1. Introduction
Breathing is not only the exchange of blood gases in the lungs but also a modulator of cardiovascular control. There are a number of key links requiring tight coordination between the cardiovascular and respiratory systems. These two systems interact with each other via hemodynamic, mechanical, and neurohumoral pathways, providing ventilation-perfusion matching, synchronization of heart rate and ventilation, and the presence of respiratory sinus arrhythmia [
1,
2,
3].
The diaphragm is the main breathing muscle directly connected to the heart by the phrenopericardial ligament. The position of the diaphragm in the thoracoabdominal cavity is not constant; it moves upward and downward depending on the respiratory phase. As a result, the movements of the diaphragm influence the position of the heart and pericardium in the mediastinum. The diaphragm's function in the cardiovascular system, although not yet fully understood, is manifested by modulating hemodynamics through acting as a respiratory pump that increases systemic venous and lymphatic return, regulating left ventricular (LV) afterload, heart rate, heart rate variability (HRV), systolic and diastolic blood pressure, as well as modulating pericardial pressure and arterial baroreflex sensitivity [
4]. However, many of these studies evaluated the effect of diaphragmatic breathing on cardiovascular parameters without directly examining diaphragm muscle function. Moreover, these studies often involved small sample sizes or single case studies and referenced single cardiac parameters. In the present paper, we aim to shed new light on this issue.
The purpose of the study was to determine the relationship of diaphragm muscle thickness, contractility, and strength with selected cardiovascular parameters, such as systolic (the force the heart exerts on the walls of the arteries each time it beats) and diastolic (the force the heart exerts on the walls of the arteries in between beats) blood pressure, Mean Arterial Pressure (average arterial pressure throughout one cardiac cycle), Pulse Pressure (difference between systolic and diastolic blood pressure), Heart Rate (number of contractions the heart makes per minute), Cardiac Output (amount of blood the heart pumps in one minute), Cardiac Power Output (hydraulic energy delivered by the left ventricle to the systemic circulation per unit time), Cardiac Index (amount of blood the heart pumps in one minute per square meter of body surface area), Stroke Volume (volume of blood pumped from the left ventricle per beat), Stroke Volume Index (amount of blood per square meter of body surface area that is mobilized with each heartbeat), Ejection Fraction Pressure (percentage of blood that's pumped out of the heart's left ventricle each time it contracts), and Pressure-Heart Rate Product (myocardial oxygen consumption). We also checked whether the presence of diaphragm muscle atrophy, paralysis, and decreased diaphragm strength would differentiate subjects in terms of cardiovascular parameters.
2. Materials and Methods
2.1. Ethical Consideration
The protocol of the present study was approved by the Bioethic Committee of Silesian Medical University, Poland (Approval number: PCN/0022/KB1/09/21). All subjects provided written informed consent to collect and use demographics and clinical data from examination. The study was conducted following the ethical principles of the Helsinki Declaration update of 2008.
2.2. Participants
30 participants qualified for the three-week post-COVID physiotherapy program at the Physical Therapy Department at the Ministry of Internal Affairs and Administration Specialist Hospital in Glucholazy, Poland, were examined. The mean age was 60.27 ± 9.76 years; the gender composition was 60% (n=18) female and 40% (n=12) male; the mean height was 167.37 ± 8.30 cm; the mean weight was 82.31 ± 18.17 kg; the mean BMI was 29.18 ± 4.90; and the mean body surface area was 1.84 ± 0.41 m². We excluded patients who had undergone previous thoracic, abdominal, or cardiac surgery, those with diseases that interfere with diaphragm function, those in whom it was difficult to sonographically visualize the diaphragm, and individuals who did not comply with commands necessary to perform proper breathing maneuvers.
2.3. Diaphragm Muscle Ultrasound
A linear probe placed perpendicular to the chest in the 9th-10th intercostal space was used to detect the diaphragm muscle as the internal hypoechoic layer between the hyperechoic pleura and peritoneum. Diaphragm thickness was measured at the end of expiration (ThExp) and inspiration (ThIns) on the left and right sides in the supine position using the liver and spleen acoustic windows, respectively. A ThExp cutoff of less than 2 mm was used to detect diaphragm atrophy. Diaphragm thickness fraction (DTF), defined as the percentage change in diaphragm thickness during inspiration, was derived from the formula: (ThIns – ThExp) / ThExp × 100%. A value of less than 20% was interpreted as diaphragmatic paralysis. To obtain the diaphragm thickening ratio (DTR), reflecting the strength of the diaphragm muscles, the inspiratory thickness value was divided by the expiratory thickness value. A cutoff value of less than 0.7 was applied to identify a decrease in diaphragm strength [
5,
6].
