1. Introduction
Pancreatic ductal adenocarcinoma (PDA) is one of the deadliest cancers, with more than 66,000 cancers diagnosed every year and the lowest 5-year survival rate (13%) among solid tumors [
1]. Limited treatment options and unreliable biomarkers to monitor response are the main reasons for such dismal outcomes, which we discussed in our previous publication [
2]. Until a decade ago, management of resected PDA (R-PDA) was straightforward, with upfront surgery (UpS) followed by adjuvant chemotherapy (AT) with FOLFIRINOX or the gemcitabine/capecitabine (Gem/Cap) combination [
3,
4]. Retrospective analysis and data from a randomized phase II/III trial, Prep-02/JSAP-05, support the use of neoadjuvant systemic therapy for resectable pancreatic cancers. However, long-term data and more randomized clinical trials are warranted[
5].
Neoadjuvant therapy (NAT) for PDA gained traction in the last 5-6 years and is now preferred in most institutions [
6,
7,
8,
9,
10]. Patient selection beyond the stage of diagnosis (resectable (Rs-PDA) vs. borderline resectable (BRs-PDA) vs. locally advanced (LA-PDA)), modality used (chemoradiation (CRT) vs. chemotherapy vs. both), chemotherapy agents (Gem vs. FOLFIRINOX vs. Gem/nab-paclitaxel (Gem-NP)), and the duration of perioperative therapy that would make a meaningful difference in the outcomes are still unclear. [
11,
12].
The biomarkers applied in current clinical practice, including imaging, carbohydrate antigen 19-9 (CA 19-9), and cell-free DNA (cfDNA) testing, are not useful in making appropriate treatment decisions [
2]. Our group previously focused on tissue mucin 5 AC (MUC5AC) as a biomarker for managing PDA and biliary tract cancer [
13,
14,
15,
16,
17]. In prior publications, we assessed its diagnostic value and provided preclinical evidence suggesting its influence on response to systemic therapy [
14,
16]. The present effort examined the role of MUC5AC in the outcomes of patients undergoing curative resection (R-PDA).
MUC5AC is a heavy glycoprotein typically produced in normal lungs and gastrointestinal tracts along with other mucins to protect them from infection, inflammation, and other physiological insults [
18,
19]. Its presence in pancreatic tissues is abnormal and is often associated with malignancy [
16,
20]. In PDA cells, MUC5AC exists in two glycoforms: less-glycosylated immature (IM) detected in the perinuclear area and heavily-glycosylated mature form (MM) detected in the apical and extracellular (EC) regions [
20]. Commercially available monoclonal antibodies (mAb) allow us to distinguish them in tissues via immunohistochemistry (IHC). The CLH2 clone detects IM, and MM is identified by 45M1, 1-13M1, 9-13, and 2-11M1 mAbs [
21,
22,
23]. Prior studies that examined the influence of IM on PDAs were for UpS patients or a mixed population (early and advanced stage PDA), and the results were inconclusive [
24,
25,
26,
27,
28]. The clinical significance of MM is not investigated well despite preclinical evidence suggesting that it has prognostic and predictive value [
29].
We believed we had been considering the wrong MUC5AC glycoform (IM) to investigate PDA outcomes or treatment resistance. We hypothesized that the MM glycoform, which is detected in apical and EC sites in PDA, affects the overall outcome and treatment resistance (prognostic and predictive value) based on the preclinical evidence. Our present study explored the clinical significance of IM and MM in resected PDA specimens (R-PDA).
2. Materials and Methods
2.1. Study Design and Population:
This study was conducted at The Ohio State University Comprehensive Cancer Center (OSU-CCC), Columbus, Ohio, after receiving appropriate approvals from the Office of the Institutional Review Board. The Total Cancer Care Program (TCCP), a division of OSUCCC, identified the patients who underwent resection for PDA at this institution and provided archived clinical and pathologic data. They also provided an H&E section and multiple unstained sections of representative primary tumor formalin-fixed paraffin-embedded (FFPE) blocks from the study period, January 2010 to June 2021. Any additional clinical or pathologic information was collected by review of medical records by the principal investigator (AM).
2.2. Immunohistochemistry
Immunohistochemical detection of MUC5AC isoforms was accomplished using mouse monoclonal antibodies CLH2 (NBP2-44455, NovoBiologicals, CO, USA) and 45M1 (ab3649, Abcam, MA, USA) on an Agilent DAKO autostainer link 48 system (Agilent Technologies, Santa Clara, CA, USA ). Formalin-fixed paraffin-embedded tissue sections were deparaffinized/rehydrated, and antigen retrieval was performed with Agilent DAKO target retrieval solution with citrate buffer (pH 6.1, S169984-2; Agilent Technologies, Santa Clara, CA, USA) at 95 °C for 27 minutes. For CLH2, the primary antibody was incubated at a dilution of 1:800 for 30 minutes at room temperature. The primary antibody was detected using a VECTASTAIN® Elite® ABC Universal PLUS Kit (PK-8200; Vector laboratories, Newark, CA) with a polymer peroxidase diaminobenzidine chromogen system. For 45M1, the primary antibody was incubated at a dilution of 1:1000 for 30 minutes at room temperature. It was detected using an Agilent Envision Flex Kit (K80002, Agilent Technologies, Santa Clara, CA, USA) and a polymer peroxidase diaminobenzidine chromogen system.
A pathologist (AE) with expertise in pancreaticobiliary pathology reviewed the immunostains. The intracellular localizations for the IM (CLH2) and MM (45M1) isoforms, including cytoplasmic and apical expression levels, were determined. Individual tissue sections were scored for the percentage of reactive tumor cells and intensity of reactivity (0, no staining; 1+ weak staining; 2+ moderately intense staining; and 3+ strong staining). The H-score was derived from the product of the percentage of tumor cells and intensity of staining, resulting in a value between 0 and 300 [
36]. Also, the presence or absence of extracellular (EC-45M1) expression for the MM isoform was noted.
