Submitted:
05 July 2024
Posted:
09 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Batch Adsorption Tests
2.3. Equilibrium and Kinetic Modeling
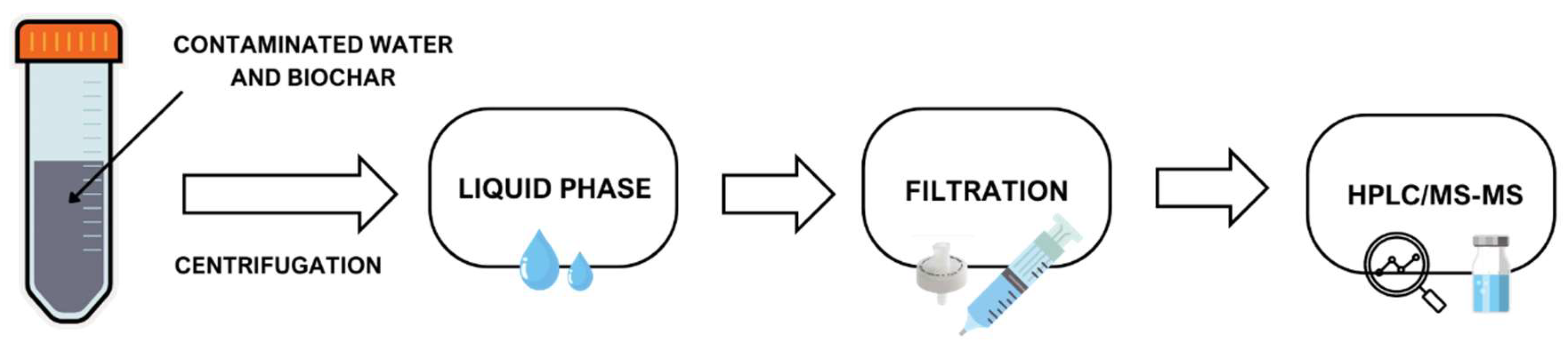
2.4. HPLC/MS-MS
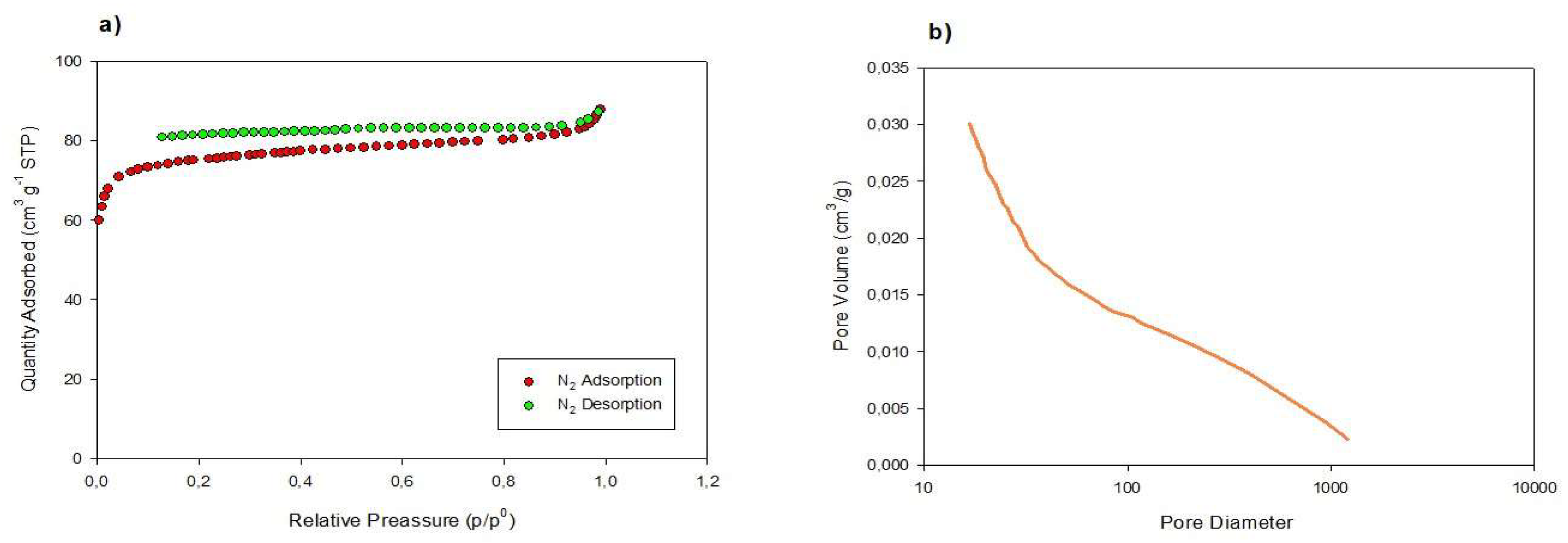
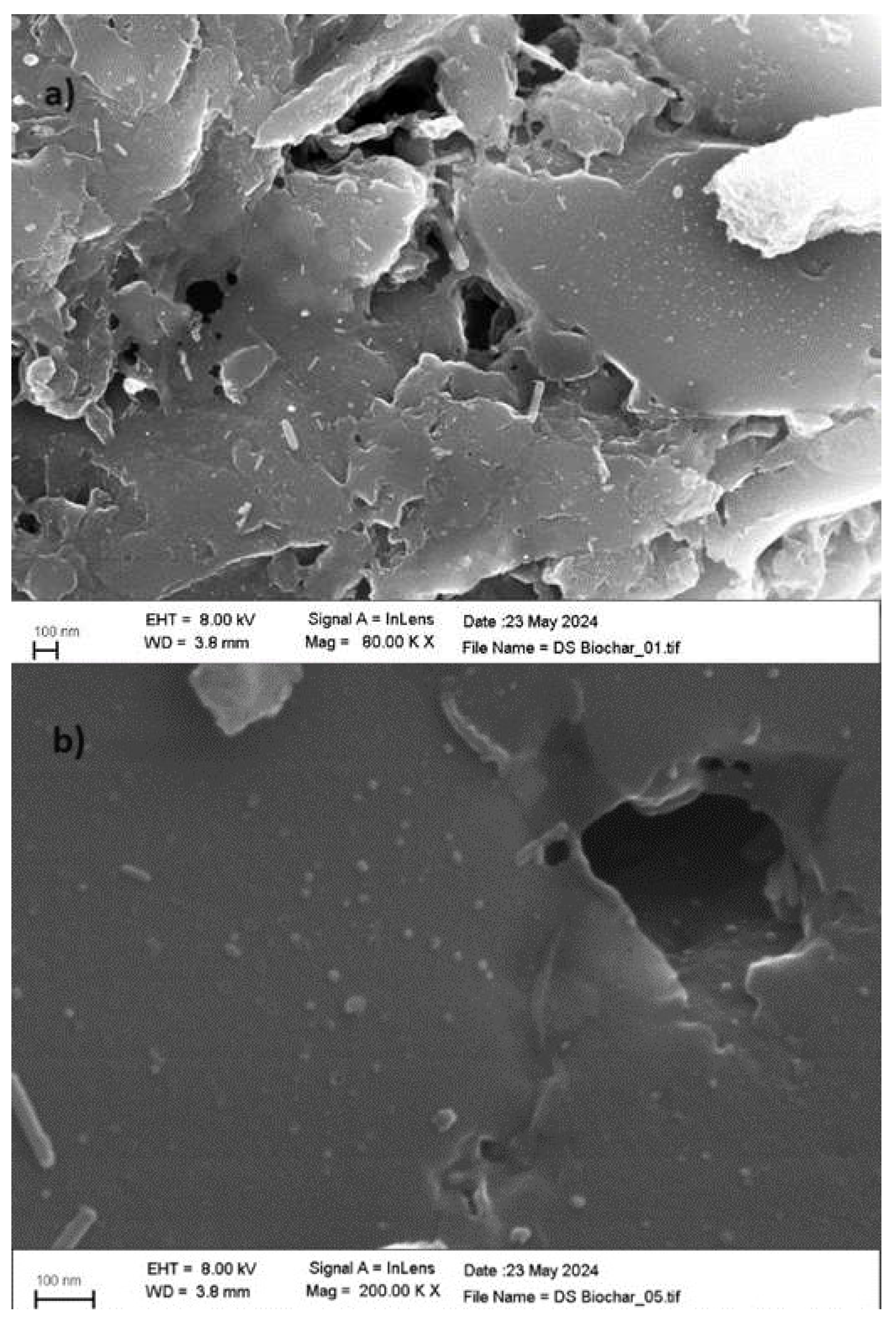
2.5. Textural Characterization and Morphology
