1. Introduction
France’s population in 2010 was approximately 65 million, with 31.5 million males and 33.5 million females [
1]. For males in 2010, the estimated all-cancer incidence rate was 363 per 100,000 person-years and the all-cancer mortality rate was 134 per 100,000 person-years [
2]. For females, the values were 252 and 73, respectively. From 2005 to 2012, all-cancer incidence and mortality rates for males decreased each year by 1.3% and 2.9%, respectively, whereas for females, the all-cancer incidence rate increased by 0.2% and all-cancer mortality rates decreased by 1.4%. Standardized death rates per 100,000 people for France in 2010 were all causes, 684; cancer, 232; cardiovascular diseases, 156; other diseases, 124; violent deaths, 64; unspecified or ill-defined causes of death, 55; and infectious and parasitic diseases , respiratory diseases, 52 [
3]. Thus, cancer was a leading cause of death.
To reduce cancer rates in France, trying to determine the causes of cancer there is worthwhile. A set of studies were conducted to estimate the causes of cancer related to lifestyle and environmental factors in France in 2015. The top seven given in
Table 3 in the summary article from that set [
4] are: tobacco smoking, 19.8% population attributable fraction (PAF); alcohol consumption, 8.0%; dietary factors, 5.4%; overweight and obesity, 5.4%; infectious agents, 4.0%; occupational exposures, 3.6%; sun exposure, 3.0%. It would be helpful if an ecological study could evaluate the cancer-specific and total risks for each of these factors. However, that is not possible due to the fact that the cancer incidence and mortality rate data for the period 2007‒2016 are only available for 12 regions with large populations. In statistical analyses, ten observations per variable is generally considered the minimum number to avoid computational difficulties [
5]. However, as will be seen, sometimes useful results can be obtained for two risk-modifying factors using only 12 geographical units, as found in this study.
Tobacco smoking has been a leading cause of cancer since the late 20th century. In the United States, lung and bronchus cancer was the leading cause of cancer death for males from 1955 to present and for females from 1988 to present [
6]. For France in 2015, the PAF of cancer cases for active tobacco smoking was estimated at about 28% for males and 8% for females [
7].
Alcohol consumption is France’s second-leading cause of cancer. For 2015, light, moderate, heavy, and former alcohol drinking were responsible for 1.5%, 1.3%, 4.4%, and 0.6% of all new cancer cases, respectively [
8]. Esophageal squamous cell carcinoma had the highest PAF (58%). An estimated 15,000 of all cancer deaths in France in 2009 were due to alcohol consumption [
9].
High body mass index (BMI) had an estimated PAF for cancer of 4.1% for males and 6.7% for females [
10]. The highest estimated PAFs were for esophageal adenosarcoma (37%) and corpus uteri cancer (34%). Data on mean BMI by sex and age group were used from the 2006 National Nutrition and Health Survey (Étude nationale nutrition santé, ENNS) (
Table 1 in Ref. 14 in [
10]). The data were for 2413 French adults between February 2006 and March 2007.
Solar radiation’s effect on cancer incidence in France was limited to cutaneous melanoma [
11]. Three data sets were used in the analysis: the 1903 South Thames birth cohort, a historical reference from France in 1980, and a geographical reference from France for 2003–2007. The PAF for all-cancer incidence varied from 1.4% to 2.8% for males and from 0.9 to 3.2% for females.
Ecological studies are observational studies that consider populations in defined geographical regions, using population-averaged health outcomes and values for risk-modifying factors. The ecological approach is only sensitive to outcomes for which the ratios of risk modifying factors to health outcome vary between the geographical populations, preferably in a quasi-linear fashion. Many ecological studies have studied the correlations between solar UVB doses and cancer incidence and mortality rates such as in the U.S. [
12,
13,
14]. Two ecological studies in the U.S. also included alcohol consumption and lung cancer rates as a proxy for smoking [
13,
15]. The most recent U.S. ecological study also include obesity prevalence [
15].
Strengths of the ecological approach include that large numbers of participants are included, and that they can be conducted efficiently since the data used are generally readily available. Thus, they can show large-scale effects analogous to satellite images of weather patterns. The limitations of ecological studies include that the population average values for the risk-modifying factors may not relate well to those who develop the health conditions of interest, such as being appropriate for different age groups, and that some important risk-modifying factors are not included in the analysis. However, these limitations have not been found to reduce the accuracy of the findings in large-scale studies. For example, in a 2002 ecological study of cancer mortality rates in the U.S., solar UVB doses were found inversely correlated with 13 types of cancer for data averaged over ~500 state economic areas in 44 states without the inclusion of any other risk-modifying factors [
12]. In the follow-on study involving 48 states and the addition of several risk-modifying factors, alcohol consumption, Hispanic heritage, lung cancer mortality rates, poverty, and urban/rural residence, the findings for solar UVB dose were essentially unchanged and the findings for the other risk factors were in general agreement with the peer-reviewed journal literature [
13].
The ecological study approach to studying the role of sunlight in reducing risk of cancer was first used in 1941 by Apperly [
16]. Garland and colleagues used this approach for colon, breast, and ovarian cancer mortality rates in the 1980‒1994 period using data from the U.S. [
17,
18,
19]. They were also first to suggest that the effect of sunlight in reducing risk of cancer was due to vitamin D production [
17].
The present study uses the geographical ecological study approach to determine the correlations between solar UVB dose, obesity prevalence, and smoking and cancer incidence and mortality rates in France for 2007–2016. Cancer incidence and mortality rate data are averaged for 12 geographical regions, as are obesity prevalence, UVB doses, and lung cancer incidence or mortality rates and statistical analysis is used to determine the correlations.
2. Materials and Methods
The present ecological study uses cancer incidence and mortality rates for 12 regions in France with respect to obesity rates, solar UVB doses, lung cancer incidence and mortality rates, and a proxy of alcohol consumption rates. Lung cancer rates are a proxy for smoking, which occurs after decades of smoking. However, as noted, second-hand smoking and air pollution can also increase the risk of lung cancer, as can diet such as from red and processed meat [
20]. Data used for the study were obtained from publicly-available datasets as discussed in the following paragraphs.
Cancer incidence and mortality rates came from a publication of the Santé publique France with the Institut national du cancer [
21]. to 2-24 in that report included incidence and/or mortality rate data for cancers at 23 anatomical sites as well as for all cancers combined. National estimates of incidence for 2007–2016 and mortality for 2007–2014 included average annual population, crude rate, standardized rate on the age structure of the world population per 100,000 person-years (the year of the data were not specified) accompanied by their prediction/95% CI, distribution of estimated departmental rates (5th and 95th percentiles: Q5–Q95), and percentage of cases in the total number of incident cases or deaths. Data for 13 regions were available. Data for Corse (Corsica) were not used in that analysis because of the few cancer cases (960 for males, 842 for females) and cancer deaths (492 for males and 351 for females) compared with the other regions (>6000 cases and 2800 deaths).
Obesity data came from a 2012 report on prevalence of obesity in 22 French regions for 2007 and 2012 [
22]. Data for 2012 were based on information for 25,714 people older than 18 years. Because the number of regions with obesity data is greater than the number of regions with cancer data, the obesity data for each region for cancer were averaged with respect to populations in the major cities and data combined for 2007 and 2012.
Table 1 gives the values used in this study.
Table 1.
