1. Case Study
Formerly titled General Chapter <661> Containers-Plastics, the following has been relocated to the following new chapters <661. 1> (Plastic Materials of Construction) and <661. 2> (Plastic Packaging Systems for Pharmaceutical Use). This reauthorization was done in December 2015, and the changes were included in USP 39 NF 34 from May 1, 2016. General Chapter <661. 1> describes the procedure of recognizing plastic materials; General Chapter <661. 2> describes the procedures of testing and the requirement for plastic packaging materials; both these chapters were published in Pharmacopeial Forum 39(5) and 40 (5) as drafts; all changes made in them are effective from May 1, 2016. Above all, there is a published Notice of Intent for a new USP General Chapter <1661>: Evaluation of Plastic Packaging Systems and Their Materials of Construction Regarding User Safety in the Pharmacopeial Forum 40(6).
1.1. USP Chapter 661.1: Plastic Materials of Construction
1.1.1. Material Composition
This chapter also classifies plastic materials that can be used in pharmaceutical operations by means such as incompatibility, stability, and effects of leaching. Some of the general types of plastics employed in the manufacturing of Pharma products include polyethylene, polypropylene, PVC, and PET, among others.
1.1.2. Chemical Compatibility
The plastic materials must be compatible with the product with which they come into direct contact; this implies that certain reactions with the active ingredients of the medication should not occur that are counterproductive to the purpose of these plastic materials. This encompasses the ability of the carrier not to allow chemical leaching, absorption or absorption of active ingredients or other parts of the CCA.
1.1.3. Extractables and Leachable
Plastic materials in pharmaceutical packaging, including plastic bottles, vials, blisters, and strips, must be tested for chemical substances that can be extracted and leached out into the product. Such interstitial concepts may be additives, processing aids, degradation products, or residual substances, which may become a threat to the quality of the final product or the patients when it comes to medications.
2. Methodology
2.1. Polyethylene High Density
2.1.1. Infrared Spectroscopy
Perform the following for Multiple Internal Reflectance. Hence, applying corrections based on the illustrated aspects elucidated in the preceding text, the corrected spectrum of the specimen manifests or points to appreciable absorption only in wavelengths relating to the specificity/identity of USP High-Density Polyethylene RS.
2.1.2. Differential Scanning Calorimetry
To conduct the thermal analysis, follow these steps: The region of the specimen’s thermal analysis curve should ideally overlap as closely as possible with the peak height of the USP High-Density Polyethylene RS curve. Also, there is a requirement that the variation between the percentage melting obtained from the specimen’s thermal analysis curve should not be more than 6.0° to the Reference Standard.
2.1.3. Heavy Metals and Nonvolatile Residue
Carry out these tests on extracts of specimens as indicated in the Physicochemical Tests section. However, bringing the sample size for each test to 20.0 mL volume of the extracting medium was observed in the control. Furthermore, in the range known as the Physicochemical Tests, the area termed the portion ought to be 60cm2, irrespective of the layer’s thickness.
2.1.4. Components Used in Contact with Oral Liquids
Execute the recommended protocols for conducting Physicochemical Analyses and assessing Buffer Capacity.
2.2. Low-Density Polyethylene
2.2.1. Infrared Spectroscopy
Go to Multiple Internal Reflectance as usual. With the correction made above, the major absorption bands are present only at wavelengths which are contained in the spectrum of the USP Low-Density Polyethylene RS.
2.2.2. Differential Scanning Calorimetry
It is recommended to continue as it is described in the Thermal Analysis section. It is necessary to check the shape of the curve obtained for the specimen’s thermal analysis to be as similar to Reference Standard – USP Low-Density Polyethylene RS as possible. The specimen’s thermal analysis curve shows that the value of the melting peak temperature must not be Greater than what the reference standard indicates by more than 8.0°.
2.2.3. Heavy Metals and Nonvolatile Residue
Keep specimen extracts ready for testing in compliance with the Physicochemical Tests, Testing Parameters and Sample Preparation. For each 20.0 mL of extracting medium in the upper slot, utilize 60 cm² of the section, irrespective of its density.
2.2.4. Heavy Metals
Containers satisfy the standards for conducting Physicochemical Tests related to Heavy Metals.
2.2.5. Nonvolatile Residue
Perform the instructions described in the method section as Physicochemical Tests Nonvolatile Residue. If, at any test condition, an extract is observed to contain both Brilliant Blue and Solvent Red 24, use the same solvent as the Blank for that test condition. The magnitude of the Sample preparation should not be more than 12 as compared to the Blank value. 0 mg when water at a temperature of 70° is used as the extracting medium, 75. 0 mg when alcohol at 70° is used as the extracting medium and 350. 0 mg when hexanes at 50° are used as the extracting medium.