3. Results and Discussion
3.1. Adsorbent Characterization of Date Biochar
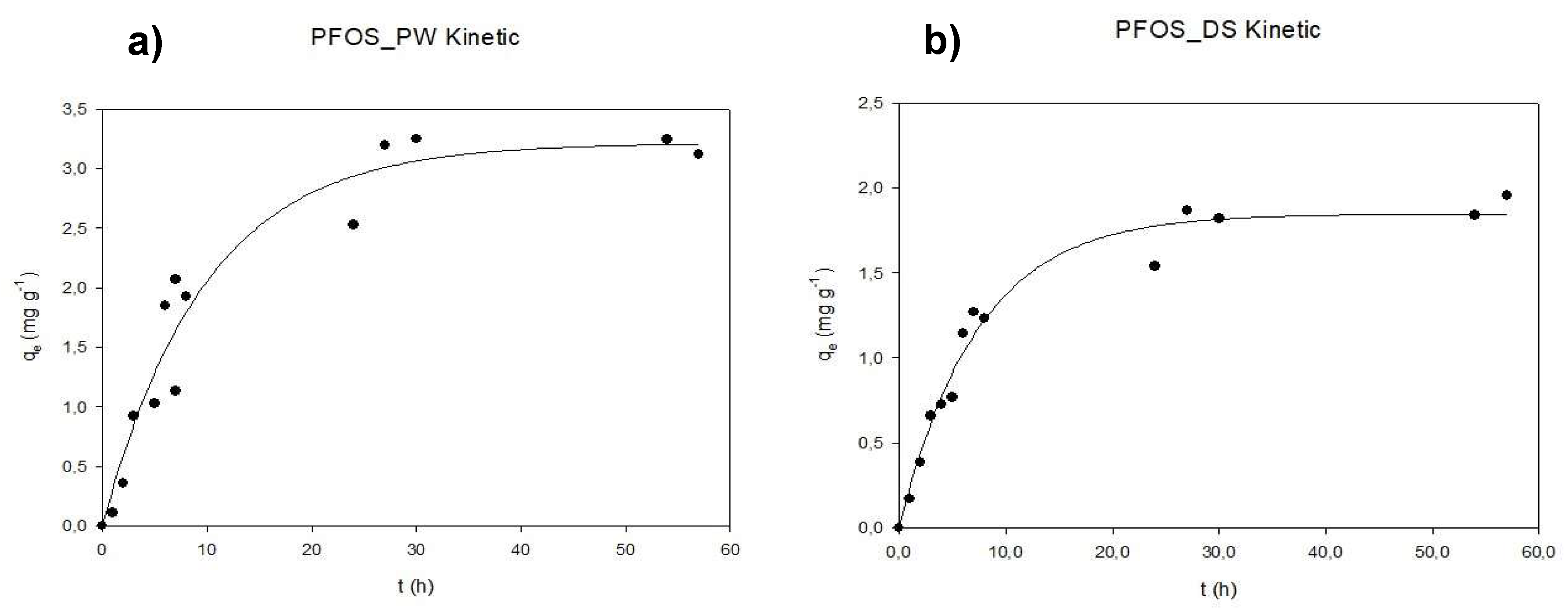
3.2. Kinetic Tests
