Submitted:
04 July 2024
Posted:
05 July 2024
You are already at the latest version
Abstract
Keywords:
Author Summary
Introduction
Material and Methods
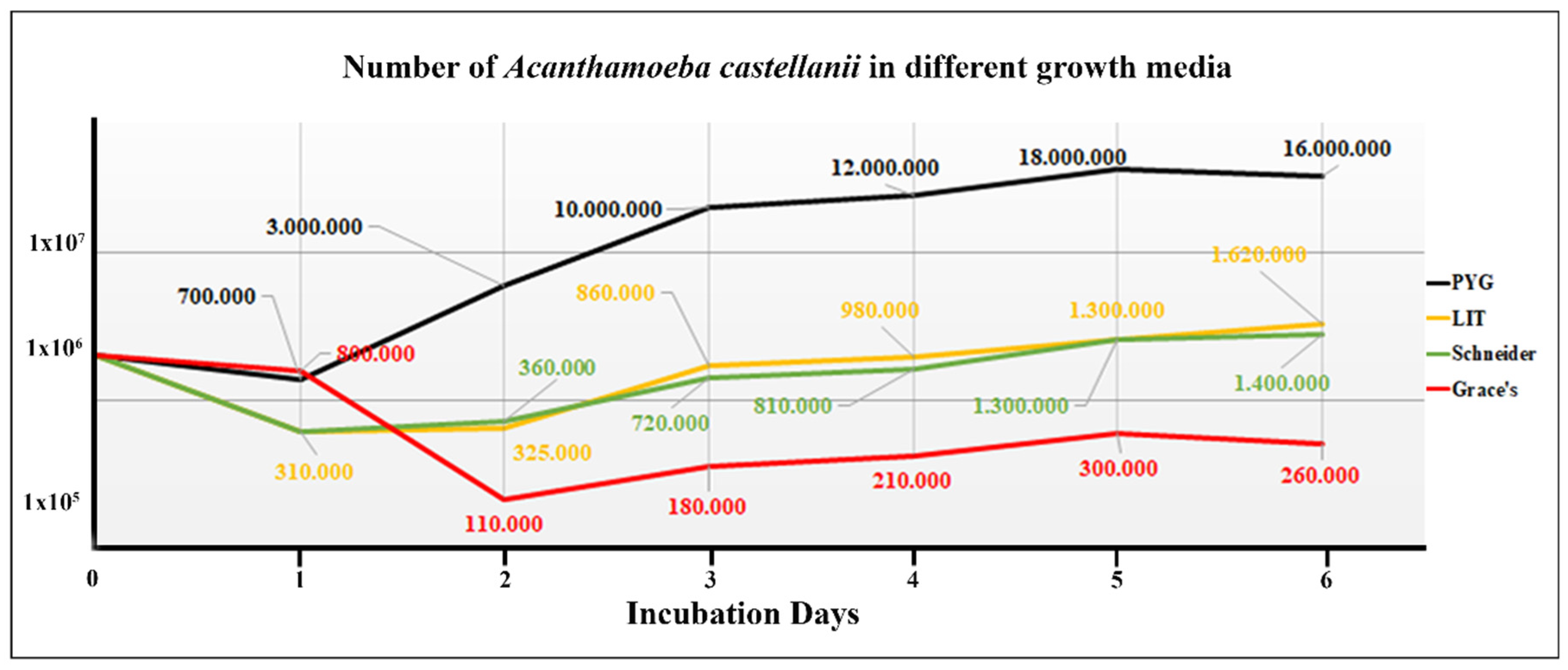
Acanthamoeba stains and cultivation conditions