Table 1.
Limits and Appropriate temperature conditions.
Table 1.
Limits and Appropriate temperature conditions.
| NMT |
Temperature |
| 12.0 mg |
70° |
| 75.0 mg |
70° |
| 350.0 mg |
50° |
2.2.6. Components Used in Contact with Oral Liquids
Execute the recommended protocols for conducting Physicochemical Analyses and assessing Buffer Capacity.
2.3. Polypropylene Containers
2.3.1. Scope
The container standards and the tests stated in this section refer to polypropylene containers, which can be made from homopolymer or copolymer and are used to package DSD and LODP. In as much as stability studies to determine the shelf life of a dosage form packed in a recommended polypropylene container have been made, any other polypropylene container meeting the above description can be used to pack the dosage form for the stated shelf life if the above studies are done covering the dosage form stability in the new polypropylene container for the same shelf life.
2.3.2. Infrared Spectroscopy
Continue in the same manner described for Multiple Internal Reflectance. Thus, the specimen’s altered spectrum should only initially contain the major absorption bands and have the same wave numbers as the Homo-polymer Polypropylene RS or copolymer polypropylene standard obtained in this method.
2.3.3. Differential Scanning Calorimeter
Follow the instructions provided for Thermal Analysis. The temperature of the melting peak in the thermal analysis graph is within a 12.0° range compared to that of USP Homo-polymer Polypropylene RS.
2.3.4. Heavy Metals
Prepare samples of specimens for these tests according to the Physicochemical Tests, Testing Parameters, and Sample Preparation. As for the extracting medium, a portion of 60 cm² is to be taken for every 20 mL, irrespective of the layer’s thickness.
2.3.5. Nonvolatile Residue
Comply with the non-volatile residue test described under Physico-chemical tests; before the final step, Blank should be the same solvent as used in all test conditions. Thus, the value of the Sample preparation should not be higher than the Blank by more than 10. for when using water at 70° as the extracting medium, not more than 0 mg; for when using alcohol at 70° as the extracting medium, not more than 60 mg; and when using hexanes at 50° as the extracting medium, not more than 225 mg. Containers can also need to provide these Nonvolatile Residue requirements for all the stated extracting media.
Table 2.
Limits and Appropriate temperature conditions.
Table 2.
Limits and Appropriate temperature conditions.
| NMT |
Temperature |
| 10.0 mg |
70° |
| 60.0 mg |
70° |
| 225.0 mg |
50° |
2.3.6. Components Used in Contact with Oral Liquids
Proceed as directed for Physicochemical Tests and Buffering Capacity.
2.4. PET Bottles and PET-G Containers
The standards and tests in this section relate to Polyethylene Terephthalate (PET) and Polyethylene Terephthalate-G (PETG) bottles, which are used in the packaging of liquid oral Dosage forms. If the stability studies have been carried out regarding determination of shelf lives of a specific liquid oral dosage form in a bottle that meets the condition of this section for the PET or PETG bottles or either of them than any other PET or PETG bottle satisfying these conditions can be used for packaging the specific dosage form given that adequate stability programs have been carried out to ensure that the identity, strength, quality, and purity of the specific dosage form are not compromised In other words, the PET or PETG bottles to be used in dispensing a specific pharma liquid OD can must be subjected to certain tests.
2.4.1. Infrared Spectroscopy
Go to the next step according to the Multiple Internal Reflectance procedure. The corrected spectrum of the specimen should contain only the first four strong wave numbers as in the spectrum of the USP Polyethylene Terephthalate RS or the USP Polyethylene Terephthalate-G RS obtained in the same way.
2.4.2. Differential Scanning Calorimetry
Proceed to the Thermal Analysis. The melting highest temperature derived from the specimen’s thermal analysis curve should not deviate from the reference by more than 4.0° from the value specified for polyethylene terephthalate in the USP Polyethylene Terephthalate RS. For polyethylene terephthalate, the specimen’s thermal analysis curve should resemble that of the reference standard USP Polyethylene Terephthalate RS. The thermal analysis curve of the specimen for polyethylene terephthalate-G may have a different pattern from the USP Polyethylene Terephthalate-G RS thermal analysis curve, but the sighting of the melting peak temperature from the specimen’s thermal analysis curve may not be different by more than 6.0°.