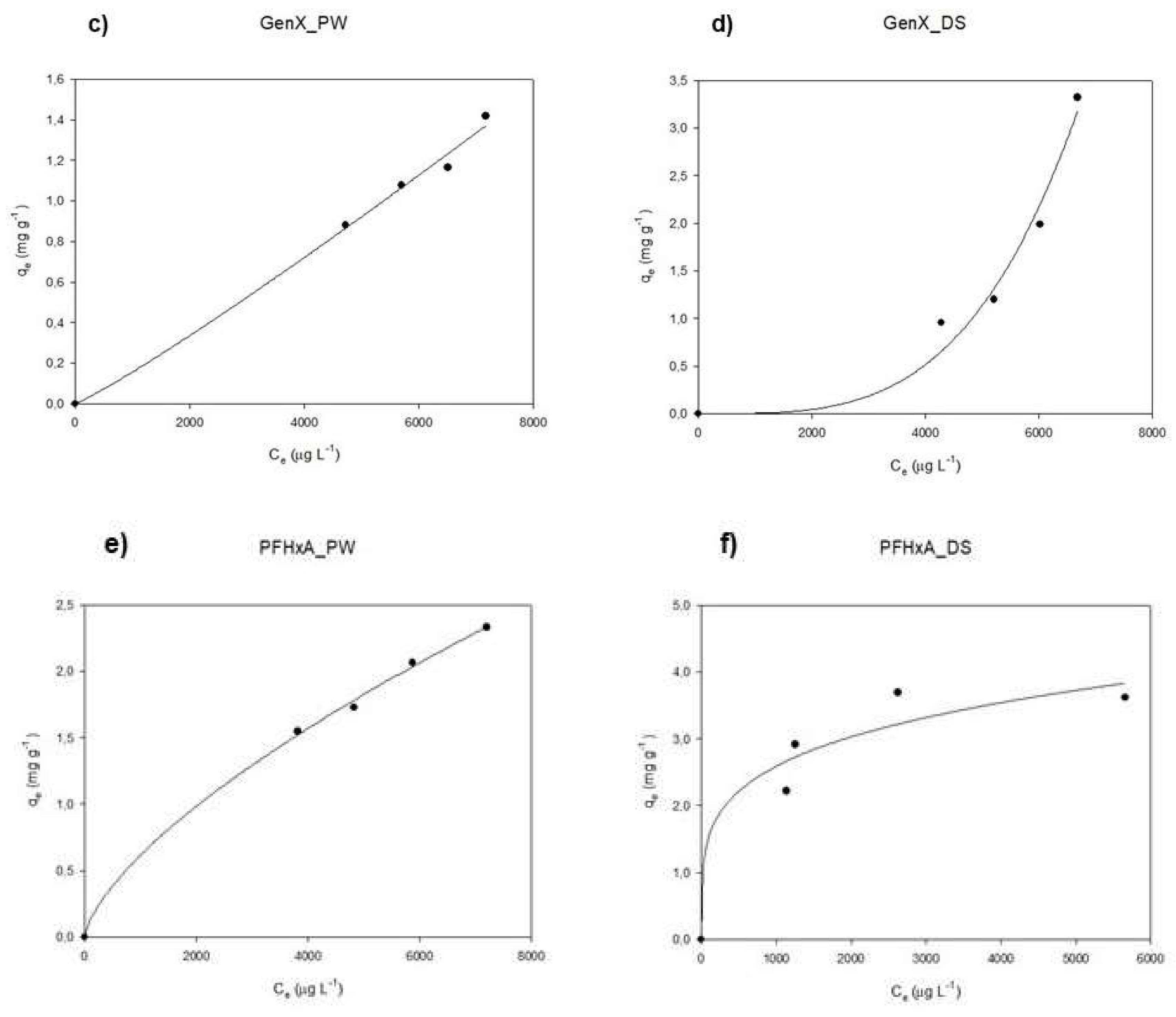
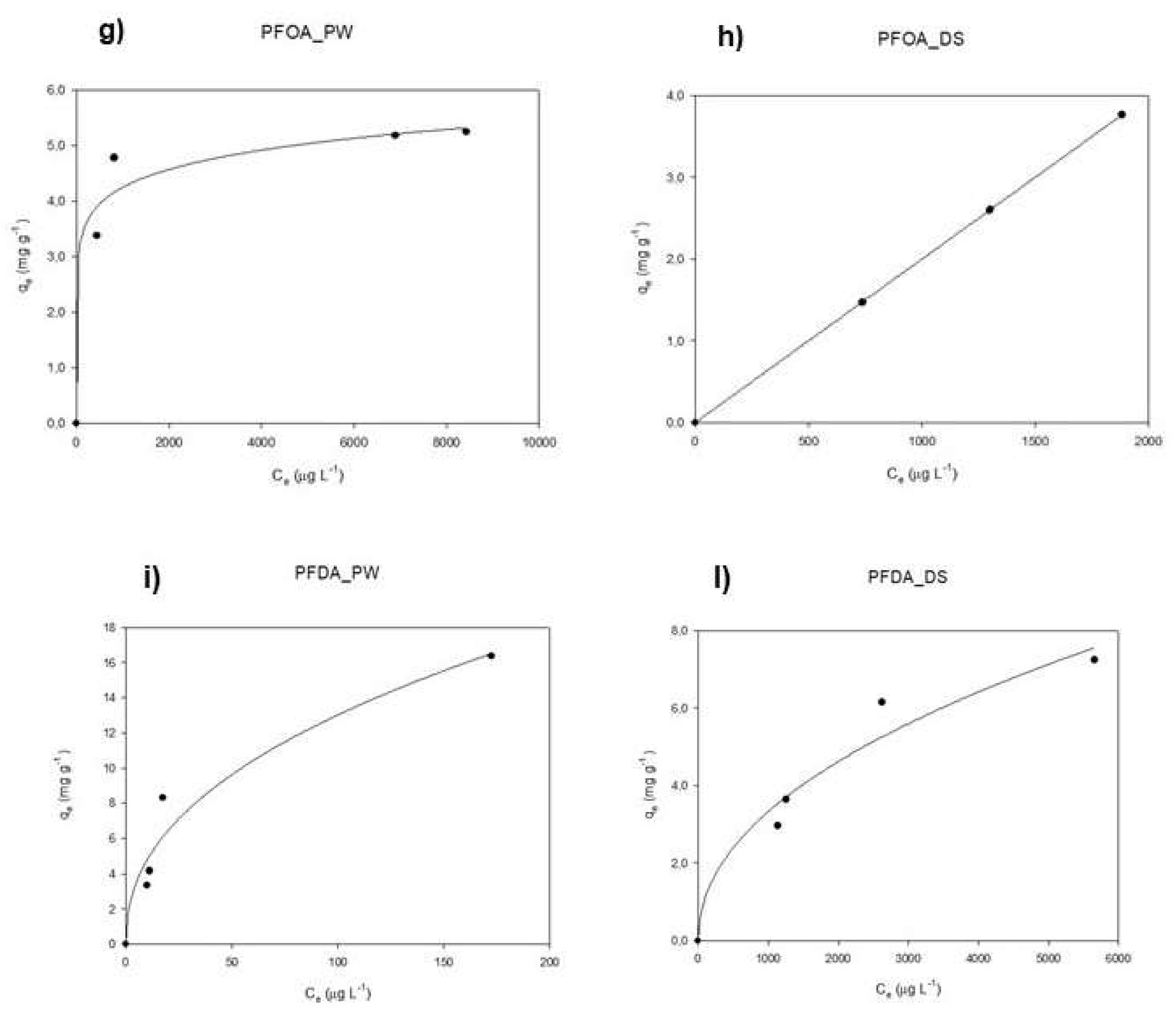
3.3. Isotherm Curves: Pinewood vs Date
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- European Chemical Agency (ECHA). Available online: https://echa.europa.eu/hot-topics/perfluoroalkyl-chemicals-pfas (Accessed on: 1 July 2024).