Values for obesity prevalence, solar UVB dose, and all-cancer incidence and mortality rates in ascending order by annual mean solar UVB doses.
Table 1.
Values for obesity prevalence, solar UVB dose, and all-cancer incidence and mortality rates in ascending order by annual mean solar UVB doses.
| Region |
Annual mean
solar UVB (Wh/m2) [23] |
Obesity prevalence (%)** [22] |
| Hauts-de-France |
5.45 |
20.4 |
| Grand Est |
5.6 |
18.0 |
| Normandie |
5.7 |
16.8 |
| Île-de-France |
* |
13.8 |
| Bretagne |
* |
12.1 |
| Centre-Val de Loire |
* |
16.8 |
| Bourgogne-Franche-Comté |
6.1 |
15.1 |
| Pays de la Loire |
* |
12.6 |
| Auvergne-Rhône-Alpes
|
6.8 |
13.3 |
| Nouvelle-Aquitaine |
7.1 |
14.6 |
| Occitanie |
8.1 |
13.6 |
| Provence-Alpes-Côte d’Azur |
* |
11.6 |
A 2011 article reports values for annual mean UVB radiation for each Mutualité sociale agricole region in France [
23]. The values were based on typical values determined for global UVB all-sky measurements from MeteoStats, Switzerland. The data were accessed from the Solar Radiation Data (SoDa) company from an apparently inactive website. However, maps are still available (
www.soda-pro.com/maps/maps-for-pay; accessed June 10, 2024), but we did not pursue that line of inquiry further due to cost and time constraints. Solar UVB doses do not change appreciably from year to year. The main cause of change is fluctuations in stratospheric ozone concentration. Measured solar UV index for Observatoire de Haute-Provence (44° N) from 1975 to 2017 showed an increase to about 1994, followed by relative stability albeit with year-to-year fluctuations [
24]. More important, air pollution such as from aerosols as well as clouds also reduces the amount of UVB reaching Earth’s surface. Aerosol loading in France is highest in northern France [
25,
26]. However, ecological studies generally do not consider those factors.
Data for alcohol consumption in 21 regions came from a 2010 report in France [
27]. To develop data for the 12 regions used in this study, we averaged data for each region for cancer with respect to populations in the major cities. Three measures were used in preliminary analyses: daily alcohol use, use at chronic risk or dependence, and repeated drunkenness. Because repeated drunkenness had the highest correlation with cancers such as esophageal cancer, we chose that as the index of alcohol consumption in the analyses.
Annual mean solar UVB doses for the regions vary from 5.45 Wh/m
2 in Hauts-de-France to 8.7 Wh/m
2 in Provence-Alpes-Côte d’Azur. Serum 25(OH)D concentrations were measured between January 2011 and February 2012 in 10 university hospitals involving 892 French white healthy adults [
28]. Mean 25(OH)D concentrations in northern France were 22 ± 8 and 25 ± 8 ng/mL in southern France.
Figure 1 in Souberbielle and colleagues [
28] shows the 10 sites where measurements were made. Using the data in
Table 1, we estimate that the mean solar UVB dose in northern France was 6.0 Wh/m
2, whereas that in southern France was 6.8 Wh/m
2. The ratio of 25(OH)D concentrations is 25/22 = 1.14, and the ratio of solar UVB doses is 1.13, showing good agreement. Although those values are only approximations, they indicate that the UVB doses are reasonable proxies for mean serum 25(OH)D concentrations in each region. The odds ratio for 25(OH)D <20 ng/mL in the north compared with the south was 1.91 (95% CI, 1.43–2.54).
Lung cancer incidence and mortality rate data also were included as an index of the cancer risk due to smoking. However, lung cancer also is affected by air pollution such as fine particulate matter (PM
2.5) in France [
25]. PM
2.5 concentrations are much higher in northern France [
25,
26] and several smaller regions throughout France. Because smoking generally takes decades to result in lung cancer, data for smoking rates would not be useful.
Data were analyzed using SigmaStat 4.0 (Grafiti, Palo Alto, CA, USA). Data plots were made using KaleidaGraph 4.5.4 (Synergy Software, Reading, PA, USA).
Table 2 gives the cross-correlation coefficients for the risk-modifying factors used in this study. Factors with significant cross-correlation coefficients should not be used in the same regression analysis. In general, 10 independent pairs of data are needed to produce useful regression results. Because only 12 data sets are used, few useful results involving any two risk-modifying factors would be expected.
Table 2.
Cross-correlation regression results (r, adjusted r2, p) for the risk-modifying factors used in this analysis.
Table 2.
Cross-correlation regression results (r, adjusted r2, p) for the risk-modifying factors used in this analysis.
| Factor |
Obesity |
LCi, males |
LCi, females |
LCm, males |
LCm, females |
| Solar UVB |
0.67, 0.40, 0.02 |
0.47, 0.14, 0.12 |
0.20, 0.00, 0.54 |
0.51, 0.19, 0.09 |
0.02, 0.00, 0.94 |
| Obesity |
|
0.83, 0.66, <0.001 |
0.34, 0.03, 0.28 |
0.87, 0.74, <0.001 |
0.00. 0.00, 0.99 |
| LCi, males |
|
|
|
0.95, 0.90, <0.001 |
0.01, 0.00, 0.97 |
| LCi, females |
|
|
|
|
0.85, 0.70, <0.001 |
3. Results
Table 3,
Table 4,
Table 5 and
Table 6 show the regression results (all
p < 0.10) for cancer incidence and mortality rates for males and females. The good agreement of the results for obesity with estimates in a model study for France [
10] supports the findings in this ecological study for UVB dose in that the same analytic method was used. However, very little effect was found for smoking when using lung cancer incidence or mortality rates as proxies for smoking. Also, no significant correlations were found for repeated drunkenness, the index of alcohol consumption.
Table 3.
Regression results for cancer incidence rates for males.
Table 3.
Regression results for cancer incidence rates for males.
