1. Introduction
The human foot, with its intricate arrangement of bones, ligaments, and muscles, serves as a remarkable biomechanical structure fundamental to human mobility. Among its movements, foot pronation, encompassing eversion, abduction, and dorsiflexion, play a pivotal role in maintaining balance, absorbing shock, and transferring energy during various activities [
1]. However, understanding the influence of fatigue on foot pronation and its potential effects on arch structure is of great interest in biomechanics [
2].
Pronation describes the medial lowering movement of the midfoot in the Chopart joints, is usually associated with a pes planus and is the counterpart of supination. The inconsistent terminology should also be noted at this point [
3]. Eversion and pronation are not synonyms. Eversion is explicitly addressed to the rearfoot with its calcaneus, even if a coupling is common, as is the case for example with a pes planovalgus [
4]. A lowering of the longitudinal arch structure thus corresponds to pronation, which can be measured by a static navicular drop (statND) and a dynamic navicular drop (dynND) representing two methods often used for objectively rating the pronation during stance and gait [
5,
6,
7]. DynND describes the degree of pronation via the lowering of the navicular bone when the foot is maximal loaded throughout gait cycle, usually occurring throughout the gait phases ‘loading response’ or ‘mid stance’. With the statND the procedure is very similar, except the height of the navicular bone is compared between a relaxed sitting position and under load in single-leg standing position, which is a modified version of Brody [
8], who first described this method.
Fatigue is a progressive decline in physical and mental performance. Especially during sustained or repetitive activities fatigue can be caused through various mechanisms, ranging from a lack of energy-providing molecules and the accumulation of metabolites within muscle fibres to inadequate motor command in the motor cortex [
9]. Different factors may lead to a reduced inter- and intramuscular coordination, illustration the complex character of fatigue. The consideration of fatigue in movement analysis represents a significant paradigm shift [
10]. Traditionally, gait analysis has predominantly focused on assessing static or baseline biomechanics and is carried out as part of preventive diagnostics in an absolute majority of cases when the patient or athlete is in a recovered state. However, it is increasingly clear that this approach may not capture the full spectrum of human movement, as it is known that fatigue-induced alterations can lead to significant changes in gait dynamics [
11,
12,
13] and foot functions [
2,
14] in sports science and clinical practice [
10,
15]. Athletes engaging in endurance events or repetitive training sessions may experience fatigue-induced deviations in foot pronation, which could increase the risk of overuse injuries and hinder performance [
16,
17]. Similarly, in clinical settings, individuals with certain foot conditions or pathologies may exhibit altered foot mechanics only when fatigue sets in, necessitating a more comprehensive understanding of these fatigue-induced changes.
Headlee et al. [
2] used a similar approach and examined healthy participants who performed 75 repetitions of isotonic dorsal flexions of the foot with a resistance of 4.55 kg and found that the navicular drop increased by an average of 1.8 mm. Gardin
, et al. [
18] also support the thesis that the navicular drop is widely believed to be an indicator to pronation-related injuries, which may be increased by fatigue. The authors fatigued the tibialis muscles of their 36 healthy participants and compared a static navicular drop between a sitting and a standing position without finding evidence of an increased drop due to fatigue. Lee, Kim and Cho [
14] examined the influence of foot muscle fatigue on balance and plantar foot pressure distribution. Although there were no significant changes in terms of balance, the medial foot pressure load increased, which can be interpreted as an indicator of increased pronation.
By examining existing research, elucidating the underlying biomechanical mechanisms, and highlighting the potential clinical and sports-related implications, this study aims to emphasize the importance of fatigue as a critical variable in movement analysis and return to play tests.
The following research questions are examined with the present study:
1st Question: Is there a difference in the static navicular drop (statND) before and after fatigue of the stabilizing foot muscles in a single leg stance?
2nd Question: Is there a difference in the dynamic navicular drop (dynND) before and after fatigue of the stabilizing foot muscles during gait cycle?
3rd Question: Is there a relation between the statND and the dynND?
The lack of a further evidence to clearly support the thesis, to achieve a clear consensus, the missing sensitization for the potential importance of fatigue for diagnostics as well as the combination of a methodical operationalization of the possible effects via the statND and the dynND at the same time distinguishes the study from the few research already existing. Ultimately, this research may contribute to the development of tailored interventions and assessment strategies, ensuring that fatigue-induced alterations in foot pronation and arch structure are recognized, understood, and managed effectively, benefiting both athletes and individuals with clinical conditions.
2. Materials and Methods
2.1. Subjects
The sample size was calculated a priori using G*Power (Version 3.1.9.6 for Macintosh, University of Kiel, Germany) for a repeated ANOVA (within-between interaction, f = 0.5, α = 0.05) A minimum group size of 20 persons was calculated (power 0.96). The study was based and carried out in accordance with the current guidelines of Declaration of Helsinki and was approved by the responsible ethics commission (No. 55). All participants signed informed consent forms, including the permission to publish the results. The authors have no conflict of interest to declare.
All 25 test persons (12♂, 13♀; 24.3 ± 2.7 years, 174.9 ± 9.09 cm; 70 ± 14.2 kg, BMI: 22.7 ± 2.8) were sport students and staff (between 18 und 35 years of age). Exclusion criteria were subjects > 35 years, illness, acute complaints or injuries, previous injuries potentially limiting the range of motion of the foot (forefoot, midfoot or rearfoot) or the ankle joint (e.g. fractures) as well as foot deformities (pes cavus, pes planus, pes planovalgus). For this purpose, the Navicular Index of all test subjects was determined, which averaged 0.27 ± 0.03 on the left side and 0.27 ± 0.03 on the right side (< 0.17 = pes planus, 0.22 – 0.31 = standard, > 0.35 = pes cavus)[
19].
