1. Introduction
Breast ptosis (BPt) is characterized by the downward nipple-areola complex displacement below the inframammary fold [
1]. The most important risk factor for developing BPt is ageing a natural phenomenon impacting the breast’s soft tissues as a consequence of a decrease in collagen synthesis via fibroblast senescence [
2] which results in both a reduction in skin elasticity and progressive weakness in support fibrous structures. In addition, low estrogen levels during menopause [
3] contribute to mammary adipose tissue reduction driving skin ageing progression [
4]. Other factors like important weight loss, macromastia, pregnancy, and both alcohol and tobacco consumption products interact with ageing and genetic factors changing the breast tissue architecture[
5], impacting the well-being and quality of life of the affected women [
6].
BPt unfolds throughout distinct stages of tissue change involving skin stretching and laxity within the ductal structures and breast-supporting ligaments [
7]. This progressive weakening sets the stage for the subsequent enlargement of mammary parenchymal volume [
8]. As the breast volume increases, support structures become ineffective and compromised, leading to typical downward breast displacement and redundant skin fold formation [
9]. On the other hand, BPt can also manifest within the context of parenchymal tissue reduction, such as that following significant weight loss [
10]. In these instances, the relative abundance of skin compared to the diminished parenchymal volume results in skin redundancy and ptosis [
11]. In this regard, a notable shift in MP demographics and associated factors has emerged in recent years. This trend is particularly evident in the increased number of mastopexies performed in younger women coinciding with the rise in anti-obesity drug prescriptions, including liraglutide, semaglutide, and tirzepatide, at least in the United States [
12,
13]. These medications induce a drastic reduction in total body fat, including mammary adipose loss and support structure weakening contributing to flaccidity and ptosis development in younger women known in the media as Ozempic breast [
14].
In this regard, mastopexy, the surgical technique aimed at restoring the breasts to their youthful position is a cornerstone of breast aesthetics. When this procedure involves glandular tissue excess removal is termed reduction mastopexy. Both procedures seek to enhance the female body’s aesthetics by repositioning the NAC to an ideal location, typically around 19-21 centimetres from the sternal notch [
4]. On the other hand, in patients with gigantomastia, breast reduction not only improves overall aesthetics but also enhances quality of life by alleviating symptoms associated with excessive breast weight like neck, head, shoulder, and back pain, among others [
2].
Several well-established breast pexy surgical techniques have been developed for correcting the NAC position, all of which inevitably involve a periareolar incision [
15]. This approach entails the areolar plaque transposition upward, acknowledging that hypertrophy is invariably accompanied by a degree of ptosis [
16]. The skin excess removal dictates the resulting scars, exemplified by the “anchor-shaped” scar, characterized by a long horizontal scar extending along the inframammary fold [
17]. This pattern, however, has the midline proximity drawback, increasing the hypertrophic scar risk. To minimize the conspicuous horizontal scar associated with traditional NAC repositioning techniques, several authors have proposed alternative approaches like the “inverted T” and “short inverted T” scar techniques aim to shorten the horizontal incision, while the radical horizontal segment elimination gives rise to the “L” and “J” scar techniques[
18,
19,
20]. These latter techniques utilize the external branch and vertical segment to accommodate the length discrepancies between the two edges [
21]. A further refinement involves the “single vertical scar” technique, which eliminates the horizontal incision [
22]. However, this approach presents the challenge of accommodating excess skin while simultaneously attempting to remove subcutaneous adipose tissue and retract the liberated layers [
22]. The resulting vertical scar exhibits maximal puckering, particularly concentrated in the lower portion (“pouch”), leading to a protracted maturation process [
23]. Lastly, to eliminate both the horizontal and vertical scars, the periareolar technique has emerged as a refined approach [
24]. This technique involves the skin excess absorbing within the periareolar scar, thereby exposing it to eccentric forces that can lead to the expansion of the areolar plaque and scar [
25]. In consequence, breast pexy, reduction mammoplasty, and breast implant placement procedures are often associated with a range of complications, particularly in the realm of wound healing [
26]. These complications can include fat necrosis, skin loss, infections, seromas, hematomas, suboptimal aesthetic outcomes, nipple sensitivity reduction, and even nipple-areola complex loss, especially when traditional techniques are employed [
27].
In response to these challenges, the past decades have witnessed the emergence of numerous techniques aimed at refining breast surgery procedures. One such advancement is laser-assisted mastopexy, which has evolved as an improvement over traditional liposuction techniques [
28]. This modality has gained widespread adoption in other plastic surgery areas like body contouring and laser-assisted lipolysis because of its efficacy and safety, resulting in reduced surgical time and a lower incidence of complications [
29,
30,
31]. As has been seen in other common procedures in plastic and cosmetic surgery, laser energy may improve breast surgical performance in technical aspects previously considered challenging, such as non-visible scars, deep-seated fat dissolving with minimal pain, rapid recovery, lesser blood loss and hematoma formation, and excellent skin retraction due to collagen synthesis stimulation [
32].
