Submitted:
08 July 2024
Posted:
09 July 2024
You are already at the latest version
Abstract
Keywords:
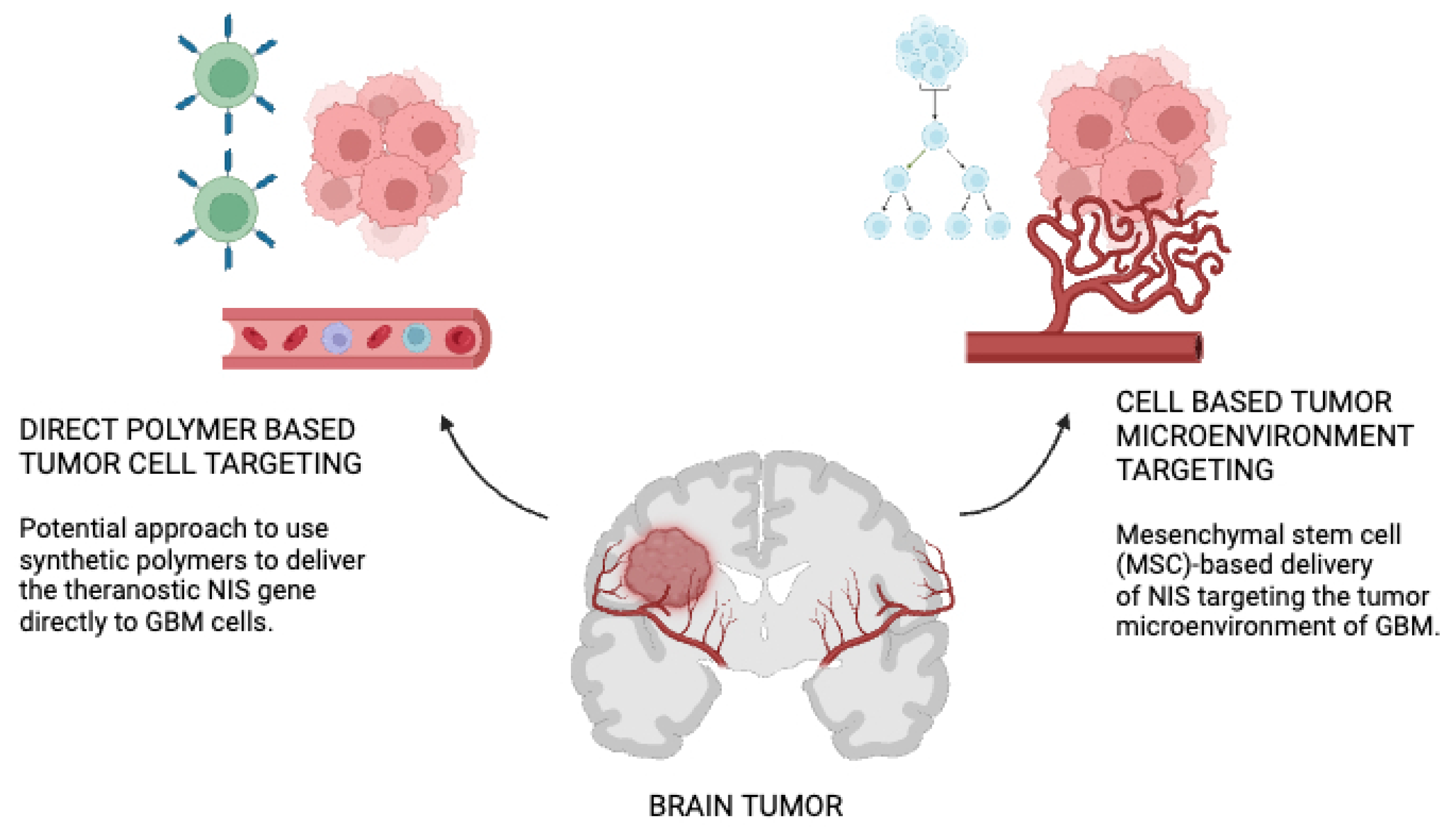
1. Introduction
2. Mesenchymal Stem Cells as Carriers of NIS Genes
2.1. Origin and Differentiation Potential
2.2. Immunomodulatory Properties
2.3. Tumor-Tropic Migration
3. Mechanism of NIS Gene Delivery
Advantages and Limitations
4. Image-Guided Approaches in Radionucleotide Therapy
5. Image-Guided NIS Radionucleotide Therapy in Glioblastoma
6. Challenges and Future Directions Discussion on Image-Guided NIS Radionucleotide Therapy for Glioblastoma
7. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shah, S. Novel Therapies in Glioblastoma Treatment: Review of Glioblastoma; Current Treatment Options; and Novel Oncolytic Viral Therapies. Med Sci (Basel). 2023, 12, 1. [Google Scholar] [CrossRef]
- Schaff, L.R.; Mellinghoff, I.K. Glioblastoma and Other Primary Brain Malignancies in Adults: A Review. JAMA. 2023, 329, 574–587. [Google Scholar] [CrossRef] [PubMed]
- Czarnywojtek, A.; Borowska, M.; Dyrka, K.; et al. Glioblastoma Multiforme: The Latest Diagnostics and Treatment Techniques. Pharmacology. 2023, 108, 423–431. [Google Scholar] [CrossRef]
- Le Rhun, E.; Preusser, M.; Roth, P.; et al. Molecular targeted therapy of glioblastoma. Cancer Treat Rev. 2019, 80, 101896. [Google Scholar] [CrossRef]
- Shah, S.; Mansour, H.M.; Aguilar, T.M.; Lucke-Wold, B. Advances in Anti-Cancer Drug Development: Metformin as Anti-Angiogenic Supplemental Treatment for Glioblastoma. Int J Mol Sci. 2024, 25, 5694. [Google Scholar] [CrossRef]
- Ohgaki, H.; Kleihues, P. The definition of primary and secondary glioblastoma. Clin Cancer Res. 2013, 19, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Hamad, A.; Yusubalieva, G.M.; Baklaushev, V.P.; Chumakov, P.M.; Lipatova, A.V. Recent Developments in Glioblastoma Therapy: Oncolytic Viruses and Emerging Future Strategies. Viruses. 2023, 15, 547. [Google Scholar] [CrossRef]
- Oh, J.M.; Ahn, B.C. Molecular mechanisms of radioactive iodine refractoriness in differentiated thyroid cancer: Impaired sodium iodide symporter (NIS) expression owing to altered signaling pathway activity and intracellular localization of NIS. Theranostics. 2021, 11, 6251–6277. [Google Scholar] [CrossRef]
- Gong, Z.; Wei, M.; Vlantis, AC.; et al. Sodium-iodide symporter and its related solute carriers in thyroid cancer. J Endocrinol. 2024, 261, e230373. [Google Scholar] [CrossRef] [PubMed]
- Spitzweg, C.; Nelson, PJ.; Wagner, E.; et al. The sodium iodide symporter (NIS): novel applications for radionuclide imaging and treatment. Endocr Relat Cancer. 2021, 28, T193–T213. [Google Scholar] [CrossRef]
- Darrouzet, E.; Lindenthal, S.; Marcellin, D.; Pellequer, J.L.; Pourcher, T. The sodium/iodide symporter: state of the art of its molecular characterization. Biochim Biophys Acta. 2014, 1838 Pt B, 244–253. [Google Scholar] [CrossRef]