- Ng, C.A.; Hungerbühler, K. Bioconcentration of perfluorinated alkyl acids: how important is specific binding? Environmental Science & Technology 2013, 47, 7214–7223. [Google Scholar] [CrossRef]
- National Institute of Environmental Health Sciences NIEHS. Available at: https://www.niehs.nih.gov/health/topics/agents/pfc/index.cfm#footnote.
- Fenton, S.E.; Ducatman, A.; Boobis, A.; DeWitt, J.C.; Lau, C.; Ng, C. Smith, J.S.; Roberts, S.M. Per- and Polyfluoroalkyl Substance Toxicity and Human Health Review: Current State of Knowledge and Strategies for Informing Future Research. Environmental Toxicology And Chemistry 2021, 40, 606–630. [Google Scholar] [CrossRef]
- Rickard, B.P.; Rizvi, I.; Fenton, S.E. Per- and poly-fluoroalkyl substances (PFAS) and female reproductive outcomes: PFAS elimination endocrine-mediated effects and disease. Toxicology 2021, 465. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.C.; Johns, L.E.; Meeker, J.D. Serum Biomarkers of Exposure to Perfluoroalkyl Substances in Relation to Serum Testosterone and Measures of Thyroid Function among Adults and Adolescents from NHANES 2011–2012. International Journal of Environmental Research and Public Health 2015, 12, 6098–6114. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Dhana, K.; Furtado, J.D.; Rood, J.; Zong, G.; Liang, L.; Qi, L.; Bray, G.A.; DeJong, L.; Coull, B.; Grandjean, P.; Sun, Q. Perfluoroalkyl substances and changes in body weight and resting metabolic rate in response to weight-loss diets: a prospective study. PLoS Medicine 2018, 15, e1002502. [Google Scholar] [CrossRef]
- Bach, C.C.; Vested, A.; Jorgensen, K.; Bonde, J.P.; Henriksen, T.B.; Toft, G. Perfluoroalkyl and polyfluoroalkyl substances and measures of human fertility: a systematic review. Critical Reviews in Toxicology 2016, 46, 735–755. [Google Scholar] [CrossRef]
- Yan, N.; Ji, Y.; Zhang, B.; Zheng, X.; Brusseau, M.L. Transport of GenX in Saturated and Unsaturated Porous Media. Environmental Science & Technology 2020, 54, 11876–11885. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, H.; Cui, Q.; Sheng, N.; Yeung, L. W. Y.; Sun, Y.; Guo, Y.; Dai, J. Worldwide distribution of novel perfluoroether carboxylic and sulfonic acids in surface water. Environmental Science & Technology 2018, 52, 7621–7629. [Google Scholar] [CrossRef]
- Brandsma, S.H.; Koekkoek, J.C.; van Velzen, M. J. M.; de Boer, J. The PFOA substitute GenX detected in the environment near a fluoropolymer manufacturing plant in the Netherlands. Chemosphere 2019, 220, 493–500. [Google Scholar] [CrossRef]
- Environmental Protection Agency (EPA). Fact sheet: Draft toxicity assessments for GenX chemicals and PFBS. U.S. Washington DC. Available online: https://www.epa.gov/chemical-research/human-health-toxicity-assessments-genx-chemicals (Accessed on: 1 July 2024).
- Ahrens, L. Polyfluoroalkyl compounds in the aquatic environment: a review of their occurrence and fate. Environmental Monitoring and Assessment 2011, 13, 20. [Google Scholar] [CrossRef]
- Environmental Protection Agency (EPA). Drinking Water. Standards and Health Advisories. EPA 822-F-18-001. Washington DC. Available online: https://www.epa.gov/sdwa/drinking-water-health-advisories-has (Accessed on: 1 July 2024).