This study aimed to evaluate the outcomes of 231 laser-assisted mastopexy procedures using a novel low-intensity multifrequency laser technology to maximise the appropriate positioning of the sternal notch-nipple complex (SNNC).
2. Materials and Methods
A non-experimental longitudinal retrospective cohort study was done on 231 women undergoing a scarless low-level laser-assisted mastopexy technique at the Clínica de Obesidad y Envejecimiento SAS, Bogotá, Colombia, assessing sternal notch-to-nipple distance (SNND), and the breast ptosis degree was by the Regnault classification system at the first, second, and third postoperative month. Additionally, the incidence and nature of complications within the first 30 days post-surgery were evaluated.
This study adhered to the legal Colombian framework for confidentiality and information privacy (Law 1581/2012 and Decree 1377/2013). All participants were informed about the surgical intervention’s nature, purpose, and technique, as well as its advantages, risks, and potential complications. The medical team addressed questions and concerns regarding the procedure using technical yet easily understandable language. All participants signed an informed consent form allowing participation in this research and clinical data use for research purposes, ensuring that individual information would not be disclosed. The anonymised dataset is available at the Harvard Dataverse Scientific repository [
33].
All patients underwent a standardized three-stage protocol:
2.1. Preoperative Stage
Each patient included in the study attended an initial medical which included measuring the SNND distance with a graduated measuring tape, both for the left and right breasts. Subsequently, breast ptosis was stratified according to the Regnault classification [
34]. All participants underwent laboratory tests consisting of complete blood count, creatinine, fasting blood glucose, urinalysis, cholesterol, triglycerides, prolactin, and thyroid-stimulating hormone. Cardiovascular pre-operatory consisted of a 12-D resting electrocardiogram for those patients under 40 years old, and for those over 40 years a stress test and a complete clinical evaluation by a cardiologist. Finally, a bilateral breast ultrasound was done on all participants.
A second medical consultation was held to review and discuss the laboratory and clinical test results to decide whether they were suitable for surgery or if prior treatment of any medical condition was required. If the patient was medically apt for surgery, a pre-surgical protocol was initiated with a dermal surface GaAIAs (semiconductor) 650-670 nm red laser application for 1 hour in each breast, three days before surgery. In addition, oral antibiotic therapy was indicated starting 2 days before surgery and continued for 14 days after surgery (Ampicillin/Sulbactam, 750 mg b.i.d orally for 16 days + metronidazole, 500 mg t.i.d orally for 7 days or Levofloxacine 500 mg b.i.d orally for 16 + metronidazole 500 mg t.i.d for 7 days in the case of beta-lactam allergy or oral intolerance). All the patient was evaluated by the anesthesiologist before the procedure.
2.2. Surgical Stage
2.2.1. Patient Marking
Given that the procedure involved lifting in three main areas, each breast was divided into three zones:
Zone 1 or Thoracic Zone: This region extends from the inferior border of the clavicle to the superior breast border. Internally it is delimited by the sternum border and externally by the anterior axillary fold.
Zone 2 or Axillary Zone: This encompasses the entire axillary area, from the anterior axillary fold, the entire axilla, and the lateral border of the breast to the 5th intercostal space, including the entire back area.
Zone 3 or Breast Zone: This comprises the breast itself, extending from the inferior mammary fold to the limits of the other two zones.
All three zones include skin, subcutaneous cellular tissue, mammary glands, ligaments, and muscle fascia. These are the layers of the skin up to the muscle where the laser can be incised. Once the marking was completed, the main surgeon proceeded to confirm both, the SNND and MP by the Regnault classification system for the right and left breast.
2.2.2. Anesthesia and Preparation
With the patient under an 8-hour fasting regimen, general anaesthesia with deep sedation was administered. The areas to be intervened were then antiseptically prepared, and sterile drapes were placed to formally initiate the surgery. The surgical procedure consisted of the following stages:
2.2.3. Breast Infiltration with Tumescent Solution
Two small incisions of 2 mm were made with an # 11 scalpel: one at the upper edge of the breast at the anterior axillary line level and a second one at the lower breast edge at the anterior axillary line for both the right and left breast. This step was followed by a # 4 atraumatic cannula insertion and an intradermal infiltration with a solution prepared with one adrenaline ampoule diluted in 1L of 0.9% NaCl solution. Each breast was perfused in a hyperhumid infiltration pattern by the injection of 2L of solution in small to normal-size breasts and 3L in the case of gigantomastia.