2.4.3. Colorant Extraction
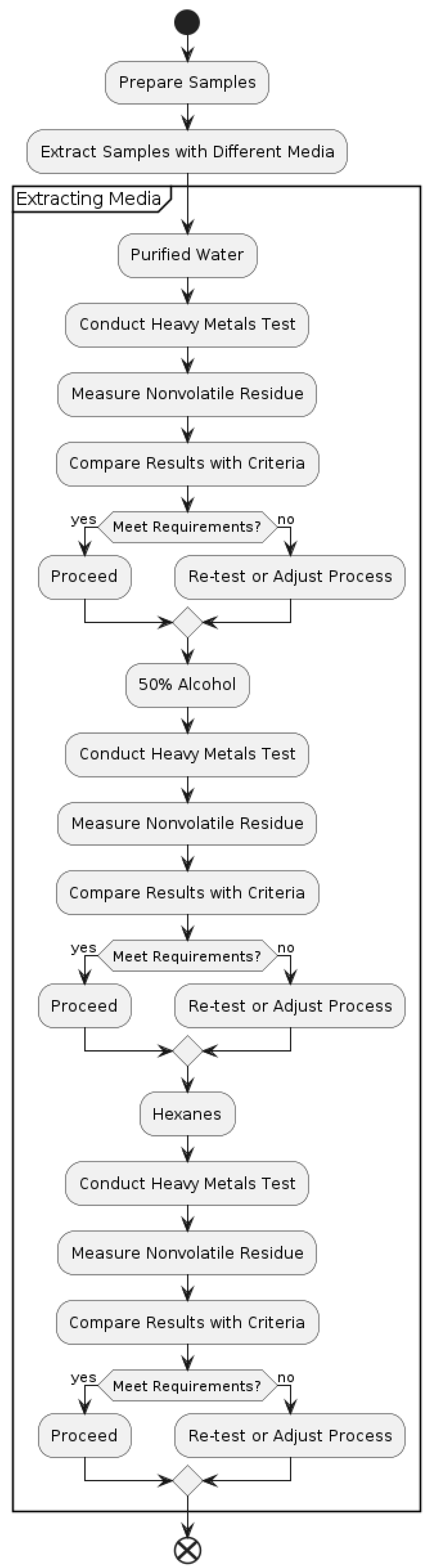
Select three test bottles. Take a small piece from the sidewall of one bottle that is not very curved and, using the utility knife, sharpen the edges to match the spectrophotometer’s sample container. Take the visible spectrum from 350 n/m to 700 n/m for collecting the visible spectrum of the sidewall. Estimate the wavelength number to the nearest two. Move to labeling and filling the other two test bottles with 50% alcohol for the PET bottles and 25% alcohol for the PETG bottles. Secure the bottles with moisture-vapor-proof material, which is aluminum foil and put on caps properly. By rendering the end of the glass bottle of similar volume to the test bottles, pour an equal volume of solvent in the bottle, cover the opening of the bottle with any watertight material like aluminum foil and seal the bottle with a suitable pull. Place the test bottles and the glass bottle into the water and keep all of them at 49° for 10 days. Take out the bottles and let them come to ambient temperature. Record the absorbance in 5 cm cuvettes of the test solutions at the wavelength max of the blue complex, utilizing the glass bottle’s solvent as the empty space according to Ultraviolet-Visible absorbance spectrum Spectro photometry (857H). The obtained absorbance values should be less than 0. 006 for solution A and 005 for solution B in the low contrast test, while for the high contrast test, you get 0.01 for both test solutions.
3. Heavy Metals, Total Ter phthaloyl Moieties, and Ethylene Glycol
3.1. Extracting Media
Purified Water
50 percent alcohol:
Prepare a solution by adding 125 mL of alcohol to 238 mL of water and thoroughly combining the two substances.
25 percent alcohol:
Prepare a solution by diluting 125 mL of alcohol-containing 50 percent concentration with water to a total volume of 250 mL, then thoroughly combine the components.
n-Heptane
3.2. General Procedure
Note: For this procedure, a 50% alcohol solution shall be used for PET bottles, while for the PETG bottles, a 25% alcohol solution of extraction shall be used. Place enough test bottles to 90% of the nominal capacity to obtain at least 30 mL of the extract. For the media blanks, have an equal number of glass bottles with Purified Water as what you used for the 50% alcohol or 25% alcohol and a separate set with n-heptane. Screw the seals on tightly, and the bottles must be sealed with a very progressive barrier, such as aluminum foil. Place the test bottles and the glass bottles in water at 49° for 10 days. After this, take the bottles out and let them come to room temperature after decanting or otherwise. Extracts should not be moved to other receptacles, either at the same or a different time that the extracting media samples are handled.
