1. Introduction
Taste and smell disorders caused by antineoplastic agents can decrease food intake in patients undergoing chemotherapy [
1,
2]. Meanwhile, malnutrition in patients with cancer can lead to weight loss, prolonged treatment, and reduced quality of life [
1,
3,
4,
5]. A study using the Italian spontaneous adverse drug reaction (ADR) reporting database identified 182 cases of taste and/or smell impairments. In particular, 75.3% of patients reported taste alterations alone, 11.0% reported smell impairments alone, and 13.7% reported that both taste and smell alterations occurred [
6]. However, our knowledge of antineoplastic agents that induce these side effects is limited. In addition, because it is difficult for patients to distinguish taste disorders from smell disorders, patients with an abnormal sense of smell might also experience an abnormal sense of taste [
7,
8]. The US Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS) database has a large number of reports because it collects reports from many countries, thereby permitting analyses of infrequent or underreported side effects. Using the FAERS database, this study aimed to understand or characterize antineoplastic agents that induce taste and smell disorders.
2. Results
2.1. Creation of Data Tables
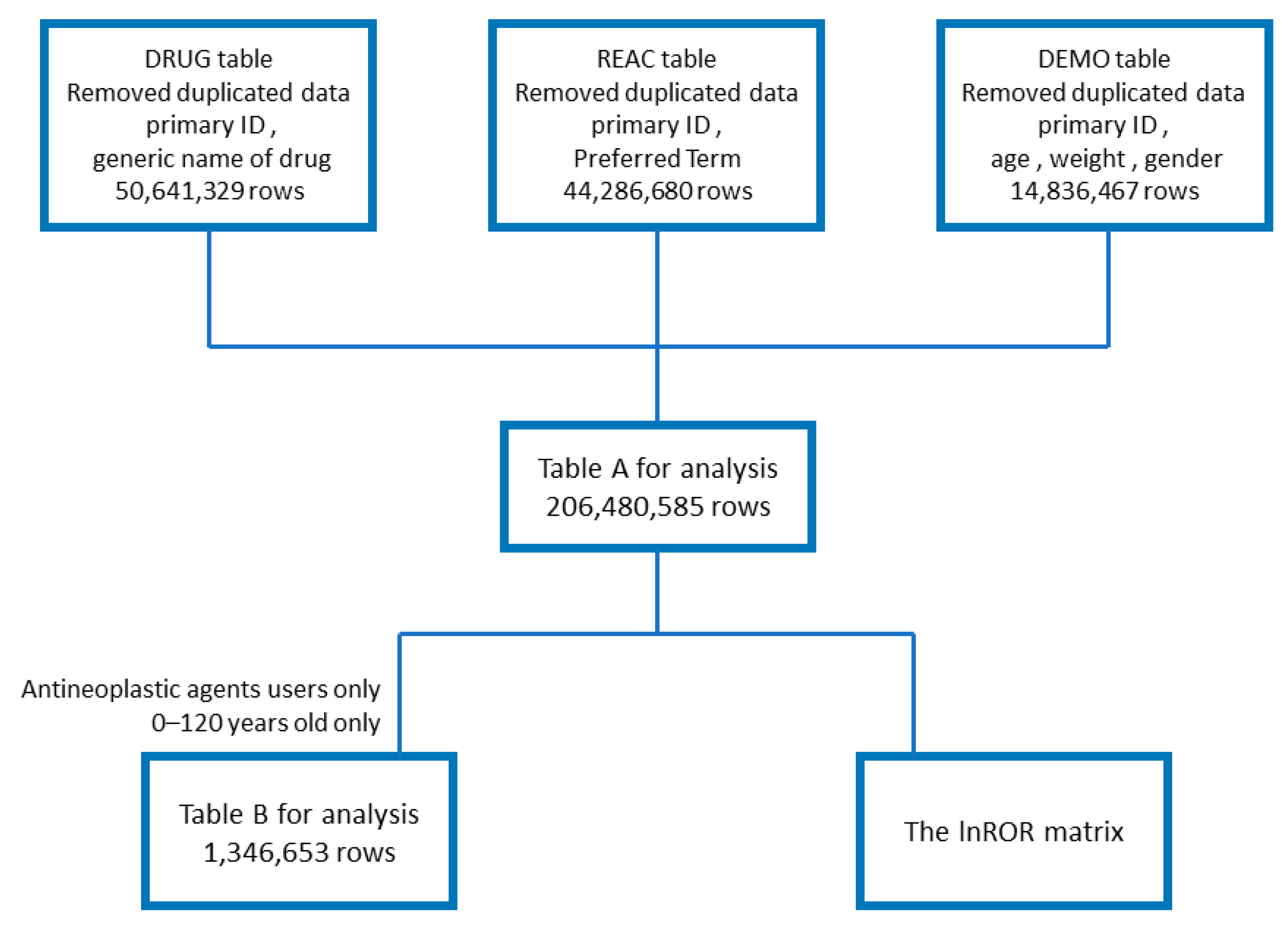
The analysis used a data table that combined the FAERS drug information (DRUG) table (50,641,329 rows), adverse reaction information (REAC) table (44,286,680 rows), and patient demographic information (DEMO) table (14,836,467 rows). Duplicate content was removed during data table creation. The flowchart of table creation is presented in
Figure 1.
Table A for analysis was created by de-duplicating each of the three tables (DRUG, REAC, and DEMO tables) and combining them using the primary ID. Table B for analysis and the lnROR matrix table were created from Table A. Table A was used to study the number of reports of taste and smell disorders and calculate p-values and RORs for drugs and adverse drug reactions. Table B presents information on the gender and age of patients who developed taste and smell disorders after antineoplastic agent treatment. The lnROR matrix was used for hierarchical cluster and principal component analyses.
2.2. Number of Reports of Taste and Smell Disorders
The data tables for analysis included 18,764 reports of abnormal taste and 1470 reports of abnormal olfaction among patients who used antineoplastic agents. There were 892 reports of combined taste and smell abnormalities (
Table 1).
2.3. Relationships of Patient Age and Gender with Taste and Smell Disorders Induced by Antineoplastic Agents
Table 2 presents data on gender and age of patients who used antineoplastic drugs. The results revealed no significant association with gender, whereas the risk of antineoplastic agent-induced taste and smell disorders was higher in patients aged ≥70 years.