| Cancer |
NTSM
(cases/100,000) |
Equation |
r, Adjusted r2, p
|
PAF,
obesity |
PAF, UVB |
| All |
355 |
316 + 3.3 × Obes |
0.57, 0.26, 0.052 |
0.01 |
|
| Bladder |
14.5 |
5.2 + 0.18 × LCi |
0.50, 0.18, 0.08 |
|
|
| Colorectal |
37.8 |
32 + 0.43 × Obes |
0.55, 0.23, 0.06 |
0.01 |
|
| Esophageal |
5.2 |
14 − 0.97 × UVB |
0.58, 0.26, 0.051 |
|
0.06 |
| Lips, mouth, pharynx |
|
−5.9 + 0.23 × LCi |
0.67, 0.40, 0.01 |
|
|
| Lips, mouth, pharynx |
|
34 − 2.9 × UVB |
0.53, 0.20, 0.07 |
|
0.07 |
| Lung |
51.8 |
29 + 1.5 × Obes |
0.83, 0.66, <0.001 |
???? |
|
| Thyroid |
4.9 |
−0.80 + 0.85 × UVB |
0.84, 0.68, <0.001 |
|
???? |
The PAF of cancer incidence and mortality rates can be estimated from the regression results from the regression equation. It is essentially the area of the triangle for the regression fit to the data from low to high value of the risk-modifying factor divided by the rectangle for the mean value of the cancer rate times the difference between high and low values for the risk-modifying factor, then multiplied by adjusted
r2 for the regression fit. For all-cancer mortality for females with respect to obesity, the PAF is:
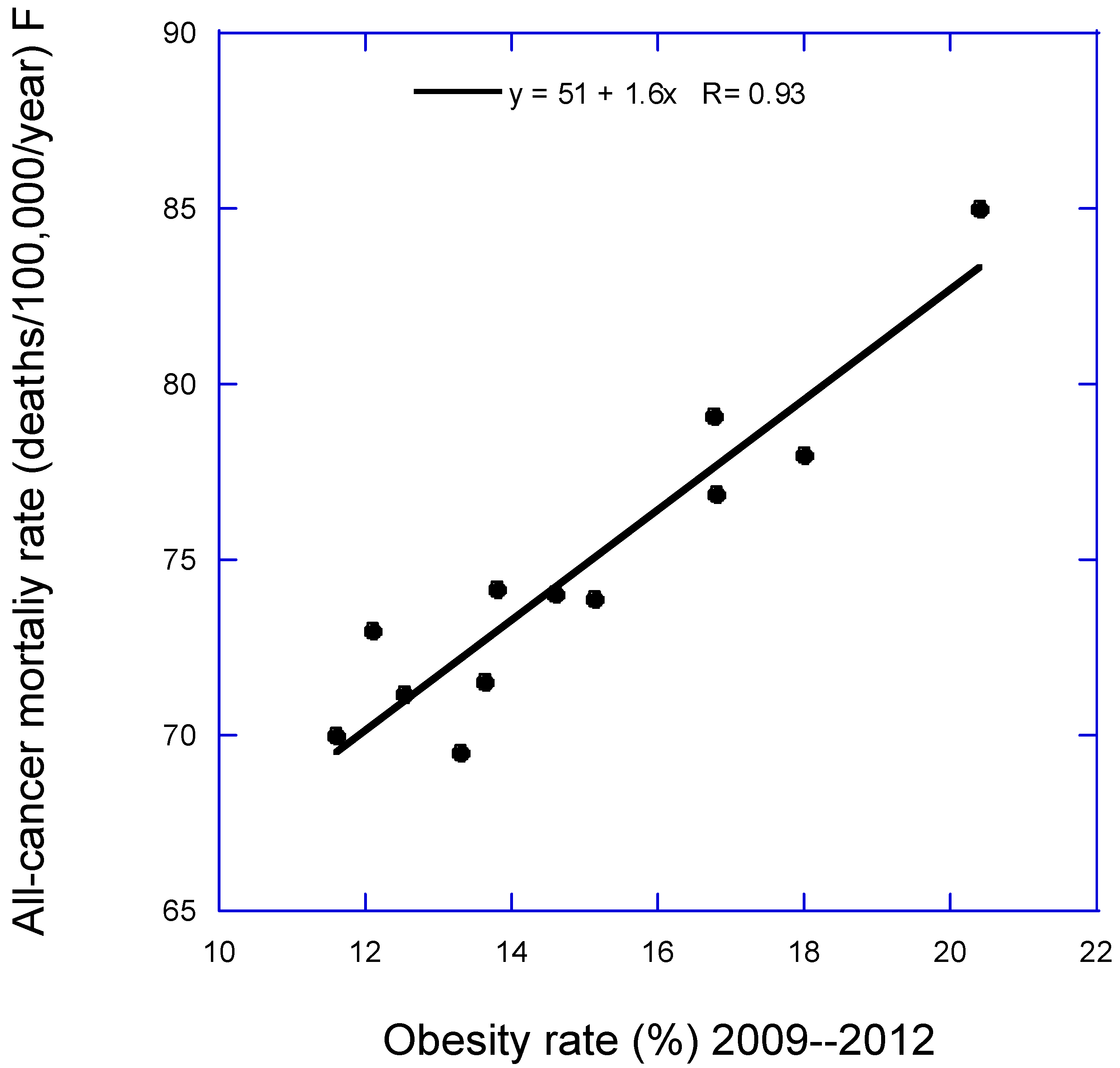
| All-cancer mortality |
51 + 1.6 × Obes |
0.94, 0.86, <0.001 |
However, that estimate is a lower limit, based only on having obesity rates in all regions set to the lowest value. If the obesity rate were set to zero, the estimate would be that the rate would be 51 deaths/100,000 per year. However, such an extrapolation cannot be made because many relationships are nonlinear, often reaching a minimum or maximum value before the zero value of the independent variable is reached. PAF values are given in Tables 3 to 6 for the types of cancer for which meaningful results were obtained.
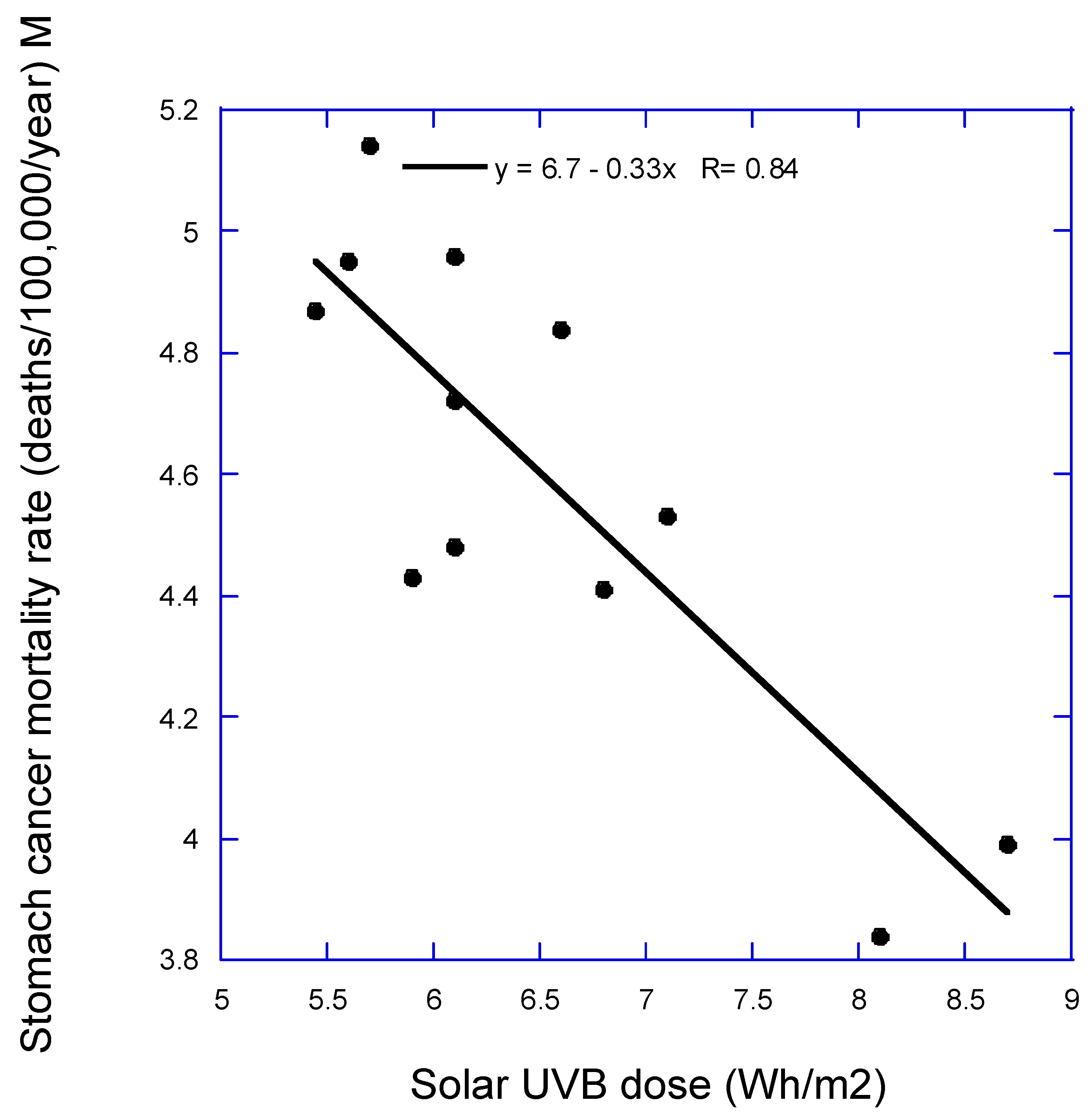
Figure 1 and
Figure 2 are scatter plots of data used in this study.