2.2. Test Procedure
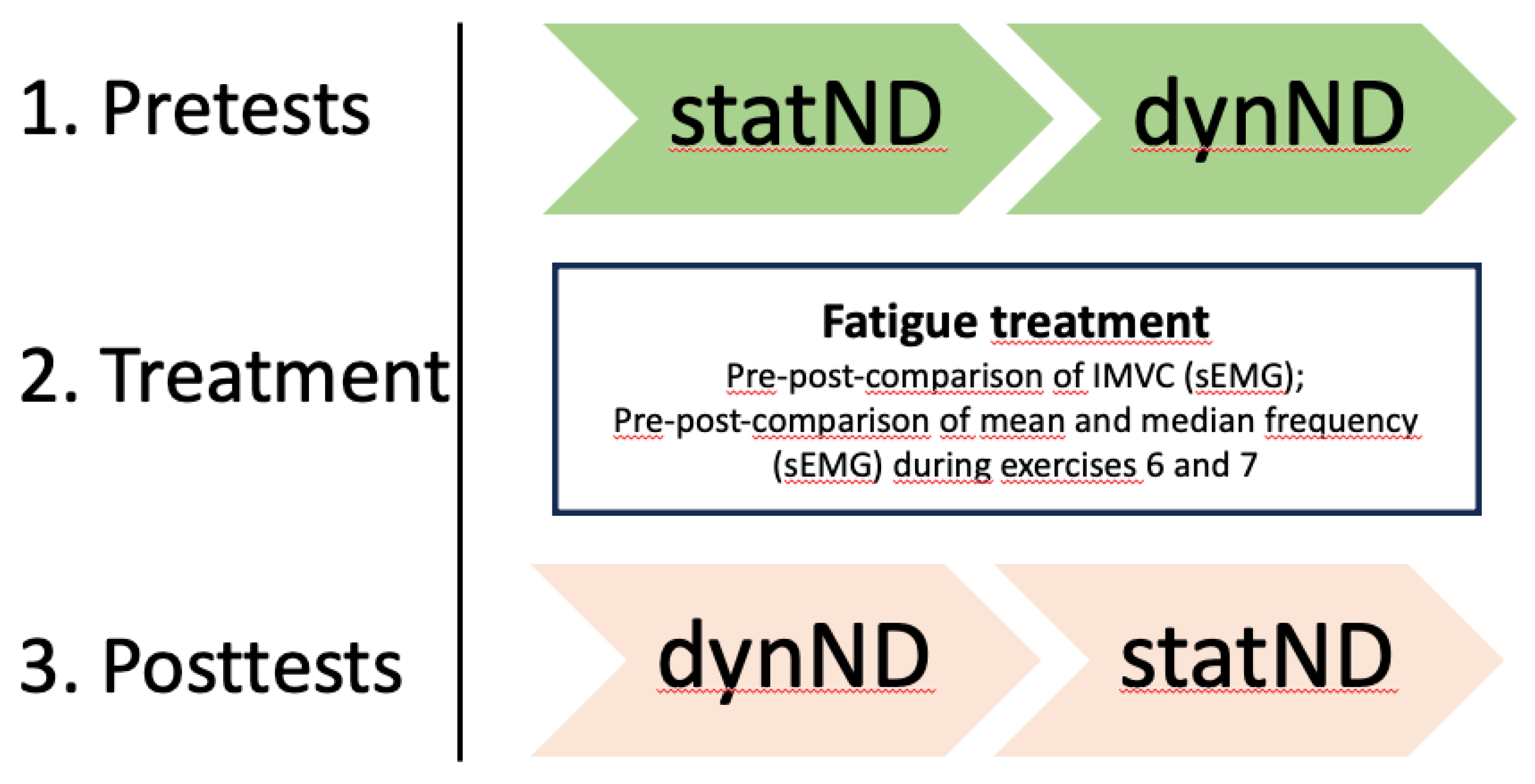
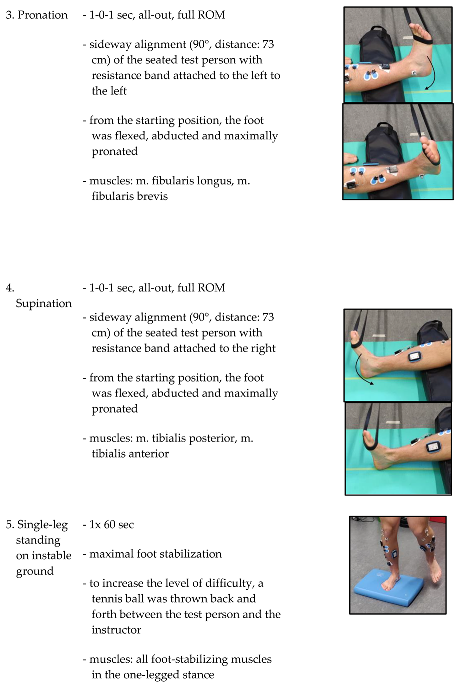
Test persons came in a rested state without intensive physical activity 48 hours before the measurements. All measurements were conducted by the same experienced researcher. All test subjects received standardized instructions on the study procedure. First, the statND was manually measured by comparing navicular height from a sitting position with a single-leg standing position. Next the subjects were marked for the three-dimensional motion tracking of the dynND during walking and surface electromyography (sEMG) was prepared to analyze muscle fatigue as well (see 2.3.2). The gait analysis was carried out over a walking distance of 800 cm, whereby only the respective gait cycles (three per side and condition) within a marked corridor of 540 cm were evaluated. This was followed by a short foot mobilization 25x full range of motion (ROM) foot rotation to the right and to the left side for both feet. Subsequently isometric maximal voluntary contraction (IMVC) measurements (external rotation, internal rotation) with sEMG were examined. Once all measurements for the pretest had been taken, the fatigue treatment with sEMG control was applied to the right foot only. Afterwards sEMG controlled IMVC measurements (external rotation, internal rotation) were repeated. Finally, the three-dimensional motion tracking for the dynND was carried out during walking. The chronology of the measurements (statND, dynND) was reversed in the posttest, as dynND was the most important for the authors and should therefore be placed closest to the treatment (
Figure 1).
2.3. Fatigue
2.3.1. Fatigue Protocol
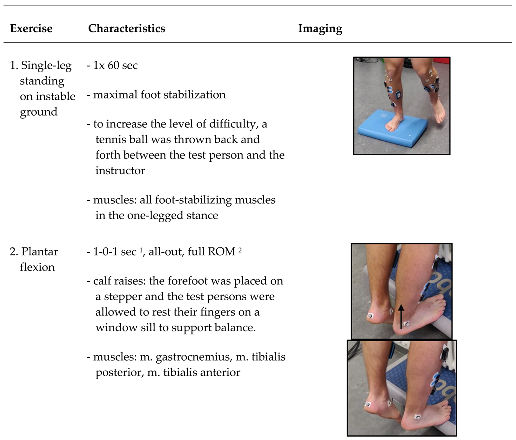
All exercises were carried out on the right side from a standing or seated position (
Table 1). During the seated position the right leg, the one to be fatigued by the exercises, was placed on a spacer. The right leg on the spacer was positioned between the lower part of m. gastrocnemius and the heel to ensure a full range of motion (ROM) of the right foot during all exercises and that the markers and electrodes were not moved or damaged by a floor contact. Before each exercise, there was a standardized exercise instruction and a test trial without the resistance band (Power Band SQMIZE, type: PB22, resistance: 24 kg, Seevetal/Hittfeld, Germany) for movement control by the instructor. Nevertheless, continuous movement control and correction was carried out by the instructor during the exercises. If the exercise could no longer be performed in accordance with the instructions, the instructor canceled the set. The specifications were the quality of movement with maximum ROM and a predefined velocity of 1-0-1 seconds (concentric - isometric – eccentric). The time between the exercises and between the measurements was kept as short as possible to minimize recovery effects. Exercises one and five were executed on an Airex mat (Airex, Sins, Switzerland). The Borg rating of perceived exertion scale (RPE scale) was used to assess the subjective feeling of stress [
20].