2.4. Identification of Antineoplastic Agents That Induce Taste and Smell Disorders
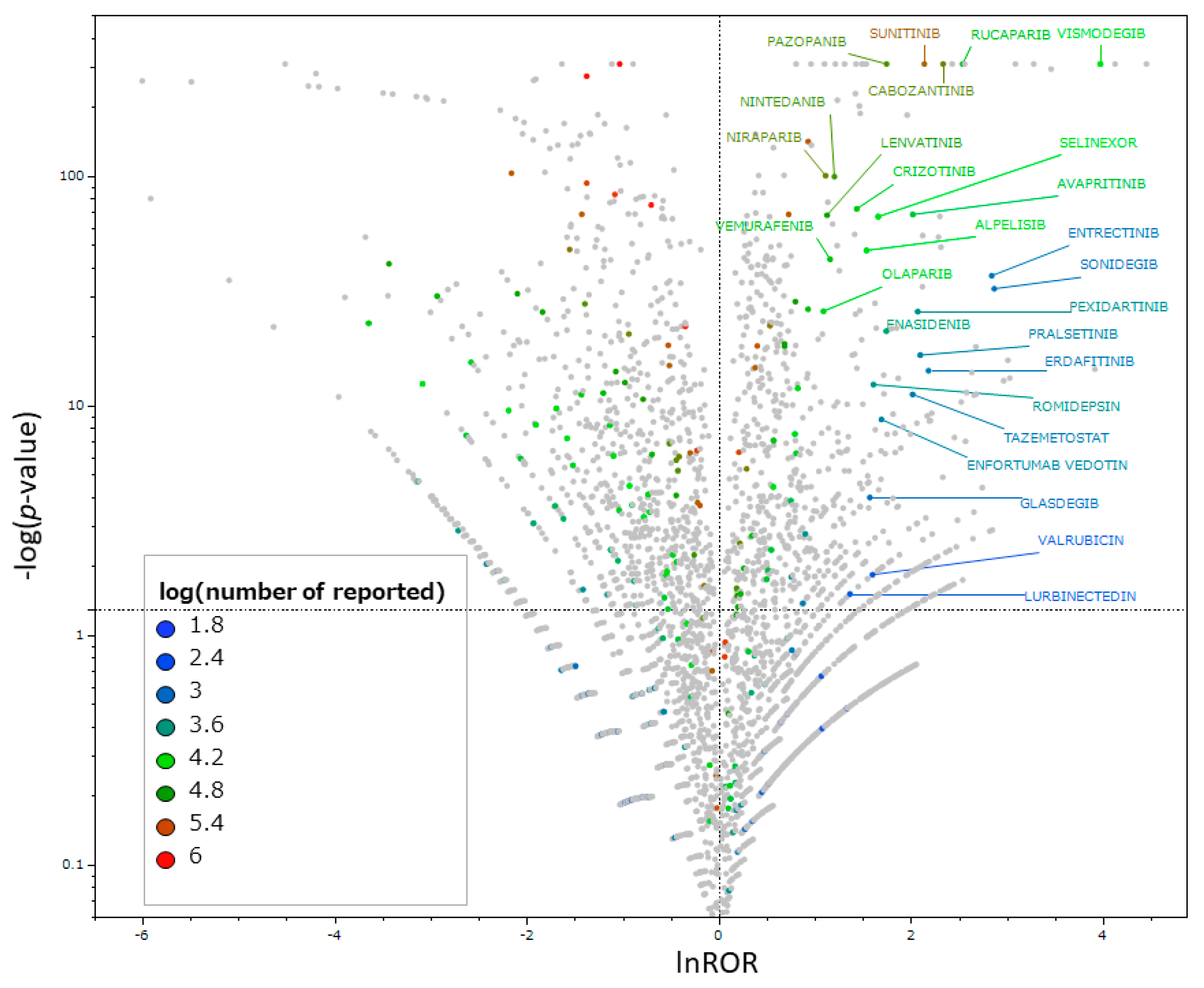
Scatter plots were created to identify antineoplastic agents that increase the risk of taste and smell disorders. As presented in
Figure 2, 56 drugs were found to potentially induce taste and smell disorders based on their reporting odds ratios (RORs) and
p-values (
Supplementary Table S1).
The vertical axis presents the statistical significance based on Fisher’s exact test, and the horizontal axis presents the risk of inducing taste and smell disorders. Drugs significantly associated with adverse reactions are indicated in the upper right corner. The scatterplot is colored for antineoplastic agents according to the number of drug reports. Non-antineoplastic agents are presented in gray, and antineoplastic agents with lnROR ≥ 1 and −logp ≥ 1.3 or greater are labeled with the drug name. Drugs with 100 or more reports are presented.
2.5. Relationships between the Class of Antineoplastic Agents and Taste and Smell Disorders
Next, the risk of taste and smell disorders was examined by Anatomical Therapeutic Chemical (ATC) class. Specifically, the percentage of antineoplastic agents that were likely to induce taste and smell disorders in each of the seven categories of antineoplastic agents was calculated (
Table 3). Based on an analysis using Fisher’s exact test, protein kinase inhibitors were found to significantly increase the risk of taste and smell disorders among antineoplastic agents (
Table 4).