2.4. Cardiovascular Parameters Assessment
Blood pressure (systolic: SBP and diastolic: DBP) and heart rate (HR) were measured by the same well-trained person in all patients using standardized procedures. To reduce measurement biases, blood pressure was assessed using an automatic oscillometric device, while heart rate was automatically measured by this device during each measurement. Before the examination, patients were required not to smoke, play sports, eat chocolate, or drink beverages containing caffeine or other psychoactive substances (i.e., alcohol or taurine). Oxygen saturation was measured using a pulse-oximetric device placed over a person's finger. Baseline measurements were recorded prior to the 6-minute walk test, while second measurements were taken immediately after the test.
From the obtained measurements, we calculated the Pulse Pressure (PP), defined as SBP minus DBP, and Mean Arterial Pressure (MAP), expressed as 1/3(SBP) + 2/3(DBP). We then estimated Cardiac Output using the formula: COest = PP x HR x 2ml, and stroke volume using the formula: SV = COest / HR [
7]. Next, we determined Pressure-Heart Rate Product (P-HRP = SBP x HR), Cardiac Power Output (CPO = MAP x COest / 451), and Ejection Fraction pressure according to the formula proposed by Kunig: EF[p] = (SBP - DBP) / SBP [
8]. Finally, after calculating the body surface area according to the Du Bois formula (BSA = 0.007184 x height^0.725 x weight^0.425) [
9], we calculated the Cardiac Index (CI = COest / BSA), Cardiac Power Index (CPI = CI x MAP / 451), and Stroke Volume Index (SVI = CI / HR x 1000).
2.5. Statistical Analysis