2.3.2. Surface Electromyography (sEMG)
sEMG was recorded using a telemetric Noraxon Desktop DTS (Noraxon, Scottdale, PA, USA; sampling frequency: 1500 Hz, lowpass filter: 500 Hz) in order to proof fatigue of foot stabilizing muscles. To record muscle activity adhesive electrodes (Ag/AgCl; Ambu Blue Sensor P: Ambub A/S, Ballerup, Denmark; diameter: 34 mm) were attached according to the SENIAM standard [
21]. For the evaluation of the pre-post-comparison for the verification of muscular fatigue, median frequency (MDF) and mean frequency (MNF) during IMVC and during exercises six and seven (see
Section 2.2;
Table 1) were analyzed using Fast Fourier Transformation (FFT) with the MR3 software (Version 12.56, Noraxon, Scottdale, PA, USA). The activity of m. fibularis longus (FL) was measured as a representative of the pronators of the foot and m. tibialis anterior (TA) for the supinators of the foot. TA was chosen, since it is not possible to derive sEMG of m. tibialis posterior, which is considered to play a more important role in stabilizing the longitudinal arch structure during the stance phase [
22].
The muscular activity for the pre-post-comparison of isometric maximal voluntary contraction (IMVC) was performed by both feet throughout external (for m. fibularis longus) and internal rotation (for m. tibialis anterior). This way the left foot serves as a control group and the right foot as the intervention group (fatigue).
1. External rotation: combination of maximal pronation of the midfoot and dorsiflexion in the ankle joint for m. fibularis longus.
2. Internal rotation: combination of maximal supination of the midfoot and dorsiflexion in the ankle joint for m. tibialis anterior.
Test persons positioned themselves frontally to the resistance band (distance to the attachment point of the resistance band (113 cm) so that a standardized tension could be ensured. Each movement (external rotation, internal rotation) was performed three times and held for five seconds with the instruction to flex the corresponding muscles to achieve this type of foot movement to the maximum. Since the IMVC is usually not reached right at the beginning, an exact three-second time window was evaluated with FFT for MNF and MDF for all runs as soon as the muscle has reached maximum activity. The test with the highest frequencies from MDF and MNF was selected for the pre-post-comparison.
Due to a technical error on one day the sample for the sEMG is reduced by five test persons to n = 20.
2.4. Pronation
2.4.1. Static Navicular Drop (statND)
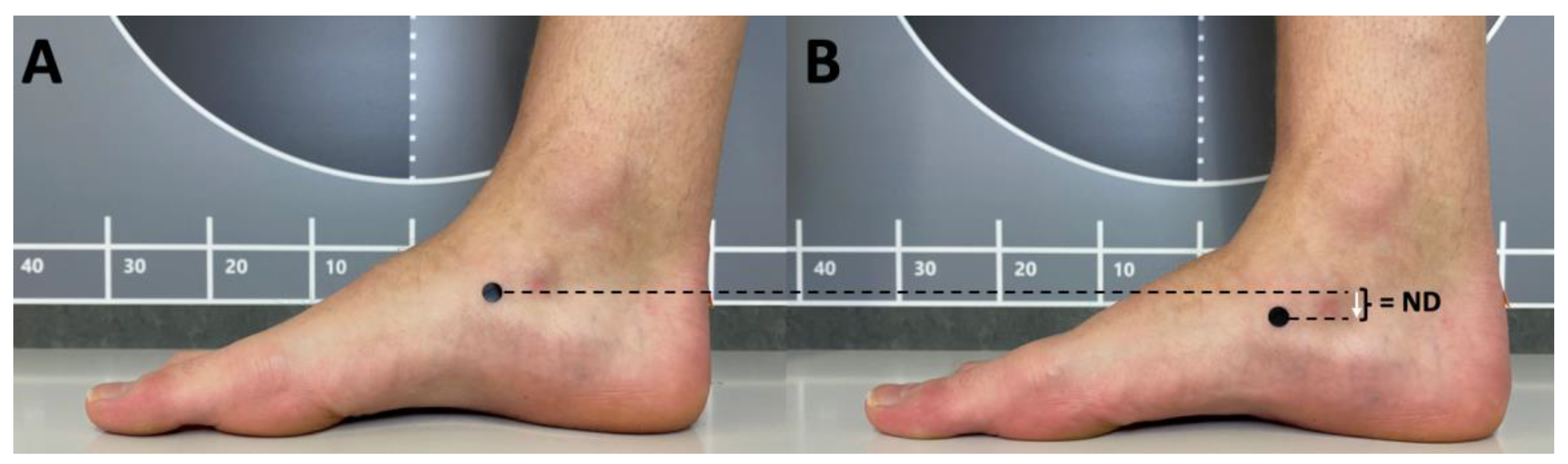
First, the navicular bone was palpated and then color-marked at its base. The height of the navicular bone was then measured manually from a seated position as in Barton, Bonanno, Levinger and Menz [
7]. The navicular height equals the vertical difference between the floor and the navicular bone. The next value is measured from a standing position in order to apply a body weight load to the foot. Similar to gait, where the foot is in a one-legged support phase for approximately 40 % [
1], the contralateral foot is lifted. This introduces a static but full load on the foot. From this position, the height of the navicular bone was measured again. The difference between the navicular height (NH) from the seated position and that from the one-legged position is statND (
Figure 2).
The statND represents sagittal plane displacement of the navicular bone. The procedure described here is a modified version of the Brody [
8].