2.6. Hierarchical Cluster Analysis
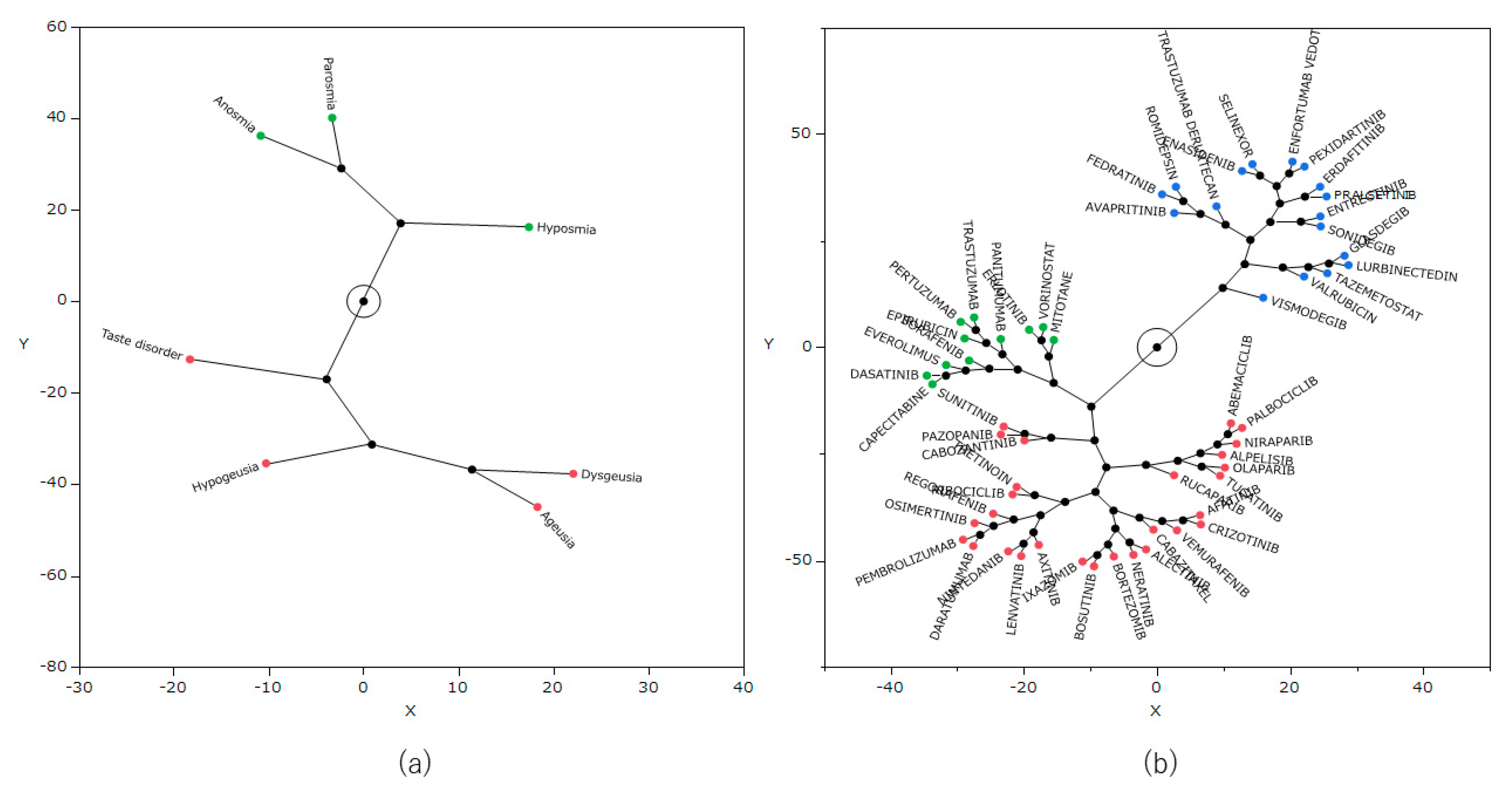
Hierarchical cluster analysis was performed using the natural logarithm of ROR (lnROR) matrix of side effects and antineoplastic agents, which identified three clusters (
Figure 3). In addition, from the results of the cluster analysis, a constellation plot of drugs and side effect words was created (
Figure 4).
The figure presents the relationship between seven side effects related to taste and smell disorders and 56 antineoplastic agents. In the color map, red indicates positive correlations, and blue indicates negative correlations.
The figure provides a visual understanding of the results of the cluster analysis. Dots indicate individual data lines, and line lengths indicate relative distances between clusters. Plots (a) and (b) are related to side effects and drugs, respectively.
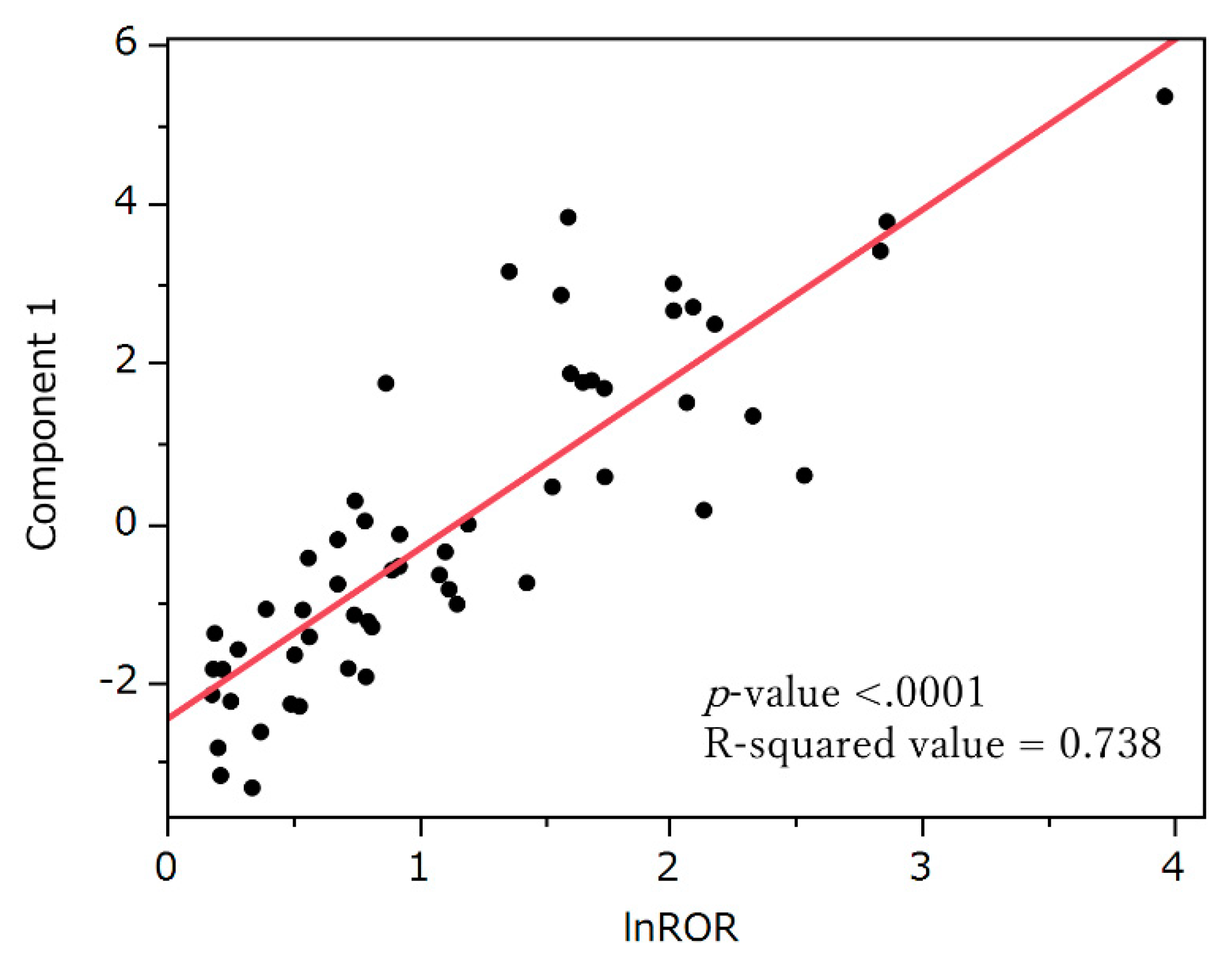
2.7. Principal Component Analysis
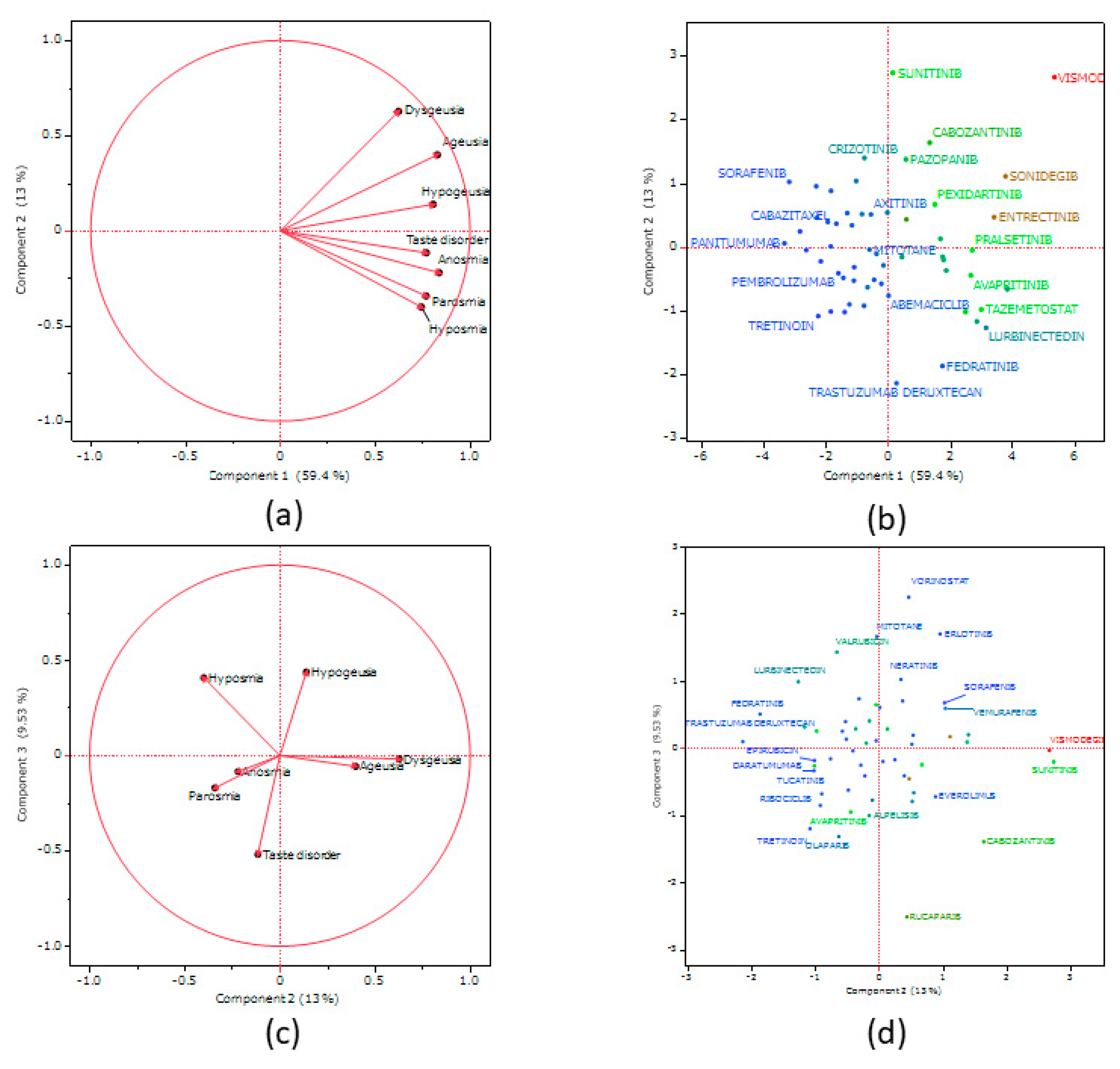
Figure 5 presents the results of principal component analysis. The contributions of principal components 1, 2, and 3 were 59.4%, 13%, and 9.53%, respectively. Principal component 1 was positively correlated lnROR in section 2.4. (
p < 0.0001, R
2 = 0.738,
Figure 6). Principal component 2 was positively correlated with the preferred terms (PTs) dysgeusia (
p < 0.0001) and ageusia (
p = 0.0023), and negatively correlated with parosmia (
p = 0.0099) and "hyposmia (
p = 0.0022). Principal component 3 was positively correlated with the PTs hypogeusia (
p = 0.0007) and hyposmia (
p = 0.0018) and negatively correlated with taste disorder (
p < 0.0001).
Loadings plots (a) and (c) present the association between adverse events related to taste and smell disorders and each principal component. Each loading vector represents an adverse effect.