Statistical significance was defined as a two-sided P value < 0.05 for all analyses. Continuous data were presented as means ± standard deviation and 95% Confidence Interval, whereas categorical data were expressed as numbers (%). As the Kolmogorov-Smirnov test showed a non-normal data distribution, a Mann-Whitney test or a Kruskal-Wallis test was used to detect differences between sample subgroups. The Rank-Spearman coefficient was used to determine correlations between analyzed variables. All data were organized and analyzed in Microsoft Excel 2020 and PQStat 2022.
3. Results
Descriptive statistics of diaphragm muscle parameters were given in
Table 1.
All patients were characterized by higher values of cardiovascular parameters after 6-minute walk test - compared to baseline data. Detailed resting and post-exercise values of cardiovascular parameters are contained in
Table 2.
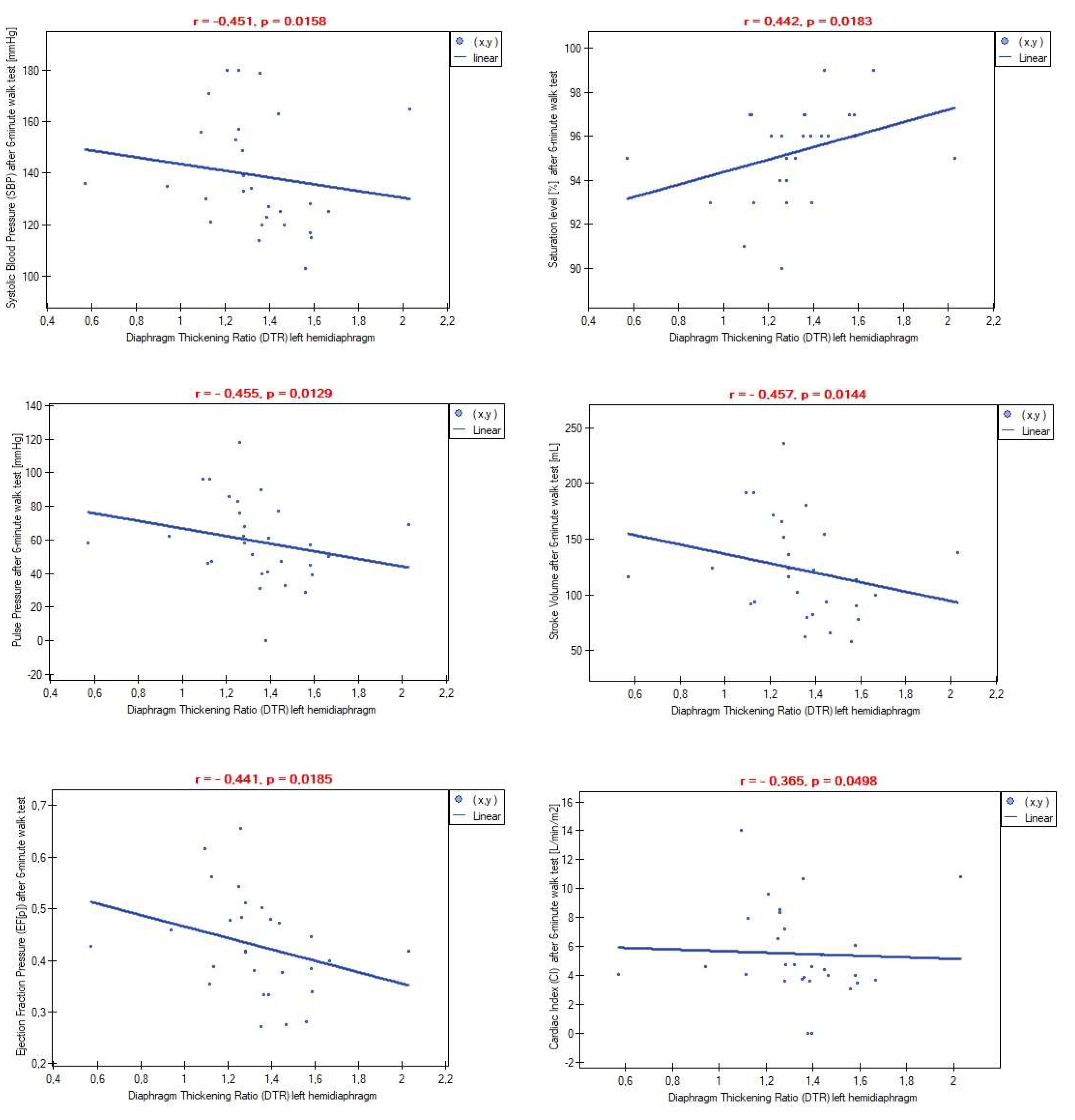
Correlation analysis showed no relationship between inspiratory and expiratory thickness and any resting cardiovascular parameters for both the left and right domes. Similar findings were observed in reference to Diaphragm Thickness Fraction (DTF) and Diaphragm Thickening Ratio (DTR). However, significant associations were found between the DTR index for the left hemidiaphragm and some post-exertion cardiovascular parameters (
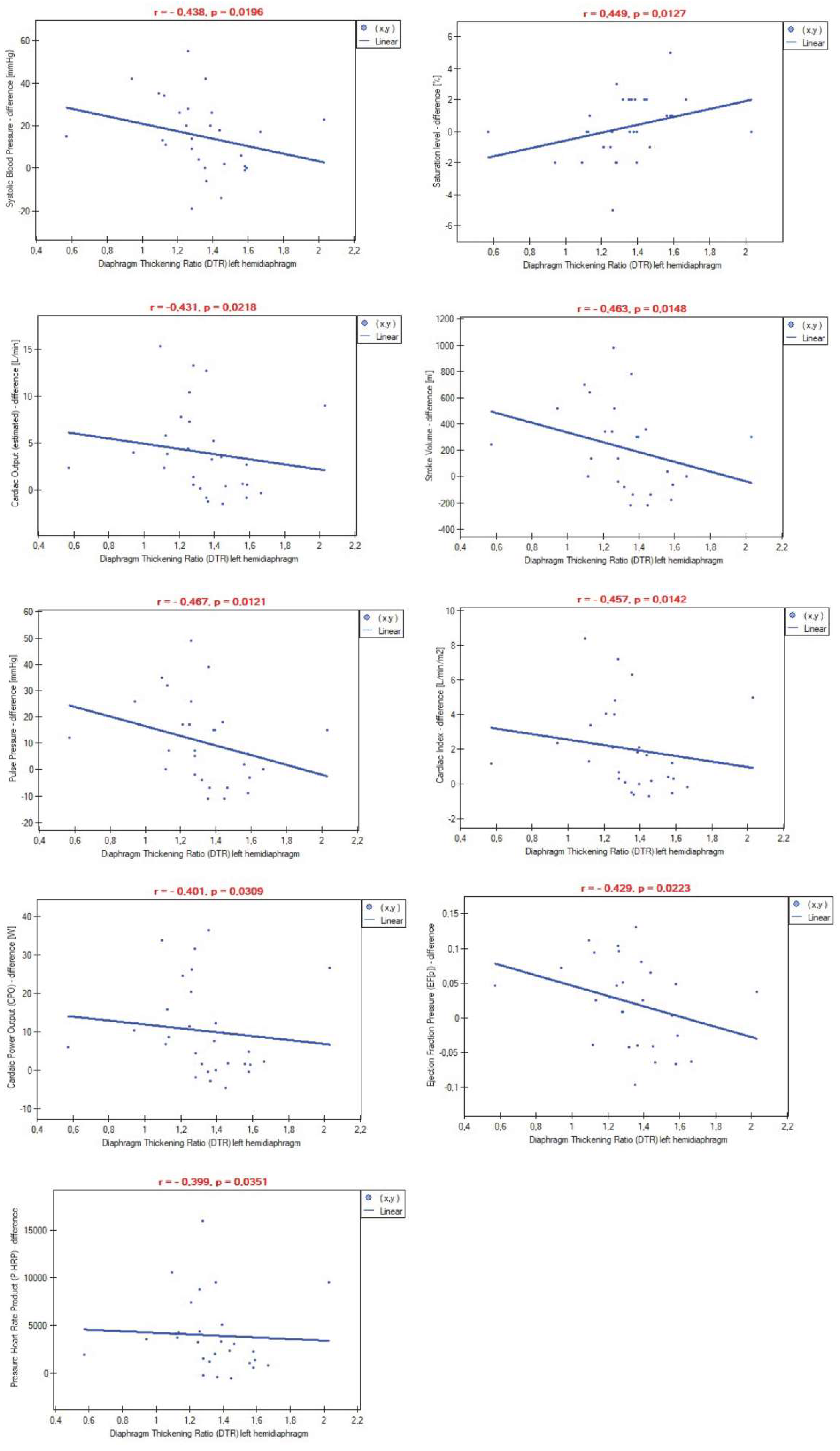
Figure 1). Additionally, a higher thickening ratio on the left side of the diaphragm was associated with lower differences in certain cardiovascular parameters measured pre- and post-6-minute walk test (
Figure 2). No dependencies were observed in relation to the right hemidiaphragm. Inspiratory and expiratory thickness, as well as the DTF index, again did not play any role in the correlations with post-exertion values or the differences in measurements.