3.3. Heavy Metals
Remove 20mL of the Purified Water extract from the test bottles. Pipette the extract, if required, into the first of two matched 50 mL pre-rinsed colour-comparison tubes and await the balance. Negative results follow this in the presence of sodium bicarbonate potassium iodide, and to test for Ethylene Glycol, use this Purified Water extract. Bring the pH of the extract to a range of 3.0 and 4.0 with 1N acetic acid or 6N ammonium hydroxy aliquoted and checked using short-range pH paper. Finally add distilled water to the mark and make it to a volume of 35 mL and mix well.
As to the second color-comparison tube, put into 2 mL of fresh standard lead solution, made by dilution of acetic acid, 1 N, and subjected to the physicochemical tests for heavy metals, as described above. The final pH value should be within the range of 3.5 to 3.8: to the mixture, add 20 mL of Purified Water extract. 3.0 and 4. 0 using short-range pH paper. Add distilled water into the volume up to about 35 mL and mix. Add 1. 2 mL of thioacetamide–glycerin base TS and 2 mL of 0.03M hydrochloric acid, pH 3. 5 ml. of the acetate buffer to each tube (Physicochemical Tests, Heavy Metals), add water to each tube up to 50 ml. and mix. The color obtained for any of the samples within 10 minutes of observation in the tube having the extract of the Purified Water from the test bottles should not be darker than the color in the tube containing the Standard lead solution when viewed from the above over the white surface. This makes sure that the lead detection limit is 1 ppm in the extract.
3.4. Total Terphthaloyl Moieties
Using the matching Extracting media as the blank, get the absorbance of the 50% alcohol or 25% alcohol extract in a 1cm cell at the wavelength of maximum absorbance, which is approximately 244 nm. According to the UV analysis, it is evident that the absorbance of the extract was NMT 0. 150, which is 1 ppm of total Ter phthaloyl moieties – These units refer to the percentage amounts of total Ter phthaloyl moieties of the catalyst.
Using n-heptane as the blank, find the absorbance of the n-heptane extract in a 1-cm cell at the wavelength of maximum absorbance, which is approximately 240 nm: according to the procedure, it is required that the absorbance of the extract is NMT 0. 150 as to one part per million of total Ter phthaloyl moieties to the weight of the nylon 6 material.
3.5. Ethylene Glycol
Dissolve the entire 125 milligrams of periodic acid in 10 milliliters of distilled water.
Gradually, while stirring, dissolve 50 mL of water and then 50 mL of sulfuric acid after that, beat the mixture to let it cool down to room temperature.
This is done by disowning 0 of the solution. A solution can be made by dissolving this in 1000ml of the solution. 100 mL of 1g/mL sodium bisulfite solution was prepared by dissolving 1g of sodium bisulfite in 10 mL of distilled water, with a validity of one week.
3.6. Disodium Chromotropate Solution:
Mix disodium chromotropate 100 milligrams in a solution of sulfuric acid 100 milliliters. Make sure that this solution is protected from light and is used within a week because, after that, its properties may change.
To prepare a standard ethylene glycol solution, accurately measure a certain volume of ethylene glycol and dissolve it in water; further, dilute the solution gradually and, if necessary, gradually to obtain a known concentration of approximately 1 µg/mL.
Purify water by using the medium extracting Extract and wash your face with water obtained from it.
Transfer 1. 0mL of the Standard solution into a 10mL volumetric flask. Similarly, transfer 10 mL of the Test solution into the second 10 mL volumetric flask using the pipette. Transfer 1. Hence, transfer 0 mL of the Purified Water Extracting medium into another 10 mL volumetric flask and label it as the 50 µg/mL stock solution.
In the first flask, mix 100 µL of Periodic acid solution gently. Allow it to stand for 1 hour before adding 1mL of Sodium bisulfite solution to each flask and stir. Put into each flask 100 µL of Disodium chromotropate solution and mix.
Note: After the addition of the Disodium chromotropate solution, all solutions should be analyzed within 1 hour.
Stir slowly, then add 6 mL of sulfuric acid to each flask. Let the solutions cool to approximately 25 °C.
Caution: If the concentrated sulfuric acid is diluted, heat is produced to the extent that the solution might boil, liberating sulfur dioxide gas. Maintain safety measures like using a fume hood.
3.7. Prepare for UV Analysis:
In 1-cm cells, measure the absorbances of the Standard and Test solutions at the wavelength of maximum absorbance, which is 575nm, as mentioned in reference number 857. Select Purified Water Extracting medium as a blank for a water mixture. This means that the absorbance of the Test solution should not be higher than the one of the Standard solution which is 1 ppm of ethylene glycol.
