Submitted:
08 July 2024
Posted:
10 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
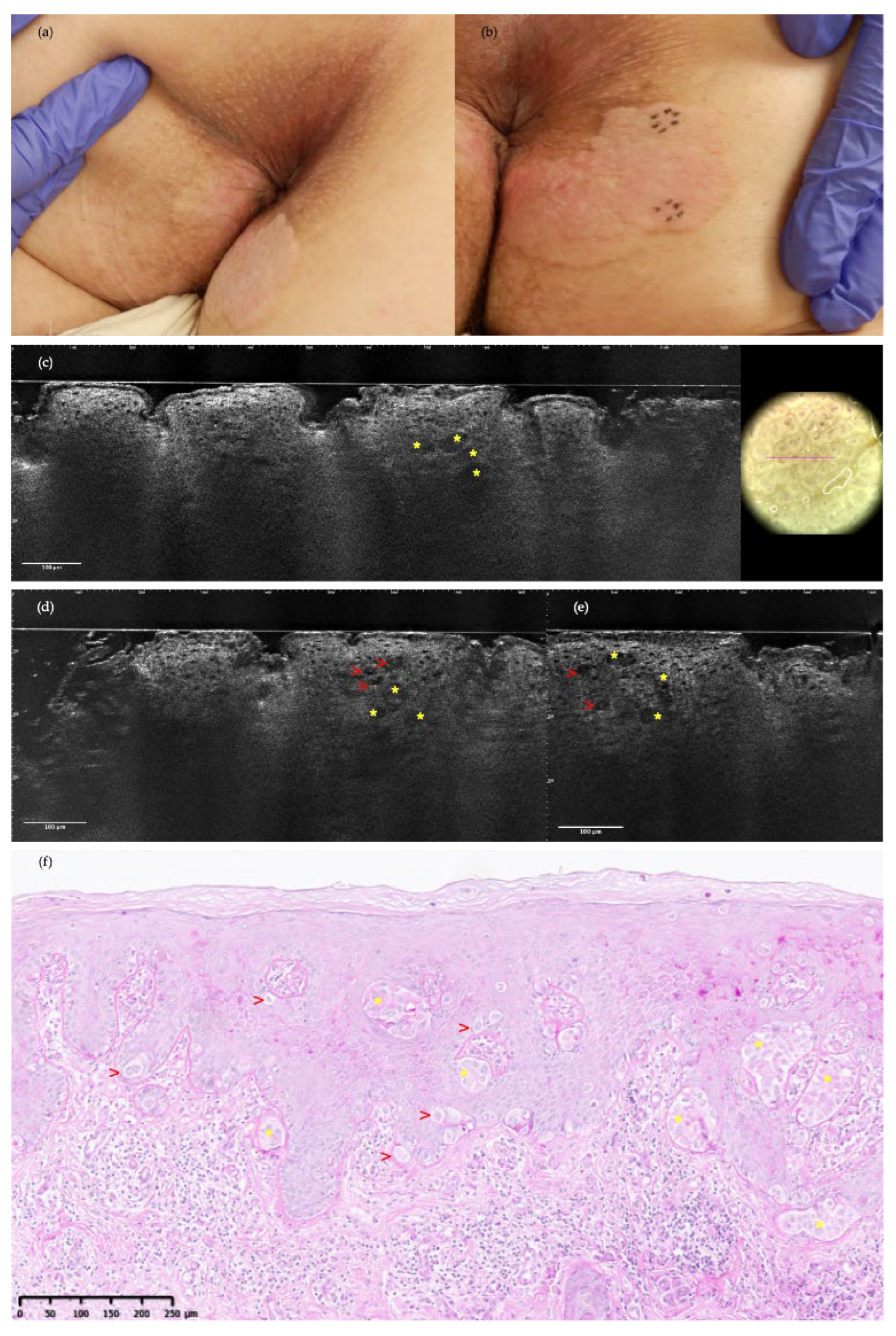
