysaccharides. in Bacterial Endotoxic Lipopolysaccharides CRC Press 1992, 171–205.
- Caroff, M.; Karibian, D. Structure of bacterial lipopolysaccharides. Carbohydr. Res. 2003, 338, 2431–2447, . [CrossRef]
- Casillo, A.; Parrilli, E.; Tutino, M.L.; Corsaro, M.M. The outer membrane glycolipids of bacteria from cold environments: isolation, characterization, and biological activity. FEMS Microbiol. Ecol. 2019, 95, . [CrossRef]
- Bryant, C.E.; Spring, D.R.; Gangloff, M.; Gay, N.J. The molecular basis of the host response to lipopolysaccharide. Nat. Rev. Microbiol. 2010, 8, 8–14, . [CrossRef]
- Kadurugamuwa, J.L.; Beveridge, T.J. Virulence factors are released from Pseudomonas aeruginosa in association with membrane vesicles during normal growth and exposure to gentamicin: a novel mechanism of enzyme secretion. J. Bacteriol. 1995, 177, 3998–4008, . [CrossRef]
- Elhenawy, W.; Bording-Jorgensen, M.; Valguarnera, E.; Haurat, M.F.; Wine, E.; Feldman, M.F. LPS Remodeling Triggers Formation of Outer Membrane Vesicles in Salmonella. mBio 2016, 7, e00940-16, . [CrossRef]
- Haurat, M.F.; Aduse-Opoku, J.; Rangarajan, M.; Dorobantu, L.; Gray, M.R.; Curtis, M.A.; Feldman, M.F. Selective Sorting of Cargo Proteins into Bacterial Membrane Vesicles. J. Biol. Chem. 2011, 286, 1269–1276, . [CrossRef]
- Elhenawy, W.; Debelyy, M.O.; Feldman, M.F. Preferential Packing of Acidic Glycosidases and Proteases into Bacteroides Outer Membrane Vesicles. mBio 2014, 5, e00909–14, . [CrossRef]
- Casillo, A.; Di Guida, R., Carillo, S., Chen, C., Kamasaka, K.,Kawamoto, J., Kurihara, T., Corsaro M.M. Structural elucidation of a novel lipooligosaccharide from the Antarctic bacterium OMVs producer Shewanella sp. HM13.Mar. Drugs 2019,17, 34.
- Di Guida, R., Casillo, A., Yokoyama, F., Kawamoto, J., Kurihara T., Corsaro., M.M. Detailed structural characterization of the lipooligosaccharide from the extracellular membrane vesicles of Shewanella vesiculosa HM13 Mar. Drugs 2020, 18, 231.
- Frias, A.; Manresa, A.; de Oliveira, E. et al. Membrane Vesicles: A Common Feature in the Extracellular Matter of Cold-Adapted Antarctic Bacteria. Microb. Ecol. 2010,59, 476–486.
- Freckelton, M.L.; Nedved, B.T.; Cai, Y.-S.; Cao, S.; Turano, H.; Alegado, R.A.; Hadfield, M.G. Bacterial lipopolysaccharide induces settlement and metamorphosis in a marine larva. Proc. Natl. Acad. Sci. 2022, 119, . [CrossRef]
- Limoli, D.H.; Jones, C.J.; Wozniak, D.J. Bacterial Extracellular Polysaccharides in Biofilm Formation and Function. Microbiol. Spectr. 2015, 3, . [CrossRef]
- Willis, L.M.; Whitfield, C. Structure, biosynthesis, and function of bacterial capsular polysaccharides synthesized by ABC transporter-dependent pathways. Carbohydr. Res. 2013, 378, 35–44, . [CrossRef]
- Nagar, S.; Antony, R.; Thamban, M. Extracellular polymeric substances in Antarctic environments: A review of their ecological roles and impact on glacier biogeochemical cycles. Polar Sci. 2021, 30, . [CrossRef]
- Schooling, S.R.; Beveridge, T.J. Membrane Vesicles: an Overlooked Component of the Matrices of Biofilms. J. Bacteriol. 2006, 188, 5945–5957, . [CrossRef]
- Whitfield, C.; Wear, S.S.; Sande, C. Assembly of Bacterial Capsular Polysaccharides and Exopolysaccharides. Annu. Rev. Microbiol. 2020, 74, 521–543, . [CrossRef]
- Gao, Y.; Widmalm, G.; Im, W. Modeling and Simulation of Bacterial Outer Membranes with Lipopolysaccharides and Capsular Polysaccharides. J. Chem. Inf. Model. 2023, 63, 1592–1601, . [CrossRef]
- Fresno, S.; Jiménez, N.; Izquierdo, L.; Merino, S.; Corsaro, M.M.; De Castro, C.; Parrilli, M.; Naldi, T.; Regué, M.; Tomás, J.M. The ionic interaction of Klebsiella pneumoniae K2 capsule and core lipopolysaccharide. Microbiology 2006, 152, 1807–1818, . [CrossRef]