We detected left side, right side, and bilateral diaphragm atrophy in 25 (83.33%), 23 (76.66%), and 25 (83.33%) participants, respectively. The percentage of diaphragm paralysis was smaller, as follows: left: n=3 (10.0%); right: n=2 (6.66%); both sides: n=1 (3.33%). Due to significant disproportion between the number of diaphragm atrophic and non-atrophic subjects, as well as diaphragm paralysis and non-paralysis subjects, we were unable to perform a comparative analysis of the impact of diaphragm dysfunction on cardiovascular parameters. Therefore, we sorted the participants into three subgroups according to quartile values of expiratory thickness (Q1 [lower quartile]: ThExp < 2.64, n=7; Q2 [interquartile range]: 2.64 ≤ ThExp ≤ 3.05, n=16; Q3 [upper quartile]: ThExp ≥ 3.06, n=7) and DTF (Q1 [lower quartile]: DTF < 26.43, n=7; Q2 [interquartile range]: 26.43 ≤ DTF ≤ 47.82, n=16; Q3 [upper quartile]: DTF ≥ 47.82, n=7). We did not observe differences between groups in any of the analyzed cardiac parameters, both for atrophy and paralysis.
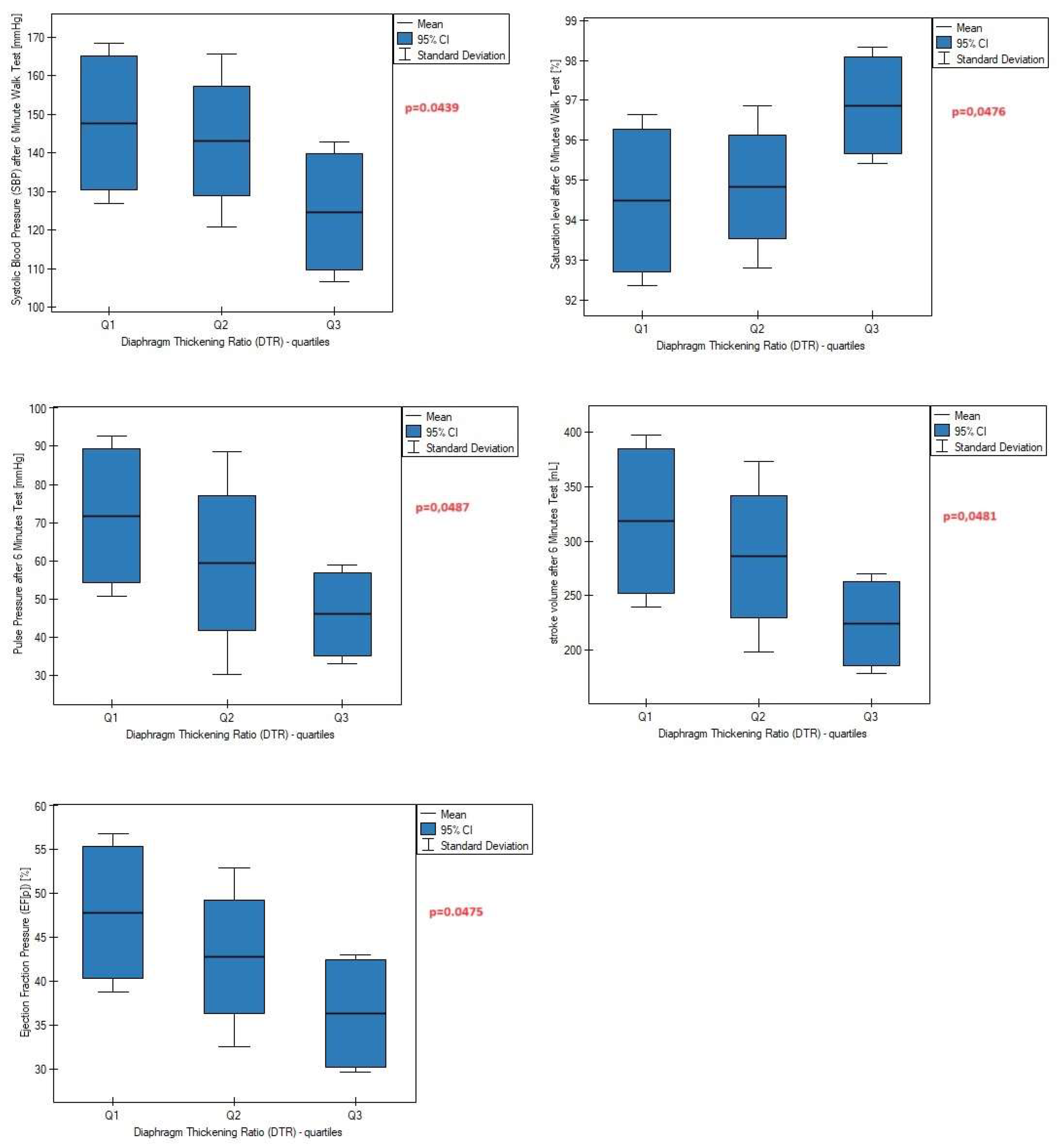
Only one patient achieved DTR values less than 0.7, indicating a significant decrease in diaphragm muscle strength. Thus, to perform the next analysis, we divided the patients into three groups again, this time according to the quartiles of the DTR index, which were as follows: (Q1 [lower quartile]: DTR < 1.25, n=7; Q2 [interquartile range]: 1.25 ≤ DTR ≤ 1.44, n=16; Q3 [upper quartile]: DTR ≥ 1.44, n=7). We found that the percentage of subjects with the highest DTR values was characterized by lower values of post-exercise systolic blood pressure, pulse pressure, and stroke volume compared to subjects with lower DTR (
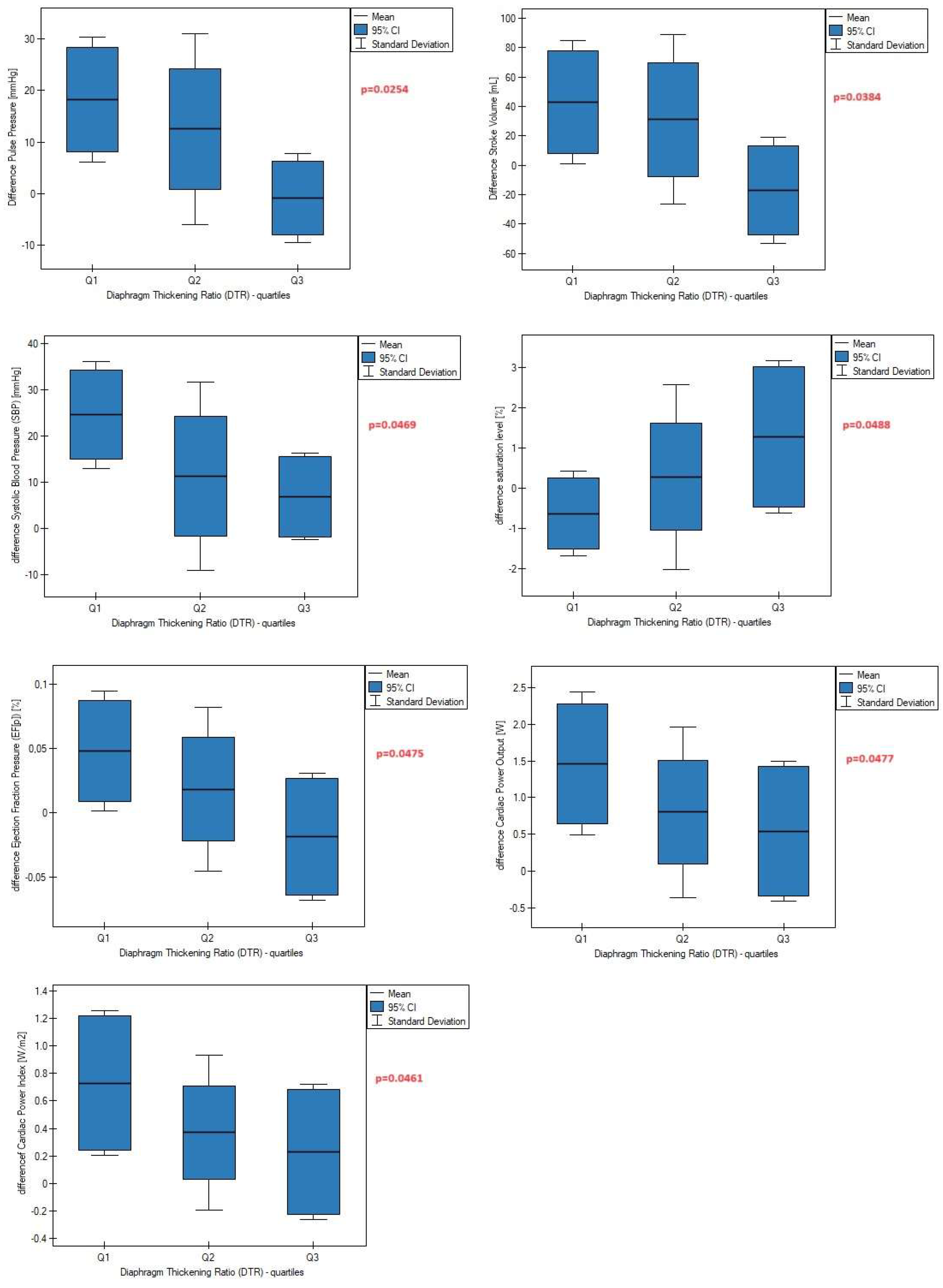
Figure 3), as well as a smaller increase in these parameters after a 6-minute walk test compared to baseline data (
Figure 4).
