1. Introduction
With the increasing demand for energy storage solutions, there is a need to develop novel and efficient electrolytes for batteries. These electrolytes should have high ionic conductivity, good stability, and compatibility with different electrode materials [
1,
2,
3]. The search for novel ionic liquid crystals as electrolytic ion charges carriers has gained momentum in recent years due to their potential in addressing the existing limitations of traditional electrolytes. This emerging class of materials offers unique properties such as tunable mesophase behavior, high ionic conductivity, and improved electrochemical stability, making them promising candidates for use in advanced energy storage devices.
Ionic liquid crystals are designed to exhibit both ionic conductivity and liquid crystalline ordering, a combination that is essential for efficient ion transport and electrode interactions within the battery system. By carefully tailoring the molecular structure and composition of these materials, researchers have been able to achieve significant advancements in enhancing their performance as electrolytes.
Furthermore, the compatibility of ionic liquid crystals with a wide range of electrode materials presents an opportunity to design custom-tailored electrolyte systems for specific battery chemistries, further expanding their potential applications in energy storage. As a result, ongoing research efforts are focused on exploring the full potential of these novel materials and optimizing their properties to meet the demands of next-generation energy storage technologies. The commercialization of lithium-ion batteries has greatly impacted our daily lives by enabling portable electronic devices, revolutionizing various industries including communications, entertainment, medicine, and more [