- Ding, D.C.; Shyu, W.C.; Lin, S.Z. Mesenchymal stem cells. Cell Transplant. 2011, 20, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Lan, T.; Luo, M.; Wei, X. Mesenchymal stem/stromal cells in cancer therapy. J Hematol Oncol. 2021, 14, 195. [Google Scholar] [CrossRef]
- Mazini, L.; Rochette, L.; Admou, B.; Amal, S.; Malka, G. Hopes and Limits of Adipose-Derived Stem Cells (ADSCs) and Mesenchymal Stem Cells (MSCs) in Wound Healing. Int J Mol Sci. 2020, 21, 1306. [Google Scholar] [CrossRef]
- Naji, A.; Eitoku, M.; Favier, B.; Deschaseaux, F.; Rouas-Freiss, N.; Suganuma, N. Biological functions of mesenchymal stem cells and clinical implications. Cell Mol Life Sci. 2019, 76, 3323–3348. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Huang, Y.; Yang, L. Research progress in the use of mesenchymal stem cells and their derived exosomes in the treatment of osteoarthritis. Ageing Res Rev. 2022, 80, 101684. [Google Scholar] [CrossRef] [PubMed]
- Herzog, E.L.; Chai, L.; Krause, D.S. Plasticity of marrow-derived stem cells. Blood 2003, 102, 3483–3493. [Google Scholar] [CrossRef]
- Angelopoulou, M.; Novelli, E.; Grove, J.E.; et al. Cotransplantation of human mesenchymal stem cells enhances human myelopoiesis and megakaryocytopoiesis in NOD/SCID mice. Experimental Hematology 2003, 31, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Matthay, M.A.; Goolaerts, A.; Howard, J.P.; Lee, J.W. Mesenchymal stem cells for acute lung injury: preclinical evidence. Crit Care Med. 2010, 38, S569–S573. [Google Scholar] [CrossRef]
- Yao, P.; Zhou, L.; Zhu, L.; Zhou, B.; Yu, Q. Mesenchymal Stem Cells: A Potential Therapeutic Strategy for Neurodegenerative Diseases. Eur Neurol. 2020, 83, 235–241. [Google Scholar] [CrossRef]
- Hua, Q.; Zhang, Y.; Li, H.; et al. Human umbilical cord blood-derived MSCs trans-differentiate into endometrial cells and regulate Th17/Treg balance through NF-κB signaling in rabbit intrauterine adhesions endometrium. Stem Cell Res Ther. 2022, 13, 301. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, B.; Zhu, X.; et al. Human umbilical cord blood-derived MSCs exosome attenuate myocardial injury by inhibiting ferroptosis in acute myocardial infarction mice. Cell Biol Toxicol. 2021, 37, 51–64. [Google Scholar] [CrossRef]
- Fu, X.; Liu, G.; Halim, A.; Ju, Y.; Luo, Q.; Song, A.G. Mesenchymal Stem Cell Migration and Tissue Repair. Cells. 2019, 8, 784. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.J.; Chailakhyan, R.K.; Gerasimov, U.V. Bone marrow osteogenic stem cells: in vitro cultivation and transplantation in diffusion chambers. Cell Tissue Kinet. 