2.3. Clinical and Pathology Data Collection
This study was conducted in compliance with all the applicable institutional ethical guidelines for the care, welfare, and use of animals. Demographic data, such as age, gender, race, social history (smoking and alcohol use), and laboratory information (CA 19-9 and total bilirubin) were collected. Clinical information regarding the date of diagnosis, clinical staging, type of surgery, residual disease (R0 vs. R1/R2), neoadjuvant and adjuvant therapy received, and performance status were collected from the medical records.
Pathology data were obtained from the pathology reports of the definitive surgical resection specimens. It included the histological grade of the tumor (well-differentiated (Grade 1), moderately differentiated (Grade 2), and poorly differentiated (Grade 3), margin status (Ms), tumor size (≤ 2 centimeters (cms) vs. > 2 cms), lymphovascular invasion (LVI), perineural invasion (PNI), perivascular-invasion (PVI), peri-pancreatic soft tissue extension (PPE), lymph node (LN) metastasis, and the presence of any reported pre-malignant lesions such as pancreatic intraepithelial neoplasia (PanIN) or intraductal papillary mucinous neoplasms (IPMN and distant metastasis.
For 70 patients, the tumor (T) staging had to be reclassified as there was a significant change in the TNM staging from the prior American Joint Committee on Cancer (AJCC) 7th edition to the currently used AJCC 8th edition. The pathological treatment response (pTR) noted on the resected samples for patients who received NAT was classified into two major groups: objective response (OR) and no response (NR). OR included patients with near complete response (nCR) and partial response (PR). nCR is characterized by single cells or a small group of cancer cells, corresponding to a tumor regression score of 1 by the modified Ryan scheme for tumor regression score (TRS) [
37]. PR was characterized by residual cancer with evident tumor regression, but with more than single cells or rare small groups of cancer cells, TRS 2. The NR group included patients with extensive tumors with no evident tumor regression or TRS 3.
2.4. Statistical Considerations
Descriptive statistics were used to summarize patient baseline characteristics. ANOVA models or Chi-square tests were applied to compare MUC5AC expression between treatment response groups. Univariate logistic regression models were employed to assess the association between pathological features and MUC5AC expression. PFS and OS analyses were performed through Kaplan-Meier curves and univariate and multivariate Cox regression models over pathological features. A Bonferroni correction was applied for p-value adjustments. All analyses used SAS 9.4 (SAS, Cary, NC, USA).
3. Results Section
3.1. Baseline Characteristics
We received 112 tumor samples (see Supplementary Figure 1). However, samples for 12 patients had to be excluded (1 had microscopic liver metastasis; 9 were samples from metastatic sites such as the lung, liver, omentum, ovary, and bladder; 1 had a neuroendocrine pancreatic tumor; and the other patient’s slides did not have tumor tissue even though there was a residual tumor in the resected sample (according to the pathology report)). We ended up with 100 patients in the study who had curative resection for PDA.
The median age of diagnosis was 65 years, with an equal number of male and female patients. A documented history of pancreatic cysts or IPMN (on imaging) before surgery was noted for 20%, and all had diabetes mellitus (DM). Forty-three (43) patients had NAT (36 FOLFIRINOX, 5 Gem-NP, and 2 FOLFOX); the other 57 had UpS. Sixteen (16) patients had neoadjuvant chemoradiation (NAT-CRT) post-NAT. Among UpS patients, 55/57 received systemic adjuvant therapy (3 FOLFIRINOX, 7 Gem-NP, 33 Gem-only, 11 Gem/Cap, 1 Cap-only). Two patients had adjuvant CRT only (one with Gem and the other with 5FU). The median number of NAT doses received by the patients in the NAT-group and FOLFIRINOX-NAT-group was the same (6 (range, 2-8)). Other pathological features noted in the resected samples are detailed in
Table 1.
3.2. MUC5AC Detection and Distribution for Patients with Resected PDA
MUC5AC detection/distribution among the samples is presented in Supplementary Table 1. Both glycoforms (45M1 and CLH2) were present in 96% of the tested samples, and there was 100% overlap for them, i.e., all the samples positive for CLH2 were also positive for 45M1. Four samples in NAT-group were negative for both. EC-45M1 was detected in 72% of the tumors, and all of these tumors had 45M1 and CLH2 expressions. CLH2 (IM) was detected only in the cytoplasm and not in the apical or EC regions (Supplementary Figure 2). The mature glycoform, 45M1 was detected only in the apical and EC regions. Mean expression levels (H-scores) for 45M1 and CLH2 were 148.5 and 145.27, respectively. 25% of (24/96, 14/43 in NAT, and 10/57 in Ups groups) PDAs that expressed 45M1 did not have EC-45M1 detected.