2.4.2. Dynamic Navicular Drop (dynND)
The dynND is an analyzing method used in gait analysis to determine the degree of pronation. DynND refers to the distance by which the navicular bone drops towards the ground under load throughout the stance phase. During gait analysis, the point of maximum drop is usually found between loading response and mid stance [
1]. The dynND was measured throughout three-dimensional motion capture by Qualisys Track Manager (Version 2.15, Göteborg, Sweden; 200 Hz). Therefore, two super-spherical reflective markers (Noraxon, Scottdale, USA, size: 8 mm diameter) were attached to the skin on the navicular bone (right & left foot) with double sided adhesive tape. The height of the marker at the beginning of the loading response, when the foot is in a fixed and flat position, and the lowest point of the marker, which usually occurs until the end of the mid stance, were used to calculate dynND. Further markers were applied according to the IOR lower body model [
23], but were only used to identify gait phases. In this case, the gait phases between the start of the loading response, which begins with the first contact of the metatarsal heads, and the end of midstance, when the heels begin to detach from the ground (heel-off) were of particular interest. The height of dynND was recorded using the Mokka Motion Kinematic & Kinetic Analyzer software (version 0.6.2)[
24].
To measure the dynND, the test subjects walked on a 800 cm walk way. The measurement took place in a middle section of 536 cm length. The walking speed was measured for all stride sequences to ensure that there were no differences between the pre-test (4.40 ± 0.40 km/h) and post-test (4.33 ± 0.42 km/h) that could influence the results of the dynND. If there was a deviation of more than 0.4 km/h, the corresponding step sequences were excluded. The difference between pre and post was 0.1 ± 0.3 km/h on average. Three step sequences were always randomly selected for each test subject and each side and a representative mean value was calculated. ND outliers with more than 1.5 of the interquartile range were eliminated, which was found in 24 out of 500 cases.
2.5. Statistics
Statistics were calculated with IBM SPSS (29.0 for Macintosh, Chicago, IL, USA). The results are stated as mean values ± standard deviations.
To proof fatigue on the right side throughout the treatment, t-tests for dependent and paired samples were applied. All test requirements for the t-test were checked and confirmed. The significance level was set at p < .025 after Bonferroni correction, since MDF and MNF were always measured in one trial. Cohen’s d is provided as an effect size for significant results (0.2 = small effect; 0.5 = medium effect, 0.8 = large effect).
To analyze pre-post-effects of fatigue and on statND (1st Question) and dynND (2nd Question) side differences by IMVC (non-fatigued (left) vs. fatigued (right)) an ANOVA with repeated measures was applied. Additionally, to analyze the relation between the two assessment methods statND and dynND (3rd Question) a mixed linear model regression was calculated. For this part of the statistical analysis, only one main effect (methods) was considered in order to answer the 4th question. ‘Side’ and ‘time’ do not provide any additional value in answering this question. All test requirements for the ANOVA and for the mixed linear model regression were checked and confirmed. For the mixed linear model regression, the sample size was increased (n = 200), as the values from the right (n = 25) and left foot (n = 25) as well as pre and posttest were combined per method (statND, dynND). The significance level was set at p < .05.
3. Results
3.1. Proof of Fatigue
To prove the validity of the fatigue treatment the muscular activity for the pre-post comparison of isometric maximal voluntary contraction (IMVC; average of 3x 3 sec) was performed at the beginning of the treatment and at the end for both feet (left = control, right = fatigue) (
Table 2). Due to a technical problem five subjects were not measured or not measured properly, which is why the sample size for the electromyographic evaluation had to be reduced to n = 20. While on the left side no significant difference for m. fibularis longus occurred, MDF and MNF for m. tibialis anterior increased significantly in the post-test. The right side (fatigue) showed significant decreased in MDF and MNF for both muscles (
Table 2).
Additionally, throughout the exercises external rotation (exercise 6; see
Table 1) and internal rotation (exercise 7; see
Table 1) of the right foot the MDF and MNF (initial 15 sec, final 15 sec) were analyzed. During the external rotation exercise the activity of m. fibularis longus, the initial sequence and the final sequence differed significantly for the MDF and the MNF. During the internal rotation exercise the activity of m. tibialis anterior, the initial sequence and the final sequence differed significantly for the MDF and the MNF (
Table 2).
Due to the test setup and procedure, a comparison with the left foot served as a control. In average the participants rated the treatment with an 18.0 ± 2.0 on Borg’s RPE which corresponds to a ‘very hard’ exertion [
20]. The sEMG values confirm the validity of the fatiguing treatment (see
Table 2).
3.2. Pronation Measured with the Static Navicular Drop (statND; 1st Question)
The static ND differed on average by 0.00 ± 1.26 mm for the left foot and by 0.2 ± 1.85 mm on the fatigued right side (
Table 3). The results show no significant difference for the within-subject factor “time of measurement” (pre vs. post) (F(1,24) = 0.267, p = .610), “side” (right vs. left foot) (F(1,24) = 2.324, p = .140) and “time*side” (F(1,24) = 0.160, p = .693).
3.3. Pronation Measured with the Dynamic Navicular Drop (dynND; 2nd Question)
For the pre-post comparison of the dynamic ND, the gait speed was determined in pre (4.40 ± 0.44 km/h) and posttest (4.33 ± 0.42 km/h), with no significant difference (p = 0.351). The dynamic ND changed in average by 0.00 ± 1.93 mm for the left control side and increased in average by 1.44 ± 2.11 mm for the right fatigued side (
Table 3). The results show a significant difference for the within-subject factor “time of measurement” (pre vs. post) (F(1,24) = 6.419, p = .018, η² = .211), “side” (right vs. left foot) (F(1,24) = 22.646, p = < .001, η² = .485) and “time*side” (F(1,24) = 6.190, p = .020, η² = .205).