- Environmental Protection Agency. (EPA). Fact sheet: PFAS National Primary Drinking Water Regulation. Available online: https://dep.nj.gov/pfas/epa-pfas-rule/ (Accessed on: 1 July 2024).
- Ross, I.; McDonough, J.; Miles, J.; Storch, P.; Kochunarayanan, P.T.; Kalve, E.; Hurst, J.; Dasgupta, S.S.; Burdick, J. A review of emerging technologies for remediation of PFASs. Remediation 2018, 28, 101–126. [Google Scholar] [CrossRef]
- Meegoda, J.N.; Kewalramani, J.A.; Li, B.; Marsh, R.W. A Review of the Applications Environmental Release and Remediation Technologies of Per- and Polyfluoroalkyl Substances. International Journal of Environmental Research and Public Health 2020, 17, 8117. [Google Scholar] [CrossRef] [PubMed]
- Rahman, F.M.; Peldszus, S.; Anderson, W.B. Behaviour and fate of perfluoroalkyl and polyfluoroalkyl substances (PFASs) in drinking water treatment: A review. Water Research 2014, 50, 318–340. [Google Scholar] [CrossRef] [PubMed]
- McCleaf, P.; Englund, S.; Ostlund, A.; Lindegren, K.; Wiberg, K.; Ahrens, L. Removal efficiency of multiple poly- and perfluoroalkyl substances (PFASs) in drinking water using granular activated carbon (GAC) and anion exchange (AE). Water Research 2017, 120, 77–87. [Google Scholar] [CrossRef]
- Liu, C.J.; David, W.; Bellona, C. Removal of per- and polyfluoroalkyl substances (PFASs) from contaminated groundwater using granular activated carbon: a pilot-scale study with breakthrough modeling. Environmental Science: Water Research & Technology 2019, 5, 1844–853. [Google Scholar] [CrossRef]
- Najm, I.; Gallagher, B.; Vishwanath, N.; Blute, N.; Gorzalski, A.; Feffer, A.; Richardson, S. Per- and polyfluoroalkyl substances removal with granular activated carbon and a specialty adsorbent: A case study. AWWA Water Science 2021, 3, e1245. [Google Scholar] [CrossRef]
- Zhang, D.; He, Q.; Wang, M.; Zhang, W.; Liang, Y. Sorption of perfluoroalkylated substances (PFASs) onto granular activated carbon and biochar. Environmental Technology, 2019; 42, 1798–1809. [Google Scholar] [CrossRef]
- Aboughaly, M.; Fattah, I. Production of Biochar from Biomass Pyrolysis for Removal of PFAS from Wastewater and Biosolids: A Critical Review. Preprints 2023, 2023040309. [Google Scholar] [CrossRef]
- Silvani, L.; Vrchotova, B.; Kastanek, P.; Demnerova, K.; Pettiti, I.; Petrangeli, Papini, M. Characterizing Biochar as Alternative Sorbent for Oil Spill Remediation. Scientific Reports 2017, 7, 43912. [Google Scholar] [CrossRef] [PubMed]
- Sonego, E.; Simonetti, G.; Di Filippo, P.; Riccardi, C.; Buiarelli, F.; Fresta, A.; Olivastri, M.; Pomata, D. Characterization of organophosphate esters (OPEs) and polyfluoralkyl substances (PFASs) in settled dust in specific workplaces. Environmental Science and Pollution Research 2022, 29, 52302–52316. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P. H.; Teller, E. Adsorption of Gases in Multimolecular Layers. Journal of the American Chemical Society 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. Journal of the American Chemical Society 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Lippens, B.C.; de Boer, J.H. Studies on pore systems in catalysts: V. The t method Journal of Catalysis 1965, 4, 319–323. [Google Scholar] [CrossRef]
- Gurvitsch, L. Physicochemical attractive force. Russian Journal of Physical Chemistry 1915, 47, 805–827. [Google Scholar]