4]. However, to meet the growing demand for energy storage in sectors such as electric vehicles and renewable power systems, there is a need for further advancements in lithium-ion technologies. One of the major challenges in lithium-ion batteries is the instability of Li metal towards electrolytes, which limits their performance and safety. To address this challenge, researchers have been investigating the use of novel electrolyte solutions comprising ionic liquid crystals. These ionic liquid crystals, with their unique properties such as high ionic conductivity and tunable mesophase behavior, have the potential to overcome the existing limitations of traditional electrolytes and enhance the performance of lithium-ion batteries. The development of these new electrolytes not only improves the stability and safety of Li-metal batteries but also enables high-performance at a wide range of temperatures.
As research and development efforts continue to explore the full potential of ionic liquid crystals as electrolytic ion charges carriers, the prospects for their widespread application in next-generation energy storage technologies appear increasingly promising. The pursuit of tailored electrolyte systems and the optimization of the properties of these novel materials are key areas of focus as the energy storage landscape continues to evolve. The utilization of ionic liquid crystals as electrolytic ion charges carriers in energy storage applications offers potential advancements in stability, performance, and safety [
5].
This research is representing two novel ionic liquid crystals from zwitterions category for assisting the lithium ion in the Lithium Battery Cells and energy storage devices. These materials had been characterized by different tools to confirm the chemical structure, mesomorphic behavior and the ionic conductivity through cyclic voltammetry [
6,
7,
8].
2. Materials and Methods
All materials were purchased are high analytical grade of purity, and n further purifications were made. 4-(Dimethylamino) pyridine, 1,4-butane sultone, 4-Benzhydrylpyridine 4 and toluene were purchased from Sigma-Aldrech, Germany.
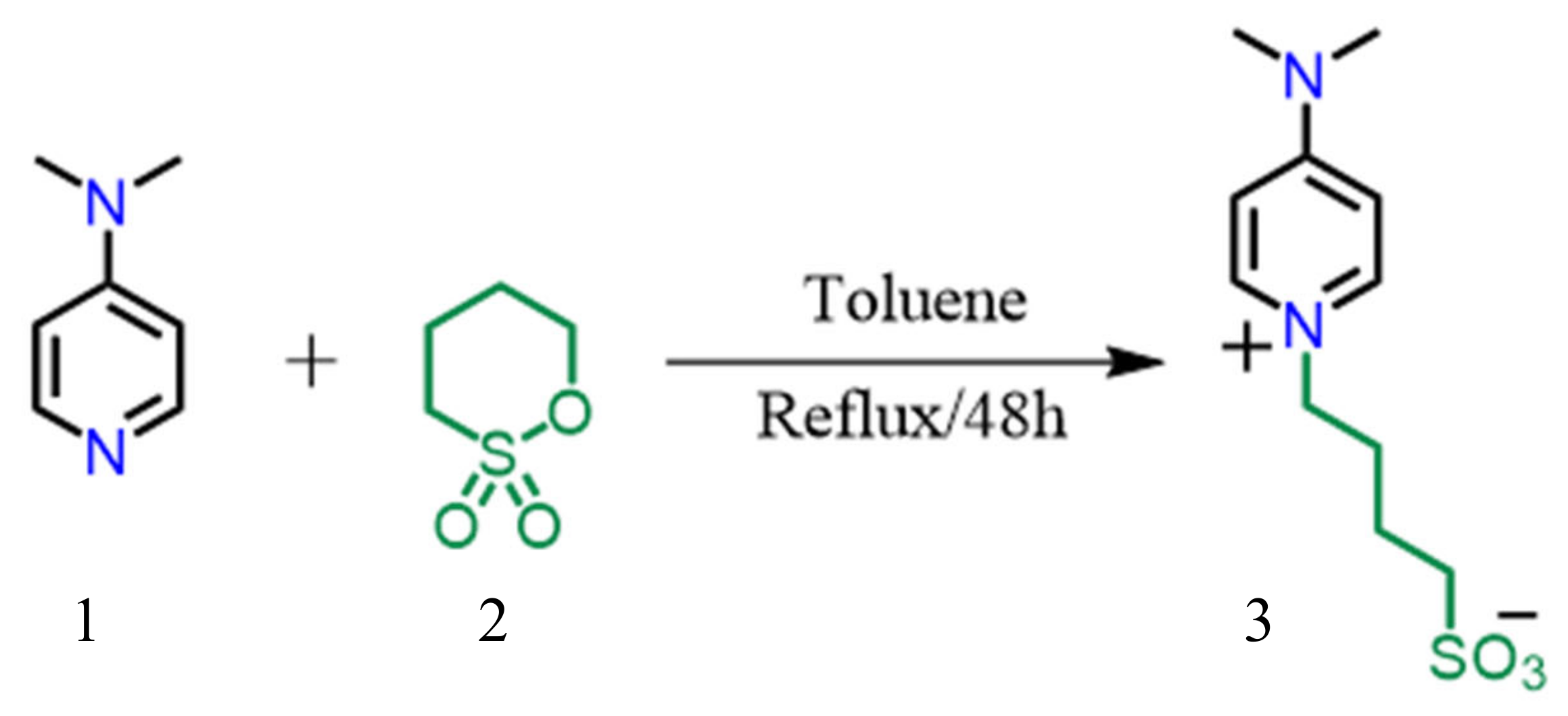
2.1. Synthesis of 4-(4-(Dimethylamino) pyridin-1-ium-1-yl)butane-1-sulfonate, will be known as (ILC1)
4-(Dimethylamino) pyridine 1 (5 g, 0.041 mmol) was dissolved in dry toluene (50 mL). To this mixture was added 1,4-butane sultone 2 (4.2 mL, 0.041 mmol) and the mixture was refluxed for 48h under nitrogen atmosphere. The white precipitate was filtered, washed with dry toluene, dry diethyl ether, and dried to afford the novel compound 3 (9.11 g).
Scheme 1.
Synthetic pathway for zwitterions ILC1 and ILC2.
Scheme 1.
Synthetic pathway for zwitterions ILC1 and ILC2.
2.2. Characterizations
Many techniques were used for confirming the structure and liquid crystals behavior such as proton- nuclear magnetic resonance H-NMR, Fourier Transformation infrared spectroscopy FT-R by Bruker, USA. The X-ray diffraction XRD was performed by D8 discovery USA, and polarizing optical microscopy POM using Leica DM750P (Leica micros-system, Switzerland) The charge and size of molecules were determined by Dynamic light scattering DLS and zeta potential were carried out by Nicomp PSS, USA.