2.2.4. Laser-Assisted Lipolysis Mastopexy
A laser lipolysis device (Lipolaser LPL9002™, Colombia) was employed throughout the procedure. This low-power cold laser device has wavelengths of 532, 650 and 980 nanometres. This equipment complies with international safety standards for electromedical devices (IEC 601-1) and laser equipment (IEC 825). Each multifrequency low-power laser fibre was placed within a 1.2 mm calibre atraumatic 30 cm long cannula. The surgical procedure usually lasts 40 to 60 minutes approximately and all regions undergo the same four-step technique as follows:
The first laser applied was the 532 nm and 900-milliwatt green laser which produces a vasoconstrictive effect ensuring little or no blood loss. The cannula was inserted through the two previous incisions followed by slow forward and backward movements in the mid-thickness of the flap, in each region for one to three minutes.
The second laser applied was the 650 nm red laser. The primary function of this laser is to induce adipocyte lysis, leading to the release of triacylglycerides in areas targeted for fat extraction, specifically in zones 1 and 2. If breast reduction is the intended procedure, this laser should be already applied in Zone 3, which encompasses the breast tissue itself. In general, the exposure time is maintained until the fat is adequately liquefied. The laser exposure time will be sufficient enough to achieve fat dilution perceived by fat consistency changes by palpation (from a solid to a liquid phase) and the absence of resistance to the laser cannula passage indicates complete adipose tissue liquefaction. Once the fat was adequately liquefied, it was meticulously aspirated mirroring the laser application sequence, ensuring removal throughout the entire thickness of the flap by slow, controlled movements, using straight and curved cannulas of 5, 4, or 3 mm in diameter connected to a suction device (Wells Johnson Co., Tucson, AZ, USA). In general, this process is minimally traumatic and results in the accumulation of liquefied, yellowish fat with minimal or no blood.
Finally, a 980 nm and 900-milliwatt infrared laser was applied for 5 minutes in each zone into the subdermal space to promote skin retraction. In this regard, Zone 2 is particularly responsive to laser-induced retraction. The rationale of these specific steps relay in that Zone 1 and Zone 2 must be left free so that Zone 3 remains unanchored and can be moved. This process facilitated the entire breast mass manipulation until a correct position was achieved. In the case of laser-assisted lipolysis mastopexy with implant placement, the prosthesis was placed after laser therapy through sub areolar incision, and then, the implant was placed in a retromuscular place.
Table 1.
Lipolaser LPL9002™ main features.
Table 1.
Lipolaser LPL9002™ main features.
| |
Laser features |
Effect |
| Red output |
Laser type: GaAIAs (semiconductor)
Wavelength: 650-670 nm
Beam diameter at focal point: 3 mm |
Fat dilution |
| Infrared output |
Laser type: GaAIAs (semiconductor)
Wavelength: 980 nm
Beam diameter at focal point: 3 mm |
Skin retraction |
| Green output |
Laser type: DPSS
Wavelength: 530
Beam diameter at focal point: 3 mm |
Vasoconstriction |
2.2.5. Postoperative Follow-Up
After the surgical procedure, immediate lymphatic drainage is performed, and the breasts are covered with a compressive bandage made of layered gauze covered with Micropore® tape. The patient is fitted with a high-pressure bra for procedures performed without prostheses and a low-pressure bra for procedures performed with prostheses.
The patient was transferred to the recovery room and vital signs (blood pressure, saturation, heart rate, respiration) were monitored for 4 hours. A liquid food tolerance test was performed, and ambulation was initiated during this period. Subsequently, the patient is discharged from the recovery room and is taken to a hyperbaric chamber session (Leader Life ACR 60-72 Monoplace Hyperbaric Chamber, Colombia) for 1 hour. Hyperbaric session produces a very pleasant analgesic effect causing a less painful recovery. Oxygen improves damaged tissue healing by improving oedema and inflammation control. Once the hyperbaric session was completed, the patient was discharged home and began post-surgical care sessions the following day, where hyperbaric treatments, external low-level laser therapy (Lipolaser LPL9002™, Colombia), pressotherapy and a 5-minute drainage routine for five days were performed. The patient underwent daily evaluations for ten days and monthly evaluations for the following three months.
2.2.6. Monthly Follow-Up Visits
Patients are evaluated monthly in consultation to monitor the procedure performed, measuring the SNND distance on both the right and left breasts in each visit.