1987, 20, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Galipeau, J.; Sensébé, L. Mesenchymal Stromal Cells: Clinical Challenges and Therapeutic Opportunities. Cell Stem Cell. 2018, 22, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Keshtkar, S.; Azarpira, N.; Ghahremani, M.H. Mesenchymal stem cell-derived extracellular vesicles: novel frontiers in regenerative medicine. Stem Cell Res Ther. 2018, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.C.; Chang, Y.H.; Shyu, W.C.; Lin, S.Z. Human umbilical cord mesenchymal stem cells: a new era for stem cell therapy. Cell Transplant. 2015, 24, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Samsonraj, R.M.; Raghunath, M.; Nurcombe, V.; Hui, J.H.; van Wijnen, A.J.; Cool, S.M. Concise Review: Multifaceted Characterization of Human Mesenchymal Stem Cells for Use in Regenerative Medicine. Stem Cells Transl Med. 2017, 6, 2173–2185. [Google Scholar] [CrossRef] [PubMed]
- Ankrum, J.A.; Ong, J.F.; Karp, J.M. Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol. 2014, 32, 252–260. [Google Scholar] [CrossRef]
- Caplan, A.I. Mesenchymal Stem Cells: Time to Change the Name! . Stem Cells Transl Med. 2017, 6, 1445–1451. [Google Scholar] [CrossRef]
- Dzobo, K. Recent Trends in Multipotent Human Mesenchymal Stem/Stromal Cells: Learning from History and Advancing Clinical Applications. OMICS. 2021, 25, 342–357. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghadban, S.; Bunnell, B.A. Adipose Tissue-Derived Stem Cells: Immunomodulatory Effects and Therapeutic Potential. Physiology (Bethesda). 2020, 35, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Xia, M.; Gao, Y.; Chen, Y.; Xu, Y. Human umbilical cord mesenchymal stem cells: an overview of their potential in cell-based therapy. Expert Opin Biol Ther. 2015, 15, 1293–1306. [Google Scholar] [CrossRef] [PubMed]
- Zupan, J. Human Synovium-Derived Mesenchymal Stem Cells: Ex Vivo Analysis. Methods Mol Biol. 2019, 2045, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Okolicsanyi, RK.; Camilleri, ET.; Oikari, LE.; et al. Human Mesenchymal Stem Cells Retain Multilineage Differentiation Capacity Including Neural Marker Expression after Extended In Vitro Expansion. PLoS One. 2015, 10, e0137255. [Google Scholar] [CrossRef] [PubMed]
- Balakrishna Pillai, A.; Mariappan, V.; JeanPierre, A.R.; Rao, S.R. Restoration of vascular endothelial integrity by mesenchymal stromal/stem cells in debilitating virus diseases. Hum Cell. 2022, 35, 1633–1639. [Google Scholar] [CrossRef] [PubMed]
- Dapkute, D.; Steponkiene, S.; Bulotiene, D.; Saulite, L.; Riekstina, U.; Rotomskis, R. Skin-derived mesenchymal stem cells as quantum dot vehicles to tumors. Int J Nanomedicine. 2017, 12, 8129–8142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Feng, Y.; Xie, X.; et al. Engineered Mesenchymal Stem Cells as a Biotherapy Platform for Targeted Photodynamic Immunotherapy of Breast Cancer. Adv Healthc Mater. 2022, 11, e2101375. [Google Scholar] [CrossRef]