3.4. Relation between Dynamic (dynND) and Static Navicular Drop (statND)(4th Question)
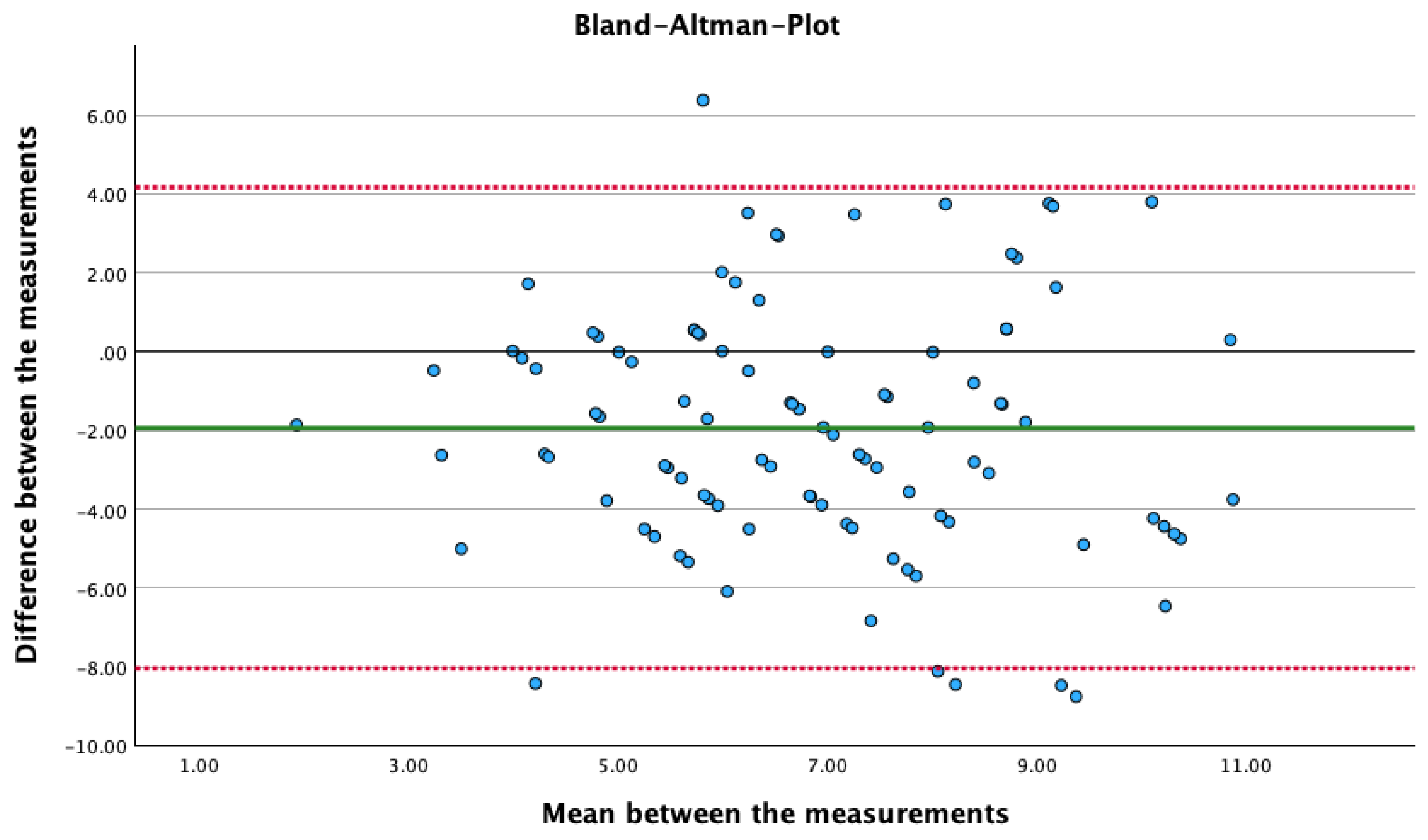
The mixed linear model regression shows a significant difference between dynND and statND (F(8,199) = 1.936, p = < .001, d = 0.2).
Figure 4 shows the Bland-Altman plot, in which the difference between two measurements is plotted against the mean values of the two measurements.
4. Discussion
The present study investigated the influence of fatigue of the ankle stabilizing muscles on the pronation of the midfoot. M. fibularis longus and brevis are important pronators of the foot and protect the foot against supination trauma, one of the most common injuries in sports [
25]. M. tibialis anterior and posterior are the antagonists and therefore supinators of the foot. They support the rearfoot rocker during gait (initial contact, loading response) and the shock absorption (loading response, mid stance) of the foot. The cushioning effect is mainly achieved by the pronation of the midfoot, in which the fibularis muscles work eccentrically. If the pronation is too strong (pes planus) [
18,
26] or too fast [
27,
28], this might lead to overuse of the anatomical structures.
The results show that the treatment led to measurable fatigue and that pronation (dynND) increased significantly as a result. The further discussion of results and methods is topic-related within the corresponding sections.
4.1. Dynamic Navicular Drop (dynND) and Fatigue (2nd Question)
Midfoot pronation, measured via dynND, increased by 1.44 mm (p < 0.001) on the fatigued side, while the control side did not change. The dynND increased by an average of 22.28 % on the fatigued side. In 5 out of 25, it even increased by > 50 %. The effect sizes underline the relevance of the changes. Depending on the authors, a dynND of 5.0 – 8.0 mm can be considered to be normal (
Table 4). Up to this level, it can be assumed that pronation is adequate and functional in terms of the metatarsal shock absorption mechanism.
Table 4 shows that ND will change in dependence of the sample and methods used. If we compare these values with the pretest values of this study, then the ND is basically within the normative range for the left foot (6.81 mm), whereas the ND of the right foot is in general a little higher (8.16 mm); although the participants had no signs of deformities, as the navicular index was assessed (see
Section 2.1). In the authors’ opinion, this could be due to a systematic bias in measurement or a systematic asymmetry (right vs. left). The minority of sources make a distinction between right and left side in their data. However, a change can be observed between pre- and posttest, which in this case even occurred in healthy (symptom-free) and athletic subjects without foot deformities. The increasing ND under the influence of fatigue could help to identify deviations which would not be observable without a fatigued state.
It is important to mention that the three-dimensional marker-based motion tracking, even if it is considered the gold standard, has a certain probability of measurement error concerning the accuracy. The mean trueness and uncertainty should be below 0.33 mm for the system used [
33]. Nevertheless, the authors interpret the systematics and proportionality of changes as potentially relevant for practice. In gait and movement analyses, a marginal change can already contribute to the occurrence of a complaint. The results of the present study match those of Headlee
, et al. [
34], in which the ND (here: statND) increased by an average of 1.8 mm following fatigue of the plantar intrinsic foot muscles. Fiolkowski
, et al. [
35] anaesthetized the intrinsic foot muscles in ten test subjects with the aid of an injection, which as a consequence increased the ND (here: statND) by 3 mm. Willems
, et al. [
36] and Weist
, et al. [
37] observed a medial shift of the foot pressure distribution in the heel area during fatigued state. Whether this observation is due to eversion of the heel or pronation was not precisely differentiated, but those two movements are often mechanically coupled. In contrast to the results of this study, Zadpoor and Nikooyan [
38] and Okamura
, et al. [
39] also found local fatigue of the intrinsic foot muscles, but observed a reduction in the heel eversion angle and a reduction in the statND, which they attributed to a kind of compensatory mechanism.