3. Results and Discussion
3.1. Structure confirmation and characterizations
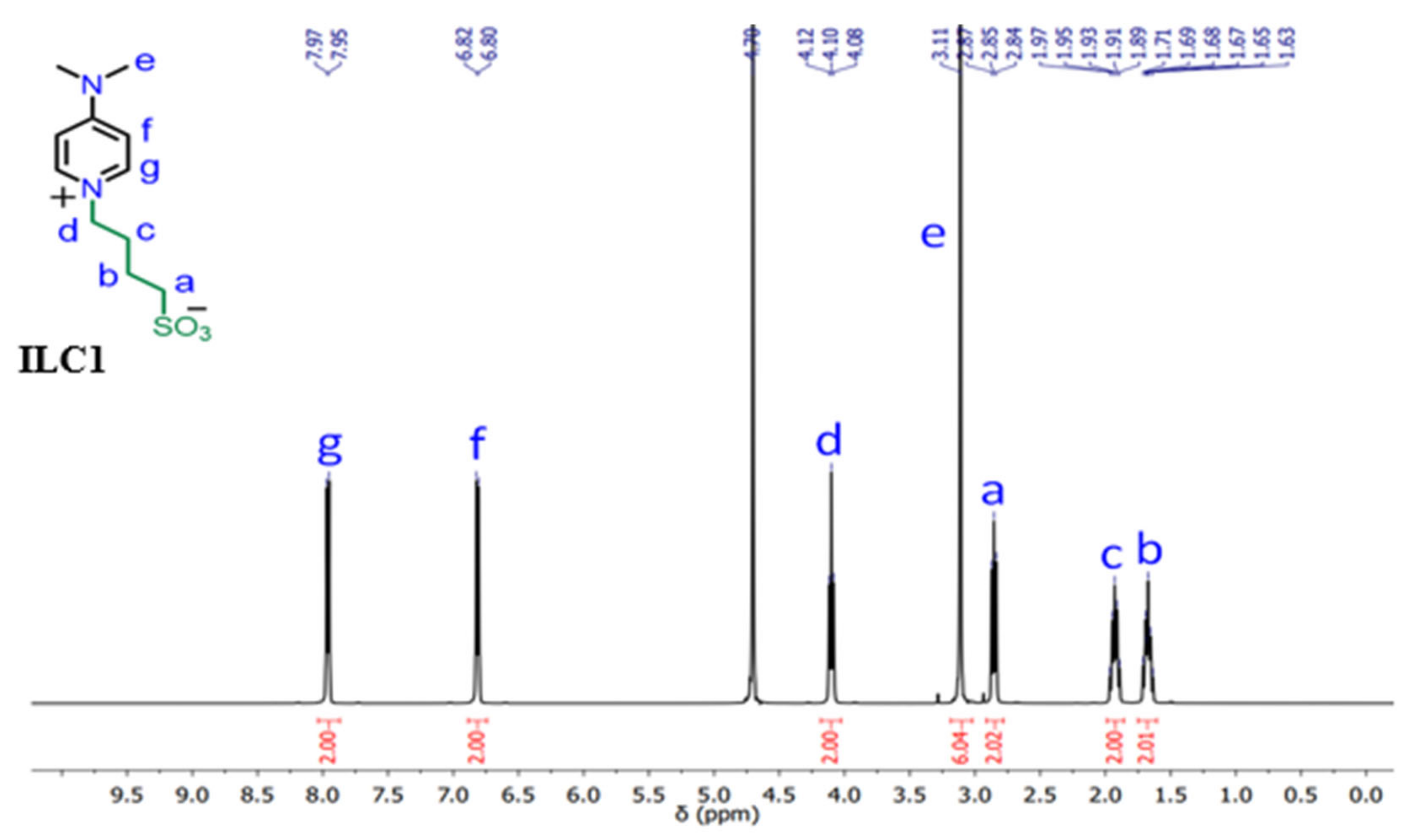
The 1H NMR spectrum of the novel compound ILC1 displayed the signals of the butyl chain at δ 1.63–1.71, 1.89–1.97, 2.84–2.87, 4.08–4.12 ppm. The methylene group CH2d appeared downfield shifted indicating direct linking to the pyridinium nitrogen atom. The signal of the two methyl groups appeared at δ 3.11 ppm. The signals of the pyridinium CH groups appeared doublet at δ 6.81 and 7.96 ppm with a coupling constant J = 7.6 Hz. The 1H NMR data of compounds ILC1 is described in details as follows:
1H-NMR (400 MHz, D2O, 25 °C): δ (ppm) = 1.63–1.71 (m, 2H, CH2), 1.89–1.97 (m, 2H, CH2), 2.84–2.87 (m, 2H, CH2), 4.08–4.12 (m, 2H, CH2), 3.11 (s, 6H, CH3), 6.81 (d, J = 7.6 Hz, 2H, ArH), 7.96 (d, J = 7.6 Hz, 2H, ArH). As shown in
Figure 1.
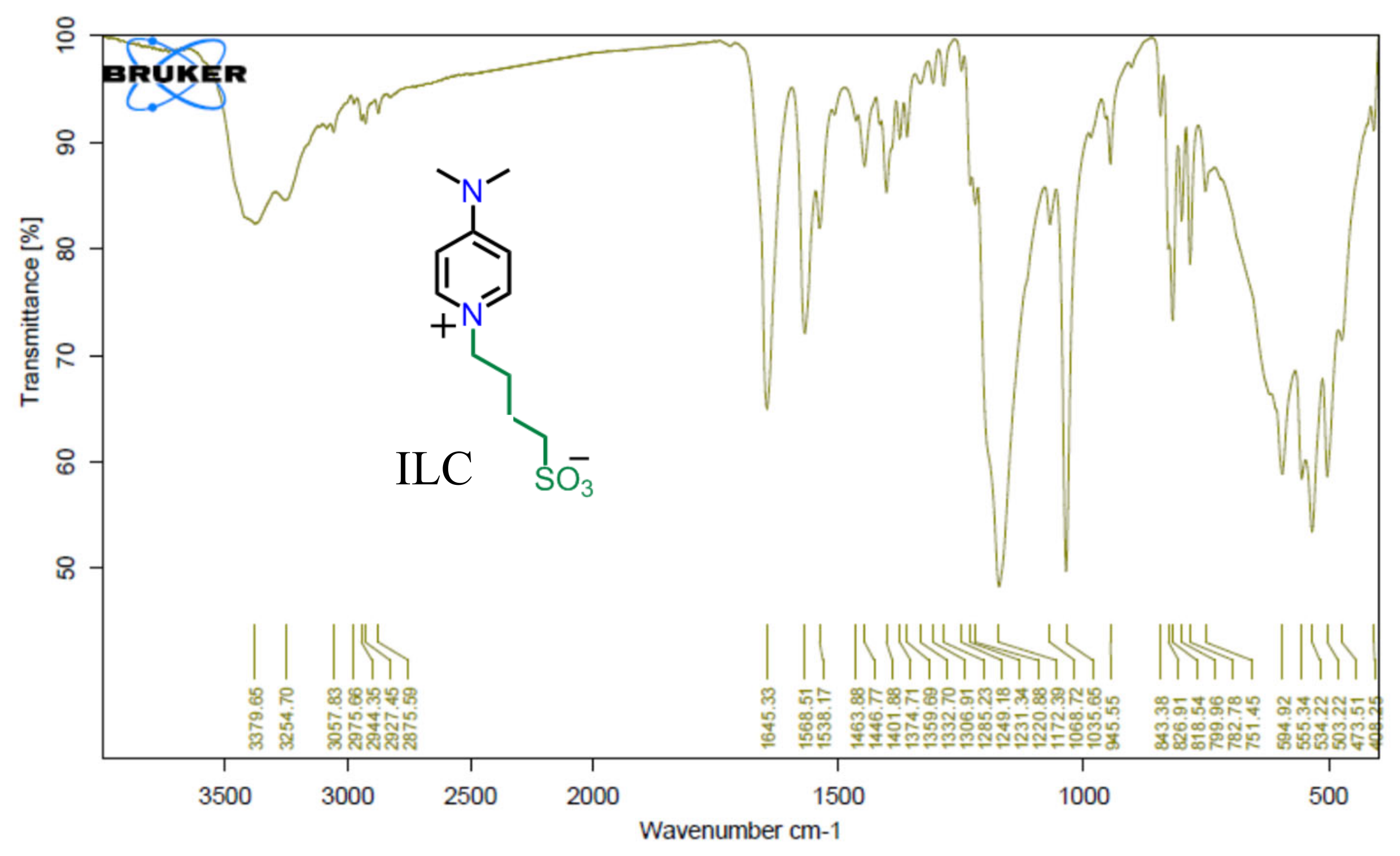
The functional group of both ILC1 and ILC2 are confirmed by FT-IR techniques, which is a powerful spectroscopic tool for this purpose. The FTIR spectrum of compound ILC1 showed an absorption band at ν 1179 cm
-1 assigned to the -SO3 stretching vibrations as demonstrated in
Figure 2. The pyridinium C=N group appeared as a strong absorptions band at ν 1645 cm
-1. The aromatic C-H stretching mode is appeared clearly at 3057 cm
-1 while the aliphatic C-H stretching peak is shown at 2975 cm
-1, 2944 cm-
1 and 2927 cm
-1. The very strong peak for C-N stretching bond in ILC1 is shown at 1035 cm