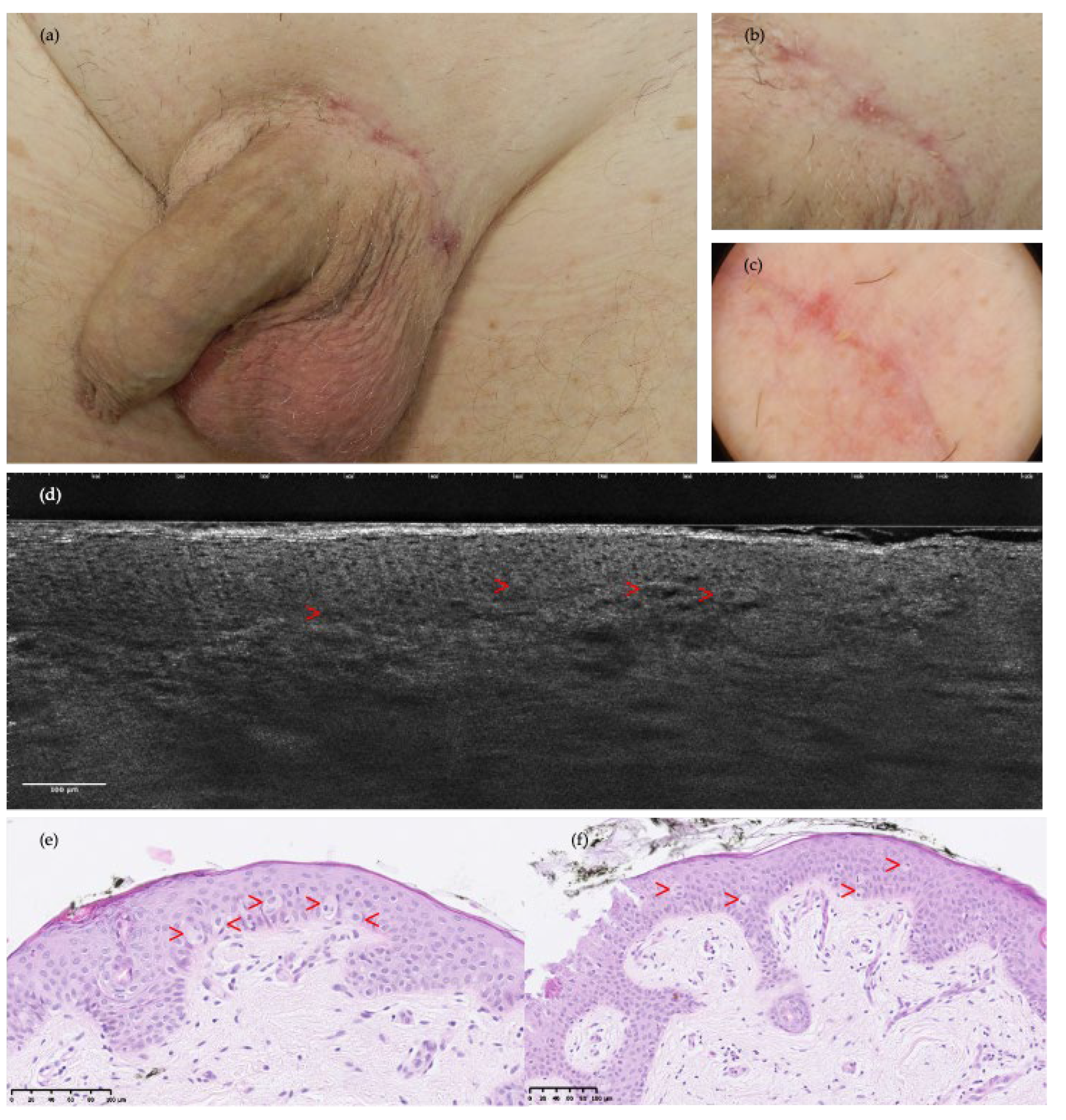
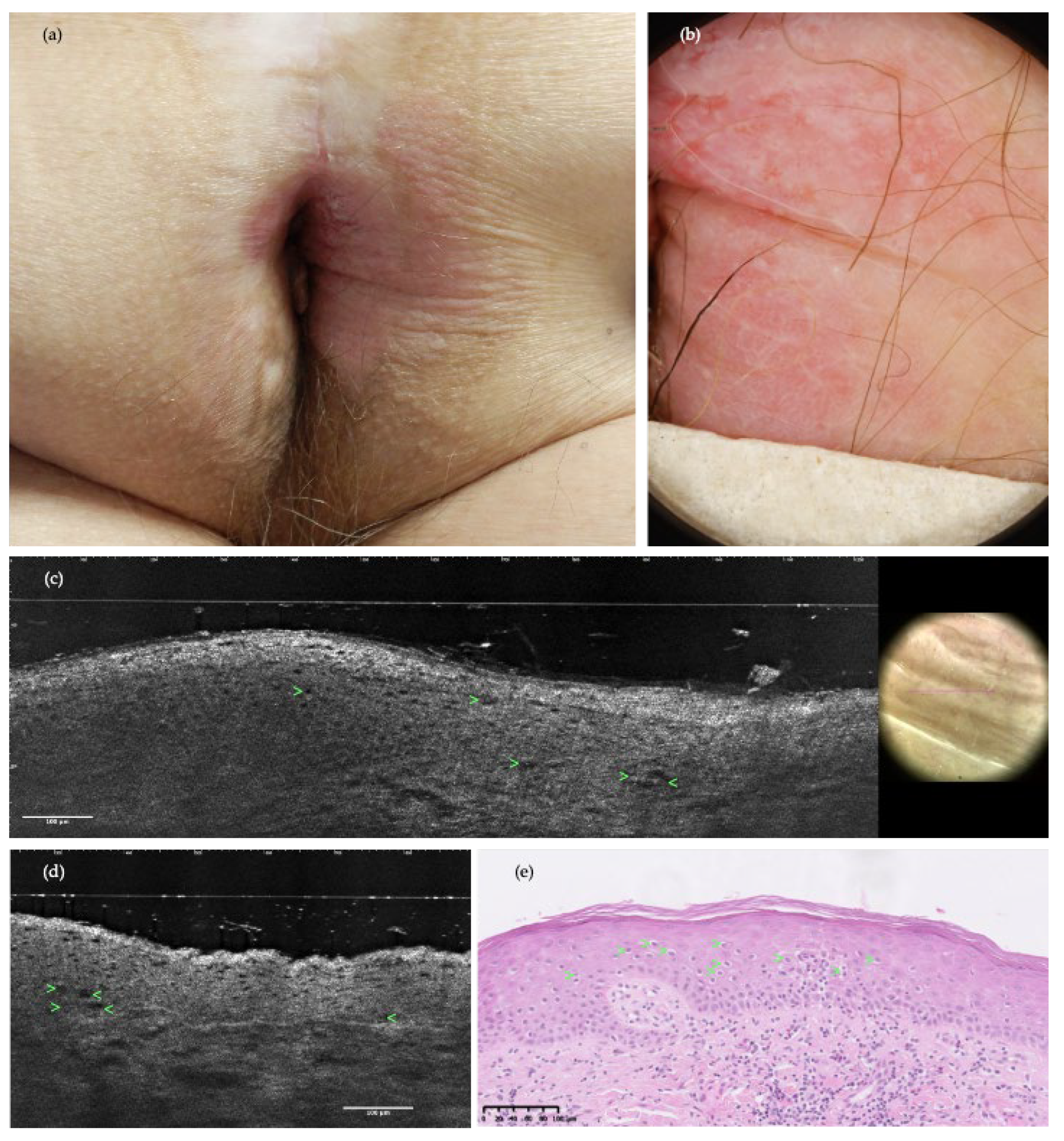
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crocker HR Paget’s Disease Affecting the Scrotum and the Penis. . Trans Pathol Soc 1888, 40, 187–191.