Given the limited number of comparable approaches, some studies show that fatigue (local, global) can influence foot kinematics and kinetics [
40]. Even if there is much to suggest that midfoot pronation would be increased, further studies should be carried out, in particular to investigate dynND, as the majority of studies used statND. Nevertheless, they all show that fatigue has an influence on the ND - regardless of the direction - and should therefore be taken into account in gait and movement analyses [
10].
4.2. Static Navicular Drop (statND) and Fatigue (1nd Question)
The midfoot pronation measured via the statND increased non-significantly by an average of 0.2 mm. In view of the statND data, it must therefore be stated that fatigue did not lead to a demonstrable change as was the case with the dynND. The statND is an efficient method for objectifying pronation, which seems to be the most used method so far but the method is significantly more error-prone and less closer to everyday life in direct comparison to the three-dimensional optical motion tracking during gait (dynND). Although the examiner was trained, the palpation and marking of the base of the navicular bone with a pencil entails potential measurement inaccuracies. Even more vulnerable in regard of the pre-post-comparison is the manual reading of the navicular height using a ruler. Furthermore, it can be assumed that the lack of dynamics and consequently comparatively low ground reaction forces or gravitational forces (standing vs. walking) could explain the difference between statND and dynND. Headlee, Leonard, Hart, Ingersoll and Hertel [
34] were able to measure a difference for the statND before and after fatigue using a similar methodology. Possible explanation could be that the measurement of statND in the present study was placed after the dynND. This resulted in a time delay of approximately two minutes, which could have been enough to stabilize the fatigued state of the right foot. In addition, the statND of Headlee, Leonard, Hart, Ingersoll and Hertel [
34] is already significantly higher in the pretest with an average of 10.0 mm than in this study with an average of 5.6 mm. For this reason, it is conceivable that the sample generally had a poorer foot status, as this ND would already be deviating from the normative values according to most authors [
31,
35,
41,
42].
4.3. Comparing Static (statND) and Dynamic (dynND) Navicular Drop Assessment (3rd Question)
The results of the present study show that there is significant difference between the two measurement methods. From the authors’ point of view, there are two main reasons for this. Firstly, the neuromuscular requirement profile for dynND is significantly higher than for statND. Compared to a dynamic measurement and the greater gravitational forces, the foot has comparatively greater stability when standing. Weaknesses, such as those caused by fatigue, can probably be better compensated while standing. Secondly, the two parameters presumably differ considerably in terms of reliability. While the dynND was measured here using a three-dimensional optical tracking system with technical support according to the best current possibilities, the statND was measured manually. Measurement inaccuracies therefore presumably have a significant influence on this part of the study. Based on the results, it would be advisable to measure pronation using the ND preferably in dynamic mode and using a two- or three-dimensional videos. Nevertheless, the practicability of the statND with sufficient reliability cannot be denied [
43], only the procedure for measuring the statND should be standardized. Some researchers compare the ND between bipedal sitting and standing [
44], others choose one-legged stance variants ([
45] or the comparison when the subtalar joint is positioned neutrally and loaded without corrections [
46]. Nevertheless, our results confirm those of Rathleff, Nielsen and Kersting [
41] who were also unable to demonstrate a predictive correlation between statND and dynND in their study. Similar results were observed by Deng, Joseph and Wong [
44] who had a similar setup of methods with 51 subjects, except they derived to statND in a sit-to-stand comparison. This is to be understood less as a final thesis, but primarily as an outlook for the necessity of further investigations of the two measurements. So far it seems like static measures of the ND don’t predict the ND during gait [
30,
44,
47].
With both methods, however, it must be taken into account that normative values are only a rough guide with the widley discussed advantages (orientation; especially for clinical conditions) and disadvantages (individuality). The extent of the ND must be set in relation to other abnormalities and the symptoms, as the ND is already strongly influenced by foot size and BMI [
48]. An increase in those parameters usually resolves in an increase of the ND.
4.4. Limitations
There are some limitations of the study that must be taken into account when interpreting the results. The fatigue treatment does not represent everyday stress. The content of the study was designed to be as standardized and economical as possible with the aim of achieving local muscle fatigue. It should also be noted that the m. tibialis posterior is actually considered to be of the greatest importance in stabilizing the pronation of the midfoot [
22]. However, this muscle cannot be derived via sEMG. For this reason, it was decided to use the partially synergistically working m. tibialis anterior. The fatigue treatment (see
Table 1) focuses more on the functions of m. tibialis anterior (dorsiflexion, supination, inversion) than on those of m. tibialis posterior (plantar flexion, supination, inversion). Consequently, a greater emphasis should have been placed on exercises combined with a form of plantar flexion combined with a supination. This could have led to a further increase in the ND.
Future studies, which also use three-dimensional motion capture, should additionally determine the rearfoot angle (eversion) at the point of maximum pronation. This will allow an even more differentiated interpretation of the foot posture and its misalignments or weaknesses.
5. Conclusions
While our results show that the statND is only affected by fatigue to a minor and non-significant extent, the dynND shows a significant increase in the navicular drop by an average of 1.44 mm. This may not seem much but for sport specific cases it could explain the causes of certain complaints in the gait and movement analyses, which we would not be able to observe with the absence of fatigue.
No relation between statND and dynND could be established in the present study, which is why further investigations are required. Future studies should focus on a clear wording and explanation of the navicular drop (e.g. statND vs. dynND). More studies should try using the dynND, since it seems to be closer to everyday life when looking at the gait cycle and its phase of single-legged support. The next step would be to investigate whether and to what extent the velocity of the internal tibial rotation increases as a result of fatigue. Internal tibial rotation is mechanically linked to metatarsal pronation and can be the cause of complaints, even if the overall range (here: navicular drop) remains unchanged [
49].
Author Contributions
Conceptualization, S.B.; methodology, S.B.; formal analysis, S.B., C.D..; investigation, R.G., D.L., S.B.; resources, M.F.; data curation, C.D., R.G., D.L.; writing—original draft preparation, S.B.; writing—review and editing, C.D., R.G., D.L., M.F., O.L.; visualization: S.B.; supervision, S.B., O.L.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Ethics Committee (No. 55) of Social Sciences at RPTU Kaiserslautern - Landau (14. Dez. 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Acknowledgments
The authors thank all participants for their contribution.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Perry, J.; Burnfield, J.M. Gait Analysis. Normal and Pathological Function, 2 ed.; SLACK Incorporated: Thorofare, 2010.