- Sadhukha, T.; O’Brien, T.D.; Prabha, S. Nano-engineered mesenchymal stem cells as targeted therapeutic carriers. J Control Release. 2014, 196, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Zeng, L.; Ding, S.; et al. Tumor-Tropic Adipose-Derived Mesenchymal Stromal Cell Mediated Bi2 Se3 Nano-Radiosensitizers Delivery for Targeted Radiotherapy of Non-Small Cell Lung Cancer. Adv Healthc Mater. 2022, 11, e2200143. [Google Scholar] [CrossRef]
- Senst, C.; Nazari-Shafti, T.; Kruger, S.; et al. Prospective dual role of mesenchymal stem cells in breast tumor microenvironment. Breast Cancer Res Treat. 2013, 137, 69–79. [Google Scholar] [CrossRef]
- Golinelli, G.; Talami, R.; Frabetti, S.; et al. A 3D Platform to Investigate Dynamic Cell-to-Cell Interactions Between Tumor Cells and Mesenchymal Progenitors. Front Cell Dev Biol. 2022, 9, 767253. [Google Scholar] [CrossRef]
- Mishra, VK.; Shih, HH.; Parveen, F.; et al. Identifying the Therapeutic Significance of Mesenchymal Stem Cells. Cells. 2020, 9, 1145. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Chen, S.; Yang, P.; Cao, H.; Li, L. The role of mesenchymal stem cells in hematopoietic stem cell transplantation: prevention and treatment of graft-versus-host disease. Stem Cell Res Ther. 2019, 10, 182. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Huang, L.; Li, Y.; et al. Mesenchymal Stem Cells and Cancer: Clinical Challenges and Opportunities. Biomed Res Int. 2019, 2019, 2820853. [Google Scholar] [CrossRef] [PubMed]
- Munir, H.; Ward, L.S.C.; McGettrick, H.M. Mesenchymal Stem Cells as Endogenous Regulators of Inflammation. Adv Exp Med Biol. 2018, 1060, 73–98. [Google Scholar] [CrossRef] [PubMed]
- Ji, K.; Ding, L.; Chen, X.; et al. Mesenchymal Stem Cells Differentiation: Mitochondria Matter in Osteogenesis or Adipogenesis Direction. Curr Stem Cell Res Ther. 2020, 15, 602–606. [Google Scholar] [CrossRef]
- Miao, J.; Ren, Z.; Zhong, Z.; et al. Mesenchymal Stem Cells: Potential Therapeutic Prospect of Paracrine Pathways in Neonatal Infection. J Interferon Cytokine Res. 2021, 41, 365–374. [Google Scholar] [CrossRef]
- Selmi-Ruby, S.; Watrin, C.; Trouttet-Masson, S.; et al. The porcine sodium/iodide symporter gene exhibits an uncommon expression pattern related to the use of alternative splice sites not present in the human or murine species. Endocrinology. 2003, 144, 1074–1085. [Google Scholar] [CrossRef]
- Knoop, K.; Kolokythas, M.; Klutz, K.; et al. Image-guided, tumor stroma-targeted 131I therapy of hepatocellular cancer after systemic mesenchymal stem cell-mediated NIS gene delivery. Mol Ther. 2011, 19, 1704–1713. [Google Scholar] [CrossRef]
- Cho, J.Y. A transporter gene (sodium iodide symporter) for dual purposes in gene therapy: imaging and therapy. Curr Gene Ther. 2002, 2, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Faivre, J.; Clerc, J.; Gérolami, R.; et al. Long-term radioiodine retention and regression of liver cancer after sodium iodide symporter gene transfer in wistar rats [published correction appears in Cancer Res. 2004 Dec 15;64, 9230]. Cancer Res. 2004, 64, 8045–8051. [Google Scholar] [CrossRef] [PubMed]