Leishmania strains and cultivation conditions of promastigotes
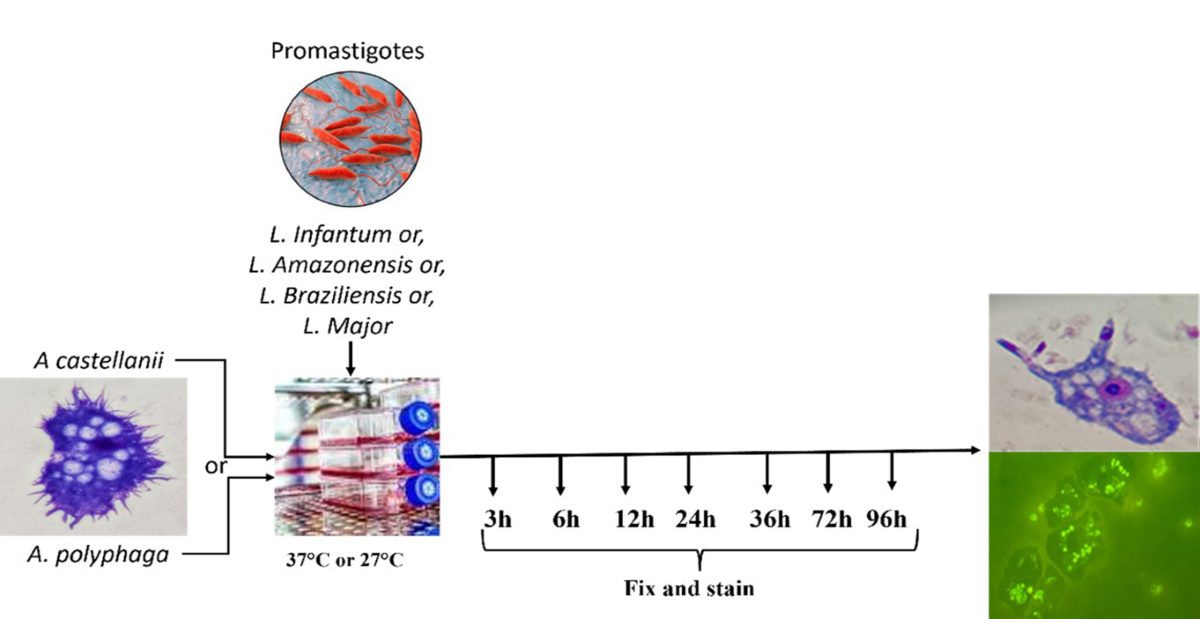
Co-culture of Leishmania spp. and Acanthamoeba spp.
The parasite release and differentiation assay