- Headlee, D.L.; Leonard, J.L.; Hart, J.M.; Ingersoll, C.D.; Hertel, J. Fatigue of the plantar intrinsic foot muscles increases navicular drop. Journal of Electromyography and Kinesiology 2008, 18, 420–425. [CrossRef]
- Ghanem, I.; Massaad, A.; Assi, A.; Rizkallah, M.; Bizdikian, A.J.; El Abiad, R.; Seringe, R.; Mosca, V.; Wicart, P. Understanding the foot’s functional anatomy in physiological and pathological conditions: the calcaneopedal unit concept. J Child Orthop 2019, 13, 134-146. [CrossRef]
- Nigg, B.; Behling, A.-V.; Hamill, J. Foot pronation. Footwear Science 2019, 11, 131-134. [CrossRef]
- Roth, S.; Roth, A.; Jotanovic, Z.; Madarevic, T. Navicular index for differentiation of flatfoot from normal foot. Int Orthop 2013, 37, 1107-1112. [CrossRef]
- Nielsen, R.G.; Rathleff, M.S.; Simonsen, O.H.; Langberg, H. Determination of normal values for navicular drop during walking: a new model correcting for foot length and gender. J Foot Ankle Res 2009, 2, 12. [CrossRef]
- Barton, C.J.; Bonanno, D.; Levinger, P.; Menz, H.B. Foot and ankle characteristics in patellofemoral pain syndrome: a case control and reliability study. Journal of Orthopaedic and Sports Physical Therapy 2010, 40, 286-296. [CrossRef]
- Brody, D.M. Techniques in the Evaluation and Treatment of the Injured Runner. Orthopedic Clinics of North America 1982, 13, 541-558. [CrossRef]
- Enoka, R.M.; Duchateau, J. Muscle fatigue: what, why and how it influences muscle function. Journal of Physiology 2008, 586, 11-23. [CrossRef]
- Becker, S.; Simon, S.; Dindorf, C.; Dully, J.; Bartaguiz, E.; Schmitz, L.; Kothe, N.; Fröhlich, M.; Ludwig, O. Fatigue as a key factor for testing knee stability with single leg drop landing for injury prevention and return to play tests. Frontiers in Sports and Active Living 2023, 5, 1-8. [CrossRef]
- Balakrishnan, A.; Medikonda, J.; Namboothiri, P.K. Analysis of the effect of muscle fatigue on gait characteristics using data acquired by wearable sensors. In Proceedings of the 2020 IEEE International Conference on Distributed Computing, VLSI, Electrical Circuits and Robotics (DISCOVER), 30-31 Oct. 2020, 2020; pp. 137-140.
- Ameli, S.; Stirling, D.; Naghdy, F.; Naghdy, G.; Aghmesheh, M. Assessing the impact of fatigue on gait using inertial sensors; 2013; pp. 307-312.
- Qu, X.; Yeo, J.C. Effects of load carriage and fatigue on gait characteristics. J Biomech 2011, 44, 1259-1263. [CrossRef]
- Lee, C.-R.; Kim, M.-K.; Cho, M.S. The Relationship between Balance and Foot Pressure in Fatigue of the Plantar Intrinsic Foot Muscles of Adults with Flexible Flatfoot. J Phys Ther Sci 2012, 24, 699-701. [CrossRef]
- Bartaguiz, E.; Dindorf, C.; Dully, J.; Becker, S.; Fröhlich, M. Effects of increasing physical load and fatigue on the biomechanics of elite cyclists. Scientific Journal of Sport and Performance 2022, 2, 59-69. [CrossRef]
- Gefen, A. Biomechanical analysis of fatigue-related foot injury mechanisms in athletes and recruits during intensive marching. Med Biol Eng Comput 2002, 40, 302-310. [CrossRef]
- Murgia, C. Overuse, fatigue, and injury: neurological, psychological, physiological, and clinical aspects. J Dance Med Sci 2013, 17, 51-52. [CrossRef]
- Gardin, F.A.; Middlemas, D.; Williams, J.L.; Leigh, S.; Horn, R.R. Navicular Drop Before and After Fatigue of the Ankle Invertor Muscles. International Journal of Athletic Therapy and Training 2013, 18, 36-39. [CrossRef]
- Murley, G.S.; Menz, H.B.; Landorf, K.B. A protocol for classifying normal- and flat-arched foot posture for research studies using clinical and radiographic measurements. Journal of Foot and Ankle Research 2009, 2, 22. [CrossRef]
- Borg, G. Borg’s perceived exertion and pain scales; Human Kinetics: Champaign, 1998.
- Hermens, H.J.; Freriks, B.; Merletti, R.; Hägg, G.G.; Stegeman, D.; Blok, J.; Rau, G.; Disselhorst-Klug, C. Seniam 8. European Recommendations for Surface Electromyography. In SENIAM, 2e édition ed.; Hermens, H.J., Merletti, R., Freriks, B., Eds.; SENIAM; Roessingh Research and Development: Enschede, The Netherlands, 1999.
- Alam, F.; Raza, S.; Moiz, J.A.; Bhati, P.; Anwer, S.; Alghadir, A. Effects of selective strengthening of tibialis posterior and stretching of iliopsoas on navicular drop, dynamic balance, and lower limb muscle activity in pronated feet: A randomized clinical trial. Phys Sportsmed 2019, 47, 301-311. [CrossRef]
- Leardini, A.; Sawacha, Z.; Paolini, G.; Ingrosso, S.; Nativo, R.; Benedetti, M.G. A new anatomically based protocol for gait analysis in children. Gait Posture 2007, 26, 560-571. [CrossRef]
- Barre, A.; Armand, S. Biomechanical ToolKit: Open-source framework to visualize and process biomechanical data. Comput Methods Programs Biomed 2014, 114, 80-87. [CrossRef]
- Kobayashi, T.; Tanaka, M.; Shida, M. Intrinsic Risk Factors of Lateral Ankle Sprain:A Systematic Review and Meta-analysis. Sports Health 2016, 8, 190-193. [CrossRef]
- Neal, B.S.; Griffiths, I.B.; Dowling, G.J.; Murley, G.S.; Munteanu, S.E.; Franettovich Smith, M.M.; Collins, N.J.; Barton, C.J. Foot posture as a risk factor for lower limb overuse injury: a systematic review and meta-analysis. Journal of Foot and Ankle Research 2014, 7, 55. [CrossRef]
- Messier, S.P.; Pittala, K.A. Etiologic factors associated with selected running injuries. Med Sci Sports Exerc 1988, 20, 501-505.