- Belmar-López, C.; Vassaux, G.; Medel-Martinez, A.; et al. Mesenchymal Stem Cells Delivery in Individuals with Different Pathologies: Multimodal Tracking, Safety and Future Applications. Int J Mol Sci. 2022, 23, 1682. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, Y.Y.; Jhiang, S.M. Cell surface targeting accounts for the difference in iodide uptake activity between human Na+/I- symporter and rat Na+/I- symporter. J Clin Endocrinol Metab. 2005, 90, 6131–6140. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Li, J.; Liu, J.; Liu, K. An Arabidopsis mitochondria-localized RRL protein mediates abscisic acid signal transduction through mitochondrial retrograde regulation involving ABI4. J Exp Bot. 2015, 66, 6431–6445. [Google Scholar] [CrossRef]
- Nowakowski, A.; Walczak, P.; Lukomska, B.; Janowski, M. Genetic Engineering of Mesenchymal Stem Cells to Induce Their Migration and Survival. Stem Cells Int. 2016, 2016, 4956063. [Google Scholar] [CrossRef] [PubMed]
- Gugjoo, M.B.; Sakeena, Q.; Wani, M.Y.; Abdel-Baset Ismail, A.; Ahmad, S.M.; Shah, R.A. Mesenchymal stem cells: A promising antimicrobial therapy in veterinary medicine. Microb Pathog. 2023, 182, 106234. [Google Scholar] [CrossRef] [PubMed]
- Siristatidis, C.; Vogiatzi, P.; Salamalekis, G.; et al. Granulocyte macrophage colony stimulating factor supplementation in culture media for subfertile women undergoing assisted reproduction technologies: a systematic review. Int J Endocrinol. 2013, 2013, 704967. [Google Scholar] [CrossRef] [PubMed]
- Bonaventura, G.; Incontro, S.; Iemmolo, R.; et al. Dental mesenchymal stem cells and neuro-regeneration: a focus on spinal cord injury. Cell Tissue Res. 2020, 379, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Etchebehere, EC.; Cendes, F.; Lopes-Cendes, I.; et al. Brain single-photon emission computed tomography and magnetic resonance imaging in Machado-Joseph disease. Arch Neurol. 2001, 58, 1257–1263. [Google Scholar] [CrossRef]
- Hade, M.D.; Suire, C.N.; Suo, Z. Mesenchymal Stem Cell-Derived Exosomes: Applications in Regenerative Medicine. Cells. 2021, 10, 1959. [Google Scholar] [CrossRef] [PubMed]
- Nose, N.; Nogami, S.; Koshino, K.; et al. [18F]FDG-labelled stem cell PET imaging in different route of administrations and multiple animal species. Sci Rep. 2021, 11, 10896. [Google Scholar] [CrossRef]