-1.
It can be seen
Figure 2, the high resolution of the stretching and bending modes and their corresponding peaks of C-H stretching aliphatic at 2972 cm
-1 and 2901 cm
-1. Whereas the stretching C-H aromatic at 3123 cm
-1 and 3049 cm
-1 for both phenyl groups. Again the SO3 at frequency ν 1178.98 cm
-1, while the pyridinium C=N group appeared as a strong absorption band at frequency ν 1640 cm
-1. The typical very strong peak for C-N stretching bond in ILC2 is shown at 1034 cm
-1. This regarding to typical aliphatic tail which has position of the zwitterion.
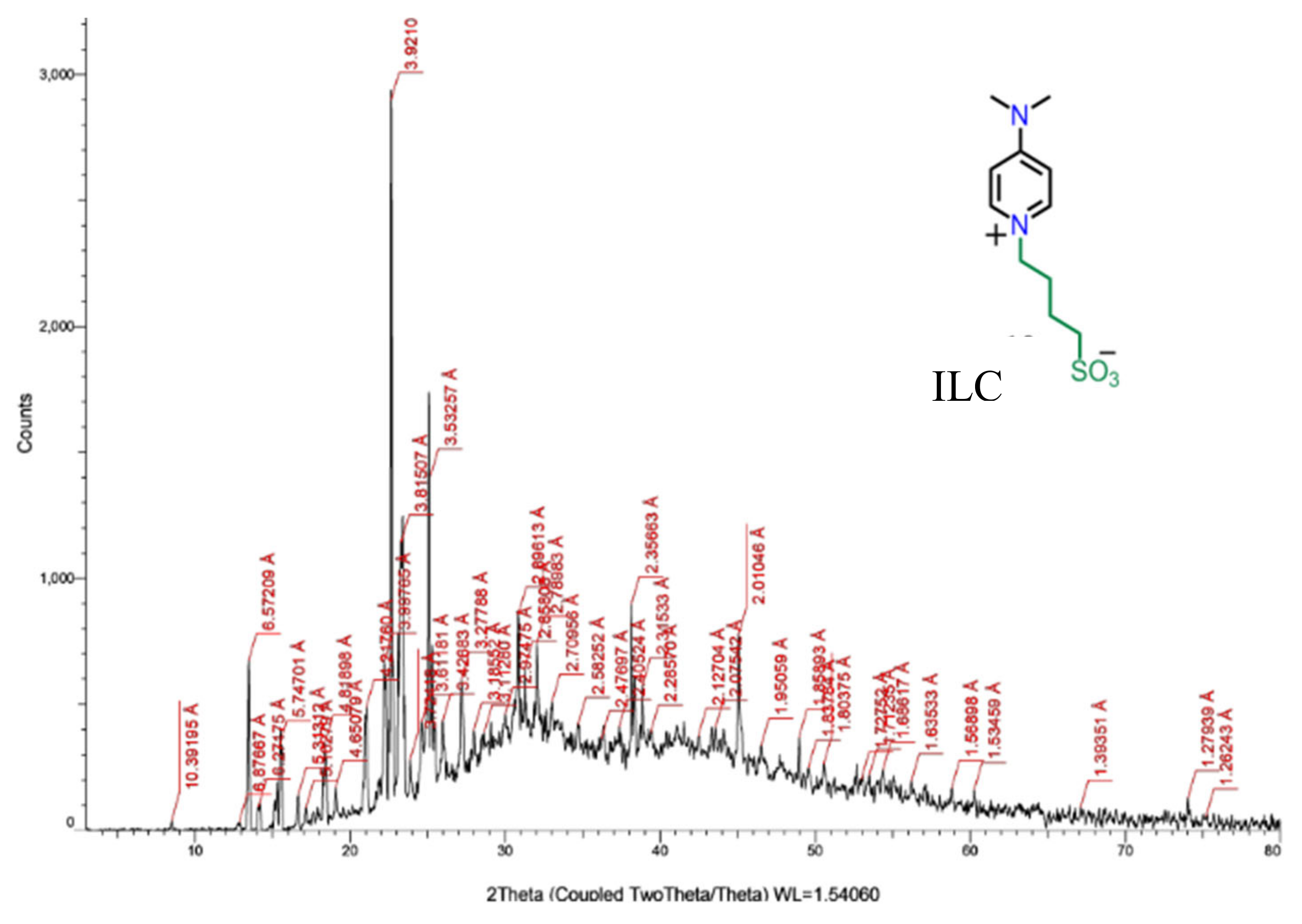
The crystallinity of these ILC1 and ILC2 has been revealed by XRD analysis, which these materials are exhibiting the crystalline phase at the room temperature. The phase structure of the pyridine derivative composing the ionic liquid crystals is shown in the
Figure 3. It seems from these patterns the phase is monoclinic [
11] as well the molecules are stacked in layers. The d-spacing are allocated on the XRD pattern as shown in
Figure 4.
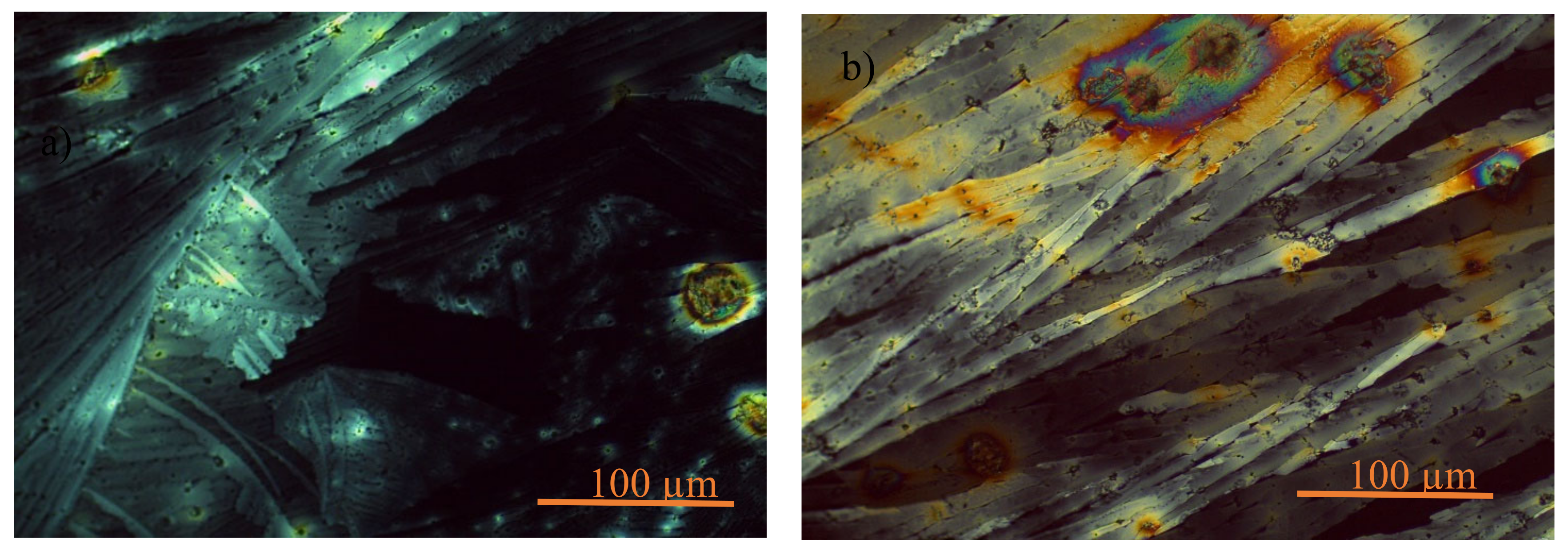
The texture of ILC1 is examined by polarizing optical microscope POM Leica DM750P, the crystals are shown in
Figure 4. The texture of both ILC1 and ILC2 is shown at the room temperature, which the phase is crystalline phase and the molecules are assembled in layers as similar as smectic phase or lamellar phase. Therefore
Figure 4 is showing the typical texture of book shelves with the same domain. This texture gives 2D pathways and channel for ion charge transfers very easily and being suitable to energy storage applications [
9,
10].