4. Test Methods
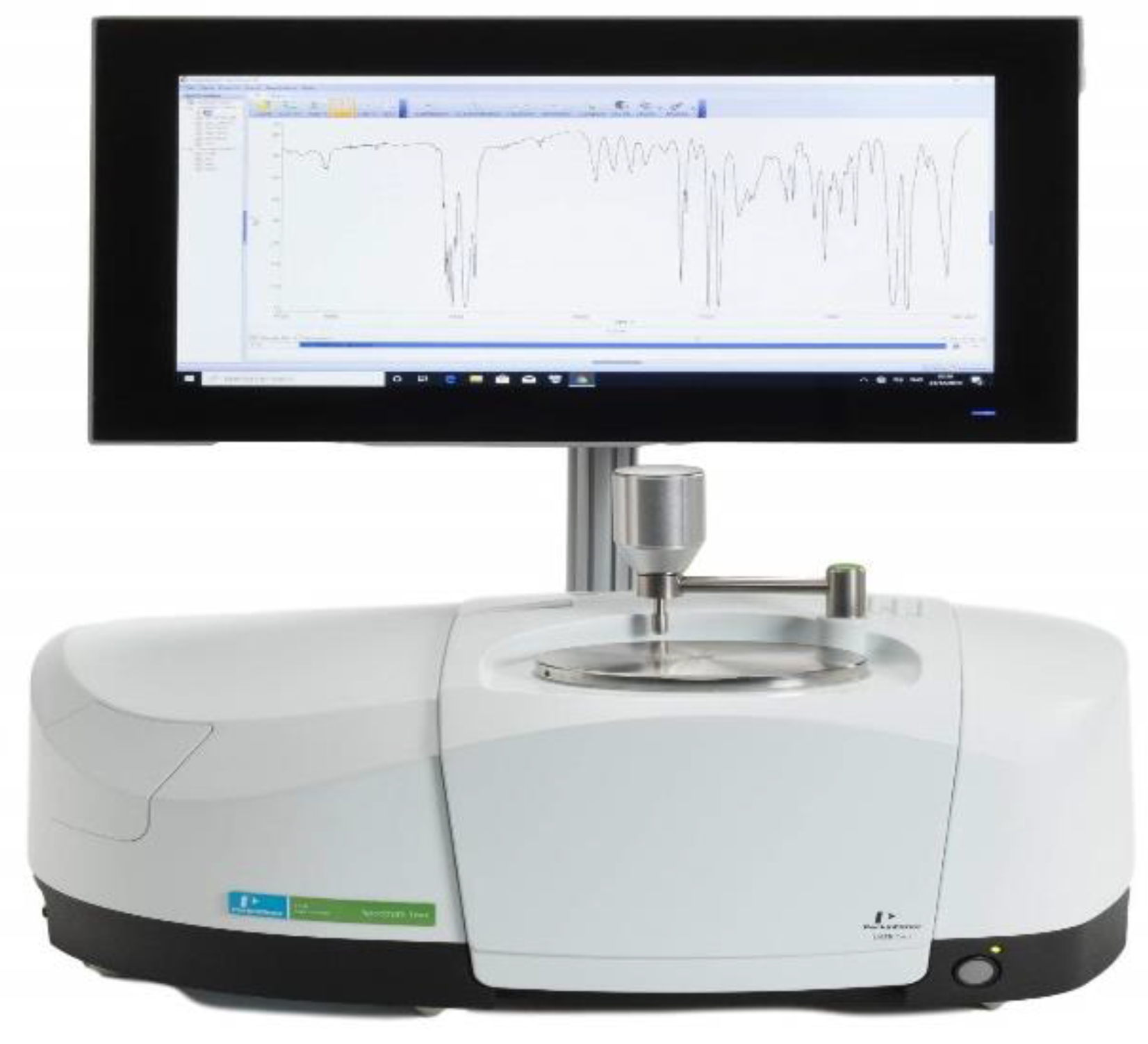
4.1. Multiple Internal Reflectance
4.1.1. Apparatus
Place an IR spectrophotometer with a multiple internal reflectance accessory-ready to obtain a spectrum that the blank spectrum should correct.
Figure 1.
FTIR Spectrophotometer.
Figure 1.
FTIR Spectrophotometer.
4.1.2. Specimen Preparation
The two thin strips of Teflon should be cut into forms of a size slightly lesser than the thickness of the average wall of the container. These strips should be trimmed so that they can easily slide into the several internal reflectance accessories. Clean the strips to ensure that no traces of substance that was measured remain on the strips; use dry paper to remove any substances; then use a piece of clean, moistened soft cloth with methanol and clean the strips; the strips should be allowed to dry. See to it that the specimens are well seated on the support for proper contact to be made. If needed, before they are mounted on the plate, heat them in high pressure of approximately 15000 psi and slightly above, at about 177°C to make them extremely thin, uniform films.