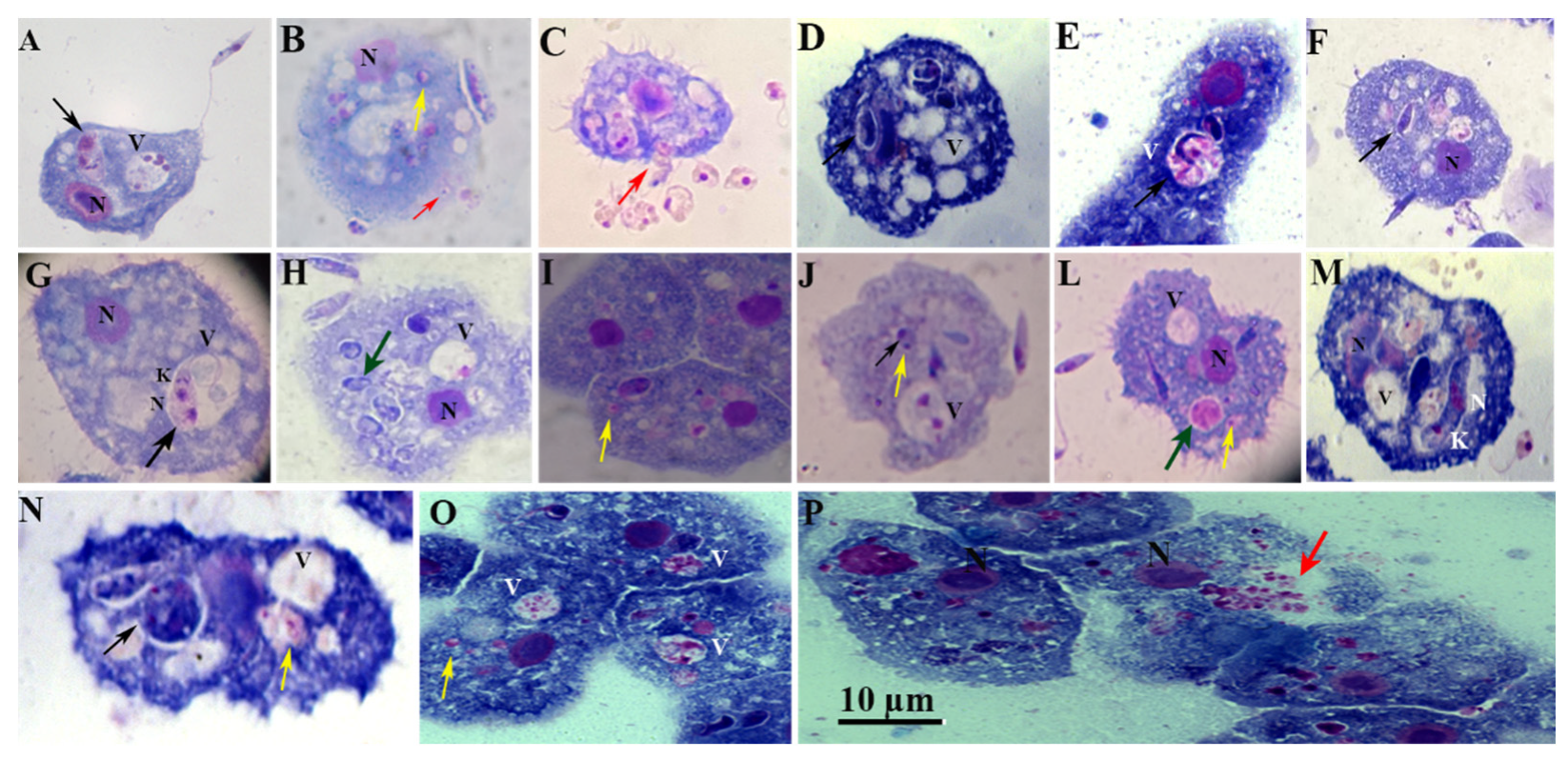
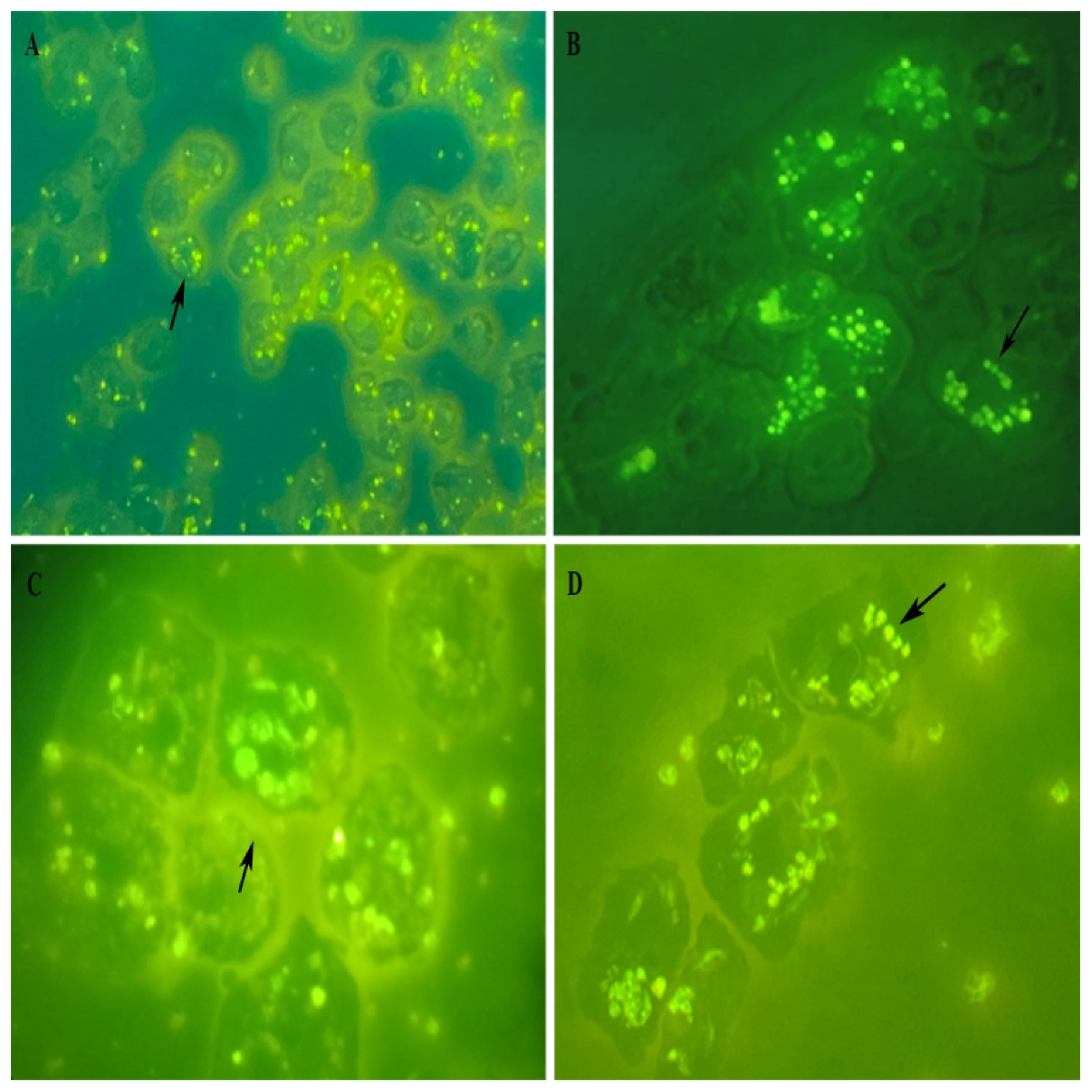
Results
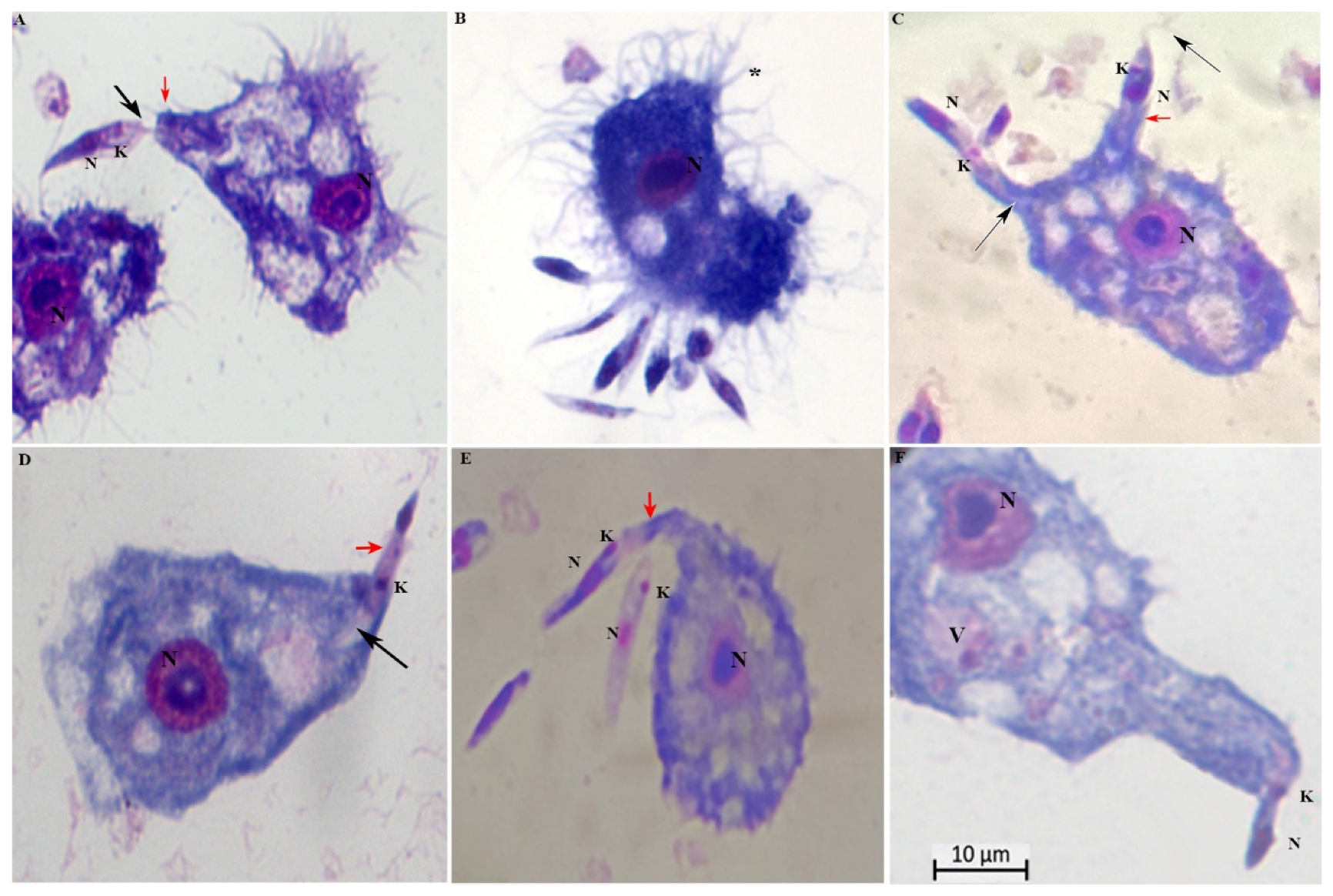
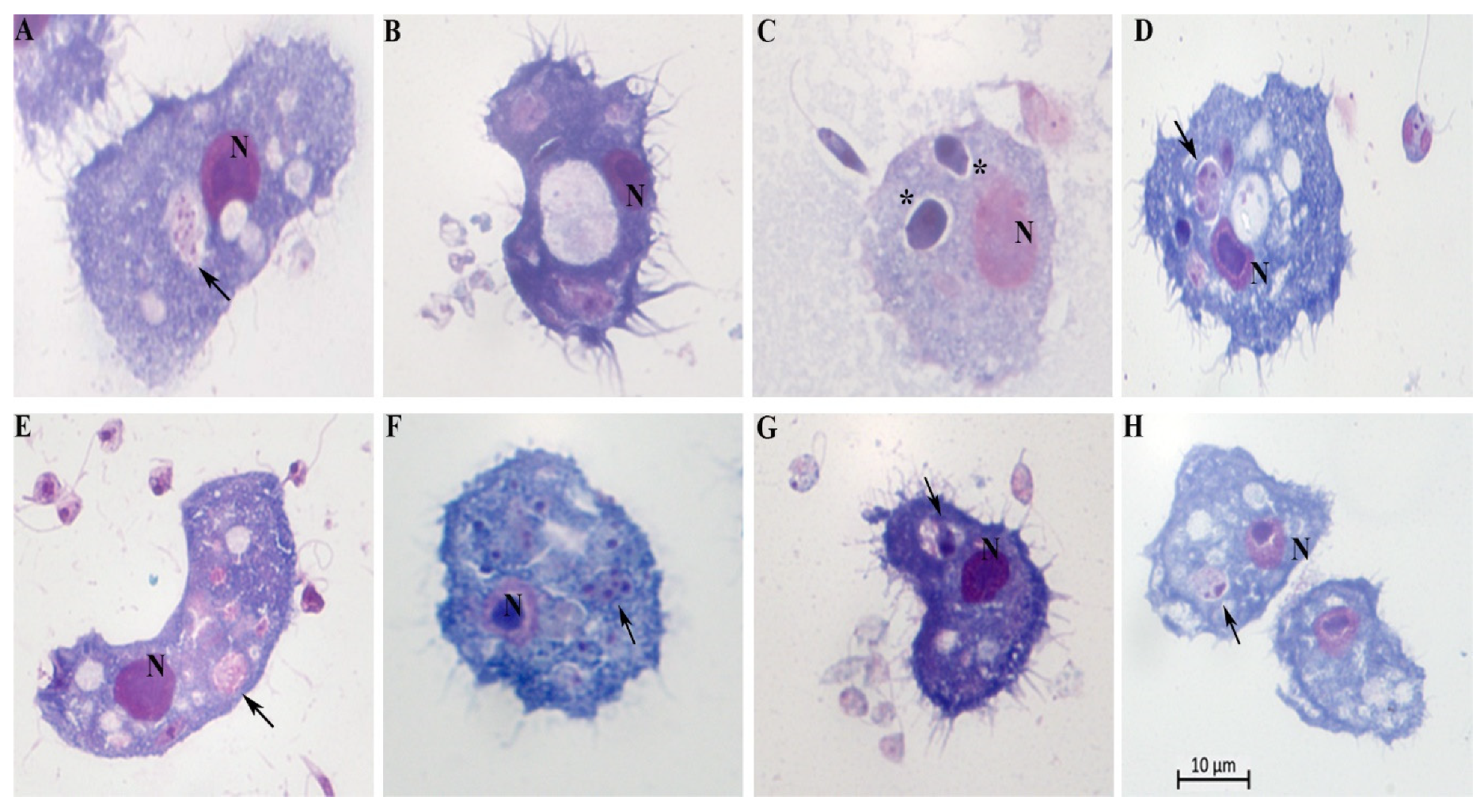
Acanthamoeba–Leishmania co-culture
Discussion
Conclusion
Funding
Data Availability
Author’s contribution
Acknowledgments
Competing Interests
References
- Mann S, Frasca K, Scherrer S, et al. A review of Leishmaniasis: current knowledge and future directions. Curr Trop Med Rep. 2021; 8:121-132.
- Gupta AK, Das S, Kamran M, Ejazi SA, Ali N. The pathogenicity and virulence of Leishmania - interplay of virulence factors with host defenses. Virulence, 2022; 13(1):903-935.