3.2. Particle sizes and charging
The molecules size play crucial role for facilitating ion transfer and charge density carrier. Therefore, the size of ILC1 is determined by using dynamic light scattering techniques DLS.
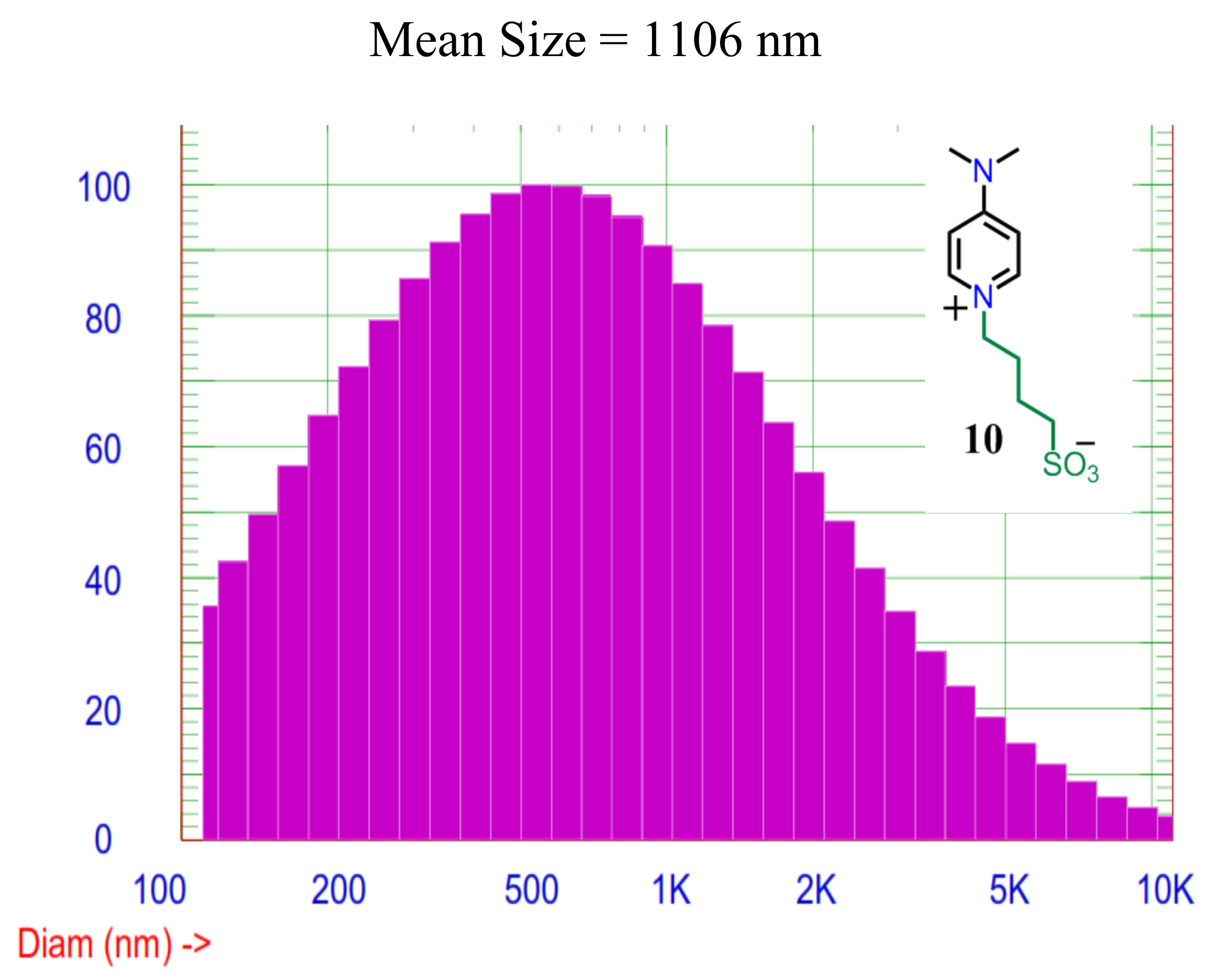
Figure 5 shows the size of molecules using laser scattering intensities. It can be noted that, the DLS technique is blind selectivity experimental type which choosing size is out control on contrary of scanned SEM or transmitted TEM electron microscopy techniques. Therefore, we used different calculations models to have accumulative idea about the self-assemblies of ILCs molecules in aqueous media [
12].