4.1.3. General Purpose
Place the multiple internal reflectance accessories on the specimen beam on the infrared spectrophotometer after cleaving and mounting the specimen segments on it. Adjust the specimen and accessory mirror settings to maximize the % transmittance of the reference beam without the use of lagging. In the case where the accessory has a double-beam instrument, tilt the intensity of the reference beam once the sample is placed on the accessory to make sure that there is full-scale deflection while scanning. Collect the IR spectrum of both polyethylene and polypropylene in the region of 3500 to 600 cm⁻¹ for PET and PETG in the range of 4000 to 400 cm⁻¹.
4.2. Thermal Analysis
If the test specimens are individually wrapped in plastic and weighed to about twelve milligrams each, put them in the test specimen pan. Make sure that the intermediate region of the pan and the side of the thermocouple come into accurate contact with each other. Perform thermal analysis under nitrogen using the following heating and cooling conditions, ensuring the equipment used is capable of these determinations: Thermal Analysis 〈891〉.
4.2.1. For Polyethylene
Find out the thermal analysis curve under nitrogen in the range of 40 and200° at the heating rate of 2 and 10°/min and cooling rate of 2 and 10°/min to 40°.
4.2.2. For Polypropylene
Identify the thermal analysis curve under nitrogen in the temperature range of RT to the melting point. 30°C. Stake at 37°C for 10 min and then cool and hold at a rate of 10° to 20°/min to 50° below the peak crystallization temperature.
4.2.3. For Polyethylene Terephthalate
First, heat the specimen from room temperature to 280° at the heating rate of about 20°/min, hold time 1 min, and then cool along with quenching the specimen to the room temperature and again heat it to 280° at a heating rate of about 5°/min.
4.2.4. For Polyethylene Terephthalate-G
Heat the specimen from room temperature to about 120° in a continuous manner at the heating rate of approximately 20° per minute. Place the specimen at 120° for 1 min and then rapidly move the specimen into the quenchant and again heat the specimen at 120° at about 10°/min.
4.3. Physicochemical Tests
The plastic material itself must be extracted for the purpose of conducting the ensuing tests on the physical and chemical properties of plastics and the extracts they produce. The specified amount of plastic must be used. It should also be possible to extract the designated surface area at the temperature that is needed.
5. Testing Parameters
5.1.1. Nonvolatile Residue
As will be seen in the specific procedures below, unless otherwise stated, use Purified Water as the Extracting medium and should be heated to 70° C when preparing the sample.
5.1.2. Blank
Where a blank is indicated in the tests that follow, the use of purified water was required.
5.1.3. Apparatus
It is recommended that the water bath be utilized with the Extraction Containers as outlined in Biological Reactivity Tests In Vivo 〈88〉, Classification of Plastics, Apparatus. Continue as mentioned in steps in the first paragraph of Classification of Plastics, Preparation of Apparatus. [Note: It is not necessary for the containers and equipment to be sterilized.]
Figure 2.
Temperature controlled water bath.
Figure 2.
Temperature controlled water bath.
5.1.4. Sample Preparation
Taken from a homogenous plastic specimen, take a portion for each 20. 0 mL of Extracting medium, for 60 cm2 of each side, which in turn is parceled out into strips of approximately 3mm in
width and as close to 5cm in length as possible. Pour the subdivided sample into a 250 ml graduated cylinder with a glass stopper of Type I glass that already contains about 150 ml of purified water. Stir the mixture for about 30 s, and then decant the liquid, discard it, and wash these structures a second time.
5.1.5. Sample Preparation Extract
Pipette the prepared Sample preparation to a suitable extraction flask, and then add the necessary quantity of Extracting medium. Obtain by heating in a water bath at the temperature indicated for the Extracting medium for 24 hours Further cooling should not be below 20°. Transfer 20 mL of the prepared extract into another suitable container as a portion. [NOTE—Use this portion in the test for Buffering Capacity.] As soon, drain the remaining extract to another, clean container and then cap it.
5.2. Nonvolatile Residue
Transfer, in reasonable amounts, 50.0 mL of the Sample preparation extract to a suitable, tared crucible (fused-silica is recommended, and if available, it should be acid-washed). Evaporate the volatile matter under the steam bath. Similarly evaporate 50.0 mL of the Blank in a second crucible. [NOTE—Inspect the crucible frequently throughout the evaporation and drying phase if an oily residue is anticipated and lower the heat if the oil seems to seep down the crucible’s sides.] Dry at 105° for 1 h: The value which should be obtained from the Sample preparation extract – the value from the Blank should not be more than 15mg.