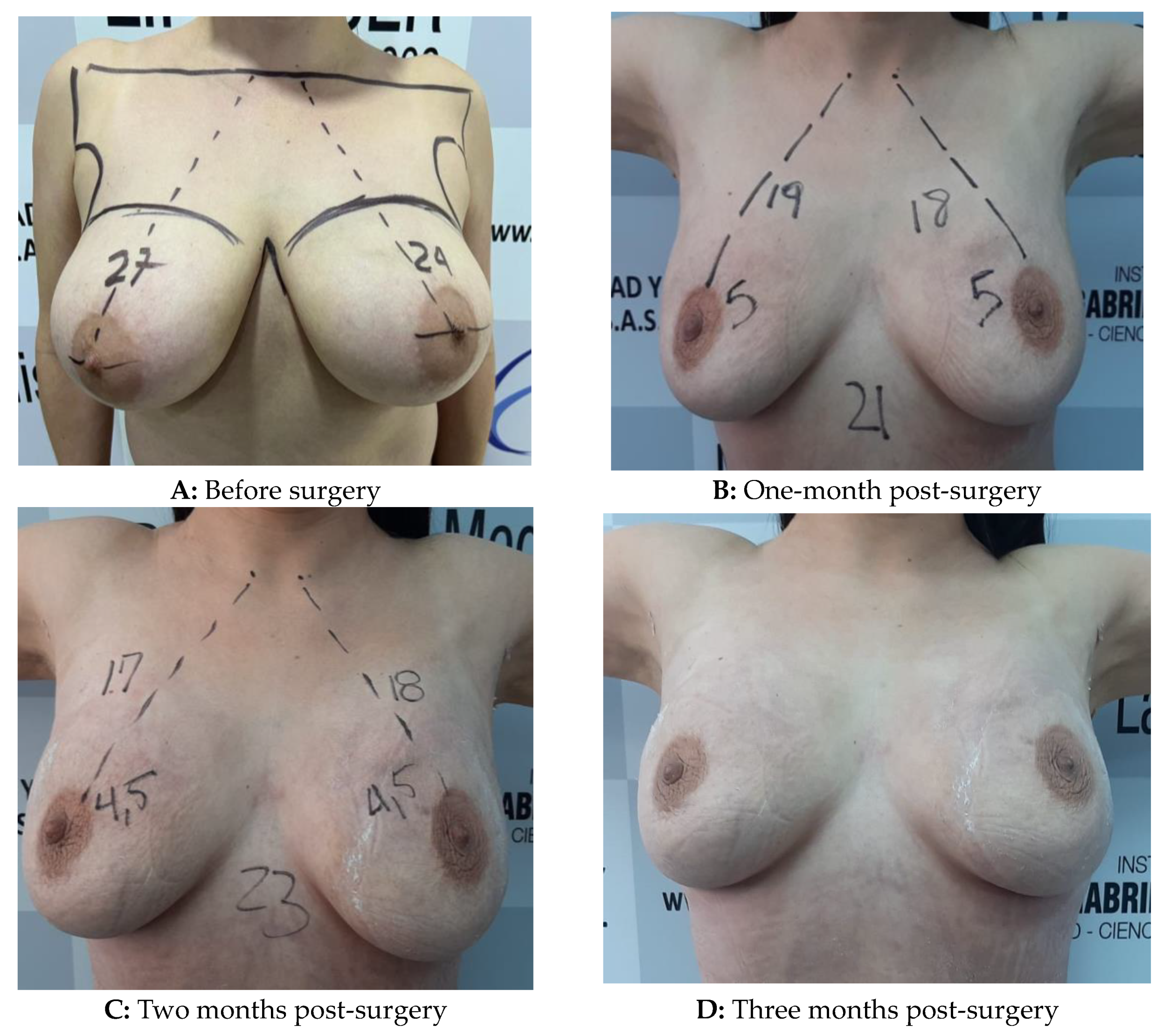
Figure 1.
Low-level laser-assisted breast mastopexy in a 37-Yo woman with grade III ptosis without prosthesis placement.
Figure 1.
Low-level laser-assisted breast mastopexy in a 37-Yo woman with grade III ptosis without prosthesis placement.
2.3. Early Surgical Complications, according to the Clavien-Dindo Classification
A surgical complication was defined as any deviation from the ideal postoperative course that is not inherent to the procedure and did not include treatment failure. This study assessed the surgical complications using the Clavien-Dindo classification system (CDCS) [
35]. CDCS is based on the therapeutic implications of perioperative surgical complications [
35]. This system has been validated in patients undergoing bariatric surgery [
36], abdominoplasty [
37], and lower body contouring surgery [
35,
38], providing a straightforward and objective means to standardize complications based on their severity and resolution. In this regard, the complication type, treatment administered, and the outcome experienced were analyzed and subsequently classified into one of the five categories proposed by the CDCS:
Grade I: Any complication that does not require medical or surgical treatment.