- Maziarka, P.; Sommersacher, P.; Wang, X.; Kienzl, N.; Retschitzegger, S.; Prins, W.; Hedin, N.; Ronsse, F. Tailoring of the pore structures of wood pyrolysis chars for potential use in energy storage applications. Applied Energy 2021, 286, 116431. [Google Scholar] [CrossRef]
- Libby, B.; Monson, P.A. Adsorption/desorption hysteresis in inkbottle pores: a density functional theory and Monte Carlo simulation study. Langmuir, 2004; 20, 4289-94. [Google Scholar] [CrossRef]
- Fan, C.; Do, D. D.; Nicholson, D. On the Cavitation and Pore Blocking in Slit-Shaped Ink-Bottle Pores. Langmuir 2011, 27, 3511–3526. [Google Scholar] [CrossRef]
- Lei, X.; Lian, Q.; Zhang, X.; Karsili, T.K.; Holmes, W.; Chen, Y.; Zappi, M.E.; Gang, D.D. A review of PFAS adsorption from aqueous solutions: Current approaches engineering applications challenges and opportunities. Environmental Pollution 2023, 321, 121138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; He, Q.; Wang, M.; Zhang, W.; Liang, Y. Sorption of perfluoroalkylated substances (PFASs) onto granular activated carbon and biochar. Environmental Technology 2019, 42, 1798–1809. [Google Scholar] [CrossRef] [PubMed]
- Ziwen, D.; Shubo, D.; Yue, B.; Qian, H.; Bin, W.; Jun, H.; Gang, Y. Adsorption behavior and mechanism of perfluorinated compounds on various adsorbents—A review. Journal of Hazardous Materials 2014, 274, 443–454. [Google Scholar] [CrossRef]
- Luft, C.M.; Schutt, T.C.; Shukla, M.K. Properties and Mechanisms for PFAS Adsorption to Aqueous Clay and Humic Soil Components. Environmental Science & Technology 2022, 56, 10053–10061. [Google Scholar] [CrossRef]
- Yu, Q.; Zhang, R.; Deng, S.; Huang, J.; Yu, G. Sorption of perfluorooctane sulfonate and perfluorooctanoate on activated carbons and resin: Kinetic and isotherm study. Water Research 2009, 43, 1150–8. [Google Scholar] [CrossRef]
- Zhang, D.; Luo, Q.; Gao, B.; Chiang, S.Y.; Woodward, D.; Huang, Q. Sorption of perfluorooctanoic acid; perfluorooctane sulfonate and perfluoroheptanoic acid on granular activated carbon. Chemosphere 2015, 144, 2336–42. [Google Scholar] [CrossRef] [PubMed]
- Lawrance, G.A. Coordinated trifluoromethanesulfonate and fluorosulfate. Chemical Reviews 1986, 86, 17e33. [Google Scholar] [CrossRef]
- Ochoa-Herrera, V.; Sierra-Alvarez, R. Removal of perfluorinated surfactants by sorption onto granular activated carbon zeolite and sludge. Chemosphere 2008, 72, 1588–1593. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.C.; Børresen, M.H.; Schlabach, M.; Cornelissen, G. Sorption of perfluorinated compounds from contaminated water to activated carbon. Journal of Soil and Sediments 2010, 10, 179–185. [Google Scholar] [CrossRef]
- Inyang, M.; Dickenson, E.R.V. The use of carbon adsorbents for the removal of perfluoroalkyl acids from potable reuse systems. Chemosphere 2017, 184, 168–175. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements; opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas; methods; instructions or products referred to in the content. |
| Pinewood | ||
|---|---|---|
| Surface area (m2 g-1) | Volume pore (m3 g-1) | |
| Total | 343±2 | 0.383 |
| Micropores | 224 | 0.136 |
| Mesopores | 119 | 0.247 |
| Date seeds | ||
| Surface area (m2 g-1) | Volume pore (m3 g-1) | |
| Total | 290±4 | 0.136 |
| Micropores | 270 | 0.110 |
| Mesopores | 20 | 0.026 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