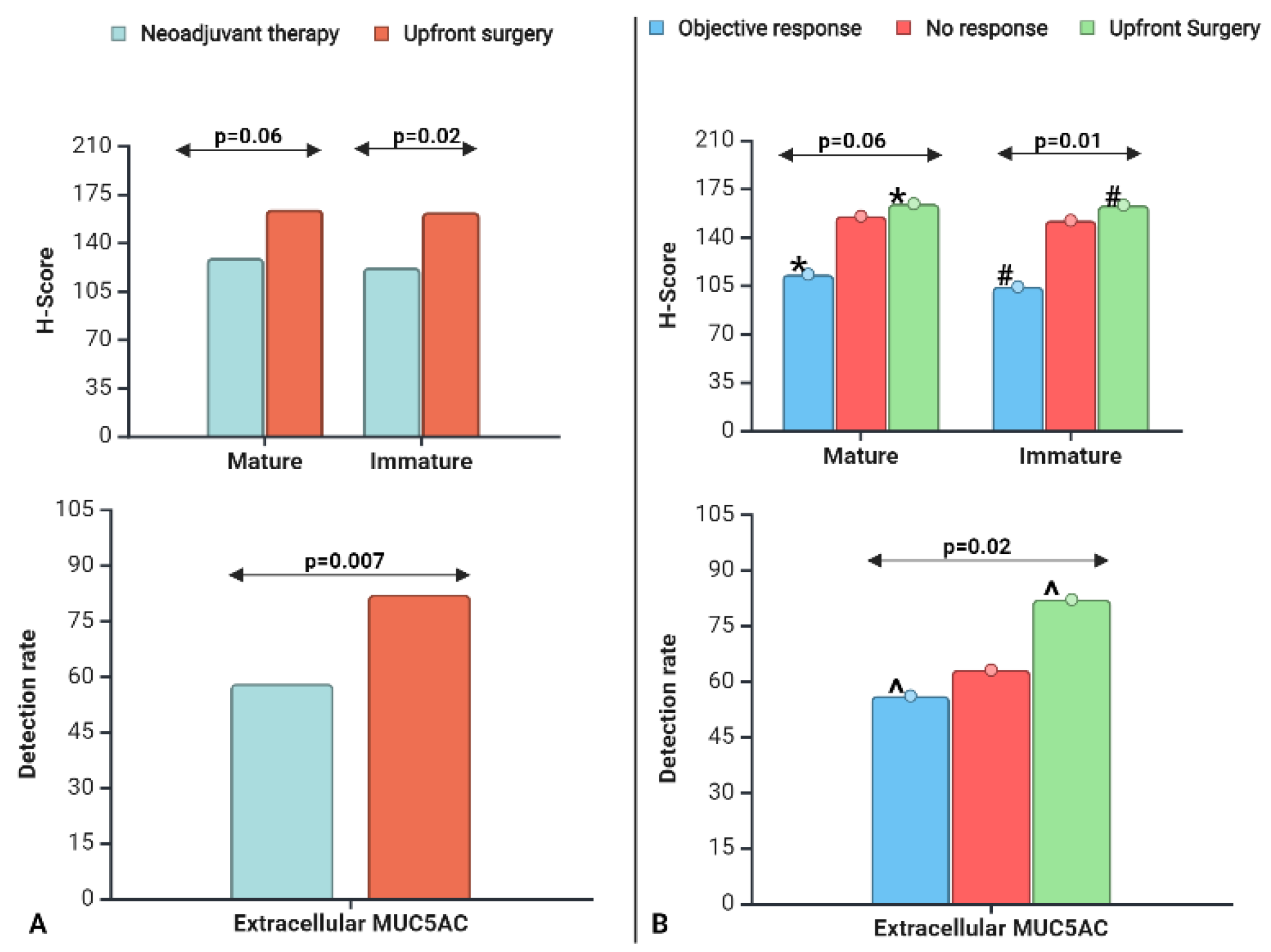
Simple linear regression analysis showed a significant (p<0.001) positive correlation between CLH2 and 45M1 for all populations (NAT, UpS, and NAT+ UpS). Simple logistic regression showed a significant correlation (p<0.001) between EC-45M1-detection and CLH2 or 45M1 expression, and the mean expression levels (H-score) were 2-3 times greater in EC-positive tumors than in EC-negative tumors (Supplementary Table 2). The mean CLH2 expression and EC-45M1 detection rates were significantly lower in the NAT group than in UpS (
Figure 1A). The 45M1 expression was lower, with a trend toward significance in the NAT group (
Table 2). We did not have enough Gem-based therapy patients (N=5) in the NAT group to learn if MUC5AC imparts more resistance to 5FU or Gem-based therapy.
3.3. MUC5AC Expression Levels and Pathological Treatment Responses in PDA post-NAT.
We first studied the effect of MUC5AC on the pTR for the entire population (N=100) and performed a sub-group analysis on the NAT group. For the first analysis, we had three groups: OR vs. NR vs. UpS. Here, the UpS population served as a control, as it represented the treatment-naïve population. We compared the mean expression of 45M1 and CLH2 and EC detection rates among the groups (
Table 2).
The mean CLH2 expression differed significantly, and 45M1 had a strong trend toward significance (p=0.06) among the three groups (
Figure 1B). The OR and UpS groups had the lowest and highest expression levels for both MUC5AC glycoforms (
Table 2). The NR group had levels close to those of the UpS group and higher than those for the OR group. In head-to-head comparisons, MUC5AC expression (mean 45M1 and CLH2) was significantly lower in the OR groups than in the UpS groups; NR vs. OR and NR vs. UpS were not significantly different.
The proportion of EC-45M1 detection (positive vs. negative) differed among the three groups (
Table 2). More subjects with OR (44%) were EC-45M1-negative than NR (37%) and UpS (18%) subjects. In head-to-head comparisons, EC-45M1-detection significantly differed, following the same trend as 45M1. The difference between OR and UpS was significant; the others were not. The detection rates for the NR group were closer to those for OR than those for the UpS group, although MUC5AC expression was closer to that of the UpS group than that for the OR group.
For the NAT group, the mean H-scores for 45M1 and CLH2 were 120 (quartile range, 30-210) and 100 (30-210), respectively. The lower CLH2 expression group (≤ 100, N=22) had a significantly (p=0.04) higher percentage of patients with OR (77% vs. 48%) than the higher CLH2 expression group (>100, N=21). There was a trend toward significance (p=0.07) for 45M1 (≤ 100 (N=22) vs. >100 (N=21), 76% vs. 50%) for the same cut-off value (H-score=100). EC-detection was significantly lower (p<0.001) for lower CLH2 (≤ 100 vs. >100, 37% vs. 90%) and 45M1 (24% vs. 91%). This evidence suggests that a threshold of 100 for H-score (for CLH2 > 45M1) could be used as a cut-off to identify patients who respond to therapy. The FOLFIRINOX NAT sub-group group (N=36) had the same trend, but the p values were only 0.07 for CLH2 and 0.08 for 45M1.
3.4. The Impact of MUC5AC on Pathological Features of Resected PDA
We examined the impact of MUC5AC by logistic regression analysis (
Table 3) for all the patients in the study, irrespective of NAT (N=100).