The zwitterion ionic liquid crystals ILC1 is soluble in Deionized water 18.0MΩ Millipore, and the result of DLS is representing the actual status of ionic liquid crystals when they are acting the electrolyte. In
Figure 5, the intensity of scattered laser beam depending on the different assemblies’ size and volume and weight after fitting to the Gaussian distribution. The mean size is in range of microscale from 1.1 µm to 2.7 µm, while from the figure, the size distribution fitted by Gaussian starts from 0.12 µm to 10 µm in the scattered intensity distribution [
13].
3.3. Zeta Potential (ζ-Potential - Charges Mobility):
The electrical potential around the charged particles or molecules suspended in solution in specific area is called zeta-potential. Thus, zeta-potential is a scientific term for electrokinetic potential in colloidal dispersion [
10]. The ζ-Potential is commonly in the mV and measured by the zeta potential analyzer by applying low electric field and tracking the intensity of light beam scattered by the charged particles.
The ILCs materials have zeta potential values which are considered as clues about the mobility and charge carriers even in the absence of the Li+ ions and/or other ions which are used in the modern batteries [
14].
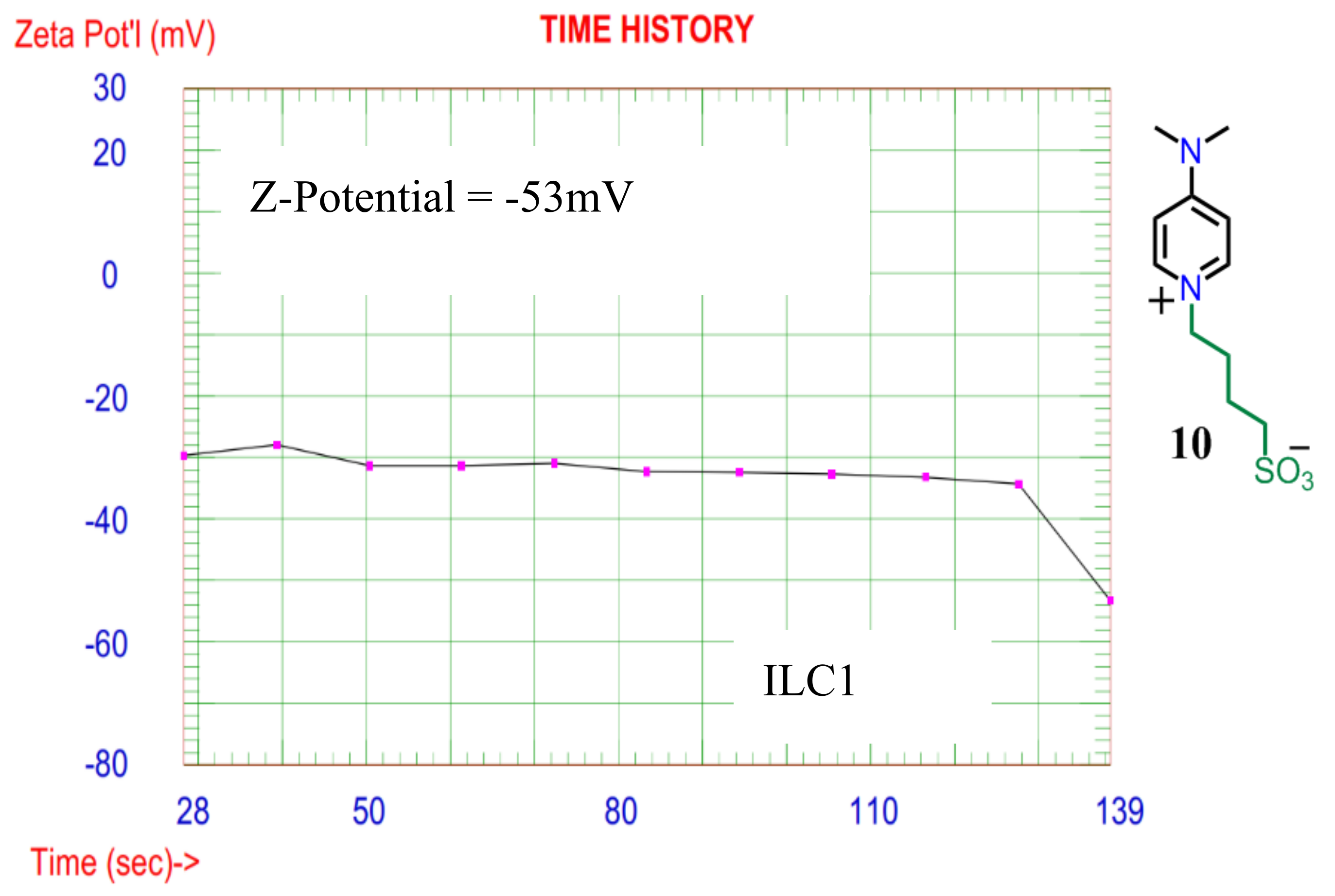
The zeta-potential plot as shown in
Figure 6, ILC1 have considerable stability as colloidal substance and its zeta value is about -53mV. This means the SO3
- is the partially dominant charge surrounding the molecules of ILC1. Thus, the material ILC1 is suitable to transport Li+ ions in the Li-polymer Battery types.