- Torres-Guerrero E, Quintanilla-Cedillo MR, Ruiz-Esmenjaud J, Arenas R. Leishmaniasis: a review. F1000Res. 2017; 6: 750.
- Yadav, P.; Azam, M.; Ramesh, V.; Singh, R. Unusual Observations in Leishmaniasis—An Overview. Pathogens 2023; 12:297.
- Sunyoto T, Boelaert M, Meheus F. Understanding the economic impact of leishmaniasis on households in endemic countries: a systematic review. Expert Rev Anti Infect Ther. 2019; 17:57-69.
- Burza S, Croft SL, Boelaert M. Leishmaniasis. Lancet. 2018; 15;392(10151):951-970.
- Hefnawy, A., Berg, M., Dujardin, J.-C. & De Muylder, G. Exploiting knowledge on Leishmania drug resistance to support the quest for new drugs. Trends Parasitol.2017; 33,162–174.
- Lindoso, J. A. L., Cunha, M. A., Queiroz, I. T. & Moreira, C. H. V. Leishmaniasis–HIV coinfection: current challenges. HIV AIDS. 2016;8, 147-156.
- Sampaio Tavares Veras P, Castro-Gomes T, Perrone Bezerra de Menezes J. Elucidating the Complex Interrelationship on Early Interactions between Leishmania and Macrophages [Internet]. Macrophages - Celebrating 140 Years of Discovery. IntechOpen; 2022. pp. 1-21. [CrossRef]
- WHO. Leishmaniasis Fact Sheet. World Health Organ (2022). Available online at: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis (accessed: July 15, 2023).
- Conceição-Silva F, Morgado FN. Leishmania Spp-Host Interaction: There Is Always an Onset, but Is There an End? Front Cell Infect Microbiol. 2019; 19 (9):330.
- Almeida, F.S.; Vanderley, S.E.R.; Comberlang, F.C.; Andrade, A.G.d.; Cavalcante-Silva, L.H.A.; Silva, E.d.S.; Palmeira, P.H.d.S.; Amaral, I.P.G.d.; Keesen, T.S.L. Leishmaniasis: Immune Cells Crosstalk in Macrophage Polarization. Trop. Med. Infect. Dis. 2023, 8, 276. [Google Scholar] [CrossRef] [PubMed]
- Alexander J, Vickerman K. Fusion of host cell secondary lysosomes with the parasitophorous vacuoles of Leishmania mexicana-infected macrophages. The Journal of Protozoology. 1975;22(4):502-508.
- Podinovskaia M, Descoteaux A. Leishmania and the macrophage: a multifaceted interaction. Future Microbiol. 2015;10(1):111-29.
- Bajgar A, Krejčová G. On the origin of the functional versatility of macrophages. Front Physiol. 2023; 23(14):1128984.
- Siddiqui, R., Khan, N.. Acanthamoeba is an evolutionary ancestor of macrophages: A myth or reality?. Experimental Parasitology. 2012; 130(2):95-97.
- Mungroo MR, Siddiqui R, Khan NA. War of the microbial world: Acanthamoeba spp. interactions with microorganisms. Folia Microbiol (Praha). 2021;66(5):689-699.
- Bowers, B., and Korn, E. D.. The fine structure of Acanthamoeba castellanii. I. The trophozoite. J. Cell Biol. 1968;39, 95-111.
- Allan J. Guimaraes, Kamilla Xavier Gomes, Juliana Reis Cortines, José Mauro Peralta, Regina H.Saramago Peralta. Acanthamoeba spp. as a universal host for pathogenic microorganisms: One bridge from environment to host virulence. Microbiological Research. 2016; 193:30-38.
- Boulais J, Trost M, Landry CR, Dieckmann R, Levy ED, Soldati T, Michnick SW, Thibault P, Desjardins M. Molecular characterization of the evolution of phagosomes. Mol Syst Biol. 2010; 19(6):423.
- Mella, C; Medina, G; Flores-Martin, S; Toledo, Z; Simaluiza, RJ; Pérez-Pérez, G; Fernández, H.Interaction between zoonotic bacteria and free living amoebas. A new angle of an epidemiological polyhedron of public health importance? Austral Journal of Veterinary Sciences. 2016; 48(1): 1-10.
- Sandstrom, G., Saeed, A., Abd, H., 2011. Acanthamoeba-bacteria: a model to study host interaction with human pathogens. Curr. Drug Targets. 2011; 12 (7):936–941.
- Segal, Gil, and Howard A. Shuman. "Legionella pneumophila utilizes the same genes to multiply within Acanthamoeba castellanii and human macrophages." Infection and immunity. 1999; 67(5): 2117-2124.