Score plots (b) and (d) present the relationship between antineoplastic agents and each principal component. Each dot represents an antineoplastic agent.
Each plot is colored by lnROR based on calculations for all drugs.
The correlation between principal component 1 and “lnROR based on calculations for all drugs” was determined, and a least-squares line was fitted.
3. Discussion
3.1. Number of Reports of Taste and Smell Disorders
Among the patients who used the antineoplastic agents shown in Table A, 100% of the patients developed either taste or smell disorder, approximately 92% of the patients developed only taste disorder, and approximately 3% developed only smell disorder. Approximately 5% of patients developed both taste and smell disorders.
In this study, more disorders related to the sense of taste were reported than those related to the sense of smell, consistent with previous findings.
3.2. Characteristics of Patients with Taste and Smell Disorders and Antineoplastic Agents
This study identified no difference in the risk of antineoplastic agent-induced taste and smell disorders according to gender. Studies on the relationship of gender with this risk have been inconsistent. Some studies identified female gender as a risk factor for the development of taste disorders [
9,
10], whereas others reported no significant differences regarding alterations in taste and smell between men and women undergoing chemotherapy [
11].
Conversely, older age was identified as a risk factor for taste and smell disorders. Similarly as noted for gender, the association between age and the risk of taste and smell disorders has been inconsistent. Some studies suggested that young people have a higher risk of taste and smell alterations [
12,
13], whereas others found that older age increased the risk [
9,
14,
15].