- Lu, CH.; Chen, YA.; Ke, CC.; et al. Preclinical Characterization and In Vivo Imaging of 111In-Labeled Mesenchymal Stem Cell-Derived Extracellular Vesicles. Mol Imaging Biol. 2021, 23, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Goh, E.L.K.; He, J.; et al. Emerging Intrinsic Therapeutic Targets for Metastatic Breast Cancer. Biology (Basel). 2023, 12, 697. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem cells: past, present, and future. Stem Cell Res Ther. 2019, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.L.; Huang, T.; Wu, B.L.; He, W.X.; Liu, D. Stem cells in cancer therapy: opportunities and challenges. Oncotarget. 2017, 8, 75756–75766. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Cruz, C.M.; Shearer, J.J.; Figueiredo Neto, M.; Figueiredo, M.L. The Immunomodulatory Effects of Mesenchymal Stem Cell Polarization within the Tumor Microenvironment Niche. Stem Cells Int. 2017, 2017, 4015039. [Google Scholar] [CrossRef] [PubMed]
- Szewc, M.; Radzikowska-Bűchner, E.; Wdowiak, P.; et al. MSCs as Tumor-Specific Vectors for the Delivery of Anticancer Agents-A Potential Therapeutic Strategy in Cancer Diseases: Perspectives for Quinazoline Derivatives. Int J Mol Sci. 2022, 23, 2745. [Google Scholar] [CrossRef]
- Attia, N.; Mashal, M.; Puras, G.; Pedraz, J.L. Mesenchymal Stem Cells as a Gene Delivery Tool: Promise, Problems, and Prospects. Pharmaceutics. 2021, 13, 843. [Google Scholar] [CrossRef]
- Cuiffo, B.G.; Karnoub, A.E. Mesenchymal stem cells in tumor development: emerging roles and concepts. Cell Adh Migr. 2012, 6, 220–230. [Google Scholar] [CrossRef]
- Rabha, B.; Bharadwaj, K.K.; Pati, S.; Choudhury, B.K.; Sarkar, T.; Kari, Z.A.; Edinur, H.A.; Baishya, D.; Atanase, L.I. Development of Polymer-Based Nanoformulations for Glioblastoma Brain Cancer Therapy and Diagnosis: An Update. Polymers. 2021, 13, 4114. [Google Scholar] [CrossRef] [PubMed]
- Yang, CT.; Lai, R.C.; Phua, V.J.X.; et al. Standard Radio-Iodine Labeling Protocols Impaired the Functional Integrity of Mesenchymal Stem/Stromal Cell Exosomes. Int J Mol Sci. 2024, 25, 3742. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Han, Y.; Zhao, G.; et al. Hypoxia-Responsive Lipid-Polymer Nanoparticle-Combined Imaging-Guided Surgery and Multitherapy Strategies for Glioma. ACS Appl Mater Interfaces. 2020, 12, 52319–52328. [Google Scholar] [CrossRef] [PubMed]
- Schug, C.; Gupta, A.; Urnauer, S.; et al. A Novel Approach for Image-Guided 131I Therapy of Pancreatic Ductal Adenocarcinoma Using Mesenchymal Stem Cell-Mediated NIS Gene Delivery. Mol Cancer Res. 2019, 17, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Nicola, J.P.; Basquin, C.; Portulano, C.; Reyna-Neyra, A.; Paroder, M.; Carrasco, N. The Na+/I- symporter mediates active iodide uptake in the intestine. Am J Physiol Cell Physiol. 2009, 296, C654–C662. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (US) and Institute of Medicine (US) Committee on the Mathematics and Physics of Emerging Dynamic Biomedical Imaging. Mathematics and Physics of Emerging Biomedical Imaging. Washington (DC): National Academies Press (US); 1996. Chapter 5, Single Photon Emission Computed Tomography. Available from: https://www.ncbi.nlm.nih.gov/books/NBK232492/.