5.3. Residue on Ignition 〈281〉
[Note: When the Nonvolatile Residue test result is NMT 5 mg, this test cannot be required.] Using additional sulfuric acid if needed but incorporating the same amount to every crucible, move forward with the residues gathered from the sample preparation extract and from the Blank in the test for Nonvolatile Residue above. The difference between the amounts of leftover on the ignition of the Sample preparation extract and the Blank shall not exceed 5 mg.
6. Heavy Metals
6.1.1. Lead Nitrate Stock Solution
Dissolve 159. 8 mg of lead nitrate in 100 mL of water, which is treated with 1 mL of nitric acid,
and after that, the volume is constituted with distilled water to the final volume of 1000 mL. This
solution should be prepared and stored in glass receptacles containing soluble lead salts.
6.1.2. Standard Lead Solution
You should dilute it on the day of use as a ratio of 10.0 mL of Lead nitrate stock solution with
water to 1000 mL. One milliliter of the Standard lead solution has an equivalent of 10 μg of lead. The
method of comparison solution prepared in 100 μL of Standard lead solution with 1 gm-substance
sample yields one ppm (part per million) of lead in the substance being tested.
6.1.3. pH 3. 5 acetate buffer
Dissolve 25.0 g of ammonium acetate in 25 mL of water, and then add 38. The control value for
the volume of 6 N hydrochloric acid. If the solution is still alkaline, adjust with 6 N ammonium
hydroxide or 6 N hydrochloric acid r the pH reaches 3. 5, then add sufficient water to make up the
volume to 100 mL, and then mix.
Transfer 20 mL of the Sample preparation extract, if necessary, filtered through the Centrifuge
tube, into one of two identical chromometer 50 mL tubes, which are designated for color comparison.
Regulate with 1 N acetic acid or 6 N ammonium hydroxide to a pH of 3. 0 and 4. 0. On top of the flask
containing the titrant, mix it gently, add short-range pH paper as the external indicator, and dilute
with water to about 35 mL
Into the secondcolour comparison tube the pipet 2 mL of Standard lead solution and then 20 mL
of the Blank. Subsequently, the samples were adjusted with 1 N acetic acid or 6 N ammonium
hydroxide to a pH of between 3. 0 and 4. 0, employing short-range pH paper as an external indicator,
and with this dilution, the contents to about 35mL and stirred to each tube add 1. Two milliliters of
thioacetamide–glycerin base TS and two milliliters of pH 3. 5 acetate buffer, dilute with water to 50
mL, and mix: When both tubes are seen downward over a white background, any brown color that
develops in the tube holding the sample preparations extract within 10 minutes is not higher than
that of the tube carrying the standard lead solution. (Sample preparation extract 1 ppm of lead).
6.1.4. Buffering Capacity
Dilute the previously collected 20-mL portion of the Sample preparation extract
potentiometrically to a pH of 7 with 0.010 N hydrochloric acid or 0. 0.010N sodium hydroxide
solution as and when needed. Treat a 20mL portion of the Blank similarly: If the same titrant is to be
used for Sample preparation extract and Blank and when the difference is NMT 10 mL If we have to
use, for instance, acid for either the Sample preparation extract or for the Blank but alkali for the
other, the total volume used for both should not be greater than 10.0 mL.
Table 3.
Material of construction and it’s description.
Table 3.
Material of construction and it’s description.
| Material |
Test |
Description |
Criteria |
| High-Density Polyethylene |
Infrared Spectroscopy |
Having corrected the spectrum, it shows only the absorption, and this is possible only at certain wavelengths. |
Matches USP High-Density Polyethylene RS |
| |
Differential Scanning Calorimetry |
The thermal analysis curve area correlates with the range of the temperature at which the material is or goes through the phase transformation with corresponding enthalpy change and melting point. |
Difference from reference standard by no more than 6.0° |
| |
Heavy Metals and Nonvolatile Residue |
Prepare extracts and test the following as stated |
NMT 12.0 mg (water), NMT 75.0 mg (alcohol), NMT 350.0 mg (hexanes) |
| Low-Density Polyethylene |
Infrared Spectroscopy |
There was only significant absorbance at wavelengths which are in the spectrum of the USP Low-Density Polyethylene RS. |
Matches USP Low-Density Polyethylene RS |
| |
Differential Scanning Calorimetry |
Typically known as the thermal analysis curve, the shape and the melting peak temperature are features of the thermal analysis curve. |
Difference from reference standard by no more than 8.0° |
| |
Heavy Metals and Nonvolatile Residue |
Prepare extraction and test as stated |
NMT 12.0 mg (water), NMT 75.0 mg (alcohol), NMT 350.0 mg (hexanes) |
| Polypropylene |
Infrared Spectroscopy |
Major absorption bands in the same wave numbers as USP Homopolymer Polypropylene RS |
Matches USP Homopolymer Polypropylene RS |
| |
Differential Scanning Calorimetry |
Highest temperature on the thermal analysis curve decreasing |
Difference from reference standard by no more than 12.0° |
| |
Heavy Metals and Nonvolatile Residue |
Prepare extracts and test as depicted |
NMT 10.0 mg (water), NMT 60.0 mg (alcohol), NMT 225.0 mg (hexanes) |
| Polyethylene Terephthalate |
Infrared Spectroscopy |
Several strong bands in the regions of USP Polyethylene Terephthalate RS |
Matches USP Polyethylene Terephthalate RS |
| |
Differential Scanning Calorimetry |
The area of the thermal analysis curve and magnitude of the melting peak temperature |
Difference from reference standard by no more than 4.0° (PET), no more than 6.0° (PETG) |
| |
Heavy Metals and Nonvolatile Residue |
Extracting media: distilled water, 50% ethanol, 25% ethanol, n-heptane |
Matches specified criteria for each extracting medium |
Figure 3.