- Morris, C.R.; Hurst, E.A. Extramammary Paget Disease: A Review of the Literature—Part I: History, Epidemiology, Pathogenesis, Presentation, Histopathology, and Diagnostic Work-up. Dermatol. Surg. 2020, 46, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, J.; Flanagan, A.M. Mammary and extramammary Paget’s disease. J. Clin. Pathol. 2000, 53, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Kanitakis, J. Mammary and extramammary Paget’s disease. J. Eur. Acad. Dermatol. Venereol. 2007, 21, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Wagner, G.; Sachse, M.M. Extramammary Paget disease – clinical appearance, pathogenesis, management. JDDG: J. der Dtsch. Dermatol. Ges. 2011, 9, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Dinnes, J.; Deeks, J.J.; Chuchu, N.; di Ruffano, L.F.; Matin, R.N.; Thomson, D.R.; Wong, K.Y.; Aldridge, R.B.; Abbott, R.; Fawzy, M.; et al. Dermoscopy, with and without visual inspection, for diagnosing melanoma in adults. Cochrane Database Syst. Rev. 2018, 2018, CD011902. [Google Scholar] [CrossRef] [PubMed]
- Dinnes, J.; Deeks, J.J.; Chuchu, N.; Matin, R.N.; Wong, K.Y.; Aldridge, R.B.; Durack, A.; Gulati, A.; Chan, S.A.; Johnston, L.; et al. Visual inspection and dermoscopy, alone or in combination, for diagnosing keratinocyte skin cancers in adults. Cochrane Database Syst. Rev. 2018, 2018, CD011901. [Google Scholar] [CrossRef]
- Adya, K.A. Dermoscopy: An Overview of the Principles, Procedure and Practice. In Dermoscopy - Histopathology Correlation; Springer Singapore: Singapore, 2021; pp. 1–13. [Google Scholar]
- Rosendahl, C.; Tschandl, P.; Cameron, A.; Kittler, H. Diagnostic accuracy of dermatoscopy for melanocytic and nonmelanocytic pigmented lesions. J. Am. Acad. Dermatol. 2011, 64, 1068–1073. [Google Scholar] [CrossRef] [PubMed]
- Bazzacco, G.; Zalaudek, I.; Errichetti, E. Dermoscopy to differentiate clinically similar inflammatory and neoplastic skin lesions. Ital. J. Dermatol. Venereol. 2024, 159, 135–145. [Google Scholar] [CrossRef]
- Bayan, C.Y.; Khanna, T.; Rotemberg, V.; Samie, F.H.; Zeitouni, N.C. A review of non-invasive imaging in extramammary Paget’s disease. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 1862–1873. [Google Scholar] [CrossRef]
- Hirokawa, D.; Lee, J.B. Dermatoscopy: An overview of subsurface morphology. Clin. Dermatol. 2011, 29, 557–565. [Google Scholar] [CrossRef]
- Mun, J.-H.; Park, S.-M.; Kim, G.-W.; Song, M.; Kim, H.-S.; Ko, H.-C.; Kim, B.-S.; Kim, M.-B. Clinical and dermoscopic characteristics of extramammary Paget disease: a study of 35 cases. Br. J. Dermatol. 2015, 174, 1104–1107. [Google Scholar] [CrossRef]
- Que, S.K.T.; Fraga-Braghiroli, N.; Grant-Kels, J.M.; Rabinovitz, H.S.; Oliviero, M.; Scope, A. Through the looking glass: Basics and principles of reflectance confocal microscopy. J. Am. Acad. Dermatol. 2015, 73, 276–284. [Google Scholar] [CrossRef]
- Guitera, P.; Scolyer, R.; Gill, M.; Akita, H.; Arima, M.; Yokoyama, Y.; Matsunaga, K.; Longo, C.; Bassoli, S.; Bencini, P.; et al. Reflectance confocal microscopy for diagnosis of mammary and extramammary Paget’s disease. J. Eur. Acad. Dermatol. Venereol. 2012, 27, e24–e29. [Google Scholar] [CrossRef]
- Guida, S.; Longhitano, S.; Ardigò, M.; Pampena, R.; Ciardo, S.; Bigi, L.; Mandel, V.D.; Vaschieri, C.; Manfredini, M.; Pezzini, C.; et al. Dermoscopy, confocal microscopy and optical coherence tomography features of main inflammatory and autoimmune skin diseases: A systematic review. Australas. J. Dermatol. 2021, 63, 15–26. [Google Scholar] [CrossRef]