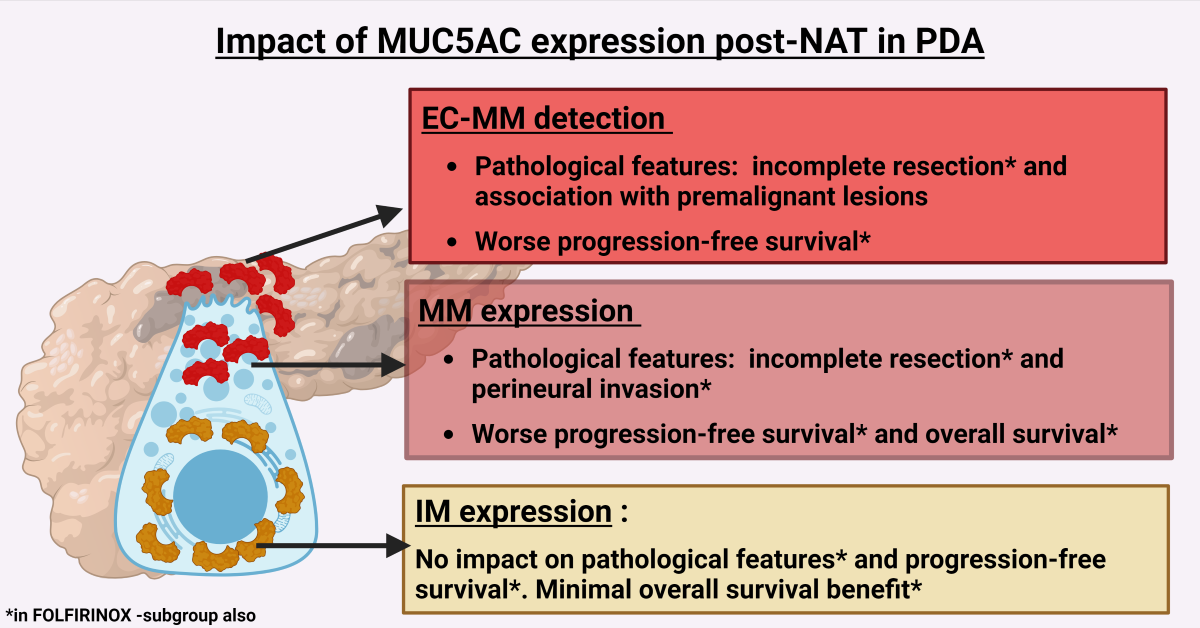
The pTR (OR vs. NR vs. UpS) was affected by 45M1 and CLH2 levels and EC-45M1 detection. CLH2 expression influenced tumor size (≤ 2 cms vs. >2 cms), and EC-45M1 detection influenced NAT and association with premalignant lesions (AwPml) such as IPMN and PanIN. A trend toward significance was noted in the effect of 45M1 on tumor size, AwPml, and NAT, and CLH2 on AwPml.
A similar analysis for the UpS group (N=57) gave information about the role of MUC5AC on treatment-naïve PDAs. For this population, 45M1 and CLH2 expression were not associated with other pathological features (data not shown here). For the NAT group (N=43), 45M1 expression levels significantly related to incomplete resection (residual disease, R0 vs. R1-R2) and PNI, but EC-45M1 detection related to AwPml (Supplementary
Table 3). An encouraging trend toward significance was noted in the effect of 45M1 on Ms, CLH2, EC-45M1-detection, and EC-45M1 CS on residual disease and PNI. In the FOLFIRINOX group (N=36), 45M1 and CLH2 affected residual disease, Ms, and PNI. The trend toward significance was noted for EC-451-detection on AwPml and residual disease and EC-45M1 CS on residual disease and PNI.
3.5. The Impact of MUC5AC on PFS and OS in R-PDA
The study population was a heterogeneous group with multiple variables (NAT, UpS, NAT-CRT, and various AT combinations). We minimized heterogeneity by assessing the relationship of MUC5AC to various groups, such as the NAT-group, patients receiving FOLFIRINOX in the NAT-group, patients receiving Gem-based AT (Gem-only, Gem/Cap, and Gem-NP), and Gem-only AT in the UpS group.
We first analyzed the impact of MUC5AC expression on the NAT-group (N=43) and on PFS and OS by UVA and MVA (
Table 4 and
Table 5). The median PFS and OS (in days) of this group were 313 (CI=212. 391) and 741 (CI=452, 857), respectively. For UVA, there was no significant relation (p>0.05) between MUC5AC expression and PFS or OS (data not shown here).
For the resected specimens, EC-45M1-negative had a better PFS on MVA (Hazard Ratio (HR) 0.2, 95 % CI: 0.059-0.766, p = 0.01). The 45M1 expression was significant (p = 0.0398) on MVA for PFS, but the HR (0.96) was not impressive enough to make it a clinically significant factor. Other features that affected PFS were pathological grade (G1-G2 vs. G3), Ms, NAT-CRT, site of recurrence, OS pathological grade (G1-G2 vs. G3), and Ms. Among 43 patients in this group, 16 (37%) did not receive adjuvant therapy, and Gem-only or Gem-based therapy was given to the others (10 Gem-only, 3 Gem-NP, 4 Gem/Cap, 7 FOLFIRINOX, 1 FOLFOX, 1 FOLFIRI, and 1 5FU-only). The CLH2 expression level did not significantly affect PFS (p=0.07 and HR of 1.02). On MVA, 45M1 and CLH2 expression impacted OS with HRs of 0.959 and 1.043, respectively (
Table 5). EC-45M1 detection did not significantly affect the OS. The pathological grade and Ms were also factors that significantly influenced OS.