Figure 1 is for all-cancer mortality rate for females vs. obesity rates.
Figure 2 is stomach cancer mortality rate for males vs. annual mean solar UVB dose. Both figures illustrate how the data is used for the PAF calculations.
4. Discussion
4.1. Solar UVB
Solar UVB dose was more strongly associated with reduced cancer risk for males than for females, possibly because, for example, males are more likely to be outdoors occupationally, e.g., in agriculture, construction, or public safety as an ecological study in Nordic countries showed that effect [
29]. Cosmetics now generally include sunscreen [
30,
31], thereby reducing vitamin D production in women.
It is interesting to observe that the results of this ecological study with a similar one for cancer incidence rates in the US for the period 2016‒2020 [
15]. There is substantial agreement for obesity prevalence and/or solar UVB doses for these cancer outcomes for males: incidence rate, all cancer, colorectal cancer (CRC), esophageal, and stomach cancer; male mortality rate, all, all-less-lung, CRC, esophageal, and stomach cancer. For females, there is substantial agreement for: incidence rate, esophageal and kidney cancer; mortality rate, all, all-less-lung, breast, CRC, esophageal, kidney, and stomach cancer. The findings that did not agree with the results for the U.S. were for bladder cancer: in the U.S, UVB was inversely correlated, in France lung cancer was directly correlated, and lung cancer for males, correlated with obesity in the French study but diabetes, obesity and UVB in the U.S. study. The U.S. study also had results for several additional cancers for males: brain and laryngeal cancer and non-Hodgkin’s lymphoma. For females, the additional cancers were laryngeal cancer and non-Hodgkin’s lymphoma. Thus, even though the French study had fewer cancer cases and geographical regions, it nonetheless produced reasonable correlations with obesity prevalence and solar UVB dose.
Solar UVB dose was not found associated with several types of cancer for which it was found in ecological studies of cancer mortality rates in the United States such as bladder and colorectal cancer [
12,
13]. The likely reason is that since people now have higher BMI, obesity becomes a more important risk factor, in part since serum 25(OH)D concentration tends to be inversely correlated with BMI and fat mass. For example, an observational study conducted in Italy involving 147 overweight and obese subjects, the regression fit for serum 25(OH)D concentration increased from 5 ng/mL for fat mass = 43 kg to 40 ng/mL for 26 kg [
32]. In multiple regression analysis using two models, fat mass was the only significant factor correlated with 25(OH)D concentration (
p < 0.01). In turn, systemic inflammation, increases cancer risk and is inversely correlated with serum 25(OH)D levels [
33]. An observational study involving 8024 individuals in the Rotterdam Study followed found that over a ten-year period, cancer risk increased from 9.7% in the lowest quartile to 14.7% in the highest quartile (
p = 0.009) [
34].
In 2010, Grant published an ecological study of French cancer incidence and mortality rates between 1998 and 2000 with respect to latitude, an index of vitamin D production from solar ultraviolet-B (UVB) radiation [
35]. Cancers with significant inverse correlations for both incidence and mortality rates included all, all excluding lung, breast, colorectal, esophageal, lung (males only), prostate, and uterine cervix and uterine corpus. Those results were similar to those from the United States [
13]. However, that analysis did not include other important cancer risk factors, such as obesity. Thus, the findings could have been due to a latitudinal gradient in obesity rather than UVB for some of the cancers.
4.1.2. Vitamin D
Solar UVB’s inverse correlation with several cancers suggests that vitamin D reduces both cancer incidence and mortality rates. Solar UV’s main effect in reducing cancer risk is through vitamin D production. A moderately important effect of solar UV exposure is an increase in serum nitric oxide concentrations through liberating it from subcutaneous nitrate stores [
36]. The main effects of this mechanism seem to be reduced risk high blood pressure [
36] and protection against viral diseases such as COVID-19 [
37]. However, there is no evidence that nitric oxide plays a role in reducing risk of cancer.
As for making causal inferences, randomized controlled trials (RCTs) are generally used for that purpose. Since modern medicine uses the RCT to determine the efficacy and adverse effects of pharmaceutical drugs, the RCT approach has been applied to studying the effect of vitamin D supplementation on risk of disease such as cancer. However, most vitamin D RCTs have been based on the guidelines for drugs. In drug trials, the only source of the drug is in the trial and only the treatment arm is given the drug. That is not appropriate for vitamin D trials as not every participant has deficient 25(OH)D concentrations. In 2014, Robert Heaney outlined the guidelines for clinical trials for nutrients such as vitamin D [
38]. The more important guidelines as applied to vitamin D include measuring 25(OH)D concentration of prospective participants and choose those with low concentrations for the outcome of interest, supplement with high enough vitamin D dose to raise concentrations to where significant reductions in adverse health outcome have been reported, then measure achieved 25(OH)D concentration and use that value in the analysis. As discussed in two recent reviews, most vitamin D RCTs reported to date have not found strong supporting evidence for vitamin D supplementation as a result of not following Heaney’s guidelines [
39,
40].
An alternate approach for assessing causality is through application of A. Bradford Hill’s criteria for causality in a biological system [
41]
. The important criteria for solar radiation and vitamin D and cancer include strength of association, consistency, temporality, biological gradient, plausibility, coherence with known science, and experiment. Not all criteria need to be satisfied, but the more that are, the stronger the case for causality. A 2009 evaluation of Hill’s criteria regarding vitamin D and cancer concluded that it largely satisfied the criteria except for experimental verification [
42]. As outlined in a 2022 review [
43], the evidence regarding consistency for many types of cancer in both geographical ecological studies and prospective observational studies and plausibility (mechanisms) has been strengthened. The main limitation is in experimental verification. Medical science uses RCTs to demonstrate efficacy. There are some preliminary results from RCTs regarding vitamin D supplementation and all-cancer mortality [
44] and all-cancer incidence for daily vitamin D supplementation for normal-weight people [
45]. Also, a recent vitamin D supplementation RCT found that gastrointestinal cancer patients who were p53 immunoreactive had greatly reduced relapse or death associated with vitamin D supplementation over nearly six years of follow [
46]. Patients were supplemented with 2000 IU/d vitamin D
3 or placebo. In the p53-Antibody (+) and p53- immunohistochemistry (3+) group, the HR for relapse or death was 0.27 (95% CI, 0.11‒0.61). In the p53-Antibody (-) group, the HR for vitamin D supplementation vs. placebo was 1.09 (95% CI, 0.65‒1.84).