- Casillo, A.; Lanzetta, R.; Parrilli, M.; Corsaro, M.M. Exopolysaccharides from Marine and Marine Extremophilic Bacteria: Structures, Properties, Ecological Roles and Applications. Mar. Drugs 2018, 16, 69. [CrossRef]
- Price, N.L.; Goyette-Desjardins, G.; Nothaft, H.; Valguarnera, E.; Szymanski, C.M.; Segura, M.; Feldman, M.F. Glycoengineered Outer Membrane Vesicles: A Novel Platform for Bacterial Vaccines. Sci. Rep. 2016, 6, 24931, . [CrossRef]
- Stevenson, T.C.; Cywes-Bentley, C.; Moeller, T.D.; Weyant, K.B.; Putnam, D.; Chang, Y.-F.; Jones, B.D.; Pier, G.B.; DeLisa, M.P. Immunization with outer membrane vesicles displaying conserved surface polysaccharide antigen elicits broadly antimicrobial antibodies. Proc. Natl. Acad. Sci. 2018, 115, E3106–E3115, . [CrossRef]
- Korenevsky, A.; Beveridge, T.J. The surface physicochemistry and adhesiveness of Shewanella are affected by their surface polysaccharides. Microbiology 2007, 153, 1872–1883, . [CrossRef]
- Carillo, S.; Casillo, A.; Pieretti, G.; Parrilli, E.; Sannino, F.; Bayer-Giraldi, M.; Cosconati, S.; Novellino, E.; Ewert, M.; Deming, J.W.; et al. A Unique Capsular Polysaccharide Structure from the Psychrophilic Marine Bacterium Colwellia psychrerythraea 34H That Mimics Antifreeze (Glyco)proteins. J. Am. Chem. Soc. 2015, 137, 179–189, . [CrossRef]
- Casillo, A.; Parrilli, E.; Sannino, F.; Mitchell, D.E.; Gibson, M.I.; Marino, G.; Lanzetta, R.; Parrilli, M.; Cosconati, S.; Novellino, E.; et al. Structure-activity relationship of the exopolysaccharide from a psychrophilic bacterium: A strategy for cryoprotection. Carbohydr. Polym. 2017, 156, 364–371, . [CrossRef]
- Deming, J.W.; Young, J.N. The role of exopolysaccharides in microbial adaptation to cold habitats. In Psychrophiles: From Biodiversity to Biotechnology; Springer: Cham, Switzerland, 2017; pp. 259–284.
- Casillo, A.; Di Guida, R.; Cavasso, D.; Stellavato, A.; Rai, D.; Yokoyama, F.; Kamasaka, K.; Kawamoto, J.; Kurihara, T.; Schiraldi, C.; et al. Polysaccharide corona: The acetyl-rich envelope wraps the extracellular membrane vesicles and the cells of Shewanella vesiculosa providing adhesiveness. Carbohydr. Polym. 2022, 297, 120036, . [CrossRef]
- Di Guida, R.; Casillo, A.; Stellavato, A., Kawai, S., Ogawa, T., di Meo, C., Kawamoto, J., Kurihara, T., Schiraldi, C., M. M. Corsaro, M.M. Capsular Polysaccharide from a fish-gut bacterium induces/promotes apoptosis of colon cancer cells in vitro through Caspases’ pathway activation Carbohydr. Polym. 2022. 278, 118908-118917.
- Kokoulin, M.S.; Kuzmich, A.S.; Romanenko, L.A.; Chikalovets, I.V. Structure and in vitro antiproliferative activity of the acidic capsular polysaccharide from the deep-sea bacterium Psychrobacter submarinus KMM 225T. Carbohydr. Polym. 2021, 262, 117941, . [CrossRef]
- Kokoulin, M.S.; Kuzmich, A.S.; Romanenko, L.A.; Chikalovets, I.V. Sulfated capsular polysaccharide from the marine bacterium Kangiella japonica inhibits T-47D cells growth in vitro. Carbohydr. Polym. 2022, 290, 119477, . [CrossRef]
- Baeza, N.; Mercade, E. Relationship Between Membrane Vesicles, Extracellular ATP and Biofilm Formation in Antarctic Gram-Negative Bacteria. Microb. Ecol. 2021, 81, 645–656, . [CrossRef]
- Wang, W.; Chanda, W.; Zhong, M. The relationship between biofilm and outer membrane vesicles: a novel therapy overview. FEMS Microbiol. Lett. 2015, 362, fnv117, . [CrossRef]
- Orench-Rivera, N.; Kuehn, M.J. Environmentally controlled bacterial vesicle-mediated export. Cell. Microbiol. 2016, 18, 1525–1536, . [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750, doi:10.1080/20013078.2018.1535750.