A similar trend was evident for patients in the NAT group treated with FOLFIRINOX (N=36). The median PFS and OS (in days) of this group were 336 (CI=162, 599) and 741(CI=334, 1003). The results are summarized as follows, a) MUC5AC did not change OS or PFS on UVA, b) EC-45M1 detection was a significant factor (HR 0.2, 95 % CI: 0.052-0.847, p = 0.02) for PFS on MVA (Supplementary Table 4), c) the 45M1 and CLH2 expressions were significant, but HRs were clinically not impressive (45M1 - 0.9 & IM -1.02), d) the LVI, Ms, Awpml, and site of recurrence were other pathological factors significantly affecting the PFS on MVA, e) MVA of factors affecting OS were (Supplementary Table 5) 45M1 and CLH2 (HRs of 0.86 and 1.12, respectively), AOI, pathological grade, LVI, Ms, tumor size, AwPml, and site of recurrence.
UpS patients who received Gem-based AT (N=51, 33 Gem-only, 11 Gem/Cap, and 7 Gem-NP) had notable results (Supplementary Table 6). 45M1 positively impacted PFS only (not OS) in MVA (HR of 1.03, p=0.03). There was a trend toward significance with CLH2 (HR of 0.97, p=0.07) on PFS. EC-45M1 did not impact PFS or OS. Other factors influencing PFS and OS are in Supplementary Table 7. MUC5AC did not change outcomes in other groups, such as UpS (N=57), Gem-only (N=33), or all patients (N=100).
The impacts of MUC5AC in the resected samples post-NAT are summarized as i) EC-45M1 detection post-NAT is a poor prognostic marker, ii) MM (45M1) expression negatively impacted PFS and OS, but the HRs were not clinically significant, iii) IM (CLH2) expression had a positive effect on OS. iv) For the UpS group, MUC5AC expression did not influence the outcomes, but MM (45M1) had a minimal positive effect on PFS in a sub-group that received Gem-based therapy.
3.6. MUC5AC Expression in Primary and Distant Metastatic Sites
In the study population, 10 patients had metastatic disease. One patient with micrometastasis to the liver identified during primary resection was excluded from this comparison (Supplementary Table 8). Four (4/9) patients (treatment-naïve) received no systemic therapy before the biopsy. The others (N=5) received systemic therapy (2-FOLFIRNIX, 1-FOLFOX, 1-Gem/nab-paclitaxel, 1-Gem only). All 9 biopsies were positive for 45M1, EC-45M1, and CLH2. The mean expressions of 45M1, EC-45M1 CS, and CLH2 were 199, 199, and 196, respectively. Among the metastatic sites, the lung had the highest H-scores, and the peritoneum had the lowest (lung (300) > other organs such as ovary, small bowel, and bladder (260) > liver (170)> primary tumor > peritoneum (100)). The 45M1 and CLH2 H scores were similar. The numbers of these two groups were too low to determine the effect of the treatment on MUC5AC expression (data not shown here). When MUC5AC expression was compared between metastatic sites (N=9) and resected specimens (N=100), CLH2 and EC-45M1 H-scores were significantly higher for metastatic sites than for primary tumors (Supplementary Table 9). The distributions (percentages of positive cells) of 45M1 and CLH2 were significantly higher for metastatic sites. We compared MUC5AC expression in treatment-naïve (N=4) and NAT-group and treated (N=5) and UpS-groups (Supplementary Table 10). There was a similar trend for those comparisons.
4. Discussion
Our study provided insight into the clinical significance of MUC5AC glycoforms in PDA and showed an association of pTR with MUC5AC glycoforms. We identified the specific population (post-systemic therapy, NAT-group in this study) and the location of MUC5AC detection (EC) that need to be explored to understand its impact on PDA. Our study reaffirms the association of MUC5AC expression with malignancy, as it is detected in all treatment-naïve patients (UpS group). The detection rates were higher than reported in other studies, including our previous TMA study [
16,
24,
25,
26,
27,
28]. The 100% concordance and positive correlation between 45M1 and CLH2 detected MUC5AC supports the theory that IM matures by glycosylation, moves to the apical region, and is released into the EC region [
20].
The inclusion of treatment-naïve (UpS) and treated (NAT) patients allowed us to examine, more effectively than in previously reported studies, the influence of treatment on MUC5AC expression and vice versa (
Table 2 and Supplementary
Table 1). MUC5AC expression was impacted by NAT and pTR (
Figure 1 and
Table 3), indicating its possible role in influencing the pathways or mechanisms of action of chemotherapeutic drugs. The countereffects of CLH2 and 45M1 for the NAT-group (
Table 4 and
Table 5, Supplementary Table 4 and Table 5) and of PFS in the Gem-based therapy sub-group of UpS (Supplementary Table 6) need to be investigated further to determine if MUC5AC expression can help in treatment selection. For most preclinical studies, the association of MUC5AC and Gem resistance was the focus of the investigations, and some potential pathways, such as the MUC5AC/β-catenin/c-Myc axis, were identified [
29,
30,
31,
32]. Our study did not have enough patients in NAT-group on Gem-based therapy (N=5) to make substantial conclusions, but MUC5AC expression could be drug-specific (Supplementary Table 11). The impact difference in HRs of intracellular MM (45M1) in the NAT group (HR of 0.96) and in the FOLFIRNOX sub-group (HR of 0.86) strengthens this hypothesis, which still needs further investigation.
The baseline MUC5AC expression did not influence major pathological features or outcomes (OS and PFS) based on the treatment-naïve patients (UpS-group). This, along with the differential impact of CLH2 (positive impact in NAT group, no impact in UpS, negative impact on Gem-based adjuvant therapy subgroup), explains the mixed results for previous IM-only-based studies of this population [
24,
25,
26]. Alternatively, post-NAT MUC5AC expression and EC detection impacted pathological features (such as pTR, incomplete resection, Ms, and PNI) and outcomes (PFS). The small difference in EC-MUC5AC detection rates between NR (63%) and OR (56%) groups, despite having larger differences in intracellular MUC5AC expression (mean H-score, OR vs. NR = 113 vs. 153) for patients who had curative resection without progression or distant metastasis, highlights the clinical significance of EC-MUC5AC in PDA, as demonstrated in preclinical studies [
29]. The number of NAT doses (median of 6) is similar to clinical practice, as reflected in most clinical trials (NORPACT, ESPAC-5, and SWOG 1505) [
11,
33,
34]. We did not have enough samples to explore further the differences between primary and metastatic lesions. However, metastatic lesions (N=9) had significantly high MUC5AC expression and a 100% EC-45M1 detection rate, implying that it has a role in disease progression and distant metastasis.