- Pan, Z.-Y.; Liang, J.; Zhang, Q.-A.; Lin, J.-R.; Zheng, Z.-Z. In vivo reflectance confocal microscopy of extramammary Paget disease: Diagnostic evaluation and surgical management. J. Am. Acad. Dermatol. 2012, 66, e47–e53. [Google Scholar] [CrossRef]
- Tan, L.; Huang, J.; Zhang, Y.; Zeng, L.; Tong, X.; Gao, L.; Zeng, J. Evaluation of in vivo reflectance confocal microscopy in the diagnosis of extramammary Paget’s disease. Microsc. Res. Tech. 2021, 85, 283–289. [Google Scholar] [CrossRef]
- Yélamos, O.; Hibler, B.P.; Cordova, M.; Hollmann, T.J.; Kose, K.; Marchetti, M.A.; Myskowski, P.L.; Pulitzer, M.P.; Rajadhyaksha, M.; Rossi, A.M.; et al. Handheld Reflectance Confocal Microscopy for the Detection of Recurrent Extramammary Paget Disease. JAMA Dermatol. 2017, 153, 689–693. [Google Scholar] [CrossRef]
- Shahriari, N.; Grant-Kels, J.M.; Rabinovitz, H.; Oliviero, M.; Scope, A. Reflectance confocal microscopy. J. Am. Acad. Dermatol. 2020, 84, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Welzel, J. Optical coherence tomography in dermatology: a review. Ski. Res. Technol. 2001, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Escobar, P.F.; Belinson, J.L.; White, A.; Shakhova, N.M.; Feldchtein, F.I.; Kareta, M.V.; Gladkova, N.D. Diagnostic efficacy of optical coherence tomography in the management of preinvasive and invasive cancer of uterine cervix and vulva. Int. J. Gynecol. Cancer 2004, 14, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Y.; Wu, Y. In vivo characterization of extramammary Paget’s disease by ultra-high cellular resolution optical coherence tomography. Ski. Res. Technol. 2020, 27, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Bard, R.L. High-Frequency Ultrasound Examination in the Diagnosis of Skin Cancer. Dermatol. Clin. 2017, 35, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Guo, L.; Yan, J.; Wang, Q.; Li, X.; Li, M.; Zhu, R.; Yang, W.; Xu, H. Ultrasound Biomicroscopy and High-Frequency Ultrasound for Evaluating Extramammary Paget Disease With Pathologic Correlation. J. Ultrasound Med. 2019, 38, 3229–3237. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Gong, X.; Wang, Q.; Wang, L.; Xu, H.; Guo, L. High-Frequency Ultrasound for Evaluation of the Pathological Invasion Level of Extramammary Paget Disease. J. Ultrasound Med. 2021, 41, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Dubois, A.; Levecq, O.; Azimani, H.; Siret, D.; Barut, A.; Suppa, M.; del Marmol, V.; Malvehy, J.; Cinotti, E.; Rubegni, P.; et al. Line-field confocal optical coherence tomography for high-resolution noninvasive imaging of skin tumors. J. Biomed. Opt. 2018, 23, 106007–9. [Google Scholar] [CrossRef] [PubMed]
- Suppa, M.; Palmisano, G.; Tognetti, L.; Lenoir, C.; Cappilli, S.; Fontaine, M.; Cano, C.O.; Diet, G.; Perez-Anker, J.; Schuh, S.; et al. Line-field confocal optical coherence tomography in melanocytic and non-melanocytic skin tumors. Ital. J. Dermatol. Venereol. 2023, 158, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Latriglia, F.; Ogien, J.; Tavernier, C.; Fischman, S.; Suppa, M.; Perrot, J.-L.; Dubois, A. Line-Field Confocal Optical Coherence Tomography (LC-OCT) for Skin Imaging in Dermatology. Life 2023, 13, 2268. [Google Scholar] [CrossRef] [PubMed]
- Gust, C.; Schuh, S.; Welzel, J.; Daxenberger, F.; Hartmann, D.; French, L.E.; Ruini, C.; Sattler, E.C. Line-Field Confocal Optical Coherence Tomography Increases the Diagnostic Accuracy and Confidence for Basal Cell Carcinoma in Equivocal Lesions: A Prospective Study. Cancers 2022, 14, 1082. [Google Scholar] [CrossRef]