A 2023 meta-analysis of vitamin D3 supplementation on cancer mortality found in the main analysis of 14 RCTs, HR = 0.94 (95% CI, 0.86‒1.02) [
47]
. However, in the ten RCTs with daily vitamin D supplementation, HR = 0.88 (95% CI, 0.78‒0.98).
A 2023 review published in 2024 concluded that the vitamin D-cancer hypothesis has not been demonstrated to be causal [
48]
.
In case the vitamin D-cancer hypothesis is correct, vitamin D supplementation for cancer prevention and treatment should be considered. A recent review recommended supplementation with 2000 IU/d of vitamin D
3 for good health [
49]. That would be a good recommendation for reducing cancer risk for people with BMI < 25 kg/m
2 of body surface area. However, as reported in secondary analyses of the VITAL study, people with BMI < 25 kg/m
2 had a 25% reduction in all-cancer incidence and those with higher BMI did not. Even though those below and above 25 kg/m
2 increased serum 25(OH)D concentrations by the same amount, 12 ng/mL, those with higher BMI did not significantly reduce risk of all-cancer incidence [
50]. The mean baseline 25(OH)D concentration of participants in that study’s vitamin D arm was near 31 ng/mL. However, 25(OH)D concentrations were not measured in those in the placebo arm or in all of the treatment group. Most likely, people with higher BMI require higher vitamin D doses as well as higher 25(OH)D concentrations to reduce cancer risk. Moreover, vitamin D supplementation has not been shown to significantly reduce inflammatory markers in overweight and obese people, as shown in a meta-analysis of 11 randomized controlled trials with a total of 504 participants [
51]. No significant change was apparent in either C-reactive protein or tumor necrosis factor concentrations related to either vitamin D dose or achieved serum 25(OH)D concentration.
Vitamin D supplementation might also be considered adjuvant treatment for people who develop cancer [
43,
52,
53]. Vitamin D is more effective in reducing cancer mortality risk than incidence [
50,
54] because vitamin D is one of the few natural compounds in the body that reduce angiogenesis around tumors and metastasis [
43].
4.2. Lung Cancer
Several reasons may explain this ecological study not having found more cancers linked to lung cancer:
Lung cancer incidence or mortality rate may not be a good proxy for effects of smoking because lung cancer risk also is related to diet, as shown here by the high correlation between obesity and lung cancer mortality rate.
Smoking rates may not vary geographically much in France.
Smoking may have been less important than before because of a trend towards lower smoking rates.
4.3. Alcohol Consumption
As for alcohol consumption, it is likely that repeated drunkenness is not a suitable measure for alcohol consumption rates or assessing their effects on cancer risk, or alcohol consumption does not vary much throughout France. Further speculation on reasons is beyond the scope of this study.
4.4. Obesity
For males, this ecological study found obesity correlated with incidence of CRC and with mortality for all-lung, bladder, CRC, and pancreatic cancer. For females, this ecological study found obesity correlated with incidence of kidney cancer and with mortality for all-lung, breast, CRC, corpus uteri, kidney, liver, ovarian, and thyroid cancer. In comparison with the article by Arnold et al. [
10], this study did not find obesity correlated with incidence or mortality rate for esophageal, gallbladder, and stomach cancers for males or females and liver cancer for males. In addition, it found obesity correlated with bladder cancer for males and females and thyroid cancer for females. Obesity has been found associated with risk of bladder cancer [
55,
56] and thyroid cancer [
57]. An analysis of the PAF for cancer incidence and mortality using data from the UK Biobank also found an association with bladder cancer, but it was the lowest of 10 for incidence and 9 for mortality [
58]. This UK Biobank study found higher BMI associated with risk of ten types of cancer, of which eight were found associated with obesity in this ecological study, the exceptions being gallbladder and stomach cancers.
Comparison of the PAF values from the two studies indicates that the ecological study found lower values. This is likely due to the fact that the ecological study approach is only sensitive to variations unless the linear regression fit to the data is compared for some value rather than minimum value for the regions. The same consideration would apply to solar UVB dose.
Many papers have discussed obesity’s role in risk of cancer incidence and mortality rates. A 2021 review estimated that 55% of cancers diagnosed in women and 24% in men were related to overweight/obesity [
59].
A 2019 review has a comprehensive discussion of mechanisms by which obesity increases risk of cancer [
60]. A 2023 review also has useful information regarding the role of adipose tissue on risk of cancer [
61].
Figure 1 in that review shows the links between the various factors linking obesity to risk of cancer. Their lists of important mechanisms are used as the framework to describe the mechanisms.
A 2021 meta-analysis of 203 studies with 6,320,365 participants evaluated the association of obesity with overall survival (OS), cancer-specific survival (CSS), and progression-free survival (PFS) or disease-free survival (DFS) in cancer patients [
62]. Overall, obesity compared to normal weight was associated with a reduced OS (hazard ratio [HR] = 1.14 [95% CI, 1.09–1.19]) and CSS (HR = 1.17 [95% CI, 1.12–1.23]). Patients also were at increased risk of recurrence (HR = 1.13 [95% CI, 1.07–1.19]). Conversely, patients with obesity and lung cancer, renal cell carcinoma, or melanoma had better survival outcomes than nonobese patients with the same cancer (lung, HR = 0.86 [95% CI, 0.76–0.98]; renal cell, HR = 0.74 [95% CI, 0.53–0.89]; melanoma, HR = 0.74 [95% CI, 0.57–0.96]).
Whether obesity can be considered a causal risk factor for some cancers is still being debated. A 2018 review discussed existing and new hypotheses for a causal connection [
63]. A 2023 article presented the evidence that metabolically (un)healthy obesity compared to metabolically healthy normal weight status for cancer at ten body sites with HRs up to 2.55 to 3.00 for three types of cancer (endometrial, liver, and renal cell) [
64].
Lung cancer risk is inversely associated with obesity. To determine why, researchers analyzed tissue differences between lung neoplasms and healthful lungs in seven gene expression data sets [
65]. Analysis showed significant enrichment of adipocytes and preadipocytes in healthful lung tissue compared with lung cancers. An understudied adipokine, omentin, was significantly and consistently lower in lung neoplasms than in healthful lungs. Omentin was consistently downregulated in lung cancers and exhibited a negative correlation with important transcription factors FOXA1, EN1, FOXC1, and ELK4. The authors suggested that omentin may serve as a prognostic factor in lung cancer and explain the “obesity paradox” in lung cancer.
4.5. Interaction between Vitamin D and Obesity/Adiposity
Vitamin D and obesity have opposite roles regarding the risk of cancer. The present ecological study found that obesity prevalence had higher correlations with all-cancer incidence rates for males and mortality rates for males and females than solar UVB doses. An interesting topic to consider is to what extent higher serum 25(OH)D concentrations reduce the effect of obesity and adipose tissue on risk of cancer. As discussed earlier, based on results from the VITAL study [
50], it appears as if 25(OH)D concentrations that reduce overall cancer do not appear to be sufficient for those who are obese. On the other hand, the VITAL study also found that when the first 1 or 2 years of follow up were excluded, the rate of death from cancer was significantly lower with vitamin D than with placebo (HR = 0.79 [95% CI, 0.63 to 0.99], and 0.75 [95% CI, 0.59 to 0.96], respectively). For cancer incidence, inflammation is an important driver, but for death, angiogenesis and metastasis are important drivers. Vitamin D seems more effective for preventing death from cancer than cancer incidence.