Prospective studies with a large sample size should validate the findings of this retrospective study. Surgical resection was a confounding factor for survival analysis for both groups (NAT and UpS). Post-operative management was not uniform in either of the groups. One-third of the NAT-group patients did not receive AT. For the rest, Gem-only or Gem-based therapy was administered. Most of the NAT group got Gem-only (33/57) or Gem-based (51/57), which is presently not the standard of care. Use of NAT was not popular during the early part of the study period. There could have been a selection bias in administering NAT. We do not have MUC5AC expression in the recurrent lesions to confirm that it is similar to that of resected samples. the NAT-CRT is another confounding factor in assessing the association between pTR and MUC5AC expression. We did not have enough sample size to make substantial conclusions for NAT or AT selection (FOLFIRINOX vs. Gem-NP).
5. Conclusions
Our study provides evidence suggesting the clinical value of MUC5AC expression in the resected PDA, specifically for patients with post-NAT. Our hypothesis that MM detected in the apical and EC regions influences the outcome more than IM was confirmed. We showed that MM, which was not studied earlier, has clinical utility (pTR and outcomes). This association has implications for deciding the duration of NAT and the timing of the surgical resection. Based on findings reported in this study and of others, the potential clinical implications of MUC5AC tissue testing could help NAT management as follows: a) the drop in tumor intracellular MM expression and conversion of EC-MUC5AC positive to negative may indicate a response to NAT and low-risk for recurrence, suggesting an ideal time for surgery; b) stable intracellular MM and conversion of intracellular MUC5AC-positive to -negative could indicate a poor response to NAT but still low-risk of recurrence; and c) stable intracellular MM and persistence EC-MUC5AC detection could indicate poor response to NAT and high-risk of recurrence.
Future research should focus on elucidating the underlying mechanisms linking MUC5AC expression to treatment outcomes of post-NAT PDA patients. Furthermore, the failure of NPC-1C antibody treatment to improve the outcome in a recent clinical trial strengthens the importance of identifying the appropriate MUC5AC glycoform for therapeutic purposes [
35]. Thus, these findings will help clinicians consider other treatment options, including changing the NAT regimen, proceeding to surgery, or using CRT, especially in the last two scenarios.
6. Patents
MUC5AC in pancreatic adenocarcinoma (PCT/US2024/012771, 1/24/24)) [patent pending]
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Supplementary Table 1: Distribution of MUC5AC expression in the tested samples; Supplementary Table 2: Comparison of intracellular MUC5AC in extracellular-positive and -negative patients; Supplementary Table 3: Univariate logistic regression model for MUC5AC expression in the neoadjuvant group (N=43); Supplementary Table 4: Multivariate analysis of clinicopathological features for progression-free survival in the FOLFIRINOX neoadjuvant group (N=36); Supplementary Table 5: Multivariate analysis of clinicopathological features for overall survival in the FOLFIRINOX neoadjuvant group (N=36); Supplementary Table 6: Multivariate analysis of clinicopathological features for progression-free survival in the gemcitabine-based adjuvant therapy in the upfront surgery group (N=57); Supplementary Table 7: Multivariate and univariate analysis of clinicopathological features for progression-free survival and overall survival in various groups; Supplementary Table 8: Site of metastasis and corresponding biopsy sites tissue provided in the study; Supplementary Table 9: Comparing MUC5AC expression between metastatic and primary tumors; Supplementary Table 10: Comparing MUC5AC distribution in primary and metastatic tissues; Supplementary Table 11: Breakdown of neoadjuvant therapy group (N=43) based on treatment; Supplementary Figure 1: Breakdown of the samples used in the project; Supplementary Figure 2: MUC5AC expression in pancreatic ductal adenocarcinoma
Author Contributions
Conceptualization, study design, chart review, and writing—original draft preparation – AM; Chart review –AS and SA; Immunohistochemistry and scoring the FFPE slides – AE; Biostatistics – LY, Writing—review and editing - UM, RKP, KH, WY, DPSS; AK, AMN, Amit, JH, SR, PM, SR, NJ, JMC, ST, AE, KP, EM, EH, KT, and MD.
Funding
The Ohio State University Intramural Research Program Award (Pelotonia, GR126178)
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of The Ohio State University (protocol number 2021C0160 and date of approval – 9/30/202.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
For ethical reasons, the data presented in this study are available upon request from the corresponding author.