- Cinotti, E.; Brunetti, T.; Cartocci, A.; Tognetti, L.; Suppa, M.; Malvehy, J.; Perez-Anker, J.; Puig, S.; Perrot, J.L.; Rubegni, P. Diagnostic Accuracy of Line-Field Confocal Optical Coherence Tomography for the Diagnosis of Skin Carcinomas. Diagnostics 2023, 13, 361. [Google Scholar] [CrossRef]
- Donelli, C.; Suppa, M.; Tognetti, L.; Perrot, J.L.; Calabrese, L.; Pérez-Anker, J.; Malvehy, J.; Rubegni, P.; Cinotti, E. Line-Field Confocal Optical Coherence Tomography for the Diagnosis of Skin Carcinomas: Real-Life Data over Three Years. Curr. Oncol. 2023, 30, 8853–8864. [Google Scholar] [CrossRef] [PubMed]
- Di Stefani, A.; Fionda, B.; Cappilli, S.; Tagliaferri, L.; Peris, K. Extramammary Paget disease imaged by LC-OCT and treated with radiotherapy. Int. J. Dermatol. 2023, 62, E503–E505. [Google Scholar] [CrossRef] [PubMed]
- Delpuech, A.; Battistella, M.; Tavernier, C.; El Zeinaty, P.; Lebbé, C.; Baroudjian, B. Intérêt de la line-field confocal optical coherence tomography (LC-OCT) pour le diagnostic non invasif de la maladie de Paget. Ann. Dermatol. Vénéréologie–FMC 2023, 3, A182. [Google Scholar] [CrossRef]
- Fukuda, K.; Funakoshi, T. Metastatic Extramammary Paget’s Disease: Pathogenesis and Novel Therapeutic Approach. Front. Oncol. 2018, 8, 38. [Google Scholar] [CrossRef]
- Morris, C.R.; Hurst, E.A. Extramammary Paget’s Disease: A Review of the Literature Part II: Treatment and Prognosis. Dermatol. Surg. 2020, 46, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Bansal, D.; A Bowman, C. Extramammary Paget’s disease masquerading as lichen sclerosus. Int. J. STD AIDS 2004, 15, 141–142. [Google Scholar] [CrossRef] [PubMed]
- Papoutsis, D.; Antonakou, A.; Sahu, B. Vulvar Paget’s disease presenting on the background of clinically diagnosed lichen sclerosus. Hippokratia 2018, 22, 94–94. [Google Scholar] [PubMed]
- Kantere, D.; Neittaanmäki, N.; Maltese, K.; Larkö, A.-M.W.; Tunbäck, P. Exploring reflectance confocal microscopy as a non-invasive diagnostic tool for genital lichen sclerosus. Exp. Ther. Med. 2022, 23, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Huisman, B.W.; Pagan, L.; Naafs, R.G.B.; Voorde, W.M.T.; Rissmann, R.; Piek, J.M.; Damman, J.; Juachon, M.J.M.; Osse, M.B.; der Kolk, T.N.-V.; et al. Dermatoscopy and Optical Coherence Tomography in Vulvar High-Grade Squamous Intraepithelial Lesions and Lichen Sclerosus: A Prospective Observational Trial. J. Low. Genit. Tract Dis. 2023, 27, 255–261. [Google Scholar] [CrossRef]
- Wang, Y.; Hao, J.; Liu, J. High-frequency ultrasound assessment of vulvar lichen sclerosus treated with photodynamic therapy. Photodiagnosis Photodyn. Ther. 2023, 41, 103277. [Google Scholar] [CrossRef]
- Verzì, A.; Broggi, G.; Micali, G.; Sorci, F.; Caltabiano, R.; Lacarrubba, F. Line-field confocal optical coherence tomography of psoriasis, eczema and lichen planus: a case series with histopathological correlation. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 1884–1889. [Google Scholar] [CrossRef] [PubMed]
- Regauer, S.; Liegl, B.; Reich, O. Early vulvar lichen sclerosus: a histopathological challenge. Histopathology 2005, 47, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Cappilli, S.; Dejonckheere, G.; Hajjar, N.; Cinotti, E.; Tognetti, L.; Perez-Anker, J.; Rubegni, P.; Puig, S.; Malvehy, J.; Perrot, J.L.; et al. Line-field confocal optical coherence tomography: a case on the importance of full-lesion examination for basal cell carcinoma. Int. J. Dermatol. 2022, 61, E248–E250. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