4. Discussion
The diaphragm is recognized as the main respiratory muscle. However, its role in the human body is much greater, with its impact on the functioning and regulation of cardiovascular parameters still greatly underestimated. It is well documented that the respiratory movement of the diaphragm muscle creates changes in intrathoracic and intraabdominal pressure. These cyclical swings are important for efficient circulatory function, enhancing venous return to the right atrium and lymph drainage to the venous system through pressure gradient differences. Increases in transdiaphragmatic pressure during diaphragm contraction additionally promote blood flow toward the right heart from the splanchnic region. The literature also portrays that diaphragm contraction increases myocardial stretch (preload), right-side stroke volume, and pericardial pressure while simultaneously reducing left ventricular stroke volume, cardiac output, and pleural pressure. During the exhalation phase, opposite processes occur [
10,
11,
12,
13,
14,
15]. To our best knowledge, no previous study has investigated whether quantitative values of diaphragm contraction affect the values of the aforementioned cardiovascular parameters. Despite demonstrating no correlations between diaphragm contractility and stroke volume at rest, we observed that a higher difference between inspiratory and expiratory left hemidiaphragm thickness (DTR index) significantly impacted the decrease of post-exertion stroke volume (
Figure 1). Furthermore, stroke volume increased more slowly during physical exertion in this patient group compared to subjects with low left hemidiaphragm contractility (
Figure 2). Similarly, a lower difference between rest and post-exercise cardiac output was found among patients with greater diaphragm thickening during the breathing cycle (
Figure 2). A new aspect of our study was to verify whether diaphragm muscle function influences other hemodynamic parameters of blood flow. For this purpose, we used cardiac index (CI) and stroke volume index (SVI). Data presented in
Figure 1 and
Figure 2 showed significant links between left hemidiaphragm muscle strength and global blood flow. These findings confirmed the previously described impact of the diaphragm muscle in modulating hemodynamic parameters and added new knowledge that the degree of diaphragm contraction is also important for regulating these parameters and their growth during exercise.
Relationships between heart rate, blood pressure, and respiration are known in the literature as cardiorespiratory coupling. Several previous studies demonstrated positive effects of diaphragmatic breathing on reducing heart rate, heart rate variability, and blood pressure. Our findings partially support previous results. As seen in
Figure 1 and
Figure 2, the diaphragm thickening ratio was positively associated with post-exercise values of systolic blood pressure. On the other hand, we found no links with diastolic blood pressure. However, muscle thickness seems to play a different role in this case. According to Ichikawa, adequate diaphragm thickness prevents an excessive drop in diastolic blood pressure during position changes from supine to standing [
16]. Similarly, we also reported no relationship between resting and exercise heart rate values with the thickness and contractility of the diaphragm. Based on this, we think that heart rate and diastolic blood pressure regulation results more from parasympathetic activity and baroreflex sensitivity after breathing exercises than the thickness or contractility of the diaphragm muscle. In another study, Bernardi et al. described the positive effect of diaphragmatic breathing on increasing resting oxygen saturation in chronic heart failure patients [
17]. In our study, the saturation level at rest was not conditioned by diaphragm muscle work, but higher left hemidiaphragm contractility was linked with better oxygen levels during physical effort (
Figure 1 and
Figure 2). Interestingly, a significant reduction in diaphragm strength was associated with a decrease in saturation level after a 6-minute walk test compared to initial data (
Figure 4).