Grade II: Complication that requires pharmacological treatment but not active intervention.
Grade III: Complication that necessitates surgical, radiological, or endoscopic treatment, either without general anaesthesia (IIIa) or with general anaesthesia (IIIb).
Grade IV: Potentially life-threatening complications requiring intensive care, such as single organ failure (including dialysis) (IVa) and multiorgan failure (IVb).
Grade V: Complications resulting in death.
In concordance with the CDCS recommendations, patients with more than one complication were classified based on the most severe complication. For this work, Grades I, II, and IIIa were considered mild, while grades IIIb, IV, and V were considered major complications.
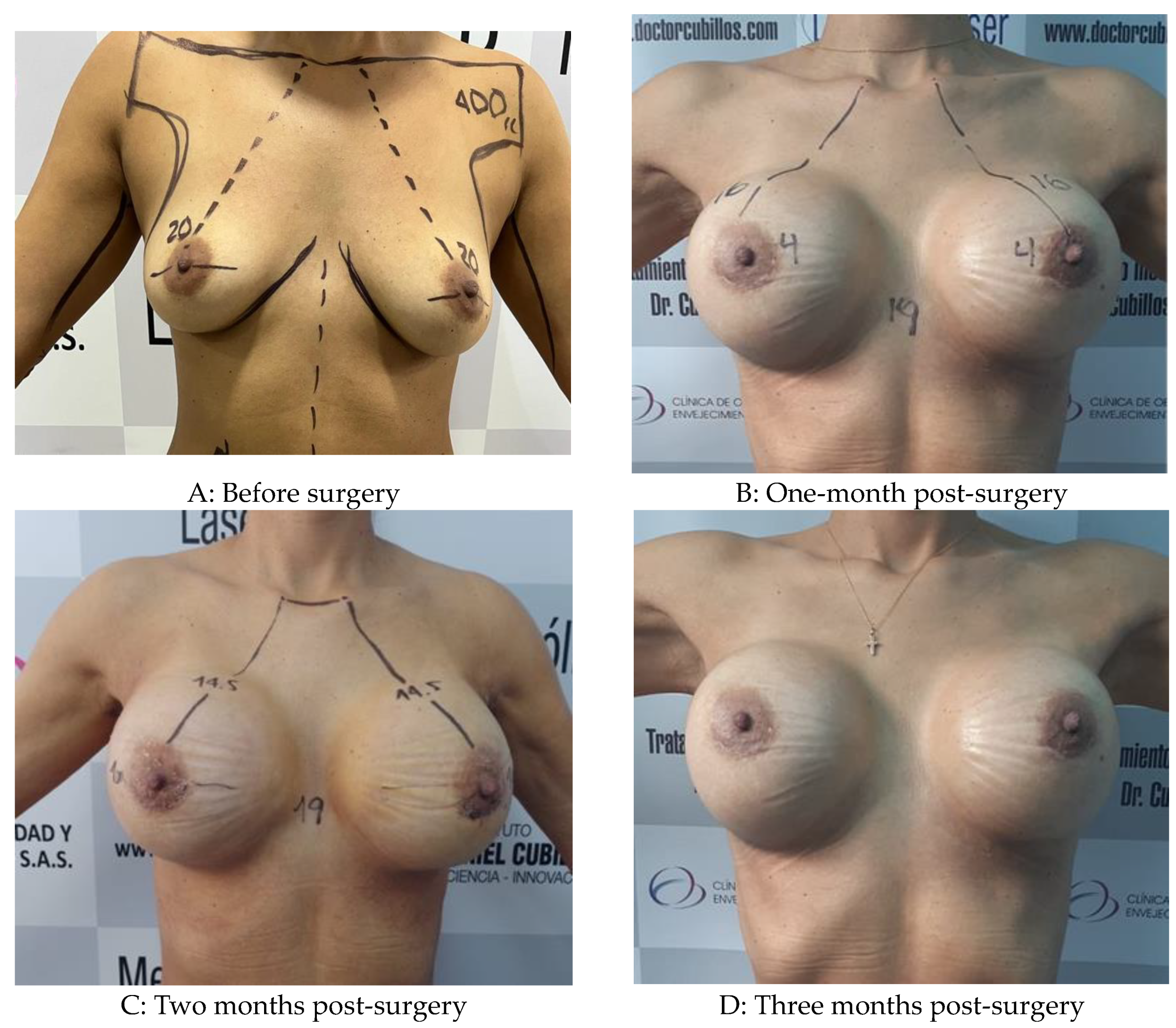
Figure 2.