- Seike, S.; Kobayashi, H.; Ueda, M.; Takahashi, E.; Okamoto, K.; Yamanaka, H. Outer Membrane Vesicles Released From Aeromonas Strains Are Involved in the Biofilm Formation. Front. Microbiol. 2021, 11, . [CrossRef]
- Koteska, D.; Wang, H.; Wagner-Döbler, I.; Schulz, S. Outer membrane vesicles of Dinoroseobacter shibae transport a volatile aldehyde. Front. Ecol. Evol. 2023, 11, 1102159.
- Lin, L.; Wang, Y.; Srinivasan, R.; Zhang, L.; Song, H.; Song, Q.; Wang, G.; Lin, X. Quantitative Proteomics Reveals That the Protein Components of Outer Membrane Vesicles (OMVs) in Aeromonas hydrophila Play Protective Roles in Antibiotic Resistance. J. Proteome Res. 2022, 21, 1707–1717, . [CrossRef]
- Teixeira, A., Loureiro, I., Lisboa, J., Oliveira, P. N., Azevedo, J. E., Dos Santos, N. M., & do Vale, A. (2023). Characterization and vaccine potential of outer membrane vesicles from Photobacterium damselae subsp. piscicida. Int. J. Mol. Sci. 2023, 24(6), 5138.
- Fischer, T.; Schorb, M.; Reintjes, G.; Kolovou, A.; Santarella-Mellwig, R.; Markert, S.; Rhiel, E.; Littmann, S.; Becher, D.; Schweder, T.; et al. Biopearling of Interconnected Outer Membrane Vesicle Chains by a Marine Flavobacterium. Appl. Environ. Microbiol. 2019, 85, . [CrossRef]
- Kwon, Y.M.; Patra, A.K.; Chiura, H.X.; Kim, S. Production of extracellular vesicles with light-induced proton pump activity by proteorhodopsin-containing marine bacteria. Microbiologyopen 2019, 8, e00808, . [CrossRef]
- Dragovic, R.A.; Gardiner, C.; Brooks, A.S.; Tannetta, D.S.; Ferguson, D.J.P.; Hole, P.; Carr, B.; Redman, C.W.G.; Harris, A.L.; Dobson, P.J.; et al. Sizing and phenotyping of cellular vesicles using Nanoparticle Tracking Analysis. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 780–788, . [CrossRef]
- Gardiner, C.; Ferreira, Y.J.; Dragovic, R.A.; Redman, C.W.G.; Sargent, I.L. Extracellular vesicle sizing and enumeration by nanoparticle tracking analysis. J. Extracell. Vesicles 2013, 2, 19671, . [CrossRef]
- Lawrie, A.S.; Albanyan, A.; Cardigan, R.A.; Mackie, I.J.; Harrison, P. Microparticle sizing by dynamic light scattering in fresh-frozen plasma. Vox Sang. 2009, 96, 206–212, . [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750, doi:10.1080/20013078.2018.1535750.
- Shao, H.; Im, H.; Castro, C.M.; Breakefield, X.; Weissleder, R.; Lee, H. New Technologies for Analysis of Extracellular Vesicles. Chem. Rev. 2018, 118, 1917–1950, . [CrossRef]
- Mobarak, H.; Javid, F.; Narmi, M.T.; Mardi, N.; Sadeghsoltani, F.; Khanicheragh, P.; Narimani, S.; Mahdipour, M.; Sokullu, E.; Valioglu, F.; et al. Prokaryotic microvesicles Ortholog of eukaryotic extracellular vesicles in biomedical fields. Cell Commun. Signal. 2024, 22, 1–19, . [CrossRef]
- McBroom, A.J.; Johnson, A.P.; Vemulapalli, S.; Kuehn, M.J. Outer Membrane Vesicle Production byEscherichia coliIs Independent of Membrane Instability. J. Bacteriol. 2006, 188, 5385–5392, . [CrossRef]
- Gardiner, C.; Di Vizio, D.; Sahoo, S.; Thery, C.; Witwer, K. W.; ́ Wauben, M.; Hill, A. F. Techniques Used for the Isolation and Characterization of Extracellular Vesicles: Results of a Worldwide Survey. J. Extracell. Vesicles 2016, 5, 32945.
- Klimentová, J.; Stulík, J. Methods of isolation and purification of outer membrane vesicles from gram-negative bacteria. Microbiol. Res. 2015, 170, 1–9, . [CrossRef]
- Bradford, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72(1-2), 248-254.
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275.