In the present study, we also examined the impact of diaphragm function on cardiovascular parameters that have not been assessed previously in the literature. For the first time, we discovered and described that diaphragm contraction regulates pulse pressure, pressure-heart rate product, cardiac power output, and ejection fraction pressure. Data presented in
Figure 4 revealed that a high left diaphragm thickening ratio was associated with a slower increase in the aforementioned parameters during physical exertion, indicating better aorta ability to distend and recoil, lower myocardial oxygen consumption, lower rate of energy output achieved by the heart during maximal stimulation, and lower percentage of blood pumped out by the left ventricle during each heart contraction, respectively. Based on these and other findings, we speculate that proper diaphragm muscle strength, measured as diaphragm thickening ratio, markedly contributes to the superior adaptation of the cardiovascular system to physical performance. Considering that the cardiac power output index is strongly related to end-organ perfusion [
18], we assume that optimal left hemidiaphragm strength should also provide adequate oxygen and nutrient delivery to the tissues.
From an anatomical perspective, the diaphragm is a musculotendinous sheet partition consisting of a central tendon and three muscular parts (sternal, costal, and lumbar with two crura). Previous studies have labeled the diaphragm as two separate muscles: costal and crural segments, but connected in one functional unit called the respiratory or thoracic diaphragm. While the costal part is mainly involved in the breathing process, the crural diaphragm is greatly engaged in gastroesophageal functions, such as swallowing, vomiting, and contributing to the antireflux barrier. This functional divergence between these two parts results from different embryological origins, nerve supply, blood supply, motor unit territories, and muscle fiber type distribution [
19,
20]. In the present study, we noted only the relationship of left diaphragm dome contractility with cardiovascular parameters. We did not observe any dependencies for the right dome of the diaphragm. It seems the results of our research provide new knowledge about another functional divergence within this muscle, this time between the left and right domes of the diaphragm. We suggest that the costal part of the left hemidiaphragm performs a dual function. Besides respiratory act, it simultaneously plays an important role in the regulation of cardiovascular parameters, which was not seen in the right hemidiaphragm. This supports the previous theory that the contracting diaphragm pulling on the phrenico-pericardial ligament stimulates the heart to beat. The aforementioned ligament starts from the pericardial sac and runs to the central tendon of the diaphragm near the midline. Most of the ligament attaches to the left leaflet of the central tendon, which explains the relationship of the left hemidiaphragm with cardiac parameters.
A strength of our study is the comprehensive assessment of diaphragm muscle function with separate consideration of the left and right domes and the assessment of its impact on numerous cardiovascular parameters at rest and after physical exertion. However, our work has two limitations. Primarily, the number of patients who took part in the study is limited. Nevertheless, we believe that the group size is sufficient to conduct statistical analysis and draw preliminary conclusions. The second limitation is the method of calculating cardiac output. We are aware that echocardiography is the most reliable measurement of this parameter. In our work, we calculated the estimated values of this parameter based on a formula available in the medical literature. A validation study of this mathematical transformation showed that this method provides an adequate measurement of CO when other methods are more difficult to implement, especially in outpatient settings, as in our case. However, we believe that despite these limitations, our work contributes new and useful knowledge to this scientific field.
5. Conclusions
The following conclusions can be drawn from the results of the study presented here:
Inspiratory and expiratory thickness values do not affect any of the analyzed cardiovascular parameters.
There is no relationship between diaphragm muscle thickness and contractility with any of the cardiovascular parameters measured at rest.
Left hemidiaphragm contractility plays an important role in the regulation and modulation of systolic blood pressure, pulse pressure, stroke volume, and ejection fraction pressure during physical effort. The impact of the right diaphragm dome on cardiovascular parameters was not observed, suggesting functional divergence between the left and right diaphragm domes.
Greater contractility of the left diaphragm is associated with a smaller increase in systolic blood pressure, pulse pressure, and stroke volume measured after exercise compared to resting data. It seems that appropriate contractility may be related to better tolerance and adaptation to physical effort through the slower achievement of maximal or submaximal values of cardiac parameters.
The diaphragm muscle should be included in the process of cardiac rehabilitation, with particular emphasis on the left hemidiaphragm.
Author Contributions
Conceptualization, J.K., M.R., J.S., M.A.; methodology, J.K., M.A and J.S.; formal analysis, J.K. and M.R.; investigation, J.K., M.R., M.A.; resources, J.S. and K.B.; data curation, J.K. and M.R.; writing—original draft preparation, J.K.; writing—review and editing, M.A.; supervision, J.S. and M.A.; project administration, J.K., J.S., K.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of the Medical University of Silesia, Katowice, Poland (protocol code: PCN/0022/KB1/09/21; date of approval: 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dick TE, Hsieh YH, Dhingra RR, Baekey DM, Galán RF, Wehrwein E, Morris KF. Cardiorespiratory coupling: common rhythms in cardiac, sympathetic, and respiratory activities. Prog Brain Res. 2014;209:191-205. PMID: 24746049; PMCID: PMC4052709. [CrossRef]
- Larsen PD, Tzeng YC, Sin PY, Galletly DC. Respiratory sinus arrhythmia in conscious humans during spontaneous respiration. Respir. Physiol. Neurobiol. 2010;174:111–118.