Conclusions
In conclusion, the utilization of ionic liquid crystals as electrolytic ion charges carriers in energy storage applications presents a promising avenue for addressing the current limitations of traditional electrolytes. The unique properties of ionic liquid crystals, including their tunable mesophase behavior, high ionic conductivity, and compatibility with a wide range of electrode materials, offer significant potential for enhancing the performance, stability, and safety of energy storage devices.
As research and development efforts continue to advance, the two tailored design of electrolyte systems using ionic liquid crystals ILC1 hold great promise for meeting the demands of next-generation energy storage technologies. The potential for improved stability and performance of lithium-ion batteries, particularly in electric vehicles and renewable power systems, underscores the significance of further exploring the capabilities of these novel materials.
Overall, the exploration of ionic liquid crystals as electrolytic ion charges carriers in energy storage applications offers a pathway towards achieving advancements in stability, performance, and safety, thereby contributing to the continued evolution of the energy storage applications.
Author Contributions
Ahmad M. Labeeb: Methodology, Conceptualization, Formal analysis, Investigation, Software, Visualization, Writing – original draft, Writing – review & editing. Hany F. Nour: Methodology, Conceptualization, Formal analysis, Software, Writing – original draft, Writing – review & editing. Tamer ElMalah: Writing – review & editing. Azza Ward: review and consultancy. Mohamed Fikry: Methodology, Conceptualization, Writing – review & editing. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
The authors do not have permission to share data.
Acknowledgments
This project was supported financially by the Science and Technology Development Fund (STDF) Egypt, Grant No. 38109.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Data availability Data will be made available on request.
References
- Abramov, B. Maiti, O. Reiser and D. Díaz Díaz, Chem. Commun., 2021, 57, 7762–7765.
- D. Liu, Y. Lu, Y. Lin and G. Jin, Chem. Eur. J., 2019, 25, 14785–14789.
- Z. Wang, N. Liu, H. Li, P. Chen and P. Yan, Eur. J. Inorg. Chem., 2017, 2017, 2211–2219.
- B. M. P. Beebeejaun-Boodoo, R. Erasmus and M. Rademeyer, CrystEngComm., 2018, 20, 4875–4887.
- Ahmad M. Labeeb, Yassmin A. Ward, and Mohamed Fikry. "Thermal Control of Tunable Photonic Optical Bandgaps in Different Cholesteric Liquid Crystals Mixtures." Journal of Molecular Liquids (2021): 117179.
- M. Labeeb, A. A. Ward, S. Ibrahim, F. Fouad, and R. M Ramadan. “Novel nanocomposites based on Tetrazine liquid crystals for energy storage application” Journal of Molecular Liquids 392, (2023), 123495.
- T. El-Malah, H. F. Nour, T. A. Khattab, S. Ibrahim, A. M. Labeeb. “Substituents role in highly symmetrical 1, 2, 3-triazoles derivatives toward self-assembly of soft liquid crystals”. Journal of Molecular Liquids 382, (2023), 121978.
- H. M. Abdulnaby, I. Elkashef, S. Ibrahim, A. M. Labeeb. “Synthesis of Silver Nanoparticles with Different Decoration Forms Dispersed in Nematic Liquid Crystals”. Egyptian Journal of Chemistry 67 (2), (2024). 601-613.
- Averett, Lacey A., Peter R. Griffiths, and Koichi Nishikida. "Effective path length in attenuated total reflection spectroscopy." Analytical chemistry 80.8 (2008): 3045-3049.
- Singh, Rajshree, et al. "Highly selective fluorescence ‘turn off’sensing of picric acid and efficient cell labelling by water-soluble luminescent anthracene-bridged poly (N-vinyl pyrrolidone)." Analyst 144.11 (2019): 3620-3634.
- Cheng, Lin, et al. "Chiral Ag (Ⅰ) Coordination Polymer Based on an α, α-L-Diaryl Prolinol-Pyridine Derivative: Circular Dichroism, SHG Response and Luminescent Property." Chinese Journal of Inorganic Chemistry 36.2 (2020): 361-367.
- Stetefeld, Jörg, Sean A. McKenna, and Trushar R. Patel. "Dynamic light scattering: a practical guide and applications in biomedical sciences." Biophysical reviews 8.4 (2016): 409-427.
- Bhattacharjee, Sourav. "DLS and zeta potential–what they are and what they are not?." Journal of controlled release 235 (2016): 337-351.
- Dutta, Debashis. "Transport of charged samples in fluidic channels with large zeta potentials." Electrophoresis 28.24 (2007): 4552-4560.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).