- Penheiter, A.R.; Russell, S.J.; Carlson, S.K. The sodium iodide symporter (NIS) as an imaging reporter for gene, viral, and cell-based therapies. Curr Gene Ther. 2012, 12, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; DeGrado, TR. [18F]Tetrafluoroborate ([18F]TFB) and its analogs for PET imaging of the sodium/iodide symporter. Theranostics. 2018, 8, 3918–3931. [Google Scholar] [CrossRef]
- Hanif, F.; Muzaffar, K.; Perveen, K.; Malhi, S.M.; Simjee, S.h.U. Glioblastoma Multiforme: A Review of its Epidemiology and Pathogenesis through Clinical Presentation and Treatment. Asian Pac J Cancer Prev. 2017, 18, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Gudkov, S.V.; Shilyagina, N.Y.; Vodeneev, V.A.; Zvyagin, A.V. Targeted Radionuclide Therapy of Human Tumors. Int J Mol Sci. 2015, 17, 33. [Google Scholar] [CrossRef]
- Shi, S.; Zhang, M.; Guo, R.; Miao, Y.; Li, B. Bone Marrow-Derived Mesenchymal Stem Cell-Mediated Dual-Gene Therapy for Glioblastoma. Hum Gene Ther. 2019, 30, 106–117. [Google Scholar] [CrossRef]
- Czarnywojtek, A.; Gut, P.; Borowska, M.; Dyrka, K.; Ruchała, M.; Ferlito, A. A NEW HYPOTHESIS IN THE TREATMENT OF RECURRENT GLIOBLASTOMA MULTIFORME (GBM). PART 1: INTRODUCTION. Pol Merkur Lekarski. 2023, 51, 430–432. [Google Scholar] [CrossRef] [PubMed]
- Czarnywojtek, A.; Gut, P.; Sowiński, J.; Ruchała, M.; Ferlito, A.; Dyrka, K. A NEW HYPOTHESIS IN THE TREATMENT OF RECURRENT GLIOBLASTOMA MULTIFORME (GBM). PART 2: IS THERE AN ALTERNATIVE THERAPY OPTION IN RECURRENT GM WHEN ALL STANDARD TREATMENTS HAVE BEEN EXHAUSTED? Pol Merkur Lekarski. 2023, 51, 433–435. [Google Scholar] [CrossRef] [PubMed]
- Yalamarty, S.S.K.; Filipczak, N.; Li, X.; et al. Mechanisms of Resistance and Current Treatment Options for Glioblastoma Multiforme (GBM). Cancers (Basel) 2023, 15, 2116. [Google Scholar] [CrossRef]
- Cho, JY.; Shen, DH.; Yang, W.; Williams, B.; Buckwalter, TL.; La Perle, KM.; et al. In vivo imaging and radioiodine therapy following sodium iodide symporter gene transfer in animal model of intracerebral gliomas. Gene Ther. 2002, 9, 1139–1145. [Google Scholar] [CrossRef]
- Guo, R.; Xi, Y.; Zhang, M.; Miao, Y.; Zhang, M.; Li, B. Human sodium iodide transporter gene-mediated imaging and therapy of mouse glioma, comparison between (188)Re and (131)I. Oncol Lett. 2018, 15, 3911–3917. [Google Scholar]
- Opyrchal, M.; Allen, C.; Iankov, I.; Aderca, I.; Schroeder, M.; Sarkaria, J.; et al. Effective radiovirotherapy for malignant gliomas by using oncolytic measles virus strains encoding the sodium iodide symporter (MV-NIS). Hum Gene Ther. 2012, 23, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Kitzberger, C.; Shehzad, K.; Morath, V.; et al. Interleukin-6-controlled, mesenchymal stem cell-based sodium/iodide symporter gene therapy improves survival of glioblastoma-bearing mice. Mol Ther Oncolytics. 2023, 30, 238–253. [Google Scholar] [CrossRef]
- Spellerberg, R.; Benli-Hoppe, T.; Kitzberger, C.; et al. Dual EGFR- and TfR-targeted gene transfer for sodium iodide symporter gene therapy of glioblastoma. Mol Ther Oncolytics 2022, 27, 272–287. [Google Scholar] [CrossRef]
- Fu, Y.; Ong, LC.; Ranganath, SH.; et al. A Dual Tracer 18F-FCH/18F-FDG PET Imaging of an Orthotopic Brain Tumor Xenograft Model. PLoS One. 2016, 11, e0148123. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