- Derengowski Lda, S., Paes, H. C., Albuquerque, P., Tavares, A. H., Fernandes, L., Silva-Pereira, I., et al. (2013). The transcriptional response of Cryptococcus neoformans to ingestion by Acanthamoeba castellanii and macrophages provides insights into the evolutionary adaptation to the mammalian host. Eukaryot. Cell. 2013; 12:761–774.
- Rayamajhee B, Subedi D, Peguda HK, Willcox MD, Henriquez FL, Carnt N. A Systematic Review of Intracellular Microorganisms within Acanthamoeba to Understand Potential Impact for Infection. Pathogens. 2021; 10(2):225. [CrossRef]
- Steenbergen, J.N., Shuman, H.A., Casadevall, A. Cryptococcus neoformans interactions with amoebae suggest an explanation for its virulence and intracellular pathogenic strategy in macrophages. Proc. Natl. Acad. Sci. U. S. A. 2001; 98(26):15245–15250.
- Roy S, Kumar GA, Jafurulla M, Mandal C, Chattopadhyay A. Integrity of the actin cytoskeleton of host macrophages is essential for Leishmania donovani infection. Biochim Biophys Acta. 2014;1838(8):2011-8.
- Valigurová, A.; Koláˇrová, I.Unrevealing the Mystery of Latent Leishmaniasis: What Cells Can Host Leishmania? Pathogens. 2023; 12, 246.
- Costa-da-Silva AC, Nascimento DO, Ferreira JRM, Guimarães-Pinto K, Freire-de-Lima L, Morrot A, Decote-Ricardo D, Filardy AA, Freire-de-Lima CG. Immune Responses in Leishmaniasis: An Overview. Trop Med Infect Dis. 2022; 31;7(4):54. [CrossRef]
- Yasmin, H.; Adhikary, A.; Al-Ahdal, M.N.; Roy, S.; Kishore, U. Host–Pathogen Interaction in Leishmaniasis: Immune Response and Vaccination Strategies. Immuno 2022; 2, 218-254.
- Tomiotto-Pellissier F, Bortoleti BTdS, Assolini JP, Gonçalves MD, Carloto ACM, Miranda-Sapla MM, Conchon-Costa I, Bordignon J and Pavanelli WR. Macrophage Polarization in Leishmaniasis: Broadening Horizons. Front. Immunol 2018; 9:2529.
- Lecoeur H, Prina E, Gutiérrez-Sanchez M, Späth GF. Going ballistic: Leishmania nuclear subversion of host cell plasticity. Trends Parasitol. 2022;38(3):205-216.
- Costa-da-Silva, A.C.; Nascimento, D.d.O.; Ferreira, J.R.M.; Guimarães-Pinto, K.; Freire-de-Lima, L.; Morrot, A.; Decote-Ricardo, D.; Filardy, A.A.; Freire-de-Lima, C.G. Immune Responses in Leishmaniasis: An Overview. Trop. Med. Infect. Dis. 2022; 7(4):54.
- Mills DB. The origin of phagocytosis in Earth history. Interface Focus. 2020; 6, 10(4):20200019.
- Bajgar A and Krejčová G. On the origin of the functional versatility of macrophages. Front. Physiol. 2023; 14:1128984.
- Ferreira MdS, Mendoza SR, Goncalves DdS, Rodrıguez-de la Noval C, Honorato L, Nimrichter L, Ramos LFC, Nogueira FCS, Domont GB, Peralta JM and.
- Guimarães AJ. Recognition of Cell Wall Mannosylated Components as a Conserved Feature for Fungal Entrance, Adaptation and Survival Within Trophozoites of Acanthamoeba castellanii and Murine Macrophages. Frontiers in Cellular and Infection Microbiology. 2022; 12: 858979.
- Casadevall A, Fu MS, Guimaraes AJ, Albuquerque P. The 'Amoeboid Predator-Fungal Animal Virulence' Hypothesis. J Fungi (Basel). 2019; 21, 5(1):10.
- Guimaraes A. J., Gomes K. X., Cortines J. R., Peralta J. M., Peralta R. H. S.. Acanthamoeba Spp. As a Universal Host for Pathogenic Microorganisms: One Bridge From Environment to Host Virulence. Microbiol. Res. 2016;193, 30–38.