- Cheifetz IM. Cardiorespiratory interactions: the relationship between mechanical ventilation and hemodynamics. Respir Care. 2014 Dec;59(12):1937-45. Epub 2014 Nov 11. PMID: 25389353. [CrossRef]
- Salah HM, Goldberg LR, Molinger J, Felker GM, Applefeld W, Rassaf T, Tedford RJ, Mirro M, Cleland JGF, Fudim M. Diaphragmatic Function in Cardiovascular Disease: JACC Review Topic of the Week. J Am Coll Cardiol. 2022 Oct 25;80(17):1647-1659. PMID: 36265961. [CrossRef]
- Sarwal A, Walker FO, Cartwright MS. Neuromuscular ultrasound for evaluation of the diaphragm. Muscle Nerve. 2013 Mar;47(3):319-29. Epub 2013 Feb 4. PMID: 23382111. [CrossRef]
- Boussuges A, Rives S, Finance J, Brégeon F. Assessment of diaphragmatic function by ultrasonography: Current approach and perspectives. World J Clin Cases. 2020 Jun 26;8(12):2408-2424. PMID: 32607319. [CrossRef]
- Koenig J, Hill LK, Williams DP, Thayer JF. Estimating cardiac output from blood pressure and heart rate: the liljestrand & zander formula. Biomed Sci Instrum. 2015;51:85-90. PMID: 25996703; PMCID: PMC5317099.
- Kunig H, Tassani-Prell P, Engelmann L. Ejection fractions and pressure-heart rate product to evaluate cardiac efficiency. Continuous, real-time diagnosis using blood pressure and heart rate. Med Klin Intensivmed Notfmed. 2014 Apr;109(3):196-9.
- Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition. 1989 Sep-Oct;5(5):303-11; discussion 312-3. PMID: 2520314.
- Harms, C. A., Babcock, M. A., McClaran, S. R., Pegelow, D. F., Nickele, G. A., Nelson, W. B., et al. (1997). Respiratory muscle work compromises leg blood flow during maximal exercise. J. Appl. Physiol. (1985) 82, 1573–1583.
- Aliverti, A., Uva, B., Laviola, M., Bovio, D., Lo Mauro, A., Tarperi, C., et al. (2010). Concomitant ventilatory and circulatory functions of the diaphragm and abdominal muscles. J. Appl. Physiol. (1985) 109, 1432–1440. [CrossRef]
- Flamm, S. D., Taki, J., Moore, R., Lewis, S. F., Keech, F., Maltais, F., et al. (1990). Redistribution of regional and organ blood volume and effect on cardiac function in relation to upright exercise intensity in healthy human subjects. Circulation 81, 1550–1559. [CrossRef]
- Miller, J. D., Pegelow, D. F., Jacques, A. J., and Dempsey, J. A. (2005a). Effects of augmented respiratory muscle pressure production on locomotor limb venous return during calf contraction exercise. J. Appl. Physiol. (1985) 99, 1802–1815. [CrossRef]
- Aliverti, A., Bovio, D., Fullin, I., Dellaca, R. L., Lo Mauro, A., Pedotti, A., et al. (2009). The abdominal circulatory pump. PLoS ONE 4:e5550. [CrossRef]
- Miller, J. D., Pegelow, D. F., Jacques, A. J., and Dempsey, J. A. (2005b). Skeletal muscle pump versus respiratory muscle pump: modulation of venous return from the locomotor limb in humans. J. Physiol. 563(Pt 3), 925–943. [CrossRef]
- Ichikawa A, Yamasaki F, Ueda M, Todaka H, Miyao E, Yoshinaga Y, Yamanaka S, Matsumura Y, Sato T. Relationship between the fall in blood pressure in the standing position and diaphragmatic muscle thickness: proof of concept study. Blood Press Monit. 2019 Dec;24(6):284-288. PMID: 31567294. [CrossRef]
- Bernardi L, Spadacini G, Bellwon J, Hajric R, Roskamm H, Frey AW. Effect of breathing rate on oxygen saturation and exercise performance in chronic heart failure. Lancet. 1998 May 2;351(9112):1308-11. PMID: 9643792. [CrossRef]
- Mishra S. Upscaling cardiac assist devices in decompensated heart failure: Choice of device and its timing. Indian Heart J. 2016 Apr;68 Suppl 1(Suppl 1):S1-4. Epub 2016 Jan 11. PMID: 27056646; PMCID: PMC4824335. [CrossRef]
- Pickering M, Jones JF. The diaphragm: two physiological muscles in one. J Anat. 2002 Oct;201(4):305-12. PMID: 12430954; PMCID: PMC1570921. [CrossRef]
- Merrell AJ, Kardon G. Development of the diaphragm -- a skeletal muscle essential for mammalian respiration. FEBS J. 2013 Sep;280(17):4026-35. Epub 2013 May 7. PMID: 23586979; PMCID: PMC3879042. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).