Low-level laser-assisted breast mastopexy in a 32-year-old woman with grade I ptosis with prosthesis placement.
Figure 2.
Low-level laser-assisted breast mastopexy in a 32-year-old woman with grade I ptosis with prosthesis placement.
Statistical analysis was performed using the R statistical computing environment [
39] running in Jamovi, a free, open-source statistical software program built in R programming language [
40]. Categorical variables were displayed in tables as absolute, relative and cumulative frequencies. Proportion comparisons were made by Pearson’s chi-square test or Fisher’s exact test. The proportion changes in MP degree along time measures were compared with Friedman’s test and the Durbin-Conover post-hoc test for pairwise contrast. On the other hand, quantitative variables were expressed as means ± SD (in parenthesis) or medians, and percentiles as appropriate. If normality and homoscedasticity were met, quantitative variables comparisons were made using Student’s t-tests (for two groups) or repeated measures ANOVA (for more than two-time measures). Welch’s t-test or Trimmed means robust Anova was employed in cases where these assumptions were unmet. A p-value < 0.05 was considered statistically significant.
3. Results
Of the 231 patients, 75% recognized themselves as ethnically mixed, 75% were in the 30-49 years age group, and 75% had some type of excess body weight (42% overweight and 33% obese). Eighty per cent of the patients reported having had at least one child (63%, 1-2 children), while 80% reported breastfeeding their children. Regarding alcoholic beverages and tobacco consumption, 90% of participants did not drink alcohol, and 94% did not smoke. The detailed distribution of these characteristics is shown in
Table 2.
The arithmetic mean of the age was 40.13 (8.71) years with a minimum age of 19 years and a maximum of 59 years. The average BMI of the patients was 28.9 (5.14) kg/m2 with an average excess weight of 16.93 (13.15) kg. The arithmetic mean of the SNND before surgery was 28.26 (3.85) cm for the right breast and 28.11 (3.90) cm for the left breast. The rest of the descriptive statistics are shown in
Table 3 including the percentile distribution of each variable.
When the patients were stratified according to the decision to receive or not to receive breast prosthesis, we found that those programmed not to receive prosthesis (30.03 Kg/m2 vs. 27.14 Kg/m2, p<0.001) and, consequently 20.21 Kg vs. 13.06 Kg, p<0.001. No significant differences were found between these two groups concerning the SNND in the right and left breasts (
Table 4).
Regarding personal pathologic history, a thorough review of the patient’s records revealed that 74% had no significant past medical conditions. However, the remaining 26% of patients presented with noteworthy comorbidities. Specifically, 24% of patients had a history of hypothyroidism, 10% had hypertension, and 10% had depression (
Table 5)
.
Before surgery, as expected, all patients had some degree of breast ptosis with a clear predominance of grade III ptosis in 53% (n=122) of the participants. After mastopexy, a significant variation in the ptosis degree was observed throughout the three months of postoperative evaluation, highlighting that the number of patients without breast ptosis increased significantly to 42% (n=97) in the first month, 58% (n=133) in the second month and 90% [209] in the third month. At the end of the follow-up, no patients with significant ptosis (grade II and III) were found. All these changes were statistically significant alongside all pre-and post-surgical times (
Table 6 and
Table 7).
Table 7 and
Table 8 show the mean and median SNND distances before and at each post-operative evaluation time. Note how the SNND distances were significantly reduced in both breasts for all times examined (p<0.01), appreciating a progressive and sustained decrease of such distance at each post-operative measurement, from 28.26 (3.85) cm to 18.79 (1.95) cm for the right breast and from 28.11 (3.90) cm to 18.64 (2.21) cm (
Table 1). It should be noted that significant differences were found when comparing all SNND measurements at the different observation times in both the right and left breast (p<0.01 in all comparisons).
The incidence of early postoperative complications in this series of patients was 4% (n=222). These complications were represented by two patients (1%) who presented generalized pain and required specialised intervention (Grade II), four patients (1.75%) with necrosis of the areola (Grade IIIb) and three seromas (Grade IIIb) (1.25%) who required surgery under general anaesthesia.
4. Discussion
BPt is characterised by CAP lowering below the breast fold, as well as skin redundancy in the lower breast pole. When the breast’s supporting structures such as pectoral muscles, skin, and connective tissues (Cooper’s ligaments) begin to lose firmness BPt develops. This condition has become an increasingly common concern, in part, because the media portrays women with youthful breast characteristics at any age. While a subset of women may retain a youthful nipple-areola complex (NAC) position throughout their lives, several well-established risk factors contribute to eventual BPt in the majority of the female population.