Concerning drug-induced taste disorder, a prior study reported a significantly higher incidence among elderly patients [
16]. This suggests that the risk of dysgeusia induced by antineoplastic agents increases with advancing age, as is usually the case in patients with drug-induced taste disorder. A study on olfaction in the elderly indicated that the number of medications affects the olfactory threshold [
17]. Future research should focus on the number of concomitant medications used by patients. In addition, the present study used an adverse drug reaction report database with a large bias, and thus, caution should be exercised in interpreting the findings. Based on the study results, appropriate monitoring of taste and smell should be conducted when administering antineoplastic agents to elderly patients.
3.3. Relationships of Antineoplastic Agents with Taste and Smell Disorders
A volcano plot was created, and 56 antineoplastic agents were found to likely induce taste and smell disorders. Some of the identified drugs, such as daratumumab, nintedanib, epirubicin, and tretinoin, did not list taste and smell disorders as side effects in their Japanese package inserts [
18]. In addition, a pseudo-positive result might have been obtained for tretinoin. Caution should be exercised in the interpretation of the detected drugs.
3.4. Relationship between the Class of Antineoplastic Agents and Taste and Smell Disorders
The results indicated that protein kinase inhibitors represent the most likely drug class to induce taste and smell disorders among antineoplastic agents. A prior study using the Italian database of spontaneous adverse drug reaction reports similarly suggested that protein kinase inhibitors are associated with taste and smell impairment [
6]. Studies on the association between protein kinase inhibitors and alterations in taste and smell suggested that oral toxicities, such as oral mucositis and xerostomia, affect taste receptor cells and olfactory receptor neurons, and neurodegeneration might be involved in the mechanism of taste and smell disorders [
19]. Decreased Wnt-β catenin signaling can affect taste by affecting cell differentiation. Sunitinib, a signal for which was detected in this study, could have a similar effect [
19,
20]. EGFR inhibitors such as erlotinib afatinib and osimertinib, FGFR inhibitors such as erdafitinib, and the VEGFR inhibitor axitinib can also affect β-catenin signaling [
19,
21,
22].
In addition, the “other antineoplastic agents” class was significantly more likely to induce taste and smell disorders. Hedgehog pathway inhibitors are believed to affect taste by inhibiting the differentiation of taste cell precursors into taste cells [
19,
23,
24]. In this study, three drugs classified as hedgehog pathway inhibitors (L01XJ) in the ATC classification, namely vismodegib, sonidegib, and glasdegib, were detected as drugs that can cause this taste disorders. However, “other antineoplastic agents” includes a wide variety of drugs. Tretinoin, which is included in this class in this study, was found to have a significant link to taste disorders (
p < 0.05, ROR > 1) with more than 100 reported cases. However, tretinoin is a vitamin A derivative that is used for various purposes other than cancer treatment. Vitamins are sometimes used to treat smell disorders, and some studies [
25] suggested that topical vitamin A can improve smell disorders. Therefore, pseudo-positives are possible, and caution should be exercised in interpreting these medications.
3.5. Hierarchical Cluster Analysis
Hierarchical cluster analysis is a method of grouping similar data to generate a classification [
26]. The result of this analysis classified the drugs into three clusters (
Figure 3), and
Table 5 lists each cluster and the included drugs. Cluster 1 displayed a strong correlation with many of the seven side effect words, and the cluster included drugs such as vismodegib and tazemetostat. Cluster 3 exhibited a positive correlation for some side effects, whereas cluster 2 displayed a negative correlation with most side effects. All of the drugs used in this cluster analysis are considered likely to induce taste and smell disorders. However, the type of side effects that are likely to occur among them can vary by drug.
Based on these results, we further clarified the detailed characteristics of antineoplastic agents that induce taste and smell disorders by performing principal component analysis.
3.6. Principal Component Analysis
Principal component analysis reduces the dimensionality of the dataset, thereby increasing the potential for interpretation while reducing information loss [
27]. Principal component 1 was positively correlated with lnROR (Figure 7). Principal component 1 represent a risk of taste and smell disorders. Principal component 2 was positively correlated with the PTs dysgeusia and ageusia, which are related to the sense of taste. It was negatively correlated with parosmia and hyposmia, which are related to the sense of smell. This suggests that principal component 2 can be plotted in a more positive direction as it relates to taste side effects and in a more negative direction as it relates to smell side effects. Principal component 3 was positively correlated with the PTs hypogeusia and hyposmia, which are related to decreases in chemosensory perception. This component was also negatively correlated with the PT taste disorder, which is related to chemosensory changes. From this result, principal component 3 can be plotted in a positive direction for side effects that decrease chemosensory perception and in a negative direction for side effects that change sensory perception. Based on this speculation, we confirmed the score plots (
Figure 6) for principal components 2 and 3. Erlotinib might be the drug likely to cause a decrease in taste, cabozantinib might be the drug likely to cause a change in taste, ribociclib might be the drug likely to cause a change in smell, and lurbinectedin might be the drug likely to cause a decrease in smell. These inferences are expected to be useful in monitoring side effects in patients using antineoplastic agents.
3.7. Limitations
This study had several limitations. First, FAERS is a spontaneous reporting database of adverse drug reactions. Spontaneous reporting of adverse drug reactions is subject to reporting bias [
28,
29], including underreporting and missing or erroneous data. In addition, because the denominator of the drug users is unknown, the ratio of adverse drug reactions cannot be calculated, making it impossible to perform an absolute risk assessment. As a countermeasure, we deleted data considered to be missing or erroneous in the analysis data tables for age and gender. In addition, the number of drugs detected was limited by the number of reports.
In addition to underreporting, other biases included the notoriety effect [
30], in which the number of reports of adverse events that have become the focus of attention increases, the ripple effect [
30], in which the number of reports on drugs of the same type and efficacy as the specific drug that was the focus of adverse event attention increases, and the Weber effect [
31], in which the number of reports increases immediately after marketing and decreases over time. In addition, another bias is the masking effect, in which certain adverse events are underestimated because of the association of other drugs [
32]. Another concern is that when multiple drugs are administered, it is difficult to identify the drug responsible for the adverse reaction [
33,
34]. Furthermore, information such as the primary disease and route of administration, which were not included in this analysis, could be confounding factors that influence the occurrence of side effects. We are considering conducting further studies that consider these influences.