Acknowledgments
Images were created using biorender.com
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J Clin 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Sheel, A.; Addison, S.; Nuguru, S.P.; Manne, A. Is Cell-Free DNA Testing in Pancreatic Ductal Adenocarcinoma Ready for Prime Time? Cancers 2022, 14, 3453. [Google Scholar] [CrossRef] [PubMed]
- Neoptolemos, J.P.; Palmer, D.H.; Ghaneh, P.; Psarelli, E.E.; Valle, J.W.; Halloran, C.M.; Faluyi, O.; O'Reilly, D.A.; Cunningham, D.; Wadsley, J.; et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet 2017, 389, 1011–1024. [Google Scholar] [CrossRef]
- Conroy, T.; Hammel, P.; Hebbar, M.; Ben Abdelghani, M.; Wei, A.C.; Raoul, J.-L.; Choné, L.; Francois, E.; Artru, P.; Biagi, J.J.; et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. New England Journal of Medicine 2018, 379, 2395–2406. [Google Scholar] [CrossRef] [PubMed]
- Unno, M.; Motoi, F.; Matsuyama, Y.; Satoi, S.; Matsumoto, I.; Aosasa, S.; Shirakawa, H.; Wada, K.; Fujii, T.; Yoshitomi, H.; et al. Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S-1 versus upfront surgery for resectable pancreatic cancer (Prep-02/JSAP-05). J Clin Oncol 2019, 37, 189–189. [Google Scholar] [CrossRef]
- Liu, S.; Li, H.; Xue, Y.; Yang, L. Prognostic value of neoadjuvant therapy for resectable and borderline resectable pancreatic cancer: A meta-analysis of randomized controlled trials. PLoS One 2023, 18, e0290888. [Google Scholar] [CrossRef]
- Kolbeinsson, H.M.; Chandana, S.; Wright, G.P.; Chung, M. Pancreatic Cancer: A Review of Current Treatment and Novel Therapies. J Invest Surg 2023, 36, 2129884. [Google Scholar] [CrossRef]
- van Dam, J.L.; Janssen, Q.P.; Besselink, M.G.; Homs, M.Y.V.; van Santvoort, H.C.; van Tienhoven, G.; de Wilde, R.F.; Wilmink, J.W.; van Eijck, C.H.J.; Groot Koerkamp, B. Neoadjuvant therapy or upfront surgery for resectable and borderline resectable pancreatic cancer: A meta-analysis of randomised controlled trials. Eur J Cancer 2022, 160, 140–149. [Google Scholar] [CrossRef]
- Versteijne, E.; van Dam, J.L.; Suker, M.; Janssen, Q.P.; Groothuis, K.; Akkermans-Vogelaar, J.M.; Besselink, M.G.; Bonsing, B.A.; Buijsen, J.; Busch, O.R.; et al. Neoadjuvant Chemoradiotherapy Versus Upfront Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Long-Term Results of the Dutch Randomized PREOPANC Trial. J Clin Oncol 2022, 40, 1220–1230. [Google Scholar] [CrossRef]
- Springfeld, C.; Ferrone, C.R.; Katz, M.H.G.; Philip, P.A.; Hong, T.S.; Hackert, T.; Büchler, M.W.; Neoptolemos, J. Neoadjuvant therapy for pancreatic cancer. Nat Rev Clin Oncol 2023, 20, 318–337. [Google Scholar] [CrossRef]
- Labori, K.J.; Bratlie, S.O.; Andersson, B.; Angelsen, J.-H.; Biörserud, C.; Björnsson, B.; Bringeland, E.A.; Elander, N.; Garresori, H.; Grønbech, J.E.; et al. Neoadjuvant FOLFIRINOX versus upfront surgery for resectable pancreatic head cancer (NORPACT-1): a multicentre, randomised, phase 2 trial. The Lancet Gastroenterology & Hepatology 2024, 9, 205–217. [Google Scholar] [CrossRef]
- Chatzizacharias, N.A.; Tsai, S.; Griffin, M.; Tolat, P.; Ritch, P.; George, B.; Barnes, C.; Aldakkak, M.; Khan, A.H.; Hall, W.; et al. Locally advanced pancreas cancer: Staging and goals of therapy. Surgery 2018, 163, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Manne, A.; Esnakula, A.; Abushahin, L.; Tsung, A. Understanding the Clinical Impact of MUC5AC Expression on Pancreatic Ductal Adenocarcinoma. Cancers 2021, 13, 3059. [Google Scholar] [CrossRef] [PubMed]
- Manne, A.; Kasi, A.; Esnakula, A.K.; Paluri, R.K. Predictive Value of MUC5AC Signature in Pancreatic Ductal Adenocarcinoma: A Hypothesis Based on Preclinical Evidence. International Journal of Molecular Sciences 2023, 24, 8087. [Google Scholar] [CrossRef] [PubMed]
- Manne, A.; Mneimneh, W.; Elkadi, O.; Escobar, D.E.; Coley, J.; Guzman, G.B.; Fnu, S.M.d.; Alkharabsheh, O.; Khushman, M.d.M. The pattern of mucin 5AC (MUC5AC) expression using immunohistochemistry and its prognostic significance in patients with pancreatic ductal adenocarcinoma. J Clin Oncol 2020, 38, e16756–e16756. [Google Scholar] [CrossRef]
- Manne, A.; Yu, L.; Hart, P.A.; Tsung, A.; Esnakula, A. Differential Expression and Diagnostic Value of MUC5AC Glycoforms in Pancreatic Ductal Adenocarcinoma. Cancers 2023, 15, 4832. [Google Scholar] [CrossRef]
- Benson, K.K.; Sheel, A.; Rahman, S.; Esnakula, A.; Manne, A. Understanding the Clinical Significance of MUC5AC in Biliary Tract Cancers. Cancers (Basel) 2023, 15. [Google Scholar] [CrossRef]
- Bansil, R.; Turner, B.S. Mucin structure, aggregation, physiological functions and biomedical applications. Current Opinion in Colloid & Interface Science 2006, 11, 164–170. [Google Scholar] [CrossRef]
- Kebouchi, M.; Hafeez, Z.; Le Roux, Y.; Dary-Mourot, A.; Genay, M. Importance of digestive mucus and mucins for designing new functional food ingredients. Food Research International 2020, 131, 108906. [Google Scholar] [CrossRef]
- Krishn, S.R.; Ganguly, K.; Kaur, S.; Batra, S.K. Ramifications of secreted mucin MUC5AC in malignant journey: a holistic view. Carcinogenesis 2018, 39, 633–651. [Google Scholar] [CrossRef]