4.6. Strengths and Weaknesses
Among this ecological study’s strengths are that it includes cancer data for geographical regions across continental France. The results regarding solar UVB radiation are generally consistent with findings in other studies. In comparison with a similar ecological study of cancer incidence rates in the US for the period 2016‒2020, there was stronger agreement between the results in this study for cancer mortality rates with results for cancer incidence rates [
66]. The differences may be due to different strengths of cancer risk-modifying factors in the two countries. The method works well for risk-modifying factors that have reasonable geographical variation as well as important impacts on cancer risk.
An important weakness of the ecological study approach is that it is only sensitive to geographical variations in cancer rates with respect to risk-modifying factors. Thus, the portion of cancer risk due to values below the minimum for the regression fit to the data cannot be determined. For example, if the mean smoking rate was similar for all regions, there would be very little variation in lung cancer by region due to smoking. Another weakness is that data for some important cancer risk–modifying factors, such as diet, were not available. In addition, the data used were representative, not inclusive. Also, because data for individual people were not used, the interaction between risk-modifying factors such as obesity and serum 25(OH)D concentrations could not be assessed. Data for obesity were for adults and not just older adults with higher cancer risk. Data for alcohol consumption did not appear to be suitable. It is noted that the 2024 U.S. ecological study [
15] included data for alcohol consumption, which was significantly correlated with several types of cancer, but did not appreciably affect the results regarding solar UVB doses of obesity prevalence. In addition, the study used no information on cancer screening, such as for breast, colorectal, and prostate cancer, or treatment. Cancer screening could increase cancer incidence rates, as it did for a few years near 1990 when the prostate-specific antigen test was widely adopted (see, e.g.,
Figure 2 in [
6]). There is also the limitation due to the multiplicity of testing. A 2004 opinion discussed how the Bonferroni rule applies to ecological studies [
67]. It appears from
Table 3 in that paper that for four repeated tests, a
p value required to reject the null hypothesis at the 0.05 level for one test is lowered to somewhere between 0.009 and 0.024. Thus, some of the regression results in
Table 3,
Table 4,
Table 5 and
Table 6 should not be considered significant just because
p is <0.05.
5. Conclusions
This ecological study finds that obesity is associated with increased risk of several types of cancer in France and that solar UVB is associated with reduced risk of some cancers. These findings are consistent with findings from other ecological and observational studies. This study suggests that cancer rates in France could be reduced through more solar UVB exposure and vitamin D supplementation as well as taking steps to reduce the rate of overweight and obesity, preferably through dietary measures.
Author Contributions
Conceptualization, W.B.G.; obtaining French datasets, P.M.; analytic method, W.B.G.; formal analysis, W.B.G.; writing—original draft preparation, W.B.G.; writing—review and editing, W.B.G. and P.M.; visualization, W.B.G.; supervision, W.B.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article or in the references provided. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors thank Barbara J. Boucher, The Blizard Institute, Barts and The London School of Medicine and Dentistry, Queen Mary University of London, for helpful comments on the manuscript.
Conflicts of Interest
W.B.G. has had funding from Bio-Tech Pharmacal, Inc., (Fayetteville, AR, USA). The funder had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. P.M. has no conflicts of interest to declare.
References
- Population, France. Availabe online: https://data.worldbank.org/indicator/SP.POP.TOTL.MA.IN?locations=FR (accessed on June 30, 2024).
- Binder-Foucard, F.; Bossard, N.; Delafosse, P.; Belot, A.; Woronoff, A.S.; Remontet, L.; French network of cancer, r. Cancer incidence and mortality in France over the 1980-2012 period: solid tumors. Rev Epidemiol Sante Publique 2014, 62, 95–108. [Google Scholar] [CrossRef] [PubMed]
- France, Causes of Death. Availabe online: https://www.ined.fr/en/everything_about_population/data/france/deaths-causes-mortality/deaths-causes/ (accessed on June 30, 2024).
- Soerjomataram, I.; Shield, K.; Marant-Micallef, C.; Vignat, J.; Hill, C.; Rogel, A.; Menvielle, G.; Dossus, L.; Ormsby, J.N.; Rehm, J. , et al. Cancers related to lifestyle and environmental factors in France in 2015. Eur J Cancer 2018, 105, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Introduction to SAS. Availabe online: https://stats.oarc.ucla.edu/sas/modules/introduction-to-the-features-of-sas/. (accessed on 22 August 2024).
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J Clin 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Hill, C.; Bonaldi, C.; Leon, M.E.; Menvielle, G.; Arwidson, P.; Bray, F.; Soerjomataram, I. Cancers attributable to tobacco smoking in France in 2015. Eur J Public Health 2018, 28, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Shield, K.D.; Marant Micallef, C.; Hill, C.; Touvier, M.; Arwidson, P.; Bonaldi, C.; Ferrari, P.; Bray, F.; Soerjomataram, I. New cancer cases in France in 2015 attributable to different levels of alcohol consumption. Addiction 2018, 113, 247–256. [Google Scholar] [CrossRef]
- Guerin, S.; Laplanche, A.; Dunant, A.; Hill, C. Alcohol-attributable mortality in France. Eur J Public Health 2013, 23, 588–593. [Google Scholar] [CrossRef]
- Arnold, M.; Touillaud, M.; Dossus, L.; Freisling, H.; Bray, F.; Margaritis, I.; Deschamps, V.; Soerjomataram, I. Cancers in France in 2015 attributable to high body mass index. Cancer Epidemiol 2018, 52, 15–19. [Google Scholar] [CrossRef]
- Arnold, M.; Kvaskoff, M.; Thuret, A.; Guenel, P.; Bray, F.; Soerjomataram, I. Cutaneous melanoma in France in 2015 attributable to solar ultraviolet radiation and the use of sunbeds. J Eur Acad Dermatol Venereol 2018, 32, 1681–1686. [Google Scholar] [CrossRef]
- Grant, W.B. An estimate of premature cancer mortality in the U.S. due to inadequate doses of solar ultraviolet-B radiation. Cancer 2002, 94, 1867–1875. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Garland, C.F. The association of solar ultraviolet B (UVB) with reducing risk of cancer: multifactorial ecologic analysis of geographic variation in age-adjusted cancer mortality rates. Anticancer Res 2006, 26, 2687–2699. [Google Scholar]
- Boscoe, F.P.; Schymura, M.J. Solar ultraviolet-B exposure and cancer incidence and mortality in the United States, 1993-2002. BMC Cancer 2006, 6, 264. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B. Cancer Incidence Rates in the US in 2016–2020 with Respect to Solar UVB Doses, Diabetes and Obesity Prevalence, Lung Cancer Incidence Rates, and Alcohol Consumption: An Ecological Study. Nutrients 2024, 16, 1450. [Google Scholar] [CrossRef] [PubMed]
- Apperly, F.L. The relation of solar radiation to cancer mortality in North America. Cancer Research 1941, 1, 191–195. [Google Scholar]
- Garland, C.F.; Garland, F.C. Do sunlight and vitamin D reduce the likelihood of colon cancer? Int J Epidemiol 1980, 9, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Garland, F.C.; Garland, C.F.; Gorham, E.D.; Young, J.F. Geographic variation in breast cancer mortality in the United States: a hypothesis involving exposure to solar radiation. Prev Med 1990, 19, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Lefkowitz, E.S.; Garland, C.F. Sunlight, vitamin D, and ovarian cancer mortality rates in US women. Int J Epidemiol 1994, 23, 1133–1136. [Google Scholar] [CrossRef]
- Wei, X.; Zhu, C.; Ji, M.; Fan, J.; Xie, J.; Huang, Y.; Jiang, X.; Xu, J.; Yin, R.; Du, L. , et al. Diet and Risk of Incident Lung Cancer: A Large Prospective Cohort Study in UK Biobank. Am J Clin Nutr 2021, 114, 2043–2051. [Google Scholar] [CrossRef]
- Chatignoux, E.; Billot-Grasset, A.; Cariou, M. Estimations régionales et départementales d’incidence et de mortalité par cancers en France, 2007-2016; Annexes aux profils regiionaux; Santé publique France, Institut national du cancer.: 2019, 2019; p 242.