4. Materials and Methods
4.1. FAERS Database
To comprehensively analyze taste and smell disorders induced by antineoplastic agents, we used the FAERS database, which contains cases from January 2004 to March 2022 (May 2022 public version) [
35]. FAERS is a large FDA-published database of spontaneous adverse drug reaction reports. FAERS consists of seven data tables: DEMO, DRUG, REAC, Outcome (OUTC), Report Sources (RPSR), Indication (INDI), and Therapy (THER). Each table can be joined. The DEMO table contains basic patient information such as age, gender, and weight. The DRUG table contains drug information such as the drug name and administration method. The REAC table contains adverse event information such as PTs. The OUTC table contains outcome information. The RPSR table contains information sources. The INDI table contains drug indication information. The THER table contains information on the duration of treatment. For this analysis, we used Table A for analysis, which contains a combination of the DRUG, REAC, and DEMO tables with the elimination of duplicates. The combining was performed by primary ID.
Because this study used anonymized data from an open access database, the requirement for ethical approval and informed consent by the Meiji Pharmaceutical University Ethics Committee was waived.
4.2. Adverse Event Terms and Drugs for Analysis
The analyzed drugs were all reported to FAERS. Antineoplastic agents were defined as drugs classified as antineoplastic agents (L01) based on the ATC classification, a classification system proposed by the World Health Organization that categorizes drugs according to their therapeutic effects and characteristics [
36]. Regarding adverse events, we used the Standardized MedDRA Query (SMQ) [
37] in MedDRA ver. 25.0, and 14 adverse events from "taste and smell disorders” (SMQ: 20000046,
Table 6) included in the analysis after excluding congenital anosmia. Specifically, the taste disorders were “dysgeusia,” “ageusia,” “taste disorder,” “hypogeusia,” “hallucination, gustatory,” “hypergeusia,” and “gustometry, abnormal.” The smell disorders were “anosmia,” “parosmia,” “hyposmia,” “hallucination, olfactory,” “olfactory nerve disorder,” “olfactory test abnormal,” and “olfactory dysfunction.”
4.3. Number of Reports of Taste and Smell Disorders
Table A was used to measure the number of taste and smell disorders in cases in which a drug classified as an antineoplastic agent (L01) in the ATC classification was used. Adverse event terms were defined using the 14 terms listed in section 4.2. Patients receiving antineoplastic agents were counted as having developed an adverse reaction if they experience at least one taste or smell disorder.
4.4. Relationships of Taste and Smell Disorders with Age and Genger Among Patients Using Antineoplastic Agents
We removed all cases other than those in which antineoplastic agents were used on the basis of the data table created in section 4.1. In the case of multiple reports with the same primary ID, if there was more than one report of a taste or smell disorder, the patient was considered to have developed a taste or smell disorder. Cells in which age was not given in years were deleted. In addition, cases with ages lower than 0 or greater than 120 were removed as likely errors. The resulting table (Table B) contained 1,346,635 rows (
Figure 1). Elderly persons were defined as those “70 years of age or older.” Patients were divided into two age groups: <70 and ≥70 years Gender was categorized as male or female. We further divided the patients into two groups according to the presence or absence of taste and smell disorders and analyzed the results using Fisher’s exact test to identify significant correlations.
4.5. Relationships between Antineoplastic Agents and Taste and Smell Disorders
Using Table A, a 2 × 2 contingency table was created (
Table 7). A value of 0.5 was added to all cells as a Haldane correction [
38,
39,
40]. RORs and
p-values were calculated for antineoplastic agents and side effect terms related to taste and smell disorders using Fisher’s exact test. We performed a relative evaluation by detecting the signal represented by the RORs of individual drugs. This imbalance analysis method allowed us to identify cases in which a high number of drug side effects are reported [
41]. Based on the calculated values, a volcano plot was created with the vertical axis being the reciprocal of the common logarithm of the
p-value (−log
p) and the horizontal axis being lnROR, the natural logarithm of the ROR [
42,
43,
44,
45,
46] (
Figure 2). Volcano plots were used to visually interpret adverse drug events. This type of scatter plot is often used to understand trends in gene expression in microarray data analysis [
47]. If the
p-value is less than 1 × 10
−308 power, it is not calculated by the statistical software, and thus, −log
p is set to 308 as an approximation. Antineoplastic agents with statistically significant associations with adverse events (ROR > 1) [
42,
43,
44,
45,
46,
48] and more than 100 reports were selected as drugs for cluster and principal component analyses.
4.6. Relationships of the Class of Antineoplastic Agents with Taste and Smell Disorders
Based on the ATC classification, antineoplastic agents include alkylating agents (L01A), antimetabolites (L01B), plant alkaloids and other natural products (L01C), cytotoxic antibiotics and related substances (L01D), protein kinase inhibitors (L01E), monoclonal antibodies and antibody drug conjugates (L01F), and other antineoplastic agents (L01X). For each of these categories, we calculated the percentage of antineoplastic agents that are likely to induce taste and smell disorders. In addition, a 2 × 2 contingency table was created for each of the seven categories of antineoplastic agents as described in section 4.5 (
Table 7).
p-values were calculated using Fisher’s exact test to identify significant correlations.
4.7. Creation of Data Tables for Principal Component and Cluster Analyses
A table of the lnROR for each taste and smell disturbance-related adverse reaction to antineoplastic agents (lnROR matrix) was created (
Figure 1). The drugs analyzed were antineoplastic agents significant at
p < 0.05 and ROR > 1 with at least 100 reported cases, as selected in section 4.6. Side effects with fewer than 3000 reports were removed. The seven PTs included it the analysis were dysgeusia, ageusia, taste disorder, anosmia, parosmia, hypogeusia, and hyposmia (
Supplementary Table S2).
4.8. Hierarchical Cluster Analysis
A hierarchical cluster analysis was performed using the tally sheets created in section 4.7. The Ward method was used for the calculation [
26].
4.9. Principal Component Analysis
Similar to the hierarchical cluster analysis, a principal component analysis was performed using the tally sheets created in section 4.7. A correlation coefficient matrix was used for the analysis focusing on principal components 1, 2, and 3.
4.10. Statistical Analysis
Statistical analyses were performed using JMP Pro 16.2 (SAS Institute Inc., Cary, NC, USA). The statistical significance level was set at P < 0.05.
5. Conclusions
Based on the results of this study, several antineoplastic agents were estimated to be likely to induce taste and smell disorders. The characteristics of each antineoplastic agent related to these side effects were also estimated by cluster and principal component analyses. We hope that these findings will serve as a basis for future epidemiological studies and clinical trials, and they will potentially be useful for selecting drugs and monitoring side effects in patients experiencing similar side effects.
Supplementary Materials
The following supporting information can be downloaded at
Preprints.org.
Author Contributions
Conceptualization Y.U.; Methodology Y.U.; Software Y.U.; Validation Y.U. and R.H.; Formal analysis R.H. and Y.U.; Investigation R.H. and Y.U.; Resources R.H. and Y.U.; Data curation R.H. and Y.U.; Writing_original draft preparation R.H.; Writing_reviewing and editing R.H. and Y.U.; Visualization R.H. and Y.U.; Supervision Y.U.; Project administration Y.U.; Funding acquisition Y.U.; All authors have read and agree to the published version of the manuscript.