- Clarke M, Lohan AJ, Liu B, Lagkouvardos I, Roy S, Zafar N, Bertelli C, Schilde C, Kianianmomeni A, Bürglin TR, Frech C, Turcotte B, Kopec KO, Synnott JM, Choo C, Paponov I, Finkler A, Heng Tan CS, Hutchins AP, Weinmeier T, Rattei T, Chu JS, Gimenez G, Irimia M, Rigden DJ, Fitzpatrick DA, Lorenzo-Morales J, Bateman A, Chiu CH, Tang P, Hegemann P, Fromm H, Raoult D, Greub G, Miranda-Saavedra D, Chen N, Nash P, Ginger ML, Horn M, Schaap P, Caler L, Loftus BJ. Genome of Acanthamoeba castellanii highlights extensive lateral gene transfer and early evolution of tyrosine kinase signaling. Genome Biol. 2013; 1;14(2):R11.
- Rayamajhee, B., Willcox, M. D. P., Henriquez, F. L., Petsoglou, C., Subedi, D., and Carnt, N. (2022). Acanthamoeba, an environmental phagocyte enhancing survival and transmission of human pathogens. Trends Parasitol. 2022; 38, 975–990.
- Khan, N.A., 2009. Acanthamoeba Biology and Pathogenesis, Caister Academic Press, ISBN 978-1-904455-43-1.
- Steenbergen J. N., Shuman H. A., Casadevall A. (2001). Cryptococcus Neoformans Interactions with Amoebae Suggest an Explanation for Its Virulence and Intracellular Pathogenic Strategy in Macrophages. Proc. Natl. Acad. Sci. U.S.A. 2001; 98, 15245–15250.
- Segal G, Shuman HA. Legionella pneumophila utilizes the same genes to multiply within Acanthamoeba castellanii and human macrophages. Infect Immun 1999; 67:2117–2124.
- Al-Khodor S, Price CT, Habyarimana F, Kalia A, Abu Kwaik Y. A Dot/Icm-translocated ankyrin protein of Legionella pneumophila is required for intracellular proliferation within human macrophages and protozoa. Mol Microbiol. 2008; Nov;70(4):908-23.
- Derengowski L., da S., Paes H. C., Albuquerque P., Tavares A. H. F. P., Fernandes L., et al.. The Transcriptional Response of Cryptococcus Neoformans to Ingestion by Acanthamoeba Castellanii and Macrophages Provides Insights Into the Evolutionary Adaptation to the Mammalian Host. Eukaryot Cell. 2013; 12, 761–774.
- Espinoza-Vergara G, Hoque MM, McDougald D, Noorian P. The Impact of Protozoan Predation on the Pathogenicity of Vibrio cholerae. Front Microbiol. 2020; 21(11):17.
- Danelishvili, L, Wu, M., Stang, B., Harriff, M., Cirillo, S.L., Cirillo, J.D., Bildfell, R., Arbogast, B., Bermudez, L.E.. Identification of Mycobacterium avium pathogenicity island important for macrophage and amoeba infection. Proceedings of the National Academy of Science USA. 2007; 104 (26):1038–11043.
- Nikolakopoulou, C., Willment, J. A., Brown, G. D.. C-Type Lectin Receptors in Antifungal Immunity. Adv. Exp. Med. Biol. 2020; 1204, 1–30.
- Allen, PG, Dawidowicz, EA. Phagocytosis in Acanthamoeba: I. A mannose receptor is responsible for the binding and phagocytosis of yeast. J Cell Physiol. 1990; 145(3):508–513. [CrossRef]
- Corsaro D. Acanthamoeba Mannose and Laminin Binding Proteins Variation across Species and Genotypes. Microorganisms. 2022; 31;10(11):2162. [CrossRef]
- Merlen T, Sereno D, Brajon N, Rostand F, Lemesre JL. Leishmania spp: completely defined medium without serum and macromolecules (CDM/LP) for the continuous in vitro cultivation of infective promastigote forms. Am J Trop Med Hyg. 1999; 60:41–50.
- Costa AFP, de Brito RCF, Carvalho LM, Cardoso JMO, Vieira PMA, Reis AB, Aguiar-Soares RDO, Roatt BM. Liver infusion tryptose (LIT): the best choice for growth, viability, and infectivity of Leishmania infantum parasites. Parasitol Res. 2020; 119(12):4185-4195.
- Dos Santos A.L, Carvalho-Kelly, LF.,Dick, CF, Meyer-Fernandes, JR. Innate immunomodulation to trypanosomatid parasite infections. Exp. Parasitol. 2016;167: 67-75.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