BPt and breast hypertrophy can cause considerable physical and emotional distress in women, such as low self-esteem, sexual embarrassment and difficulty in sports and exercise. back, shoulder and neck pain, as well as intertrigo. In fact, a study by Ibrahim et al. found that the burden of living with breast ptosis requiring surgical intervention was comparable to that of breast hypertrophy, uni- and bilateral mastectomy, cleft lip and cleft palate [
41]. These data explain why the demand for breast surgery in both men and women is on the rise, including breast augmentation and correction of gynecomastia. According to the ISAPS mastopexy emerged as the fifth most common surgical procedure for women, with a remarkable increase of 31.4% between 2020 and 2021 [
35].
The objectives of mastopexy are breast reshaping, volume redistribution and the NAC reposition, while reductive mastoplasty (with or without pexy) aims to achieve the same aesthetic results adding a breast size reduction for functional purposes [
37]. Both interventions aim to produce an aesthetically pleasing and long-lasting appearance, minimising scarring, improving recovery and preventing complications [
36,
42]. In the realm of ideal breast aesthetics, the nipple should occupy the most prominent position of the breast mound, just superior to the inframammary fold (IMF). Additionally, the breast mound itself should exhibit a superior predominance, exhibited as a well-defined prominence above the IMF on the chest wall. [
26,
43]. However, the method requires a careful balance between an expansive and a reductive force, all in a compromised blood supply environment. Early studies demonstrated the poor predictability of the procedure [
38,
44] and high complication rates, ranging from 8% to 16% [
38,
45] including nipple/skin necrosis, seroma, hypertrophic scars and high patient dissatisfaction. Despite its long history as a complicated operation, debate continues about the ideal technique and the need for staging [
46,
47].
Laser-assisted techniques in aesthetic surgery have been widely developed in other areas of cosmetic surgery. Nevertheless, few studies have been focussed on breast lift with or without prosthesis placement. These approaches offer various advantages over traditional surgical methods, including improved precision, reduced recovery time, and potentially better aesthetic outcomes [
28,
48,
49,
50,
51]. In this study, we present the results of a new mastopexy technique involving minimal visible scares in addition to low-level multifrequency laser in a cohort of 231 patients with breast ptosis concerning 1). The different proportions in breast ptosis before and 1st, 2nd and third month after the laser-assisted mastopexy; 2) The SNND distance before and after 1st, the 2nd and 3rd month after the laser-assisted mastopexy and 3) The early surgical complications incidence (<30 days) according to Clavien-Dindo system.
To the best of our knowledge, this is the first study exploríng the effect of multifrequency low-level laser-assisted mastopexy in a large patient cohort and quantifying the surgical success by both, evaluating the number of patients achieving a non-breast ptosis state and by monthly SNND measuring during three months. In this regard, at the start of the study, all patients (100%) presented with any breast ptosis degree at the initial evaluation (pre-surgery). Interestingly we observed that after surgery there was a progressive and significant increase month by month in the proportion of patients without breast ptosis from 42% in the first month, 58% in the second month and 90% in the third month (p<0.01), demonstrating that breast ptosis correction by low-level multifrequency laser treatment is a dynamic process that progress throughout the three-month observational window. Moreover, SNDD serial measurements confirmed the NAC repositioning by 30% in both breasts, from 27 cm at baseline to 19 cm at three months after surgery, this change represents, on average, a progressive lifting of 8 cm in 3 months as can be seen in
Table 8.
In this study, any traditional surgical technique for volume reduction or tissue rearrangement is employed, suggesting the important lifting effect observed is attributable to the unique properties of the LPL9002™ lipo laser system. This system employs a combination of three specific wavelengths (533 nm, 650 nm, and 980 nm) that interact with various anatomical structures within the breast to achieve the desired lifting effect. The rationale behind these wavelengths is the specific effects on adipose tissue, skin and fibrous structures [
52]. In this sense, the green 530 nm DPSSL laser stimulates clothing in small vessels, thus promoting hemostasis during surgical procedures [
53]. Its ability to be absorbed by haemoglobin within blood vessels allows for precise and controlled small blood vessel sealing, minimizing blood loss, contributing to hematomas and ecchymosis prevention, faster recovery, and enhancing patient comfort [
54]. Additionally, the DPSSL laser enables greater precision when working in areas near important blood vessels, especially in the breast. In this sense, the minimal thermal effect on the surrounding tissues ensures a better surgical experience and improved recovery [
55]. On the other hand, the GaAlAs 650-670 nm red laser causes selective fat cell lysis during the lipolysis procedure, allowing fat extraction with minimal tissue trauma [
31,
56]. Alternatively, although the infrared GaAlAs 980 nm laser has been employed to break selectively fat cell membranes, this wavelength’s main feature is stimulating skin’s collagen formation, contributing to a skin-tightening effect observed in this study [
57,
58,
59].