- Marsh, J.B.; Weinstein, D.B. Simple charring method for determination of lipids. J. Lipid Res. 1966, 7, 574–576.
- Christopoulos, T. K. Nucleic acid analysis. Analytical Chemistry 1999, 71(18), 425-438.
- Nowotny, A., Nowotny, A. Carbohydrate determination by phenol-sulfuric acid. In: Basic Exercises in Immunochemistry. 1979 Springer, Berlin, Heidelberg.
- Biermann, C. J., McGinnis, G. D. Analysis of Carbohydrates by GLC and MS 1988. CRC press.
- Tian, C.-M.; Yang, M.-F.; Xu, H.-M.; Zhu, M.-Z.; Zhang, Y.; Yao, J.; Wang, L.-S.; Liang, Y.-J.; Li, D.-F. Emerging role of bacterial outer membrane vesicle in gastrointestinal tract. Gut Pathog. 2023, 15, 1–21, . [CrossRef]
- Shen, Y.; Torchia, M.L.G.; Lawson, G.W.; Karp, C.L.; Ashwell, J.D.; Mazmanian, S.K. Outer Membrane Vesicles of a Human Commensal Mediate Immune Regulation and Disease Protection. Cell Host Microbe 2012, 12, 509–520, . [CrossRef]
- Kulp, A.; Kuehn, M.J. Biological Functions and Biogenesis of Secreted Bacterial Outer Membrane Vesicles. Annu. Rev. Microbiol. 2010, 64, 163–184, . [CrossRef]
- Cai, L.; Li, H.; Deng, J.; Zhou, R.; Zeng, Q. Biological interactions with Prochlorococcus: implications for the marine carbon cycle. Trends Microbiol. 2023, 32, 280–291, . [CrossRef]
- Zheng, W.; Bergman, B.; Chen, B.; Zheng, S.; Xiang, G.; Rasmussen, U. Cellular responses in the cyanobacterial symbiont during its vertical transfer between plant generations in the Azolla microphylla symbiosis. New Phytol. 2009, 181, 53–61, . [CrossRef]
- Yoon, S. H.; Lee, S. H. Marine bacterial vesicles as a novel class of nanomaterials. Front. Mar. Sci. 2019, 6, 457.
- Ellis, T.N.; Kuehn, M.J. Virulence and Immunomodulatory Roles of Bacterial Outer Membrane Vesicles. Microbiol. Mol. Biol. Rev. 2010, 74, 81–94, . [CrossRef]
- Devoe, I. W.; Gilchrist, J. E. Release of alkaline phosphatase by Pseudomonas aeruginosa during growth. J. Bacteriol. 1073, 116(2), 395-401.
- Alves, N. J., Turner, K. B., DiVito, K. A., Walper, S. A., Zabetakis, D. Bacterial nanobiotechnology: bioengineered bacterial outer membrane vesicles for therapeutic applications. Cur. Opin. Biotechnol.2015, 35, 76-84.
- Turner, L.; Bitto, N.J.; Steer, D.L.; Lo, C.; D’costa, K.; Ramm, G.; Shambrook, M.; Hill, A.F.; Ferrero, R.L.; Kaparakis-Liaskos, M. Helicobacter pylori Outer Membrane Vesicle Size Determines Their Mechanisms of Host Cell Entry and Protein Content. Front. Immunol. 2018, 9, 1466, . [CrossRef]
- Ferreira, M.; Ogren, M.; Dias, J.N.R.; Silva, M.; Gil, S.; Tavares, L.; Aires-Da-Silva, F.; Gaspar, M.M.; Aguiar, S.I. Liposomes as Antibiotic Delivery Systems: A Promising Nanotechnological Strategy against Antimicrobial Resistance. Molecules 2021, 26, 2047, . [CrossRef]
- Caracciolo, G.; Farokhzad, O.C.; Mahmoudi, M. Biological Identity of Nanoparticles In Vivo : Clinical Implications of the Protein Corona. Trends Biotechnol. 2017, 35, 257–264, . [CrossRef]
- Shi, R., Dong, Z., Ma, C., Wu, R., Lv, R., Liu, S., Liu, J. High-Yield, Magnetic Harvesting of Extracellular Outer-Membrane Vesicles from Escherichia coli. Small 2022, 18(48), 2204350.
- Tzipilevich, E.; Habusha, M.; Ben-Yehuda, S. Acquisition of Phage Sensitivity by Bacteria through Exchange of Phage Receptors. Cell 2017, 168, 186–199.e12, . [CrossRef]
- Zhang, Y.; Dou, Y.; Liu, Y.; Di, M.; Bian, H.; Sun, X.; Yang, Q. Advances in Therapeutic Applications of Extracellular Vesicles. Int. J. Nanomed. 2023, 18, 3285–3307, . [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).