Funding
This work was partly supported by JSPS KAKENHI Grant Numbers 21K06653, 22K06707.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cohen, J.; Wakefield, C.E.; Laing, D.G. Smell and Taste Disorders Resulting from Cancer and Chemotherapy. Curr. Pharm. Des. 2016, 22, 2253–2263. [CrossRef]
- Hutton, J.L.; Baracos, V.E.; Wismer, W.V. Chemosensory dysfunction is a primary factor in the evolution of declining nutritional status and quality of life in patients with advanced cancer. J. Pain Symptom Manag. 2007, 33, 156–165. [CrossRef]
- Wickham, R.S.; Rehwaldt, M.; Kefer, C.; Shott, S.; Abbas, K.; Glynn-Tucker, E.; Potter, C.; Blendowski, C. Taste changes experienced by patients receiving chemotherapy. Oncol Nurs Forum. 1999, 26, 697–706.
- Comeau, T.B.; Epstein, J.B.; Migas, C. Taste and smell dysfunction in patients receiving chemotherapy: a review of current knowledge. Support Care Cancer. 2001, 9, 575–580. [CrossRef]
- Brisbois, T.D.; de Kock, I.H.; Watanabe, S.M.; Baracos, V.E.; Wismer, W.V. Characterization of chemosensory alterations in advanced cancer reveals specific chemosensory phenotypes impacting dietary intake and quality of life. J Pain Symptom Manage. 2011, 41, 673–683. [CrossRef]
- Tuccori, M.; Lapi, F.; Testi, A.; Ruggiero, E.; Moretti, U.; Vannacci, A.; Bonaiuti, R.; Antonioli, L.; Fornai, M.; Giustarini, G.; Scollo, C.; Corona, T.; Ferrazin, F.; Sottosanti, L.; Blandizzi, C. Drug-induced taste and smell alterations: a case/non-case evaluation of an italian database of spontaneous adverse drug reaction reporting. Drug Saf. 2011, 34, 849–859. [CrossRef]
- Deems, D.A.; Doty, R.L.; Settle, R.G.; Moore-Gillon, V.; Shaman, P.; Mester, A.F.; Kimmelman, C.P.; Brightman, V.J.; Snow, J.B. Jr. Smell and taste disorders, a study of 750 patients from the University of Pennsylvania Smell and Taste Center. Arch Otolaryngol Head Neck Surg. 1991, 117, 519–528. [CrossRef]
- Goodspeed, R.B.; Gent, J.F.; Catalanotto, F.A. Chemosensory dysfunction. Clinical evaluation results from a taste and smell clinic. Postgrad Med. 1987, 81, 251–257, 260. [CrossRef]
- Buttiron Webber, T.; Briata, I.M.; DeCensi, A.; Cevasco, I.; Paleari, L. Taste and Smell Disorders in Cancer Treatment: Results from an Integrative Rapid Systematic Review. Int J Mol Sci. 2023, 24, 2538. [CrossRef]
- Malta, C.E.N.; de Lima Martins, J.O.; Carlos, A.C.A.M.; Freitas, M.O.; Magalhães, I.A.; de Vasconcelos, H.C.A.; de Lima Silva-Fernandes, I.J.; de Barros Silva, P.G. Risk factors for dysgeusia during chemotherapy for solid tumors: a retrospective cross-sectional study. Support Care Cancer. 2022, 30, 313–325. [CrossRef]
- Amézaga, J.; Alfaro, B.; Ríos, Y.; Larraioz, A.; Ugartemendia, G.; Urruticoechea, A.; Tueros, I. Assessing taste and smell alterations in cancer patients undergoing chemotherapy according to treatment. Support Care Cancer. 2018, 26, 4077–4086. [CrossRef]
- Zabernigg, A.; Gamper, E.M.; Giesinger, J.M.; Rumpold, G.; Kemmler, G.; Gattringer, K.; Sperner-Unterweger, B.; Holzner, B. Taste alterations in cancer patients receiving chemotherapy: a neglected side effect? Oncologist. 2010, 15, 913–920. [CrossRef]
- Eravcı, F.C.; Uçar, G.; Özcan, K.M.; Çolak, M.; Ergün, Y.; Açıkgöz, Y.; Ikincioğulları, A.; Uncu, D.; Dere, H.H. The effect of chemotherapy on olfactory function and mucociliary clearance. Support Care Cancer. 2021, 29, 1635–1641. [CrossRef]
- Pugnaloni, S.; Vignini, A.; Borroni, F.; Sabbatinelli, J.; Alia, S.; Fabri, M.; Taus, M.; Mazzanti, L.; Berardi, R. Modifications of taste sensitivity in cancer patients: a method for the evaluations of dysgeusia. Support Care Cancer. 2020, 28, 1173–1181. [CrossRef]
- Riga, M.; Chelis, L.; Papazi, T.; Danielides, V.; Katotomichelakis, M.; Kakolyris, S. Hyposmia: an underestimated and frequent adverse effect of chemotherapy. Support Care Cancer. 2015, 23, 3053–3058. [CrossRef]
- Ikeda, M.; Ikui, A.; Komiyama, A.; Kobayashi, D.; Tanaka, M. Causative factors of taste disorders in the elderly, and therapeutic effects of zinc. J Laryngol Otol. 2008, 122, 155–160. [CrossRef]
- Ottaviano, G.; Savietto, E.; Scarpa, B.; Bertocco, A.; Maculan, P.; Sergi, G.; Martini, A.; Manzato, E.; Marioni, G. Influence of number of drugs on olfaction in the elderly. Rhinology. 2018, 56, 351–357. [CrossRef]
- Search for Ethical Drug Information on the Website of the Pharmaceuticals and Medical Devices Agency (PMDA). Available online: https://www.pmda.go.jp/PmdaSearch/iyakuSearch/ (accessed on 26 June 2024).