Testing Procedures for Heavy Metals and Nonvolatile Residues.
Figure 3.
Testing Procedures for Heavy Metals and Nonvolatile Residues.
7. USP Chapter 661.2: Plastic Packaging Systems for Pharmaceutical Use
8. Implementation Challenges and Considerations
Since USP Chapters 661 and 671 give detailed rules on testing the quality of the containers and their performance, the requirements’ practical application may be complicated for pharmaceutical producers. Some considerations include the feasibility of testing services, competency in the type of methods to be used, and the evaluation of the results. Finally, innovations in the field of containers and changes in the legislation may require one to reconsider and update the testing methods periodically.
9. Result and Discussion
Pharmaceutical industry can find rich information in Chapters 661 and 671 of USP regarding the quality and performance of the material used in the packaging of drugs. Thus, manufacturers comply with these standards to maintain the reliability and safety of the pharmaceutical products and satisfy the legal norms. It is crucial for the personnel of industries, regulators, and standard makers to carry on with the cooperation to develop and implement even more effective and generalizable guidelines for the frames of the contemporary picture of pharmaceutical production.
References
- D. Giron, Applications of Thermal Analysis and Coupled Techniques in Pharmaceutical Industry, Journal of Thermal Analysis and Calorimetry, vol. 68, no. 2, pp. 335-357, 2002. [CrossRef]
- Giron, D. Thermal Analysis, Microcalorimetry and Combined Techniques for the Study of Pharmaceuticals, Journal of Thermal Analysis and Calorimetry, Volume 56, pages 1285–1304, (1999). [CrossRef]
- <661= Plastic Packaging Systems and Their Materials of Construction, 2017. Online. [Available]:https://www.uspnf.com/sites/default/files/usp_pdf/EN/USPNF/revisions/661_rb_notice.pdf.
- USP <667= Permeation Testing. Online. [Available]:Available]:https://csanalytical.com/functional-physicalperformance-testing/usp-permeation-testing/.
- 〈1661〉 Evaluation of Plastic Packaging Systems and Their Materials of Construction with Respect to Their User Safety Impact, USP 39 page 1827 and PF 40(6) [Nov.–Dec. 2014]. https://www.usp.org/sites/default/files/usp/document/workshops/1661_evaluation_of_plastic_packaging_systems_and_their_materials_of_construction_with_respect_to_their_user_safety_impact_pf_42.pdf.
- Jenke, Dennis R., Daniel L. Norwood, and Desmond G. Hunt. “USP plastic packaging general chapters: an overview.” Report 39 (2013): 1-19.
- <661= Containers, USP29. http://ftp.uspbpep.com/v29240/usp29nf24s0_c661_viewall.html.
- USP compared with USP and USP , 2021. https://www.smithers.com/resources/2021/august/usp-661-compared-with-usp-661-1-and-usp-661-2. 2021.
- Bottom, Rod. Thermal analysis: Solving problems in the pharmaceutical industry, Pharmaceutical Technology Europe, vol. 13, no. 11, Nov. 2001, pp. 37+, 2001. https://go.gale.com/ps/i.do?id=GALE%7CA81170313&sid=googleScholar&v=2.1&it=r&linkaccess=abs&iss
n=17537967&p=AONE&sw=w&userGroupName=anon%7E9f9e070f&aty=open-web-entry. 1753.
- Bernard Roullet, C. R. E. M., B. France, and C. R. E. M. Olivier Droulers. “Pharmaceutical packaging color and drug expectancy.” Advances in consumer research 32 (2005): 164-171. https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=501ffefac6e85acd49f4cff27e0dabc15db5eda0.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).