- Rodrigues, P.; TenBroek, T.; Van Emmerik, R.; Hamill, J. Evaluating runners with and without anterior knee pain using the time to contact the ankle joint complexes’ range of motion boundary. Gait Posture 2014, 39, 48-53. [CrossRef]
- Cornwall, M.W.; McPoil, T.G. Relative movement of the navicular bone during normal walking. Foot Ankle Int 1999, 20, 507-512. [CrossRef]
- Dicharry, J.M.; Franz, J.R.; Della Croce, U.; Wilder, R.P.; Riley, P.O.; Kerrigan, D.C. Differences in static and dynamic measures in evaluation of talonavicular mobility in gait. J Orthop Sports Phys Ther 2009, 39, 628-634. [CrossRef]
- Kim, T.; Park, J.C. Short-term effects of sports taping on navicular height, navicular drop and peak plantar pressure in healthy elite athletes: A within-subject comparison. Medicine (Baltimore) 2017, 96, e8714. [CrossRef]
- Nielsen, R.G.; Rathleff, M.S.; Moelgaard, C.M.; Simonsen, O.; Kaalund, S.; Olesen, C.G.; Christensen, F.B.; Kersting, U.G. Video based analysis of dynamic midfoot function and its relationship with Foot Posture Index scores. Gait Posture 2010, 31, 126-130. [CrossRef]
- Eichelberger, P.; Ferraro, M.; Minder, U.; Denton, T.; Blasimann, A.; Krause, F.; Baur, H. Analysis of accuracy in optical motion capture - A protocol for laboratory setup evaluation. Journal of biomechanics 2016, 49 10, 2085-2088.
- Headlee, D.L.; Leonard, J.L.; Hart, J.M.; Ingersoll, C.D.; Hertel, J. Fatigue of the plantar intrinsic foot muscles increases navicular drop. J Electromyogr Kinesiol 2008, 18, 420-425. [CrossRef]
- Fiolkowski, P.; Brunt, D.; Bishop, M.; Woo, R.; Horodyski, M. Intrinsic pedal musculature support of the medial longitudinal arch: an electromyography study. J Foot Ankle Surg 2003, 42, 327-333. [CrossRef]
- Willems, T.M.; De Ridder, R.; Roosen, P. The effect of a long-distance run on plantar pressure distribution during running. Gait & Posture 2012, 35, 405-409. [CrossRef]
- Weist, R.; Eils, E.; Rosenbaum, D. The influence of muscle fatigue on electromyogram and plantar pressure patterns as an explanation for the incidence of metatarsal stress fractures. Am J Sports Med 2004, 32, 1893-1898. [CrossRef]
- Zadpoor, A.A.; Nikooyan, A.A. The effects of lower extremity muscle fatigue on the vertical ground reaction force: a meta-analysis. Proc Inst Mech Eng H 2012, 226, 579-588. [CrossRef]
- Okamura, K.; Kanai, S.; Oki, S.; Tanaka, S.; Hirata, N.; Sakamura, Y.; Idemoto, N.; Wada, H.; Otsuka, A. Does the weakening of intrinsic foot muscles cause the decrease of medial longitudinal arch height? J Phys Ther Sci 2017, 29, 1001-1005. [CrossRef]
- Hazzaa, W.A.; Hottenrott, L.; Kamal, M.A.; Mattes, K. The Influence of General and Local Muscle Fatigue on Kinematics and Plantar Pressure Distribution during Running: A Systematic Review and Meta-Analysis. Sports (Basel) 2023, 11. [CrossRef]
- Rathleff, M.S.; Nielsen, R.G.; Kersting, U.G. Navicula drop test ad modum Brody: does it show how the foot moves under dynamic conditions? J Am Podiatr Med Assoc 2012, 102, 34-38. [CrossRef]
- Christensen, B.H.; Andersen, K.S.; Pedersen, K.S.; Bengtsen, B.S.; Simonsen, O.; Kappel, S.L.; Rathleff, M.S. Reliability and concurrent validity of a novel method allowing for in-shoe measurement of navicular drop. J Foot Ankle Res 2014, 7, 12. [CrossRef]
- Mcpoil, T.G.; Cornwall, M.W.; Abeler, M.G.; Devereaux, K.J.; Flood, L.J.; Merriman, S.E.; Sullivan, S.R.; Laan, M.J.v.D.; Villadiego, T.A.; Wilson, K. The Optimal Method to Assess the Vertical Mobility of the Midfoot: Navicular Drop versus Dorsal Arch Height Difference? Clinical research on foot & ankle 2013, 2013, 1-7.
- Deng, J.; Joseph, R.; Wong, C.K. Reliability and validity of the sit-to-stand navicular drop test: Do static measures of navicular height relate to the dynamic navicular motion during gait. Journal of Student Physical Therapy Research 2010, 2, 21-28.
- Raissi, G.R.; Cherati, A.D.; Mansoori, K.D.; Razi, M.D. The relationship between lower extremity alignment and Medial Tibial Stress Syndrome among non-professional athletes. Sports Med Arthrosc Rehabil Ther Technol 2009, 1, 11. [CrossRef]
- Billis, E.; Katsakiori, E.; Kapodistrias, C.; Kapreli, E. Assessment of foot posture: Correlation between different clinical techniques. The Foot 2007, 17, 65-72. [CrossRef]
- Rathleff, M.S.; Olesen, C.G.; Moelgaard, C.M.; Jensen, K.; Madeleine, P.; Olesen, J.L. Non-linear analysis of the structure of variability in midfoot kinematics. Gait Posture 2010, 31, 385-390. [CrossRef]
- Nielsen, R.G.; Rathleff, M.S.; Simonsen, O.H.; Langberg, H. Determination of normal values for navicular drop during walking: a new model correcting for foot length and gender. Journal of Foot and Ankle Research 2009, 2, 12. [CrossRef]
- Benca, E.; Listabarth, S.; Flock, F.K.J.; Pablik, E.; Fischer, C.; Walzer, S.M.; Dorotka, R.; Windhager, R.; Ziai, P. Analysis of Running-Related Injuries: The Vienna Study. J Clin Med 2020, 9. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).