- Eschwege, E.; Charles, M.A.; Basdevant, A. Enquête épidémiologique nationale sur le surpoids et l'obésité; INSERM, Kantar Health and Roche: 2012.
- Orton, S.M.; Wald, L.; Confavreux, C.; Vukusic, S.; Krohn, J.P.; Ramagopalan, S.V.; Herrera, B.M.; Sadovnick, A.D.; Ebers, G.C. Association of UV radiation with multiple sclerosis prevalence and sex ratio in France. Neurology 2011, 76, 425–431. [Google Scholar] [CrossRef]
- Zerefos, C.; Fountoulakis, I.; Eleftheratos, K.; Kazantzidis, A. Long-term variability of human health-related solar ultraviolet-B radiation doses for the 1980s to the end of the 21st century. Physiol Rev 2023, 103, 1789–1826. [Google Scholar] [CrossRef]
- Kulhanova, I.; Morelli, X.; Le Tertre, A.; Loomis, D.; Charbotel, B.; Medina, S.; Ormsby, J.N.; Lepeule, J.; Slama, R.; Soerjomataram, I. The fraction of lung cancer incidence attributable to fine particulate air pollution in France: Impact of spatial resolution of air pollution models. Environ Int 2018, 121, 1079–1086. [Google Scholar] [CrossRef]
- Tchicaya, A.; Lorentz, N.; Omrani, H.; de Lanchy, G.; Leduc, K. Impact of long-term exposure to PM(2.5) and temperature on coronavirus disease mortality: observed trends in France. Environ Health 2021, 20, 101. [Google Scholar] [CrossRef]
- Beck, F.; Guignard, R.; Léon, C.; Richard, J.R. Atlas des usages de substances psychoactives 2010; Institut national de prévention et d’éducation pour la santé: Saint-Denis Cedex France, 2013; p. 106. [Google Scholar]
- Souberbielle, J.C.; Massart, C.; Brailly-Tabard, S.; Cavalier, E.; Chanson, P. Prevalence and determinants of vitamin D deficiency in healthy French adults: the VARIETE study. Endocrine 2016, 53, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B. Role of solar UVB irradiance and smoking in cancer as inferred from cancer incidence rates by occupation in Nordic countries. Dermatoendocrinol 2012, 4, 203–211. [Google Scholar] [CrossRef]
- Shanbhag, S.; Nayak, A.; Narayan, R.; Nayak, U.Y. Anti-aging and Sunscreens: Paradigm Shift in Cosmetics. Adv Pharm Bull 2019, 9, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Couteau, C.; Philippe, A.; Galharret, J.M.; Metay, E.; Coiffard, L. UV filters in everyday cosmetic products, a comparative study. Environ Sci Pollut Res Int 2024, 31, 2976–2986. [Google Scholar] [CrossRef] [PubMed]
- De Pergola, G.; Martino, T.; Zupo, R.; Caccavo, D.; Pecorella, C.; Paradiso, S.; Silvestris, F.; Triggiani, V. 25 Hydroxyvitamin D Levels are Negatively and Independently Associated with Fat Mass in a Cohort of Healthy Overweight and Obese Subjects. Endocr Metab Immune Disord Drug Targets 2019, 19, 838–844. [Google Scholar] [CrossRef]
- Bellia, A.; Garcovich, C.; D'Adamo, M.; Lombardo, M.; Tesauro, M.; Donadel, G.; Gentileschi, P.; Lauro, D.; Federici, M.; Lauro, R. , et al. Serum 25-hydroxyvitamin D levels are inversely associated with systemic inflammation in severe obese subjects. Intern Emerg Med 2013, 8, 33–40. [Google Scholar] [CrossRef]
- Fest, J.; Ruiter, R.; Mulder, M.; Groot Koerkamp, B.; Ikram, M.A.; Stricker, B.H.; van Eijck, C.H.J. The systemic immune-inflammation index is associated with an increased risk of incident cancer-A population-based cohort study. Int J Cancer 2020, 146, 692–698. [Google Scholar] [CrossRef]
- Grant, W.B. An ecological study of cancer incidence and mortality rates in France with respect to latitude, an index for vitamin D production. Dermatoendocrinol 2010, 2, 62–67. [Google Scholar] [CrossRef]
- Weller, R.B.; Wang, Y.; He, J.; Maddux, F.W.; Usvyat, L.; Zhang, H.; Feelisch, M.; Kotanko, P. Does Incident Solar Ultraviolet Radiation Lower Blood Pressure? J Am Heart Assoc 2020, 9, e013837. [Google Scholar] [CrossRef]
- Cherrie, M.; Clemens, T.; Colandrea, C.; Feng, Z.; Webb, D.J.; Weller, R.B.; Dibben, C. Ultraviolet A radiation and COVID-19 deaths in the USA with replication studies in England and Italy. Br J Dermatol 2021, 185, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Heaney, R.P. Guidelines for optimizing design and analysis of clinical studies of nutrient effects. Nutr Rev 2014, 72, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Trummer, C.; Theiler-Schwetz, V.; Grubler, M.R.; Verheyen, N.D.; Odler, B.; Karras, S.N.; Zittermann, A.; Marz, W. Critical Appraisal of Large Vitamin D Randomized Controlled Trials. Nutrients 2022, 14, 303. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Boucher, B.J.; Al Anouti, F.; Pilz, S. Comparing the Evidence from Observational Studies and Randomized Controlled Trials for Nonskeletal Health Effects of Vitamin D. Nutrients 2022, 14, 3811. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.B. The Environment and Disease: Association or Causation? Proc R Soc Med 1965, 58, 295–300. [Google Scholar] [CrossRef]
- Grant, W.B. How strong is the evidence that solar ultraviolet B and vitamin D reduce the risk of cancer?: An examination using Hill's criteria for causality. Dermatoendocrinol 2009, 1, 17–24. [Google Scholar] [CrossRef]
- Muñoz, A.; Grant, W.B. Vitamin D and Cancer: An Historical Overview of the Epidemiology and Mechanisms. Nutrients 2022, 14, 1448. [Google Scholar] [CrossRef]
- Kim, S.R.; Kim, K.; Lee, S.A.; Kwon, S.O.; Lee, J.K.; Keum, N.; Park, S.M. Effect of Red, Processed, and White Meat Consumption on the Risk of Gastric Cancer: An Overall and Dose(-)Response Meta-Analysis. Nutrients 2019, 11. [Google Scholar] [CrossRef]
- Keum, N.; Chen, Q.Y.; Lee, D.H.; Manson, J.E.; Giovannucci, E. Vitamin D supplementation and total cancer incidence and mortality by daily vs. infrequent large-bolus dosing strategies: a meta-analysis of randomised controlled trials. Br J Cancer 2022, 127, 872–878. [Google Scholar] [CrossRef]