- Nollet, S.; Forgue-Lafitte, M.E.; Kirkham, P.; Bara, J. Mapping of two new epitopes on the apomucin encoded by MUC5AC gene: expression in normal GI tract and colon tumors. Int J Cancer 2002, 99, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Nollet, S.; Escande, F.; Buisine, M.P.; Forgue-Lafitte, M.E.; Kirkham, P.; Okada, Y.; Bara, J. Mapping of SOMU1 and M1 epitopes on the apomucin encoded by the 5' end of the MUC5AC gene. Hybrid Hybridomics 2004, 23, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Bara, J.; Chastre, E.; Mahiou, J.; Singh, R.L.; Forgue-Lafitte, M.E.; Hollande, E.; Godeau, F. Gastric M1 mucin, an early oncofetal marker of colon carcinogenesis, is encoded by the MUC5AC gene. Int J Cancer 1998, 75, 767–773. [Google Scholar] [CrossRef]
- Takano, Y.; Ohike, N.; Tajiri, T.; Asonuma, K.; Harada, K.; Takahashi, H.; Morohoshi, T. Gastric- and intestinal-type marker expression in invasive ductal adenocarcinoma of the pancreas. Hepatobiliary Pancreat Dis Int 2012, 11, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Jinfeng, M.; Kimura, W.; Hirai, I.; Sakurai, F.; Moriya, T.; Mizutani, M. Expression of MUC5AC and MUC6 in invasive ductal carcinoma of the pancreas and relationship with prognosis. Int J Gastrointest Cancer 2003, 34, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Aloysius, M.M.; Zaitoun, A.M.; Awad, S.; Ilyas, M.; Rowlands, B.J.; Lobo, D.N. Mucins and CD56 as markers of tumour invasion and prognosis in periampullary cancer. Br J Surg 2010, 97, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Takikita, M.; Altekruse, S.; Lynch, C.F.; Goodman, M.T.; Hernandez, B.Y.; Green, M.; Cozen, W.; Cockburn, M.; Sibug Saber, M.; Topor, M.; et al. Associations between selected biomarkers and prognosis in a population-based pancreatic cancer tissue microarray. Cancer Res 2009, 69, 2950–2955. [Google Scholar] [CrossRef] [PubMed]
- Higashi, M.; Yokoyama, S.; Yamamoto, T.; Goto, Y.; Kitazono, I.; Hiraki, T.; Taguchi, H.; Hashimoto, S.; Fukukura, Y.; Koriyama, C.; et al. Mucin expression in endoscopic ultrasound-guided fine-needle aspiration specimens is a useful prognostic factor in pancreatic ductal adenocarcinoma. Pancreas 2015, 44, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Krishn, S.R. Secretory Mucin MUC5AC in Gastrointestinal Malignancies. Theses & Dissertations 2016, 110.
- Ganguly, K.; Cox, J.L.; Ghersi, D.; Grandgenett, P.M.; Hollingsworth, M.A.; Jain, M.; Kumar, S.; Batra, S.K. Mucin 5AC–Mediated CD44/ITGB1 Clustering Mobilizes Adipose-Derived Mesenchymal Stem Cells to Modulate Pancreatic Cancer Stromal Heterogeneity. Gastroenterology 2022, 162, 2032–2046, e2012. [Google Scholar] [CrossRef]
- Ganguly, K.; Bhatia, R.; Rauth, S.; Kisling, A.; Atri, P.; Thompson, C.; Vengoji, R.; Ram Krishn, S.; Shinde, D.; Thomas, V.; et al. Mucin 5AC Serves as the Nexus for β-Catenin/c-Myc Interplay to Promote Glutamine Dependency During Pancreatic Cancer Chemoresistance. Gastroenterology 2022, 162, 253–268, e213. [Google Scholar] [CrossRef]
- Ganguly, K.; Krishn, S.R.; Jahan, R.; Atri, P.; Rachagani, S.; Rauth, S.; Xi, H.; Lu, Y.; Batra, S.; Kaur, S. Abstract 65: Gel-forming mucin MUC5AC as the nexus for cell-adhesion molecules governing pancreatic cancer aggressiveness and chemoresistance. Cancer Research 2019, 79, 65–65. [Google Scholar] [CrossRef]
- Ghaneh, P.; Palmer, D.; Cicconi, S.; Jackson, R.; Halloran, C.M.; Rawcliffe, C.; Sripadam, R.; Mukherjee, S.; Soonawalla, Z.; Wadsley, J.; et al. Immediate surgery compared with short-course neoadjuvant gemcitabine plus capecitabine, FOLFIRINOX, or chemoradiotherapy in patients with borderline resectable pancreatic cancer (ESPAC5): a four-arm, multicentre, randomised, phase 2 trial. The Lancet Gastroenterology & Hepatology 2022. [CrossRef]
- Sohal, D.; Duong, M.T.; Ahmad, S.A.; Gandhi, N.; Beg, M.S.; Wang-Gillam, A.; Wade, J.L.; Chiorean, E.G.; Guthrie, K.A.; Lowy, A.M.; et al. SWOG S1505: Results of perioperative chemotherapy (peri-op CTx) with mfolfirinox versus gemcitabine/nab-paclitaxel (Gem/nabP) for resectable pancreatic ductal adenocarcinoma (PDA). J Clin Oncol 2020, 38, 4504–4504. [Google Scholar] [CrossRef]
- Huffman, B.M.; Basu Mallick, A.; Horick, N.K.; Wang-Gillam, A.; Hosein, P.J.; Morse, M.A.; Beg, M.S.; Murphy, J.E.; Mavroukakis, S.; Zaki, A.; et al. Effect of a MUC5AC Antibody (NPC-1C) Administered With Second-Line Gemcitabine and Nab-Paclitaxel on the Survival of Patients With Advanced Pancreatic Ductal Adenocarcinoma. JAMA Network Open 2023, 6, e2249720. [Google Scholar] [CrossRef] [PubMed]
- Meyerholz, D.K.; Beck, A.P. Principles and approaches for reproducible scoring of tissue stains in research. Lab Invest 2018, 98, 844–855. [Google Scholar] [CrossRef]
- Ryan, R.; Gibbons, D.; Hyland, J.M.; Treanor, D.; White, A.; Mulcahy, H.E.; O'Donoghue, D.P.; Moriarty, M.; Fennelly, D.; Sheahan, K. Pathological response following long-course neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Histopathology 2005, 47, 141–146. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).