Unfortunately, due to the novel nature of this technique, there are few comparable studies. In this regard, 12 consecutive patients reported by Ingram et al. addressed laser energy as an adjunct to liposuction using the “Slim Lipo” Nd: YAG laser (Palomar ASPIRE, USA) with 924 and 975 nm wavelengths after the liposuction step. The baseline sternal notch to nipple distance was on average 28 cm (median: 28 cm, range: 26-31 cm) and postoperative, all patients exhibited a significative elevation of both nipples (3.36 cm, as above, and the degree of nipple elevation on one side was strongly correlated with that on the other side [
28]. Regrettably, the authors do not report the NAC beyond the immediate post-operatory period. Another study by Sánchez et al. reported the mirrored “D” technique and laser-assisted liposuction in 46 female patients between 20 and 66 years old. Their results showed there was no surgical revision, postoperative infection or necrosis of the papillary areolar plaque, 5 cases of papillary areolar plaque epidermolysis, were treated with flavonoids and horse chestnut without sequelae. A breast-QTM evaluation showed a high satisfaction degree with the procedure (86%), but regrettably, there were no SNND measuring changes. It should be emphasised, that the mirror D-technique leaves a visible 6 cm on average [
60], whereas our technique leaves only a minimal scar on the lower edge of the areola in the case of laser-assisted mastopexy with prosthesis placement and no visible scars in the case of mastopexy without implant placement. In another study made on 94 women (188 breasts) with a new non-laser technique (electrically assisted liposuction with loop placement), Abboud et al. [
50] reported a 7.3 cm in NAC elevation. However, it should be noted that our low-level laser technique did not require the use of loops to achieve NAC elevation, as neo-collagen synthesis and skin tightening effectively achieved breast tissue reconfiguration.
Laser-assisted lipolysis has been most frequently used in gynecomastia and pseudogynecomastia. In a study conducted by Trelles et al. [
51], laser-assisted lipolysis was evaluated in patients with Simon’s Grades II and III. The authors reported a significant reduction in breast volume along with significant skin tightening, and good satisfaction with the surgical results. Similarly, Fusco et al. reported the efficacy and safety of laser-assisted lipolysis for pseudo-gynecomastia management. The comparison between baseline and post-treatment pictures by a physician evaluator 4 months after treatment revealed significant improvement in all patients. Three subjects (33%) showed grade 4 improvement; 4 (44%) showed grade 3 improvement, and 2 (22%) showed grade 2 improvement. Breast sizes decreased in all patients 4 months after 1444-nm LAL and none of them experienced postoperative complications [
61].
Concerning surgical complications, mastopexy and breast implant placement are associated with general and specific complications inherent to any surgical procedure such as hematoma, infection, wound healing delay, wound dehiscence, areola-skin necrosis, implant disruption and silicon leakage, anaplastic large cell lymphoma, and poor scars quality, including hypertrophic scar [
1,
30]. In a meta-analysis of thirty-four studies published from 1980 through 2016 conducted by Di Summa et al. assessing non-implant mastopexy techniques outcomes in 1888 patients, the overall complication rate was 10.4%. In this study, the most common complications were scar-related (3%, including hypertrophic or unesthetic appearance) and nipple–areola-related problems (2.9%, including distortion, asymmetry, and reduction sensation). On the other hand, our study found a complication incidence according to the Clavien-Dindo system of only 4%. In this sense, laser-assisted mastopexy was associated with very low and non-life-threatening early surgical complications within 30 days. These complications were represented by two Grade II cases presenting generalized pain requiring specialized intervention and four IIIb cases requiring re-intervention under general anaesthesia (4 areola necrosis and 3 seromas).
Author Contributions
Conceptualization, G.C-V., N.V., A.M.O.P., and V.B.; methodology, D.R-P. and V.B.; validation, G.C-V., N.V., A.M.O.P., D.R-P. and V.B.; formal analysis, D.R-P. and V.B.; investigation, G.C-V., N.V. and A.M.O.P.; resources, G.C-V., N.V., A.M.O.P., D.R-P. and V.B.; data curation, D.R-P. and V.B.; writing—original draft preparation, G.C-V., N.V., A.M.O.P., D.R-P. and V.B.; writing—review and editing, G.C-V., N.V., A.M.O.P., D.R-P. and V.B.; supervision, G.C-V., N.V., A.M.O.P., and V.B.; funding acquisition, G.C-V., D.R-P. and V.B. All authors have read and agreed to the published version of the manuscript.