- Kanno, K.; Akutsu, T.; Ohdaira, H.; Suzuki, Y.; Urashima, M. Effect of Vitamin D Supplements on Relapse or Death in a p53-Immunoreactive Subgroup With Digestive Tract Cancer: Post Hoc Analysis of the AMATERASU Randomized Clinical Trial. JAMA Netw Open 2023, 6, e2328886. [Google Scholar] [CrossRef]
- Kuznia, S.; Zhu, A.; Akutsu, T.; Buring, J.E.; Camargo, C.A., Jr.; Cook, N.R.; Chen, L.J.; Cheng, T.D.; Hantunen, S.; Lee, I.M. , et al. Efficacy of vitamin D(3) supplementation on cancer mortality: Systematic review and individual patient data meta-analysis of randomised controlled trials. Ageing Res Rev 2023, 87, 101923. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Shui, I.; Giovannucci, E. Chapter 85 - Vitamin D Status and cancer incidence, mortality, and prognosis. Feldman and Pike's Vitamin D (Fifth Edition) 2024, 2, 719–739. [Google Scholar] [CrossRef]
- Pludowski, P.; Grant, W.B.; Karras, S.N.; Zittermann, A.; Pilz, S. Vitamin D Supplementation: A Review of the Evidence Arguing for a Daily Dose of 2000 International Units (50 microg) of Vitamin D for Adults in the General Population. Nutrients 2024, 16, 391. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.E.; Cook, N.R.; Lee, I.M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Gordon, D.; Copeland, T.; D'Agostino, D. , et al. Vitamin D Supplements and Prevention of Cancer and Cardiovascular Disease. N Engl J Med 2019, 380, 33–44. [Google Scholar] [CrossRef]
- Gouveia, H.J.C.B.; da Dilva, M.M.; de Castro, F.M.; da Silva, L.K.T.M.; da Silva-Calado, C.M.S.; da Silva-Araujo, E.R.; Silva, M.d.C.; Toscano, A.E. Vitamin D supplementation does not alter inflammatory markers in overweight and obese individuals: A systematic review and meta-analysis of randomized controlled trials. Nutrition Research, 2024. [Google Scholar] [CrossRef]
- El-Sharkawy, A.; Malki, A. Vitamin D Signaling in Inflammation and Cancer: Molecular Mechanisms and Therapeutic Implications. Molecules 2020, 25. [Google Scholar] [CrossRef]
- Negri, M.; Gentile, A.; de Angelis, C.; Monto, T.; Patalano, R.; Colao, A.; Pivonello, R.; Pivonello, C. Vitamin D-Induced Molecular Mechanisms to Potentiate Cancer Therapy and to Reverse Drug-Resistance in Cancer Cells. Nutrients 2020, 12, 1798. [Google Scholar] [CrossRef]
- Chandler, P.D.; Chen, W.Y.; Ajala, O.N.; Hazra, A.; Cook, N.; Bubes, V.; Lee, I.M.; Giovannucci, E.L.; Willett, W.; Buring, J.E. , et al. Effect of Vitamin D3 Supplements on Development of Advanced Cancer: A Secondary Analysis of the VITAL Randomized Clinical Trial. JAMA Netw Open 2020, 3, e2025850. [Google Scholar] [CrossRef]
- Sun, J.W.; Zhao, L.G.; Yang, Y.; Ma, X.; Wang, Y.Y.; Xiang, Y.B. Obesity and risk of bladder cancer: a dose-response meta-analysis of 15 cohort studies. PLoS One 2015, 10, e0119313. [Google Scholar] [CrossRef]
- Zhao, L.; Tian, X.; Duan, X.; Ye, Y.; Sun, M.; Huang, J. Association of body mass index with bladder cancer risk: a dose-response meta-analysis of prospective cohort studies. Oncotarget 2017, 8, 33990–34000. [Google Scholar] [CrossRef]
- Abiri, B.; Ahmadi, A.R.; Valizadeh, A.; Abbaspour, F.; Valizadeh, M.; Hedayati, M. Obesity and thyroid cancer: unraveling the connection through a systematic review and meta-analysis of cohort studies. J Diabetes Metab Disord 2024, 23, 461–474. [Google Scholar] [CrossRef]
- Parra-Soto, S.; Cowley, E.S.; Rezende, L.F.M.; Ferreccio, C.; Mathers, J.C.; Pell, J.P.; Ho, F.K.; Celis-Morales, C. Associations of six adiposity-related markers with incidence and mortality from 24 cancers-findings from the UK Biobank prospective cohort study. BMC Med 2021, 19, 7. [Google Scholar] [CrossRef] [PubMed]
- Argyrakopoulou, G.; Dalamaga, M.; Spyrou, N.; Kokkinos, A. Gender Differences in Obesity-Related Cancers. Curr Obes Rep 2021, 10, 100–115. [Google Scholar] [CrossRef]
- Avgerinos, K.I.; Spyrou, N.; Mantzoros, C.S.; Dalamaga, M. Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metabolism 2019, 92, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Pati, S.; Irfan, W.; Jameel, A.; Ahmed, S.; Shahid, R.K. Obesity and Cancer: A Current Overview of Epidemiology, Pathogenesis, Outcomes, and Management. Cancers (Basel) 2023, 15. [Google Scholar] [CrossRef]
- Petrelli, F.; Cortellini, A.; Indini, A.; Tomasello, G.; Ghidini, M.; Nigro, O.; Salati, M.; Dottorini, L.; Iaculli, A.; Varricchio, A. , et al. Association of Obesity With Survival Outcomes in Patients With Cancer: A Systematic Review and Meta-analysis. JAMA Netw Open 2021, 4, e213520. [Google Scholar] [CrossRef] [PubMed]
- Stone, T.W.; McPherson, M.; Gail Darlington, L. Obesity and Cancer: Existing and New Hypotheses for a Causal Connection. EBioMedicine 2018, 30, 14–28. [Google Scholar] [CrossRef]
- Sun, M.; Fritz, J.; Haggstrom, C.; Bjorge, T.; Nagel, G.; Manjer, J.; Engeland, A.; Zitt, E.; van Guelpen, B.; Stattin, P. , et al. Metabolically (un)healthy obesity and risk of obesity-related cancers: a pooled study. J Natl Cancer Inst 2023, 115, 456–467. [Google Scholar] [CrossRef]
- Parida, S.; Siddharth, S.; Sharma, D. Role of Omentin in Obesity Paradox in Lung Cancer. Cancers (Basel) 2021, 13. [Google Scholar] [CrossRef]
- Grant, W.B. Cancer Incidence Rates in the US in 2016-2020 with Respect to Solar UVB Doses, Diabetes and Obesity Prevalence, Lung Cancer Incidence Rates, and Alcohol Consumption: An Ecological Study. Nutrients 2024, 16, 1450. [Google Scholar] [CrossRef]
- Garcia, L.V.; de Geoecologia, D. Escaping the Bonferroni iron claw in ecological studies. OIKOS 2004, 105, 657–663. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).