- van der Werf, A.; Rovithi, M.; Langius, J.A.E.; de van der Schueren, M.A.E.; Verheul, H.M.W. Insight in taste alterations during treatment with protein kinase inhibitors. Eur J Cancer. 2017, 86, 125–134. [CrossRef]
- Chen, L.; Xu, P.; Xiao, Q.; Chen, L.; Li, S.; Jian, J.M.; Zhong, Y.B. Sunitinib malate inhibits intestinal tumor development in male ApcMin/+ mice by down-regulating inflammation-related factors with suppressing β-cateinin/c-Myc pathway and re-balancing Bcl-6 and Caspase-3. Int Immunopharmacol. 2021, 90, 107128. [CrossRef]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014, 15, 178–196. [CrossRef]
- Zhang, W.; Zhang, H.; Wang, N.; Zhao, C.; Zhang, H.; Deng, F.; Wu, N.; He, Y.; Chen, X.; Zhang, J.; Wen, S.; Liao, Z.; Zhang, Q.; Zhang, Z.; Liu, W.; Yan, Z.; Luu, H.H.; Haydon, R.C.; Zhou, L.; He, T.C. Modulation of β-catenin signaling by the inhibitors of MAP kinase, tyrosine kinase, and PI3-kinase pathways. Int J Med Sci. 2013, 10, 1888–1898. [CrossRef]
- Barlow, L.A. Progress and renewal in gustation: new insights into taste bud development. Development. 2015, 142, 3620–3629. [CrossRef]
- Miura, H.; Kusakabe, Y.; Sugiyama, C.; Kawamatsu, M.; Ninomiya, Y.; Motoyama, J.; Hino, A. Shh and Ptc are associated with taste bud maintenance in the adult mouse. Mech Dev. 2001, 106, 143–145. [CrossRef]
- Hummel, T.; Whitcroft, K.L.; Rueter, G.; Haehner, A. Intranasal vitamin A is beneficial in post-infectious olfactory loss. Eur Arch Otorhinolaryngol. 2017, 274, 2819–2825. [CrossRef]
- Everitt, B.S.; Landau, S.; Leese, M.; Stahl, D. Cluster Analysis, 5th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2011; ISBN 978-0-470-74991-3.
- Jolliffe, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20150202. [CrossRef]
- Maeda, R. JADER from pharmacovigilance point of view. Jpn. J. Pharmacoepidemiol. Yakuzai Ekigaku 2014, 19, 51–56.
- Noguchi, Y.; Tachi, T.; Teramachi, H. Detection algorithms and attentive points of safety signal using spontaneous reporting systems as a clinical data source. Brief. Bioinform. 2021, 22, bbab347. [CrossRef]
- Pariente, A.; Gregoire, F.; Fourrier-Reglat, A.; Haramburu, F.; Moore, N. Impact of safety alerts on measures of disproportionality in spontaneous reporting databases: the notoriety bias. Drug Saf. 2007, 30, 891–898. [CrossRef]
- Hartnell, N.R.; Wilson, J.P. Replication of the Weber effect using postmarketing adverse event reports voluntarily submitted to the United States Food and Drug Administration. Pharmacotherapy. 2004, 24, 743–749. [CrossRef]
- Wang, H.W.; Hochberg, A.M.; Pearson, R.K.; Hauben, M. An experimental investigation of masking in the US FDA adverse event reporting system database. Drug Saf. 2010, 33, 1117–1133. [CrossRef]
- Pariente, A.; Avillach, P.; Salvo, F.; Thiessard, F.; Miremont-Salamé, G.; Fourrier-Reglat, A.; Haramburu, F.; Bégaud, B.; Moore, N.; Association Française des Centres Régionaux de Pharmacovigilance (CRPV). Effect of competition bias in safety signal generation: analysis of a research database of spontaneous reports in France. Drug Saf. 2012, 35, 855–864. [CrossRef]
- Poleksic, A.; Xie, L. Database of adverse events associated with drugs and drug combinations. Sci Rep. 2019, 9, 20025. [CrossRef]
- FDA Adverse Event Reporting System (FAERS). Available online: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-adverse-event-reporting-system-faers (accessed on 26 June, 2024).
- Lumini, A.; Nanni, L. Convolutional Neural Networks for ATC Classification. Curr Pharm Des. 2018, 24, 4007–4012. [CrossRef]
- MedDRA Japanese Maintenance Organization. Available online: https://www.meddra.org/ (accessed on 26 June, 2024).
- Watanabe, H.; Matsushita, Y.; Watanabe, A.; Maeda, T.; Nukui, K.; Ogawa, Y.; Sawa, J.; Maeda, H. Early detection of important safety information. Recent methods for signal detection. Jpn. J. Biomet. 2004, 25, 37–60.
- Ohyama, K.; Sugiura, M. Evaluation of the association between topical prostaglandin F2α analogs and asthma using the JADER database: Comparison with β-blockers. Yakugaku Zasshi. 2018, 138, 559–564. [CrossRef]
- Greenland, S.; Schwartzbaum, J.A.; Finkle, W.D. Problems due to small samples and sparse data in conditional logistic regression analysis. Am. J. Epidemiol. 2000, 151, 531–539. [CrossRef]
- Gravel, C.A.; Douros, A. Considerations on the use of different comparators in pharmacovigilance: A methodological review. Br. J. Clin. Pharmacol. 2023, 89, 2671–2676. [CrossRef]
- Hosoya, R.; Uesawa, Y.; Ishii-Nozawa, R.; Kagaya, H. Analysis of factors associated with hiccups based on the Japanese Adverse Drug Event Report database. PLoS One. 2017, 12, e0172057. [CrossRef]
- Kawabe, A.; Uesawa, Y. Analysis of Corticosteroid-Induced Glaucoma Using the Japanese Adverse Drug Event Reporting Database. Pharmaceuticals. 2023, 16, 948. [CrossRef]
- Okunaka, M.; Kano, D.; Matsui, R.; Kawasaki, T.; Uesawa, Y. Comprehensive Analysis of Chemotherapeutic Agents That Induce Infectious Neutropenia. Pharmaceuticals. 2021, 14, 681. [CrossRef]
- Kan, Y.; Nagai, J.; Uesawa, Y. Evaluation of antibiotic-induced taste and smell disorders using the FDA adverse event reporting system database. Sci Rep. 2021, 11, 9625. [CrossRef]
- Nakao, Y.; Asada, M.; Uesawa, Y. Comprehensive Study of Drug-Induced Pruritus Based on Adverse Drug Reaction Report Database. Pharmaceuticals. 2023, 16, 1500. [CrossRef]
- Chen, J.J.; Wang, S.J.; Tsai, C.A.; Lin, C.J. Selection of differentially expressed genes in microarray data analysis. Pharmacogenomics J. 2007, 7, 212–220. Epub 2006 Aug 29. [CrossRef]
- Van Puijenbroek, E.P.; Bate, A.; Leufkens, H.G.M.; Lindquist, M.; Orre, R.; Egberts, A.C. A Comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiol. Drug. Saf. 2002, 11, 3–10. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).